Abstract
Background.
Whether cytomegalovirus (CMV) DNA exists in plasma as virion-associated or free DNA is uncertain.
Methods.
An assay combining DNase I digestion and CMV quantitative polymerase chain reaction (DNase-CMV-qPCR) was developed to differentiate free naked DNA from virion DNA. One hundred three frozen and 10 fresh CMV DNA–positive plasma samples from solid-organ transplant recipients (SOTRs) were tested. Three sets of paired qPCR (P-qPCR) assays with amplicons of variable length were used to study CMV DNA fragmentation in 20 SOTR plasma samples, viral stocks (Towne, Merlin, AD169) and the first World Health Organization (WHO) international standard (IS) for CMV DNA.
Results.
In all plasma samples, 98.8%–100% of CMV DNA was free DNA; this was the only form in 93 of 103 (90.3%) frozen and all 10 fresh samples tested using DNase-CMV-qPCR. Low levels of virion CMV DNA were found in 10 of 103 (9.7%) samples with higher total DNA load. Cytomegalovirus DNA results were highly reproducible for 3 CMV virus stocks and WHO IS (P > .80), tested by three sets of paired q-PCR. However, for the 20 SOTR plasma samples, the smaller amplicon assay result was 2.6-fold, 3.4-fold, and 6.5-fold higher than the longer amplicion result (P < .001).
Conclusions.
Cytomegalovirus DNA in SOTR plasma is almost exclusively free DNA, highly fragmented, and not virion associated.
Keywords: cytomegalovirus, free CMV DNA, virion-associated DNA, CMV DNA fragmentation, plasma CMV DNA, DNase I, Quantitative PCR.
Plasma is a commonly used matrix for the quantitative measurement of cytomegalovirus (CMV) DNA in both clinical and research settings. Results from quantitative CMV nucleic acid testing (QNAT) assays, which often use real-time quantitative polymerase chain reaction (qPCR) technology, have been used successfully to prevent and diagnose CMV disease and to monitor the efficacy of antiviral treatment in solid-organ transplant recipients (SOTRs) and hematopoietic stem-cell transplant recipients [1, 2]. Dynamic changes in CMV DNA levels, usually measured in whole blood (WB), to which the plasma compartment contributes, have been used to study CMV replication kinetics in vivo [3, 4]. Because blood donors seroconverting to CMV have low but detectable CMV DNA present in plasma for long time periods, investigators have suggested that CMV DNA–positive plasma might be a source of transfusion-transmitted CMV infection when using leukoreduced blood products [5, 6]. Although the biologic form of CMV DNA in plasma is uncertain, clinicians and investigators interpreting results often assume that CMV DNA is encapsidated (ie, virion associated) in this cell-free compartment [1, 3, 6].
However, more than a decade ago, Boom et al studied CMV DNA fragment sizes in the plasma of 3 kidney SOTRs and concluded that the CMV DNA was highly fragmented and unlikely associated with infectious virions [7]. In a recent study that examined result concordance among CMV QNAT assays used to test plasma samples from SOTRs, assays with smaller amplicons gave quantitatively higher results than those with larger amplicons [8], consistent with the hypothesis that CMV DNA in clinical plasma samples is fragmented.
Cytomegalovirus DNA may be present in human plasma in a number of biologic forms, including naked DNA, DNA complexed with chromatin or nucleosomes or integrated into vesicles (exosomes or apoptotic bodies), as well as encapsidated in infectious or noninfectious virions. We used 2 types of assays to study the biologic form of CMV DNA in SOTR plasma. The first combined DNase I treatment and a qPCR assay to differentiate free CMV DNA from encapsidated CMV DNA; the second used paired qPCR assays with amplicons of variable length to assess CMV DNA fragmentation in plasma. We report the results of these studies.
METHODS
Tissue-Cultured Cytomegalovirus Strains
Viral stocks that contained both free CMV DNA and infectious/noninfectious CMV DNA in virions were prepared from 3 CMV strains (Merlin, AD169, Towne) cultured in MRC-5 cells [9]. The first World Health Organization (WHO) international standard (IS) for CMV DNA (5 × 106 IU/mL) was obtained from National Institute for Biological Standards and Control (Ridge, Hertfordshire, UK) [10]. Cytomegalovirus DNA purified from the 3 viral stocks and the WHO IS using the QIAamp DNA mini kit (Qiagen, Hilden, Germany) was the source of noninfectious free CMV DNA for spiking experiments and for CMV DNA fragmentation experiments.
Development and Optimization of the DNase I Digestion and CMV Quantitative Polymerase Chain Reaction
DNase I enzymatic digestion [11] was combined with an in-house–developed CMV-qPCR [12] to develop a quantitative assay to differentiate free CMV DNA from CMV DNA encapsidated in virions. The amount of free DNA was defined as the difference between results reported for the total CMV DNA (untreated) and the virion DNA (DNase I treated) aliquots of each sample. DNase I supplied by different vendors, DNA input doses, incubation times, and reaction volumes were evaluated to optimize DNase digestion. D-Phenylalanyl-L-prolyl-L-arginine chloromethyl ketone (PPACK), a direct thrombin inhibitor, was used to inhibit ethylenediaminetetraacetic acid (EDTA) plasma clotting during digestion. A commercial salmon testes DNA (sDNA; Sigma Aldrich, Ontario, Canada) was spiked into samples as an internal inhibitor control and as a quality control for DNA extraction and PCR performance. The optimized DNase treatment reaction mixture (200 μL) contained 20 μL of 10X reaction buffer, 10 μL of DNase I (Promega, Madison, WI), 50 μL of plasma, 5 × 104 copies of sDNA, and 1 μg of PPACK (EMD Millipore, Darmstadt, Germany). Enzymatic digestion was carried out at 37°C overnight (16–18 hours) with a boost of 10 μL of DNase added at 2 hours of incubation. The undigested sample was processed in parallel, omitting the DNase and the reaction buffer from the mixture. The QIAamp DNA mini kit was used for DNA extraction. Cytomegalovirus DNA and the sDNA (both reported as genome copies per milliliter) were quantified as previously described [12,13]. The CMV assay’s limit of detection and limit of quantification were 1.69 and 2.69 log10 copies/mL, respectively. The conversion factor for the assay is 5.65 genome copies/mL = 1 IU/mL.
Validation of the DNase I Digestion and CMV Quantitative Polymerase Chain Reaction
The completeness of free DNA degradation by DNase was evaluated by spiking known amounts of CMV DNA (extracted from Merlin virus stock) into 10 CMV DNA–negative SOTR plasma samples, resulting in CMV DNA concentrations of 6.0, 7.0, and 8.0 log10 copies/mL that were tested using the DNase-CMV-qPCR assay. In addition, serial dilutions (1:4, 1:20, 1:100, 1:500) of the 3 virus stocks (Merlin, AD169, Towne) were used to test the reproducibility of the DNase-CMV-qPCR assay at different virion concentrations.
Frozen Towne stock was thawed and retested after 2 years of storage at −70oC. To assess whether virions could be disrupted during the period between venipuncture and plasma separation, 5 μL of 1:5 diluted Towne virus stock (9.90 log10 copies/mL) was spiked into 500 μL of EDTA WB aliquots from a single CMV-negative SOTR and then stored at 4°C. Plasma was processed from the spiked WB at days 0, 1, and 2, and tested in triplicate.
To evaluate the impact of freeze/thaw (F/T) cycles and different matrices, the 3 CMV virus stocks were spiked into CMV-negative plasma and cell-culture medium (minimal essential medium, 10% fetal bovine serum; 1:100 final) and then subjected to 4 F/T cycles. Results were compared with those for samples stored at 4°C.
Testing of Clinical Plasma Samples Using the DNase I Digestion and CMV Quantitative Polymerase Chain Reaction
A total of 103 residual plasma samples (group 1), which had been stored at −70oC and which had tested CMV DNA– positive at the Provincial Laboratory for Public Health, Edmonton, Canada, using the CMV-qPCR assay [12], were thawed and tested using the DNase-CMV-qPCR. These samples were collected as EDTA WB from 20 SOTRs as part of routine clinical care. To evaluate the impact of freezer storage on clinical samples, an additional 10 fresh plasma samples (group 2) with CMV DNA levels ranging 2.80–5.89 log10 copies/mL collected from 10 SOTRs were each aliquoted into 2 tubes. One aliquot was stored at 4°C (fresh); the other aliquot was stored at −70°C for >24 hours (frozen). Fresh and frozen samples were assayed in parallel using the DNase-CMV-qPCR.
Characteristics of group 1 and group 2 patients (allograft type, pretransplant donor [D]/recipient [R] CMV serostatus) and samples (antivial therapy at the time of collection and storage time) are summarized in Table 1.
Table 1.
Characteristics of Solid Organ Transplant Patients and Plasma Samples
| Patient information | Plasma sample information | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Median (range) age, y; Sex | CMV donor (D) / recipient (R) serostatus | Allograft type | No. of sample tested | Median (range) time after transplant, mo | Median (range) sample numbers/ patient | Median (range) CMV DNA load, log10 copies/mL |
Median (range) storage time, y | Receiving antiviral therapy, no. (%)a | |
| Group1 n = 20 |
57 (8–76) F = 6 M = 14 |
D+/R−: n = 10; D+/R+: n = 7; D−/R+: n = 2; D−/R−: n = 1 |
Kidney: n = 9; Lung: n = 6; Heart: n = 3; Liver: n = 2 |
n = 103 | 4 (1–6) | 5 (3–9) | 4.46 (2.97–6.99) |
4.5 (1–7) | 70 (68%) (40 R− and 30 R+) |
| Group 2 n = 10 |
60 (3–69) F = 6 M = 4 |
D+/R−: n = 6; D+/R+: n = 3; D−/R+: n = 1 |
Kidney: n = 2; Lung: n = 5; Liver: n = 2; Heart: n = 1 |
n = 10 | 6 (2–72) | 1 | 3.26 (2.80–5.89) |
Not applicable | 5 (50%) (4 R− and 1 R+) |
| Group 3 n = 12 |
55 (4–73) F = 7 M = 6 |
D+/R−: n = 7; D+/R+: n = 3; D−/R−: n = 1; D−/R+: n = 1 |
Kidney: n = 6; Lung: n = 3; Heart: n = 2; Liver: n = 1 |
n = 20 | 12 (2–54) | 1 (1–3) | 6.00 (4.88–6.40) |
1.5 (0.1–6) | 11 (55%) (6 R− and 5 R+) |
aAt the time of sample collection, the patient was on intravenous ganciclovir, intravenous foscarnet, or oral valganciclovir.
Development of Paired qPCR Assays With Variable Amplicon Sizes for Measurement of Fragmentation
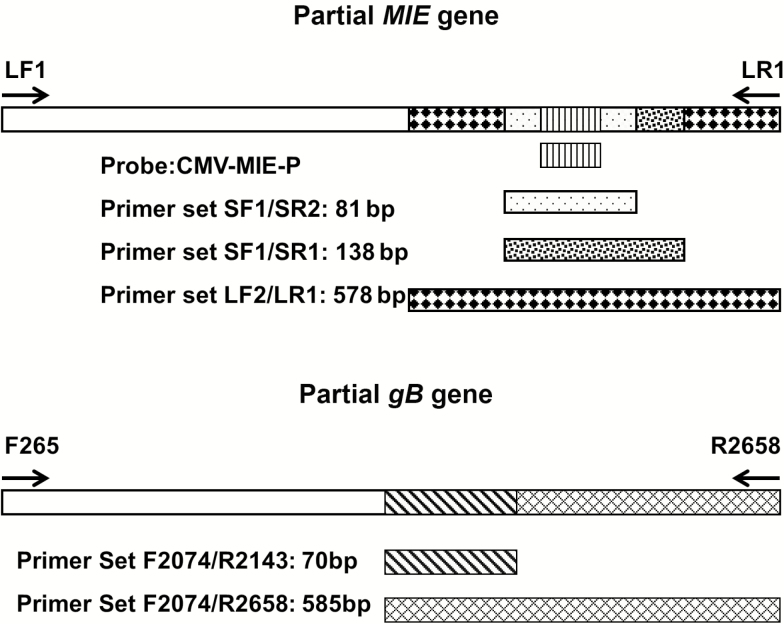
Three paired qPCR assays (P-qPCRs) were designed so that the 2 sets of primers within each assay pair generated a long amplicon and an interposed short amplicon from the same gene. The amplicon sizes and target genes for the 3 P-qPCRs are as follows: P-qPCR-1, TaqMan qPCR detecting 81-bp/138-bp amplicons of the major immediate-early (MIE) gene; P-qPCR-2, SYBR Green qPCR detecting 138-bp/578-bp amplicons of the MIE gene [7] ; and P-qPCR-3, SYBR Green qPCR detecting 70-bp/585-bp amplicons of glycoprotein B (gB) gene. Details regarding the primers and probes used are summarized in Figure 1 and Supplementary Table 1. To ensure that the assays within each pair demonstrated the same sensitivity for CMV DNA detection and similar PCR amplification efficiency despite the differences in amplicon size, results generated using each pair were compared with serial 10-fold dilutions of 2 nonfragmented lengths of CMV DNA (1640 bp and 2394 bp) amplified from the MIE and gB genes and purified from agarose gel. Dilutions of these fragments were also used as calibrators to allow reporting of quantitative results.
Figure 1.
Schematic chart of the design of the different primer pairs for the paired quantitative polymerase chain reaction assays. For MIE gene, LF1/LR1 = 1640 bp, LF2/LR1 = 578 bp, SF1/SR1 = 138 bp, SF1/SR2 = 81 bp; probe was located in between SF1 and SR2. For gB gene, F265/R2658 = 2394 bp, F2074/R2658 = 585 bp, F2074/R2143 = 70 bp.
Testing of Clinical Plasma Samples and Viral Stocks for Fragmentation
Twenty residual plasma samples (group 3), stored at −70oC with high CMV viral load (VL) (>5.0 log10 copies/mL) collected from 12 SOTRs were tested using the 3 P-qPCRs. Characteristics of group 3 patients and samples are summarized in Table 1. In addition, a fresh high CMV VL clinical plasma sample was tested using the 3 P-qPCRs in the same run as a frozen high CMV VL clinical plasma sample and frozen Merlin-spiked plasma. Characteristics of these patients and samples are summarized in Table 2.
Table 2.
Results of Testing a Fresh Plasma Sample and a Frozen Plasma Sample From 2 Solid-Organ Transplant Recipients Compared With a Control Sample Using the 3 Paired Quantitative Polymerase Chain Reaction Assays
| Sample type | Patient/ sample | Sample storage | P-qPCR-1 (Taqman MIE), log10 copies/mL | P-qPCR-2 (SYBR MIE), log10 copies/mL | P-qPCR-3 (SYBR gB), log10 copies/mL | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 81 bp |
138 bp |
Log10 differencea | 138 bp |
578 bp |
Log10 difference | 70 bp |
585 bp |
Log10 difference | |||
| Fresh plasma | D+/R− 11 months after kidney transplant/no antiviral therapy |
4oC 48 h |
6.78 | 5.53 | 1.25 | 6.33 | 4.79 | 1.54 | 6.92 | 5.44 | 1.48 |
| Frozen plasma | D+/R− 3 months after multivisceral transplant/no antiviral therapy |
−70oC 3 months |
6.56 | 6.36 | 0.20 | 6.17 | 5.95 | 0.22 | 6.71 | 5.79 | 0.92 |
| Frozen control | CMV negative plasma spiked with Merlin virus stock | −70oC 6 months |
5.43 | 5.37 | 0.06 | 5.21 | 5.27 | –0.06 | 5.57 | 5.41 | 0.16 |
Abbreviations: CMV, cytomegalovirus; D, donor; P-qPCR, paired quantitative polymerase chain reaction; R, recipient.
alog10 difference = smaller amplicon qPCR measurement result - larger amplicon qPCR measurement result.
Cytomegalovirus DNA extracted from the 3 virus stocks and the WHO IS was also tested using the 3 P-qPCRs. The impact of matrix and F/T cycles on CMV DNA fragmentation was assessed by comparing results of the 3 P-qPCRs for spiked samples prepared as described above (DNase-CMV-qPCR).
Ethics Review
The use of the clinical samples for these studies was approved by the University of Alberta Human Research Ethics Review Board (project 00042807).
Statistical Methods
All CMV DNA measurement results were converted to log10 copies per milliliter before analysis. Results were compared using analysis of variance (ANOVA) and t test where appropriate; P < .05 was the threshold for significance. Statistical testing was performed using SPSS v21 (Chicago, IL).
RESULTS
Validation of the DNase I Digestion and CMV Quantitative Polymerase Chain Reaction
Purified free CMV DNA spiked into CMV DNA–negative plasma (final concentration 6.0 log10 copies/mL) was completely degraded. When larger amounts were spiked (final concentration 7.0–8.0 log10 copies/mL), residual detectable but nonquantifiable CMV DNA was found in 1 of 10 plasma samples tested. The completeness of free CMV DNA degradation by DNase in our assay was calculated to be 100% at CMV DNA concentrations ≤6.0 log10 copies/mL and 99.97%–100% at concentrations >7.0 log10 copies/mL in 1:4 diluted plasma.
The mean ± standard deviation (SD) of the proportions of DNase-resistant CMV DNA (assumed to be virion associated) across all serial dilutions (1:4, 1:20, 1:100, 1:500) of Towne, AD169, and Merlin stocks was 76.2% ±10.6%, 49.4% ± 6.1%, and 36.5% ± 7.7%, respectively. These proportions suggest the presence of a significant amount of both free CMV DNA and virion CMV DNA in these stocks. As indicated by the SD, the DNase-resistant proportion of CMV DNA was reproducible in serial dilutions, confirming the presence of excess enzyme (DNase) over substrate (DNA) and that the DNase-resistant CMV DNA is likely encapsidated in virions.
Delays in separating plasma from WB over 48 hours did not impact the amount of virion CMV DNA detected. The means ± SDs of virion CMV DNA detected in plasma processed on day 0, day 1, and day 2 from Towne-spiked CMV DNA–negative WB were 6.38 ± 0.07, 6.33 ± 0.17, and 6.34 ± 0.05 log10 copies/mL, respectively (ANOVA, P > .80).
No significant difference was found in total CMV DNA (9.92 ± 0.06 vs 9.62 ± 0.07 log10 copies/mL) and virion CMV DNA (9.80 ± 0.10 vs 9.27 ± 0.05 log10 copies/mL) in the Towne stock over 2 years of storage at −70°C (t test, P > .05).
Virion CMV DNA quantitation was not significantly impacted by the matrix (culture medium vs plasma) or after 4 F/T cycles when these matrices were spiked with the 3 CMV virus stocks (ANOVA; P > .80) (Supplementary Table 2).
Biological form of Cytomegalovirus DNA in Solid-Organ Transplant Recipient Plasma Samples
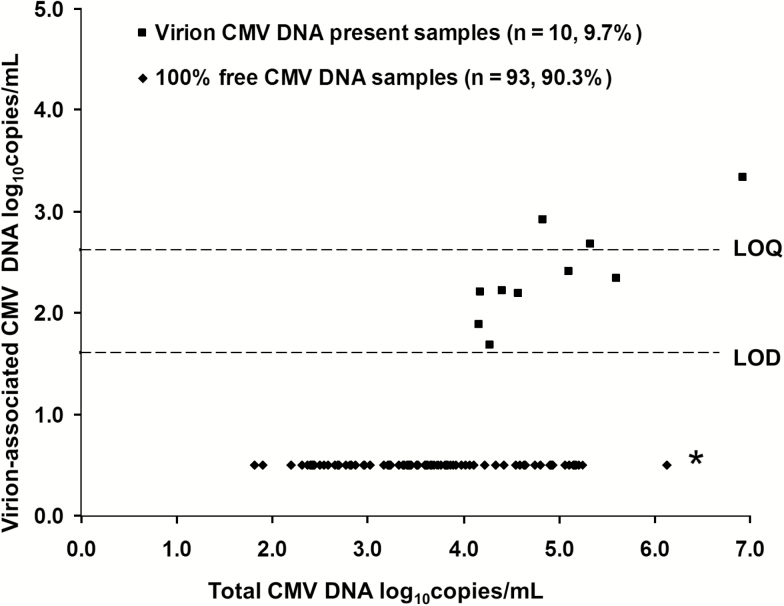
The DNase-CMV-qPCR testing results for the 103 archived plasma samples obtained from 20 SOTRs (group 1) are summarized in Figure 2. Free CMV DNA constituted 98.8%–100% of the total CMV DNA in all samples. In 93 of 103 (90.3%) samples, CMV DNA was exclusively free DNA. Virion DNA was detected in 10 of 103 (9.7%) samples collected from 8 unique patients, all of whom had additional samples tested containing only free CMV DNA. Samples in which virion CMV DNA was detected had higher VLs (4.94 ± 0.86) than those in whom only free CMV DNA was detected (3.65 ± 0.91 log10 copies/mL; t test, P < .001), but virion detection was not influenced by the presence of primary infection versus reactivation infection or receipt of antiviral therapy (t test, P = .35 and P = .41, respectively). Only 3 of 10 samples containing CMV virions, each from a unique patient, had CMV DNA consistently quantifiable at low levels (2.69–3.34 log10 copies/mL) after DNase exposure. The 7 remaining samples had CMV DNA that was detectable but not quantifiable on repeat testing. Exclusively free DNA was found in all fresh samples also tested as frozen samples from group 2.
Figure 2.
Quantitative measurements of virion-associated cytomegalovirus (CMV) DNA in solid-organ transplant recipient (SOTR) plasma samples detected using the DNase I digestion and CMV quantitative polymerase chain reaction (DNase-CMV-qPCR) in relationship to total CMV DNA in the sample. Samples with no virions detected and with virions detected were grouped separately. Limit of quantification = 2.69 log10copies/mL. Limit of detection = 1.69 log10copies/mL. *: All samples had free CMV DNA detected. Abbreviations: CMV, cytomegalovirus; LOD, limit of detection; LOQ, limit of quantification.
Evaluation of Cytomegalovirus DNA Fragmentation in Cultured Cytomegalovirus Strains and Clinical Samples by Paired Quantiative Polymerase Chain Reaction Assays
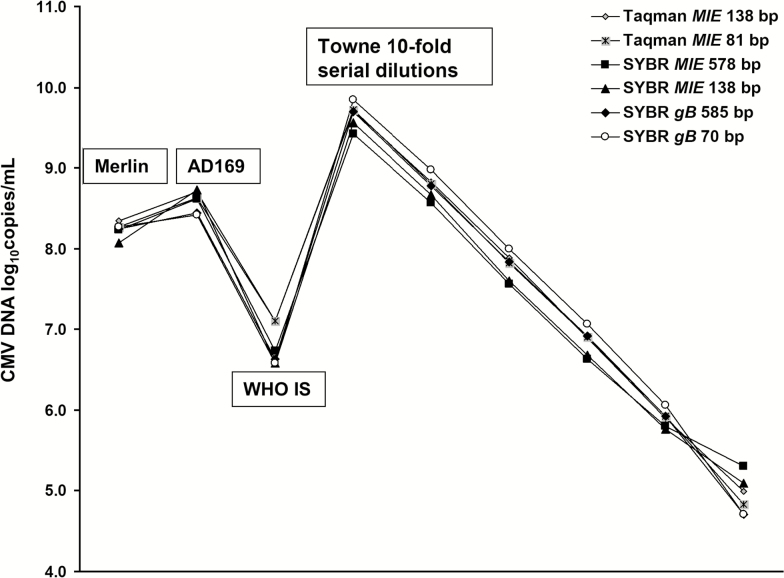
All three P-qPCRs showed similar analytical sensitivity and amplification efficiency (data not shown). Good linearity results were observed across all 3 qPCR pairs on testing serial 10-fold dilutions of CMV DNA extracted from the Towne viral stock (Figure 3). Quantitative results were highly reproducible among the 3 pairs and for the short and long amplicons within each pair. When the 3 viral stocks and WHO IS were tested, mean ± SD VLs (log10 copies per milliliter) for all 6 qPCR assays were as follows: 8.24 ± 0.09 (Merlin), 8.62 ± 0.10 (AD169), 9.66 ± 0.15 (Towne), and 6.79 ± 0.25 (WHO IS) (t test, P > .80). The reproducibility of these results, independent of assay used, indicates the CMV DNA in these sample types is not highly fragmented.
Figure 3.
Testing results for 3 cytomegalovirus (CMV) virus stocks and the World Health Organization international standard using 3 paired quantitative polymerase chain reaction (qPCR) assays with long and short amplicons. Six 10-fold dilutions of Towne viral stock were tested. No significant difference was found in CMV DNA measurement among all assays (log10 copies per milliliter; t test, P > .80). Abbreviations: CMV, cytomegalovirus; IS, international standard; WHO, World Health Organization.
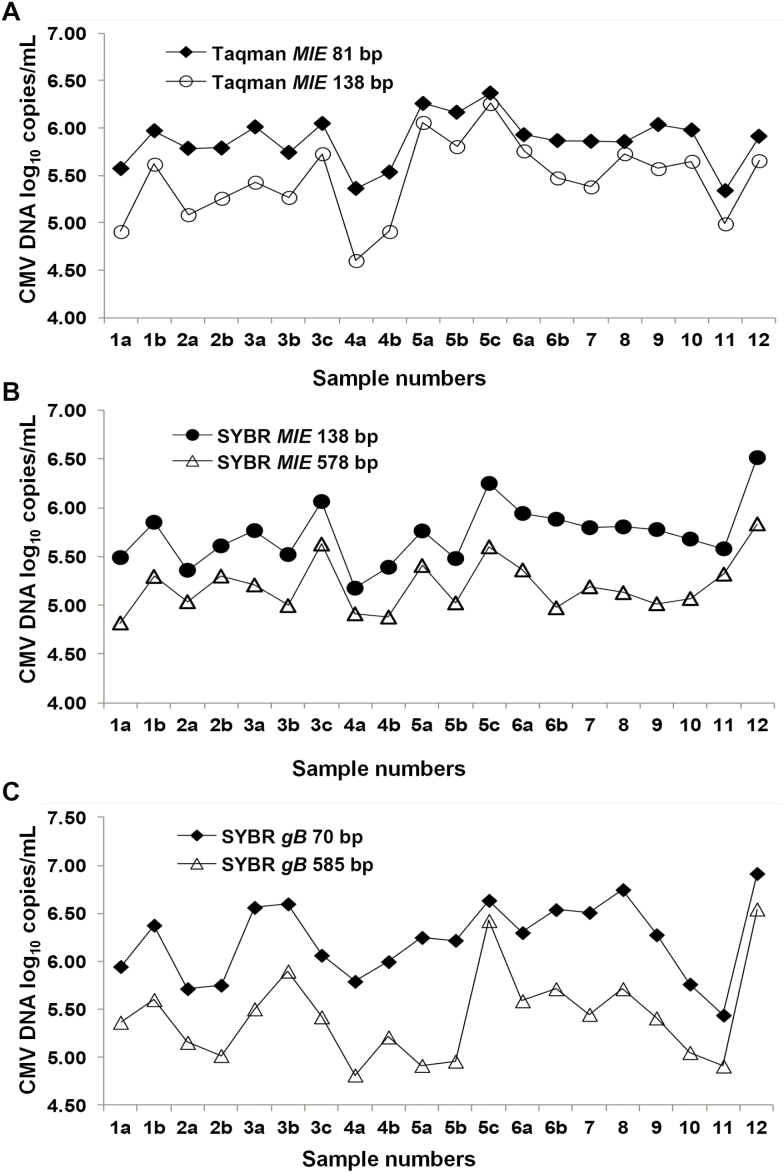
However, a significantly different pattern of CMV DNA results was observed in SOTR plasma tested using the P-qPCRs when compared with the viral stocks and WHO IS. The mean ± SD CMV DNA loads (log10 copies per milliliter) in 20 plasma samples were 5.46 ± 0.41 versus 5.87 ± 0.27, 5.20 ± 0.27 versus 5.75 ± 0.31, and 5.41 ± 0.46 versus 6.23 ± 0.41 for the long versus short amplicon assays within the P-qPCR-1, P-qPCR-2, and P-qPCR-3 assays, respectively (t test, P < .001) (Figure 4). The CMV DNA results for the short amplicon assays were a mean of 2.6-fold, 3.4-fold, and 6.5-fold greater than those detected with the corresponding long amplicon for P-qPCR-1, P-qPCR-2, and P-qPCR-3, respectively. When tested in a single run, higher quantitative results for the smaller amplicon in each of the 3 P-qPCRs were observed for both a fresh and a frozen clinical plasma sample; results across all P-qPCRs were reproducible for the frozen Merlin-spiked plasma sample (Table 2).
Figure 4.
Results of cytomegalovirus (CMV) DNA quantitation of 20 clinical plasma samples collected from 12 solid organ transplant recipients using 3 paired quantitative polymerase chain reaction assays with long and short amplicons. Each unique patient is identified by a number; serial samples collected from the same patient are identified as a, b, and c. The mean, median, and (range) of CMV viral load expressed as log10 copies per milliliter for the 20 samples were as follows: Taqman MIE 138 bp, 5.46, 5.52 (4.60–6.26) versus Taqman MIE 81 bp, 5.87, 5.89 (5.34–6.37) (A); SYBR MIE 578 bp, 5.20, 5.16 (4.82–5.84) versus SYBR MIE 138 bp, 5.74, 5.76 (5.18–6.51) (B); SYBR gB 585 bp, 5.41, 5.42 (4.82–6.55) versus SYBR gB 70 bp 6.23, 6.26 (5.44–6.92) (C). The mean log10 copies per milliliter difference between the short and long amplicon assays in A, B, and C were 0.41 (P < .001), 0.54 (P < .001), and 0.81 (P < .001), respectively. Abbreviation: CMV, cytomegalovirus.
No significant difference was observed in CMV DNA results between the short and long amplicon assays of each P-qPCR when the 3 viral stocks were spiked into either plasma or culture medium, suggesting that matrix did not impact fragmentation. Similarly, 4 F/T cycles did not change the CMV DNA results observed in either plasma or culture medium (ANOVA, P > .80) (Supplementary Table 2).
DISCUSSION
We optimized and validated an assay for CMV used by previous investigators to differentiate free Epstein-Barr virus (EBV) DNA (DNase-sensitive) from EBV DNA encapsidated in virions (DNase-resistant) in the plasma of immunocompetent subjects with primary EBV infection and patients with EBV-associated malignancies that exploited the ability of DNase I to destroy naked DNA [14, 15]. Recent interest in detecting cell-free DNA in plasma for fetal genetic testing and as tumor markers has expanded information related to preanalytic factors contributing to DNA yield and integrity in plasma [16, 17]. The anticoagulant EDTA used in our study samples efficiently complexes bivalent cations essential for DNase activity and protects cell-free DNA from WB’s DNase preanalytical impact [17]. Our study confirmed that CMV DNA levels in EDTA WB remain stable up to 48 hours before plasma separation; others have shown CMV DNA’s stability in EDTA plasma for up to 14 or 21 days at 4°C [18, 19]. However, to optimize our DNase-CMV-qPCR an additional alternate anticoagulant (PPACK) was required to prevent plasma clotting when buffer with divalent cations was added to allow DNase digestion to proceed. Plasma dilution (1:4) was also required to minimize DNase inhibitor effects in plasma.
A limitation of our study is that many of the samples had been frozen at –70°C. These temperatures are used to store infectious virus stocks and thus can preserve intact virions. We observed no significant decrease in total CMV DNA and virion percentage over 2 years of storage of Towne viral stock. Although we cannot rule out that some CMV DNA fragmentation or virion disruption occurred during freezer storage, this is unlikely to account for the almost total absence of virions in both our frozen and fresh samples and the consistent presence of a high degree of DNA fragmentation regardless of freezer storage time. Although direct study of CMV DNA fragmentation in fresh plasma is limited to a single sample in our study and 3 patients studied by Boom et al [7], results suggest the presence of highly fragmented CMV DNA in these samples, resembling patterns observed in frozen samples. Further study of fresh samples would be useful.
We believe that the biologic form of CMV DNA observed in frozen plasma likely reflects its in vivo form at the time of venipuncture. Although we observed DNase-resistant CMV DNA, presumably virion-associated, in a few plasma samples with high VL, contamination of plasma with virions from the lysis of CMV-infected leukocytes during specimen transport prior to plasma separation cannot be excluded.
Our study results are consistent with a body of clinical and laboratory observations. There has been no documented transmission of CMV infection from transfused plasma, even in patients at high risk of acquiring CMV infection from cellular blood components [20]. Even when CMV DNA plasma loads are high, infectious virus remains localized to the cellular compartment and has not been cultured from plasma despite numerous attempts in both human [21–24] and mouse studies [25]. An exception was a study of AIDS patients with very high CMV VL in plasma [26]. A single viral CMV plaque was observed on culture of plasma in 2 of 11 patients; contamination of plasma from leukocyte lysis could not be excluded. Older studies of serially monitored immunocompetent subjects with acute primary CMV infection reported prolonged detection of CMV DNA in plasma and the white blood cell fraction [27–29]. Although it has been argued that tissue culture techniques lacked sensitivity, when simultaneous viral culture of blood components was performed, infectious virus was always limited to the leukocyte fraction and not detected in plasma [21, 27].
For clinical care, plasma CMV DNA measurement is an excellent surrogate of viral replicative activity when used for disease prevention and management [1, 2]. However, our study suggests virion load is not being directly measured in this compartment. Free DNA released from CMV-infected cells undergoing apoptosis as a result of replicative viral infection or immune-mediated killing is the predominant and perhaps exclusive biologic form of CMV DNA present. This should be considered when interpreting unusual patterns of CMV DNA clearance in plasma or modeling viral kinetics in vivo.
It was hoped that the use of calibrators traceable to the new WHO IS in CMV DNA plasma assays used in clinical laboratories would improve harmonization of results that historically had been highly variable [1, 2]. In a recent study comparing CMV DNA QNAT results among 10 assays calibrated to the WHO IS, result harmonization was observed when plasma spiked with the WHO IS (derived from tissue-cultured Merlin strain) was tested, but testing results for SOTR clinical plasma samples demonstrated significant ongoing variability [8]. Moreover, assays with smaller amplicon sizes trended toward higher quantitative CMV DNA measurements than assays with larger amplicon sizes when testing clinical samples, but not for the WHO IS. Our study confirms these observations.
Our study confirms and extends the observations of Boom et al [7] but does not enable estimation of the proportion of different-sized CMV DNA fragments in plasma. However, Boom et al [7] examined this more directly by using CMV DNA QNAT assays with amplicons 578 bp and 138 bp in size to study DNA eluted from serial agarose gel slices after fractionating DNA from the plasma of 2 SOTRs by size using electrophoresis. The majority of CMV DNA fragments from patient plasma were significantly <2000 bp. CMV DNA levels were highest in the shortest DNA fragments and most often detected with only the 138-bp assay. This suggests CMV DNA in patient plasma is highly fragmented. We found a 2.6-fold difference in CMV VL even when the amplicon sizes in our P-qPCR test pair were both very small (81 bp/138 bp), suggesting that a portion of the CMV DNA in plasma is present in extremely small fragments <138 bp.
Our study results have implications for assay design to make the international goal of result harmonization among QNAT assays for CMV DNA achievable. Although most new commercial assays have gene targets <150 bp in size, many older assays, including a recently US Food and Drug Administration–approved commercial assay, have significantly larger gene targets. Result harmonization might be improved if standards required smaller targets within a narrow acceptable size range. The nucleic acid extraction procedure also influences CMV DNA QNAT results [2]. In our study, DNA extraction was performed using a Qiagen commercial product that has a lower yield and partial loss of DNA fragments <150 bp in size [30]. Differences in QNAT results between assay pairs may have been even greater with a nucleic acid extraction system more efficient for small fragments. For result harmonization of CMV QNAT plasma assays, it would be important to ensure that nucleic acid extraction systems used demonstrate high and comparable efficiency in isolating small DNA fragments.
Our study observations should not be extrapolated to WB, although plasma CMV DNA contributes to this matrix. Further studies of the size distribution of CMV DNA fragments in various blood compartments in a variety of CMV disease states before and after antiviral therapy and in the presence of variable degrees of immune response are warranted.
In conclusion, CMV DNA in the plasma of SOTR is almost exclusively free DNA and highly fragmented. These observations have significant implications for the infectivity of plasma in the setting of blood transfusion, the interpretation of dynamic changes in serial CMV VLs in SOTRs, and the design of qPCR assays for CMV DNA measurement.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Acknowledgments. The authors thank the molecular diagnostic laboratory, ProvLab, for providing clinical samples for this study. The authors also thank Maria Eloisa Hasing and Candice McEachren for manuscript review and Min Cao for technical assistance.
Financial support. This work was supported by a Canadian Blood Service research and development grant (RES0018943).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Kraft CS, Armstrong WS, Caliendo AM. Interpreting quantitative cytomegalovirus DNA testing: understanding the laboratory perspective. Clin Infect Dis 2012; 54:1793–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Razonable RR, Hayden RT. Clinical utility of viral load in management of cytomegalovirus infection after solid organ transplantation. Clin Microbiol Rev 2013; 26:703–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Emery VC, Asher K, Sanjuan Cde J. Importance of the cytomegalovirus seropositive recipient as a contributor to disease burden after solid organ transplantation. J Clin Virol 2012; 54:125–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Emery VC, Cope AV, Bowen EF, Gor D, Griffiths PD. The dynamics of human cytomegalovirus replication in vivo. J Exp Med 1999; 190:177–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ziemann M, Krueger S, Maier AB, Unmack A, Goerg S, Hennig H. High prevalence of cytomegalovirus DNA in plasma samples of blood donors in connection with seroconversion. Transfusion 2007; 47:1972–83. [DOI] [PubMed] [Google Scholar]
- 6. Drew WL, Roback JD. Prevention of transfusion-transmitted cytomegalovirus: reactivation of the debate? Transfusion 2007; 47:1955–8. [DOI] [PubMed] [Google Scholar]
- 7. Boom R, Sol CJ, Schuurman T, et al. Human cytomegalovirus DNA in plasma and serum specimens of renal transplant recipients is highly fragmented. J Clin Microbiol 2002; 40:4105–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Preiksaitis JK, Hayden RT, Tong Y, et al. Are we there yet? Impact of the first international standard for cytomegalovirus DNA on the harmonization of results reported on plasma samples. Clin Infect Dis 2016; 63:583–9. [DOI] [PubMed] [Google Scholar]
- 9. Wroblewska Z, Wellish MC, Wolinsky JS, Gilden D. Comparison of human cytomegalovirus growth in MRC-5 human fibroblasts, brain, and choroid plexus cells in vitro. J Med Virol 1981; 8:245–56. [DOI] [PubMed] [Google Scholar]
- 10. Fryer JF, Health AB, Minor PD, et al. A collaborative study to establish the 1st WHO International Standard for human cytomegalovirus for nucleic acid amplification technology. Biologicals 2016; 44(4):242–51. [DOI] [PubMed] [Google Scholar]
- 11. N’soukpoé-Kossi CN, Diamantoglou S, Tajmir-Riahi HA. DNase I - DNA interaction alters DNA and protein conformations. Biochem Cell Biol 2008; 86:244–50. [DOI] [PubMed] [Google Scholar]
- 12. Pang XL, Chui L, Fenton J, LeBlanc B, Preiksaitis JK. Comparison of LightCycler-based PCR, COBAS amplicor CMV monitor, and pp65 antigenemia assays for quantitative measurement of cytomegalovirus viral load in peripheral blood specimens from patients after solid organ transplantation. J Clin Microbiol 2003; 41:3167–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Haugland RA, Siefring SC, Wymer LJ, Brenner KP, Dufour AP. Comparison of Enterococcus measurements in freshwater at two recreational beaches by quantitative polymerase chain reaction and membrane filter culture analysis. Water Res 2005; 39:559–68. [DOI] [PubMed] [Google Scholar]
- 14. Ryan JL, Fan H, Swinnen LJ, et al. Epstein-Barr Virus (EBV) DNA in plasma is not encapsidated in patients with EBV-related malignancies. Diagn Mol Pathol 2004; 13:61–8. [DOI] [PubMed] [Google Scholar]
- 15. Suwiwat S, Pradutkanchana J, Ishida T, Mitarnun W. Quantitative analysis of cell-free Epstein-Barr virus DNA in the plasma of patients with peripheral T-cell and NK-cell lymphomas and peripheral T-cell proliferative diseases. J Clin Virol 2007; 40:277–83. [DOI] [PubMed] [Google Scholar]
- 16. El Messaoudi S, Rolet F, Mouliere F, Thierry AR. Circulating cell free DNA: preanalytical considerations. Clin Chim Acta 2013; 424:222–30. [DOI] [PubMed] [Google Scholar]
- 17. Barra GB, Santa Rita TH, de Almeida Vasques J, Chianca CF, Nery LF, Santana Soares Costa S. EDTA-mediated inhibition of DNases protects circulating cell-free DNA from ex vivo degradation in blood samples. Clin Biochem 2015; 48:976–81. [DOI] [PubMed] [Google Scholar]
- 18. Abdul-Ali D, Kraft CS, Ingersoll J, Frempong M, Caliendo AM. Cytomegalovirus DNA stability in EDTA anti-coagulated whole blood and plasma samples. J Clin Virol 2011; 52:222–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Xie L, Liang XN, Deng Y, et al. Effects of storage time on cytomegalovirus DNA stability in plasma determined by quantitative real-time PCR. J Virol Methods 2014; 207:196–9. [DOI] [PubMed] [Google Scholar]
- 20. Bowden R, Sayers M. The risk of transmitting cytomegalovirus infection by fresh frozen plasma. Transfusion 1990; 30:762–3. [DOI] [PubMed] [Google Scholar]
- 21. Rinaldo CR, Jr, Black PH, Hirsch MS. Interaction of cytomegalovirus with leukocytes from patients with mononucleosis due to cytomegalovirus. J Infect Dis 1977; 136:667–78. [DOI] [PubMed] [Google Scholar]
- 22. Fiala M, Payne JE, Berne TV, et al. Epidemiology of cytomegalovirus infection after transplantation and immunosuppression. J Infect Dis 1975; 132:421–33. [DOI] [PubMed] [Google Scholar]
- 23. Hamprecht K, Steinmassl M, Einsele H, Jahn G. Discordant detection of human cytomegalovirus DNA from peripheral blood mononuclear cells, granulocytes and plasma: correlation to viremia and HCMV infection. J Clin Virol 1998; 11:125–36. [DOI] [PubMed] [Google Scholar]
- 24. Lipson SM, Shepp DH, Match ME, Axelrod FB, Whitbread JA. Cytomegalovirus infectivity in whole blood following leukocyte reduction by filtration. Am J Clin Pathol 2001; 116:52–5. [DOI] [PubMed] [Google Scholar]
- 25. Roback JD, Su L, Zimring JC, Hillyer CD. Transfusion-transmitted cytomegalovirus: lessons from a murine model. Transfus Med Rev 2007; 21:26–36. [DOI] [PubMed] [Google Scholar]
- 26. Spector SA, Merrill R, Wolf D, Dankner WM. Detection of human cytomegalovirus in plasma of AIDS patients during acute visceral disease by DNA amplification. J Clin Microbiol 1992; 30:2359–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Revello MG, Zavattoni M, Sarasini A, Percivalle E, Simoncini L, Gerna G. Human cytomegalovirus in blood of immunocompetent persons during primary infection: prognostic implications for pregnancy. J Infect Dis 1998; 177:1170–5. [DOI] [PubMed] [Google Scholar]
- 28. Zanghellini F, Boppana SB, Emery VC, Griffiths PD, Pass RF. Asymptomatic primary cytomegalovirus infection: virologic and immunologic features. J Infect Dis 1999; 180:702–7. [DOI] [PubMed] [Google Scholar]
- 29. Lilleri D, Zelini P, Fornara C, Comolli G, Revello MG, Gerna G. Human cytomegalovirus-specific CD4+ and CD8+ T cell responses in primary infection of the immunocompetent and the immunocompromised host. Clin Immunol 2009; 131:395–403. [DOI] [PubMed] [Google Scholar]
- 30. Fleischhacker M, Schmidt B, Weickmann S, et al. Methods for isolation of cell-free plasma DNA strongly affect DNA yield. Clin Chim Acta 2011; 412:2085–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.