ABSTRACT
Background
Behavioral lifestyle interventions during pregnancy can prevent excessive gestational weight gain (GWG) in women with normal weight; however, effective interventions to reduce GWG in ethnically diverse women with obesity are lacking.
Objective
A randomized controlled trial was conducted to test whether a behavioral lifestyle intervention with partial meal replacement reduces GWG rate in Hispanic and non-Hispanic women with overweight or obesity relative to enhanced usual care.
Design
Participants (n = 257) were recruited in San Luis Obispo, California, and Providence, Rhode Island, between November 2012 and May 2016. Participants were pregnant (mean ± SD: 13.6 ± 1.8 wk of gestation) with overweight or obesity and had a mean age of 30.3 y; 41.6% of participants were Hispanic. Women were randomly assigned within site and by ethnicity to enhanced usual care (n = 128) or to a behavioral lifestyle intervention with partial meal replacement (n = 129). The primary outcome was GWG per week of observation. Secondary outcomes were proportions exceeding Institute of Medicine (IOM) guidelines for total GWG, changes in weight-control behaviors and cardiovascular disease risk factors, and incidence of pregnancy complications. Study retention was 99.6% (256 of 257).
Results
The intervention compared with usual care resulted in less mean ± SD weekly GWG (0.33 ± 0.25 compared with 0.39 ± 0.23 kg/wk; P = 0.02) and total GWG (9.4 ± 6.9 compared with 11.2 ± 7.0 kg; P = 0.03) and reduced the proportion of women who exceeded IOM guidelines for total GWG (41.1% compared with 53.9%; P = 0.03). No significant group × time × demographic subgroup (ethnicity, BMI, age, parity, and income) interactions were observed. Among intervention participants, greater meal replacement intake was related to reduced GWG rate (β = −0.07; 95% CI:−0.12, −0.03; P = 0.002). The intervention compared with usual care increased weight-control strategies (P < 0.0001) and cognitive restraint (P < 0.0001) and reduced triglycerides (P = 0.03).
Conclusion
Prenatal behavioral intervention with partial meal replacement significantly reduced GWG in Hispanic and non-Hispanic women with overweight or obesity. This trial was registered at www.clinicaltrials.gov as NCT01545934.
Keywords: prenatal intervention, meal replacements, randomized clinical trial, lifestyle intervention, obesity, gestational weight gain, Institute of Medicine
INTRODUCTION
Excessive gestational weight gain (GWG) is an established independent risk factor for high postpartum weight retention and future weight gain, cardiovascular disease, and type 2 diabetes in women (1). The National Academy of Sciences Institute of Medicine (IOM) has formulated specific GWG recommendations in an effort to help prevent adverse maternal and neonatal outcomes (1). However, ∼35% of women with normal weight and 60% of women with obesity gain more than is recommended (2). Behavioral lifestyle interventions during pregnancy can prevent excessive GWG in women with normal weight (3); however, interventions to reduce GWG in women with obesity, including a previous study that we conducted (3), did not find significant effects on GWG (4–9). The most effective programs have involved moderate caloric restriction and frequent patient-provider contact, but adherence has been problematic (10–13) and many were tested outside of the US health care system (14–19). Furthermore, Hispanic women were the largest minority population in the United States in 2010 (20) and had higher obesity and fertility rates (21) than non-Hispanic whites, but few studies, to our knowledge, have included a significant proportion of Hispanic women.
Meal replacement programs with additional intake provided by solid foods (i.e., partial meal replacement) have been shown to effectively promote weight loss and improvements in metabolic disease risk factors in nonpregnant adults with obesity (22). In pregnancy, meal replacements have been shown to improve nutritional and metabolic variables in undernourished women (23, 24), but to our knowledge, no study to date has incorporated meal replacements as a means of slowing GWG rate.
The purpose of the Healthy Beginnings/Comienzos Saludables study was to determine the efficacy of a multicomponent behavioral lifestyle intervention with partial meal replacement on GWG rates in Hispanic and non-Hispanic women with overweight or obesity. The primary hypothesis was that the rate of GWG per week of observation would be reduced among participants assigned to the intervention relative to enhanced usual care. Secondary hypotheses were that, relative to enhanced usual care, the intervention would reduce the incidence of excessive GWG, defined by using the 2009 IOM recommendations for total GWG (1), and produce greater improvements in maternal weight-control behaviors (i.e., diet, physical activity, sleep, weight-control strategies, and eating behaviors) and metabolic risk factors without adversely affecting pregnancy outcomes.
METHODS
Design
Healthy Beginnings/Comienzo Saludables was a randomized controlled clinical trial conducted at California Polytechnic State University, San Luis Obispo, California, and at the Miriam Hospital with Women and Infants Hospital in Providence, Rhode Island. This trial was registered at www.clinicaltrials.gov as NCT01545934. In addition, Healthy Beginnings/Comienzo Saludables is part of the Lifestyle Interventions for Expectant Moms (LIFE-Moms) consortium. The LIFE-Moms consortium is a collaboration between 7 clinical trials, a research coordinating unit, and NIH sponsoring institutes and centers. The goals and methods of the consortium have been published elsewhere (25). Each trial, including Healthy Beginnings/Comienzo Saludables, conducts a separate study testing a different lifestyle intervention but has common core measures, procedures, and eligibility criteria that are consistent across all trials.
Participants
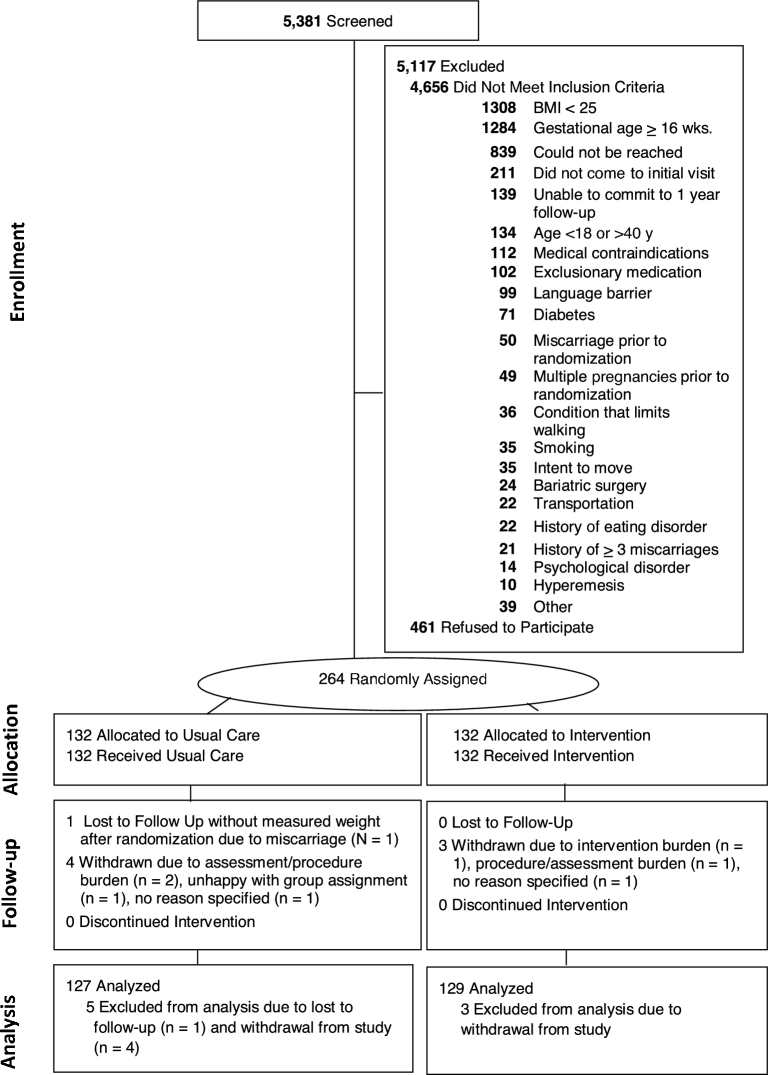
Procedures were approved by institutional review boards (IRBs) at California Polytechnic State University, California State University at Northridge (for Dignity Health-affiliated hospitals and clinics in Central California), the Miriam Hospital, and Women & Infants Hospital. Participants provided written informed consent. Recruitment occurred at multiple obstetrician-gynecologists’ offices in California and Rhode Island between November 2012 and October 2015; the study's final assessment was conducted in May 2016. Eligibility criteria included gestational age between 9 and 16 wk, as assessed by ultrasound; BMI (in kg/m2) ≥25 on the basis of study entry measured weight and height; English or Spanish speaking; age ≥18 y; and singleton pregnancy. Participants with glycated hemoglobin ≥6.5 were excluded. In addition, participants with self-reported major health diseases (e.g., heart disease, cancer, renal disease, and diabetes), current substance abuse, current treatment for a serious psychological disorder (schizophrenia, bipolar disorder), contraindications to aerobic exercise, or who had repeated no-shows or loss of contact during initial screening and other less frequent criteria (Figure 1) were excluded. As shown in Figure 1, most of the women were deemed ineligible due to normal-weight status (28.0%) or being ≥16 wk of gestation (27.5%). Women who withdrew (n = 7) could not be included in the data analysis.
FIGURE 1.
Participant Flow and Retention into Healthy Beginnings/Comienzos Saludables. In the Healthy Beginnings/Comienzos Saludables randomized trial, 264 women were enrolled and randomly assigned. After randomization, 4 enhanced-usual-care and 3 intervention participants withdrew participation and 1 participant was lost to follow-up, leaving an analytic sample of 256.
Interventions
In this 2-site trial, randomization was computer-generated by the study statistician, and women were randomly assigned within site and by ethnicity (Hispanic or non-Hispanic) to 1 of the 2 treatment conditions: 1) enhanced usual care or 2) behavioral lifestyle intervention with partial meal replacement.
Enhanced usual care
Participants in the enhanced–usual care group received all aspects of usual care offered by their prenatal care providers, including physicians, nurses, nutritionists, or counselors from the Women, Infants, and Children's Special Supplemental Nutrition Program (WIC) (26). Usual prenatal care visits typically occur monthly until 28 wk of gestation, biweekly for 28–36 wk of gestation, and weekly until delivery. In addition, in this group at the time of study randomization, participants attended an ∼20-min welcome visit with a study interventionist, who provided general information about healthy eating, physical activity, and the IOM recommendations for total GWG (3). Study interventionists were bilingual registered dietitians or counselors with degrees in nutrition, community health, psychology, kinesiology, or a related field. Participants received study newsletters with general information about pregnancy-related health (e.g., prenatal vitamins, quitting smoking, planning to breastfeed, maternity clothes) at 2-mo intervals that were designed to improve retention in the study.
Behavioral lifestyle intervention with partial meal replacement during pregnancy
Participants in the intervention group received all aspects of enhanced usual care plus a behavioral lifestyle intervention designed to prevent excessive weight gain during pregnancy. The intervention was rooted in social learning theory (27) and conceptualized pregnancy as a “teachable moment” for behavior change (28). The program was based on our previous intervention (3) but was expanded to include face-to-face visits and a structured partial meal replacement plan. Each woman received an ∼20-min individual, face-to-face counseling session with a study interventionist every 2 wk until 20 wk of gestation and then monthly visits until delivery. Women were encouraged to gain ∼0.5 pounds (0.23 kg)/wk on the basis of the 2009 IOM guidelines for healthy weight gain during the second and third trimesters of pregnancy for women with overweight or obesity. Women whose GWG was over or under this amount during any 1-mo interval received additional visits with the interventionist (2 visits/mo) to support a return to the recommended GWG rate. All of the visits were conducted at our research centers or at affiliate locations near recruitment clinics.
To promote weight control, women were provided with a structured meal plan (29) that was individually tailored to meet each participant's self-reported dietary needs, including food aversions, cravings, lactose intolerance, and specialized diets such as vegetarianism. The partial meal replacement plan provided a caloric prescription of ∼18 kcal/kg body weight at study entry (10, 30) and consisted of 30% of calories from fat, 15–20% from protein, and 50–55% from carbohydrates (31). Women were instructed to replace 2 meals with the provided meal replacement shakes or bars and to consume ≥1 meal of regular foods and 2–4 healthy snacks/d. The meal replacement products were provided free of charge at every intervention visit and in the quantities needed until the next scheduled intervention visit. The study's meal replacement options were selected at the study onset by investigators after an analysis of various meal plan scenarios that considered the micronutrient and macronutrient composition of specific meal replacement products, the use of prenatal vitamins, the intervention's calorie and nutritional goals, and the current micronutrient and macronutrient recommendations for pregnant women (31). Options included organic and lactose-free drinks and bars in a variety of flavors and brands (Supplemental Table 1).
Participants were encouraged to aim for a goal of 30 min of activity on most days of the week (32). They were provided with a pedometer and advised to gradually increase the number of steps walked each day until reaching an ultimate goal of 10,000 steps/d. In addition, at each visit, women were provided with a personalized graph of their weight gain with feedback. Other behavioral strategies included daily recording of food, drink, and caloric intake and physical activity; stimulus control techniques; problem-solving skills; goal-setting; self-reinforcement for goal attainment; and daily self-monitoring of weight by using a scale provided by the study. At every visit, diet and meal replacement records were reviewed by the interventionist and the self-reported intake of the meal replacements was discussed to facilitate problem-solving and adherence. Automated postcards designed to further promote behavioral skills, healthy eating, and exercise habits were mailed weekly.
Outcome assessments
Assessments were conducted early in pregnancy (between 9 and 16 wk), at 24–27 and at 35–36 wk of gestation, and at the hospital after delivery. Participants received $25 for completing each assessment, except for the visit immediately after delivery. Assessment staff was masked to randomization to minimize potential bias. The primary outcome was GWG per week of study observation.
Weight and height were assessed in duplicate to the nearest 0.1 kg or 0.1 cm by using a calibrated standard digital scale and stadiometer with the participant wearing light-weight clothing without shoes. The rate of GWG was computed as the difference between weights measured at study entry (9–16 wk of gestation) and the final pregnancy visit (35–36 wk of gestation), divided by the number of weeks between entry and the final pregnancy visit. If the measure at gestational week 35–36 was unavailable (n = 28), the most proximal clinic visit–measured weight was used. For the secondary outcome of adherence to IOM recommendations, women were categorized as exceeding or not exceeding IOM guidelines for total GWG (1). The IOM defines overweight as a BMI of 25–29.9 and obesity as a BMI ≥30 and recommends that women with overweight at the time of conception limit total GWG to 7–11.5 kg (15–25 pounds) and women with obesity (all classes) to 5–9 kg (11–20 pounds). Thus, for this computation, prepregnancy weight, which was based on self-report at the time of the last menstrual period (3, 33), was subtracted from weight measured at the visit at 35–36 wk of gestation (or the most proximal), and all women were categorized as exceeding or not exceeding 2009 IOM guidelines for total GWG. Women without a prepregnancy self-reported weight (n = 4) or a study- or clinic-measured final pregnancy weight (n = 1) were assumed to have exceeded the guidelines.
Dietary intake was assessed at study entry and at 35 wk of gestation by using 24-h recalls on 2 random days over 1 wk and completed by using the National Cancer Institute Automated Self-Administered 24-h recall (ASA-24; http://riskfactor.cancer.gov/tools/instruments/asa24.html) (34). Variables included daily total calories and percentages of calories from carbohydrate, fat, and protein (35). Daily meal replacement intake was also assessed via the ASA-24 and quantified as the total number of meal replacement products, including shakes and bars, that were consumed each day, on average, during the assessment period. The proportion of participants reporting ≥0.9 or <0.9 products consumed/d was further computed. The wrist-worn ActiGraph GT3X+ (ActiGraph) accelerometer was used at study entry and at 35 wk of gestation to provide estimates of time in physical activity and sleep (36). Acceptable wear time was classified as ≥1 d wear time with ≥19.2 h/d. Time spent sleeping was estimated by using an algorithm that defined sleep as the absence of change in arm angle >5° for ≥5 min (37). Three separate random forest methods were used to predict time spent in 1) moderate and vigorous intensity physical activity, 2) sedentary or nonsedentary behavior, and 3) locomotion or nonlocomotion. The method relies on probabilities assigned to leaf nodes of 500 decision trees created from random summary variables that include the mean and SD of the vector magnitude (not subtracting gravity) and angle of acceleration as well as features derived from fast Fourier transform analysis of the signal. Minor changes to code were made to account for the data collection in 50 Hz compared with 80 Hz in the original models. Additional details of this method are available (38). We also processed the data by using the GGIR package in R (3.3.1), which uses a linear threshold-based method to estimate time spent in activity-intensity categories.
The Weight Control Strategies Scale (39) was used to assess the extent to which participants practiced behavioral weight-control strategies. Subscales include dietary strategies (e.g., “I had several servings of fruit and/or vegetables each day”), self-monitoring strategies (e.g., “I kept a record of the type and amount of food I ate”), physical activity (e.g., “I engaged in moderate-intensity exercise like brisk walking or something similar to brisk walking for at least 30 minutes a day”), and psychological coping (e.g., “If I had negative thoughts about my weight loss progress, I tried to catch myself and stop that kind of thinking”). The Eating Inventory (40) was used to assess dietary restraint (i.e., self-initiated, cognitive attempts to restrict food intake) and disinhibition (i.e., loss of control over eating).
Maternal systolic and diastolic blood pressure and fasting glucose, triglycerides, total cholesterol, HDL cholesterol, LDL cholesterol, insulin, C-peptide, and leptin were measured at study entry and at 35–36 wk of gestation. HOMA-IR was calculated (41). At 24–27 wk of gestation, a 2-h 75-g oral-glucose-tolerance test was performed by research staff, and gestational diabetes was confirmed by using the International Association of Diabetes and Pregnancy Study Groups criteria (42). If a study-measured oral-glucose-tolerance test was not obtained (n = 96), chart-abstracted, clinic measures of gestational diabetes were used that varied across clinics [i.e., based on the American College of Obstetricians and Gynecologists–endorsed, 2-step approach (43), a 1-h 50-g value ≥200 mg/dL, or a clinical chart indication of “diabetes”].
Infant birth weight measurements were performed between 24 h and 1 wk of life by trained research staff; length was obtained on a hard-surface infant measuring board. Obstetric records were abstracted after delivery to obtain other maternal or infant complications, including the number of women with preeclampsia, gestational hypertension, preterm delivery, and cesarean delivery. Race and ethnicity were assessed by self-report by using questionnaires with fixed categories. Marital status, income, education, employment status, and childbearing history were also assessed by self-report questionnaires. Gestational age in weeks at study entry was measured via clinical ultrasound.
Statistical methods
Sample size
The study's target sample size was 260 participants (130 participants/condition). The study's initial IRB-approved protocol in 2012 had a recruitment target of 350 participants. However, in 2014, the recruitment target was revised from 350 to 260 participants and approved by the IRB and the NIH and was consistent with the sample sizes in the other LIFE-Moms trials (25). The target sample size of 130 participants/condition (total n = 260) was projected to provide ≥80% power to detect an intervention effect on our primary outcome based on the previous studies (3, 15) (d ≥ 0.35) at the α = 0.05 level by using a 2-sided test of significance and assuming 10% attrition and the exclusion of formal withdrawals. With 130 participants in each group, the study was also projected to have ≥80% power to detect a ≥20% difference between the intervention and enhanced-usual-care groups in proportions exceeding GWG guidelines at the α = 0.05 level with the use of a 2-sided test of significance.
Analysis plan
To compare participants in the 2 groups and completers with noncompleters, the Wilcoxon test for continuous variables, and Pearson’s chi-square test or exact tests for categorical variables were used. For the primary aim, a multiple linear regression model was used to examine the impact of the intervention on GWG per week of observation, simultaneously adjusting for prespecified potential effect modifiers that included weeks of gestation at randomization, age, ethnicity (Hispanic compared with non-Hispanic), parity (multiparity compared with primiparity), study entry BMI category (overweight compared with obese), and household family income (≥$50,000/y compared with <$50,000/y); site (California compared with Rhode Island) was also included as a fixed effect. Similar models were used that included group × time × demographic subgroup (i.e., age, ethnicity, parity, study entry BMI category, and household family income) terms to examine whether treatment effects differed across demographic subgroups.
For our secondary aim, a multiple logistic regression analysis was used to examine the effect of treatment group on the proportion of women who exceeded BMI-specific IOM recommendations for total GWG, simultaneously adjusting for the same a priori covariates. To test intervention effects on the study's other secondary aims (i.e., diet, physical activity, sleep, weight-control strategies, eating behaviors, metabolic factors), likelihood-based, linear mixed-effects models were used (and planned a priori) to accommodate participants with any missing data. Although missing or excluded data were rare in this study, instances are noted in Figure 1 and in the footnotes of the tables. The models included treatment group and a group × time interaction terms (fixed effect) to test if the change over time in the dependent variable differed significantly for the 2 study groups, including the same a priori–defined covariates. Similar models were used that included group × time × demographic subgroup terms.
Multiple linear regression models with covariates were used to examine the relation between changes in GWG rate and intervention adherence measures (i.e., meal replacement products per day, attendance) and to determine the effect of the intervention compared with enhanced usual care on maternal and child complications. Similar regression models were used to explore treatment group effects on GWG rate after adjusting for changes in meal replacement products per day and other behavioral changes. Significance was set at P < 0.05. R (3.3.1), SPSS (23.0.0), and JMP (12.2.0) statistical packages were used for all of the analyses. Accelerometer data processing was conducted in R by using the GGIR package version 1.5-9, and random Forest version 4.6-12.
RESULTS
Figure 1 summarizes the participant flow and retention into Healthy Beginnings/Comienzos Saludables study. Participant characteristics were well balanced by randomly assigned group (Table 1). As shown, 41.6% of the women were Hispanic/Latino, and 40% and 60% were overweight and obese, respectively. At the visit at 35–36 wk of gestation, 99.6% (n = 256 of 257) of participants either attended the visit (88.7%; n = 228 of 257) or provided a clinic-measured weight (10.9%; n = 28 of 257). Demographic characteristics did not significantly differ between participants who attended and those who did not attend the visit at 35–36 wk of gestation.
TABLE 1.
Baseline characteristics of participants by condition
| Total | Enhanced usual | Intervention | |
|---|---|---|---|
| Characteristic | (n = 257) | care (n = 128) | (n = 129) |
| Age, y | 30.3 ± 5.421 | 29.7 ± 5.5 | 30.7 ± 5.3 |
| Hispanic/Latino, n (%) | |||
| Yes | 107 (41.6) | 54 (42.2) | 53 (41.1) |
| No | 150 (58.4) | 74 (57.8) | 76 (58.9) |
| Race/ethnicity (participants could select multiple), n (%) | |||
| American Indian or Alaskan Native | 8 (3.1) | 3 (2.3) | 5 (3.9) |
| Asian | 4 (1.6) | 1 (0.8) | 3 (2.3) |
| Black or African American | 15 (5.8) | 7 (5.4) | 8 (6.2) |
| Native Hawaiian or Pacific Islander | 6 (2.3) | 3 (2.3) | 3 (2.3) |
| White | 156 (60.7) | 79 (62.2) | 77 (59.7) |
| Other | 78 (30.4) | 36 (28.1) | 42 (32.6) |
| Marital status, n (%) | |||
| Married or living with significant other | 220 (85.6) | 112 (87.5) | 108 (83.7) |
| Never married/divorced/widowed | 37 (14.4) | 16 (12.5) | 21 (16.3) |
| Annual household income, n (%) | |||
| <$24,999 | 71 (27.6) | 39 (30.5) | 32 (24.8) |
| $25,000–49,999 | 72 (28.0) | 33 (25.8) | 39 (30.2) |
| $50,000–99,999 | 70 (27.2) | 33 (25.8) | 37 (28.7) |
| ≥$100,000 | 44 (17.1) | 23 (18.0) | 21 (16.3) |
| Education, n (%) | |||
| High school or less | 67 (26.1) | 39 (30.5) | 28 (21.7) |
| Some college/completed college | 154 (59.9) | 70 (54.7) | 84 (65.1) |
| Postgraduate work | 36 (14.0) | 19 (14.8) | 17 (13.2) |
| Employment, n (%) | |||
| Employed full time (≥35 h/wk) | 141 (54.9) | 71 (55.5) | 70 (54.3) |
| Employed part time (<35 h/wk) | 46 (17.9) | 26 (20.3) | 20 (15.5) |
| Unemployed | 70 (27.2) | 31 (24.2) | 39 (30.2) |
| Parity, n (%) | |||
| Primiparous | 69 (27.3) | 31 (24.6) | 38 (29.9) |
| Multiparous | 184 (72.7) | 95 (75.4) | 89 (70.1) |
| Weeks of gestation at study entry | 13.6 ± 1.8 | 13.4 ± 1.9 | 13.7 ± 1.6 |
| Weight at study entry, kg | 85.1 ± 16.4 | 86.0 ± 17.5 | 84.1 ± 15.2 |
| BMI at study entry, kg/m2 | 32.5 ± 5.3 | 32.6 ± 5.3 | 32.3 ± 5.2 |
| Weight status, n (%) | |||
| Overweight | 102 (39.7) | 48 (37.5) | 54 (41.9) |
| Obese | 155 (60.3) | 80 (62.5) | 75 (58.1) |
| Preconception weight,2 kg | 83.1 ± 16.4 | 83.8 ± 17.6 | 82.5 ± 15.3 |
| Preconception weight status,2n (%) | |||
| Overweight | 114 (44.4) | 52 (45.6) | 62 (54.4) |
| Obese | 139 (54.1) | 73 (52.5) | 66 (47.5) |
| Weight gain from preconception to study entry, kg | 1.9 ± 4.3 | 2.0 ± 5.0 | 1.8 ± 3.5 |
1Mean ± SD (all such values).
2Preconception weight was based on self-report and available from 125 enhanced usual care and 128 intervention participants (i.e., 3 enhanced-usual-care participants and 1 intervention participant did not report this information).
GWG
Weight-change variables are summarized in Table 2. Women in the intervention group had significantly lower weekly GWG rates compared with enhanced usual care (0.33 compared with 0.39 kg/wk, respectively; P = 0.02). The intervention group gained an average of 0.07 kg/wk (95% CI: −0.13, −0.02 kg/wk) less than the enhanced-usual-care group (P = 0.02, overall model r2 = 0.19). Across the entire pregnancy, from preconception to weeks 35–36, women in the intervention group gained a mean ± SD of 9.4 ± 6.9 kg compared with 11.2 ± 7.0 kg in the enhanced-usual-care group (P = 0.03). No significant group × time × demographic subgroup (ethnicity, BMI status, parity, age, income) interactions were observed. Overall, Hispanic ethnicity (β = −0.10; 95% CI:−0.17, −0.04; P < 0.001), obesity (β = −0.11; 95% CI:−0.17, −0.05; P < 0.0001), and multiparity (β = 0.07; 95% CI: 0.002, 0.13; P = 0.04) were related to a lower weekly GWG rate. No other significant main effects were observed. In additional analyses, the intervention was effective in reducing the weekly GWG rate from study entry until 24–26 wk of gestation (β = −0.12; 95% CI:−0.17, −0.06; P < 0.0001) but did not significantly (P = 0.85) affect GWG rate between 24–26 wk of gestation and 35 wk of gestation (Table 2).
TABLE 2.
GWG by treatment group1
| Group2 | |||||
|---|---|---|---|---|---|
| Enhanced usual | Intervention | Unstandardized coefficient | P for | ||
| care (n = 127) | (n = 129) | (SE)3 or OR4 | 95% CI | coefficient | |
| Rate of GWG,3 kg/wk | |||||
| Study entry until 35–36 wk of gestation | 0.39 ± 0.23 | 0.33 ± 0.25 | −0.07 (0.03) | −0.13, −0.02 | 0.02 |
| Study entry until 24–26 wk of gestation | 0.37 ± 0.23 | 0.27 ± 0.23 | −0.12 (0.03) | −0.17, −0.06 | 0.0001 |
| 24–26 wk until 35–36 wk of gestation | 0.43 ± 0.29 | 0.42 ± 0.26 | −0.006 (0.04) | −0.08, 0.06 | 0.85 |
| IOM guidelines for total GWG4 | |||||
| Exceed IOM, n (%) | 69 (53.9) | 53 (41.1) | 0.57 | 0.33, 0.95 | 0.03 |
| Mean gain, kg | 16.2 ± 4.7 | 15.5 ± 5.7 | |||
| Within IOM, n (%) | 30 (23.4) | 37 (28.7) | 1.34 | 0.75, 2.39 | 0.32 |
| Mean gain, kg | 8.3 ± 1.5 | 8.0 ± 1.4 | |||
| Below IOM, n (%) | 29 (22.7) | 39 (30.2) | 1.54 | 0.87, 2.79 | 0.13 |
| Mean gain, kg | 2.2 ± 3.1 | 2.3 ± 2.8 | |||
| Total weight gain from preconception to 35–36 wk of gestation,5 kg | 11.2 ± 7.0 | 9.4 ± 6.9 | −1.89 (0.87) | −3.60, −0.18 | 0.03 |
1GWG, gestational weight gain; IOM, Institute of Medicine.
2Values are means ± SDs or percentages reported without adjustment for covariates.
3Linear regression models were adjusted for total weeks of gestation at study entry, age, income, ethnicity, parity, study entry BMI category, and site. The estimates of regression coefficients were based on analyses that included the covariates. The rate of GWG from entry to delivery was defined as the difference between study entry and 35–36 wk (or 24–26 wk) of gestation-measured weights divided by the number of weeks between observations. The mean ± SD number of weeks of observation from study entry until 35–36 wk of gestation was 23.6 ± 3.9 wk and from study entry until 24–26 wk of gestation was 13.4 ± 2.2 wk.
4ORs from logistic regression models examining IOM recommendations for total GWG by treatment group with adjustments for age, income, ethnicity, parity, pregravid BMI category, and site. IOM guidelines for GWG were based on self-reported pregravid weight and measured height (used to compute BMI status as overweight or obese) and the difference between self-reported pregravid weight and 35–36 wk of gestation or the most proximal measured weight.
5Results from linear regression analysis for total GWG from preconception (self-reported weight) through 35 wk of gestation (measured weight) adjusted for age, income, ethnicity, parity, pregravid BMI category, and site. Results reflect the unstandardized coefficient (SE) based on the linear regression that included the covariates.
Also shown in Table 2, women in the intervention group were 43% less likely to exceed IOM recommendations for total GWG than were those in the enhanced–usual care group (OR: 0.57; 95% CI: 0.34, 0.95; P = 0.03; Table 2). No significant group × time × demographic subgroup (ethnicity, BMI status, parity, age, income) interactions were observed. Overall, women with prepregnancy obesity had higher odds of exceeding IOM guidelines for total GWG than did women with overweight (OR: 1.9; 95% CI: 1.1, 3.2; P = 0.02), but no other significant main effects were observed in relation to the IOM total GWG outcome.
Intervention adherence
From study entry to 35–36 wk of gestation, the intervention compared with enhanced usual care significantly increased the average number of meal replacement products consumed each day by an additional 0.57 products/d (95% CI: 0.45, 0.68 products/d) (P < 0.0001; Table 3). Within the intervention group alone, increased use of meal replacements was significantly related to decreased GWG rate (β = −0.07; 95% CI: −0.12, −0.03; P = 0.002). The proportion of participants who consumed ≥1 replacement/d from study entry to 35 wk of gestation increased by 38.2 percentage points (from 4.0% to 42.2%) in intervention participants but only modestly changed (2.4-percentage point increase, from 3.1% to 5.5%) among usual-care participants (P < 0.0001). The effect of the intervention group on lowering the GWG rate was reduced to a trend (P = 0.063) when changes in daily meal replacement intake were included in the model. Examining attendance, intervention participants attended a mean ± SD of 6.5 ± 2.5 visits; attendance was not significantly related to GWG rate (P = 0.49).
TABLE 3.
Changes in behavioral strategies by treatment group1
| Least-squares mean2 (95% CI) | |||||||
|---|---|---|---|---|---|---|---|
| Enhanced usual care (n = 128) | Intervention (n = 129) | P 3 | |||||
| Baseline | 35 wk | Baseline | 35 wk | Group | Time | Group × time | |
| Diet4 | |||||||
| Meal replacement products, n/d | 0.12 (0.04, 0.19) | 0.17 (0.09, 0.26) | 0.13 (0.05, 0.20) | 0.75 (0.66, 0.84) | 0.0001 | 0.0001 | 0.0001 |
| Total energy, kcal/d | 1777 (1681, 1873) | 1785 (1682, 1888) | 1743 (1649, 1837) | 1673 (1570, 1776) | 0.17 | 0.46 | 0.35 |
| Carbohydrates, % of energy/d | 49.41 (47.97, 50.86) | 49.62 (48.1, 51.17) | 51.47 (50.06, 52.89) | 53.21 (51.65, 54.78) | 0.0003 | 0.15 | 0.25 |
| Protein, % of energy/d | 17.33 (16.72, 17.93) | 17.06 (16.41, 17.71) | 16.18 (15.59, 16.78) | 16.80 (16.14, 17.44 | 0.03 | 0.53 | 0.11 |
| Fat, % of energy/d | 36.08 (33.99, 38.17) | 35.05 (33.11, 36.99) | 36.87 (34.8, 38.95) | 34.05 (32.15, 35.95) | 0.93 | 0.01 | 0.23 |
| Physical activity5 | |||||||
| Sleep time, min/d | 454.0 (443.9, 464.2) | 430.1 (419.1, 441.1) | 450.2 (440.2, 460.2) | 421.6 (409.8, 433.4) | 0.27 | 0.0001 | 0.45 |
| Total physical activity, VM sum/d | 5853.7 (5847, 5857) | 5846.6 (5843, 5854) | 5852.8 (5845, 5855) | 5845.6 (5838, 5850) | 0.87 | 0.21 | 0.99 |
| Nonsedentary activity, min/d | 432.7 (416.6, 448.8) | 424.5 (407.9, 441.1) | 430.2 (414.3, 446.2) | 439.9 (422.9, 456.7) | 0.80 | 0.92 | 0.65 |
| Locomotion, min/d | 14.5 (12.6, 16.4) | 12.6 (10.1, 14.0) | 15.0 (13.0, 16.7) | 12.0 (10.0, 14.0) | 0.97 | 0.03 | 0.62 |
| MVPA, min/d | 135.1 (128.5, 146.2) | 126.8 (117.9, 136.2) | 133.2 (125.9, 143.4) | 123.4 (116.5, 135.2) | 0.63 | 0.10 | 0.88 |
| Weight-control strategies6 | |||||||
| Total score | 40.13 (38.2, 42.1) | 37.3 (35.4, 39.2) | 41.3 (35.3, 39.2) | 66.2 (64.1, 68.2) | 0.37 | 0.78 | 0.0001 |
| Dietary strategies | 24.3 (23.0, 25.7) | 24.3 (23.1, 25.6) | 24.8 (23.4, 26.3) | 27.6 (26.3, 29.0) | 0.62 | 0.31 | 0.005 |
| Self-monitoring strategies | 3.9 (2.9, 4.9) | 2.8 (1.6, 3.9) | 3.9 (2.8, 4.9) | 17.9 (16.7, 19.1) | 0.99 | 0.56 | 0.0001 |
| Physical activity strategies | 7.0 (5.9, 8.1) | 5.3 (4.1, 6.4) | 7.3 (6.1, 8.5) | 11.8 (10.6, 13.0) | 0.76 | 0.96 | 0.0001 |
| Psychological coping | 4.9 (4.0, 5.9) | 4.9 (3.8, 6.0) | 5.3 (4.3, 6.3) | 8.8 (7.6, 9.9) | 0.62 | 0.29 | 0.0001 |
| Eating behaviors7 | |||||||
| Dietary restraint | 12.1 (11.3, 12.9) | 11.0 (10.1, 11.9) | 12.0 (11.1, 12.9) | 14.7 (13.8, 15.6) | 0.88 | 0.81 | 0.0001 |
| Dietary disinhibition | 5.8 (5.3, 6.4) | 5.2 (4.7, 5.7) | 5.3 (4.7, 5.9) | 4.7 (4.2, 5.3) | 0.18 | 0.09 | 0.78 |
1ASA-24, National Cancer Institute Automated Self-Administered 24-h recall; MVPA, moderate to vigorous physical activity; VM, vector magnitude.
2The least-squares mean group values were estimated with covariates in the model (weeks of gestation at study entry, age, income, ethnicity, parity, BMI category, site) and presented by group along with the corresponding 95% CIs and P values.
3Linear mixed-effects models included group, time, weeks of gestation at study entry, age, income, ethnicity, parity, BMI category, site, and the time × group interaction.
4Diet was assessed via the ASA-24; 99.2% (n = 254 of 256) of participants completed this assessment at baseline and 82.4% (n = 211 of 256) at 35 wk of gestation.
5Physical activity was based on objective assessment for a 1-wk period; 95.7% (n = 245 of 256) of participants completed this at baseline and 78.5% (n = 201 of 256) at 35 wk of gestation. Of these, proportions with valid data (i.e., as >1 d of wear time with >19.2 h/d, >1200 min/d for sedentary time, and <400 min/d for MVPA) of the analytic sample included 230 of 256 participants (89.8%) at baseline and 187 of 256 participants (73.0%) at 35 wk of gestation.
6The Weight Control Strategies Scale (39) was used to assess the extent to which participants practiced behavioral weight-control strategies. Subscales include dietary strategies (e.g., “I had several servings of fruit and/or vegetables each day”), self-monitoring strategies (e.g., “I kept a record of the type and amount of food I ate”), physical activity (e.g., “I engaged in moderate-intensity exercise like brisk walking or something similar to brisk walking for at least 30 minutes a day”), and psychological coping (e.g., “If I had negative thoughts about my weight loss progress, I tried to catch myself and stop that kind of thinking”). In total, 100% (n = 256 of 256) of participants completed this assessment at baseline and 82.0% (n = 210 of 256) completed it at 35 wk of gestation.
7The Eating Inventory (40) was used to assess restraint (i.e., self-initiated, cognitive attempts to restrict food intake) and disinhibition (i.e., loss of control over eating). In total, 100% (n = 256 of 256) of participants completed this assessment at baseline and 82.8% (n = 212 of 256) completed it at 35 wk of gestation.
Behavioral changes
We examined the impact of the intervention on calorie and macronutrient intakes, physical activity, weight-control strategies, and eating behaviors. There were no significant group × time interactions on calorie and macronutrient intakes (Table 3). In both groups over time, the percentage of calories from fat decreased. In addition, there were no significant group × time interactions in sleep and physical activity. In both groups over time, sleep and locomotion time declined (Table 3).
From study entry to 35–36 wk of gestation, the intervention significantly increased practice of weight-control strategies, including self-monitoring, diet and exercise strategies, and psychological coping. The intervention also significantly increased cognitive restraint (Table 3) but had no significant effect on dietary disinhibition. The effect of the intervention group on lowering GWG rate was removed (P = 0.29) when change in weight-control strategies (total score) was included in the model and was attenuated but not removed (unstandardized B = −0.067; 95% CI: −0.127, −0.007); P = 0.028) with the inclusion of dietary restraint.
Metabolic changes and pregnancy complications
From study entry to 35–36 wk of gestation the intervention compared with enhanced usual care significantly reduced triglycerides (P = 0.03) and resulted in trend reductions in leptin (P = 0.06), fasting glucose (P = 0.09), and systolic blood pressure (P = 0.06) (Table 4). Independent of group assignment, greater increases in GWG rate were related to increases in insulin (2.63 μU/mL; 95% CI: 1.63, 4.23 μU/mL; P < 0.0001), HOMA-IR (2.84; 95% CI: 1.67, 4.85; P < 0.0001), and C-peptide (1.52 ng/mL; 95% CI: 1.15, 2.01 ng/mL; P < 0.0001) but not triglycerides (P = 0.30), HDL cholesterol (P = 0.38), LDL cholesterol (P = 0.33), diastolic blood pressure (P = 0.81), systolic blood pressure (P = 0.70), or total cholesterol (P = 0.26). The incidence of pregnancy complications was similar between groups (Table 5).
TABLE 4.
Cardiovascular disease risk factors by treatment group
| Least-squares mean1 (95% CI) | |||||||
|---|---|---|---|---|---|---|---|
| Enhanced usual care (n = 127) | Intervention (n = 129) | P 2 | |||||
| Baseline | 35 wk | Baseline | 35 wk | Group | Time | Group × time | |
| Fasting glucose, mg/dL | 84.00 (85.54, 88.46) | 84.78 (83.20, 86.35) | 87.30 (85.89, 88.72) | 83.05 (81.40, 84.89) | 0.39 | 0.0001 | 0.09 |
| Fasting insulin, μU/mL | 14.37 (12.67, 16.06) | 19.33 (17.50, 21.16) | 13.32 (11.67, 14.97) | 18.09 (16.19, 20.00) | 0.25 | 0.0001 | 0.88 |
| HOMA-IR3 | 2.442 (2.188, 2.726) | 3.428 (3.049, 3.853) | 2.422 (2.178, 2.693) | 3.215 (2.851, 3.625) | 0.58 | 0.0001 | 0.46 |
| Fasting cholesterol, mg/dL | 188.6 (180.8, 196.5) | 242.2 (233.9, 250.6) | 189.7 (182.1, 197.3) | 238.9 (230.4, 247.4) | 0.81 | 0.0001 | 0.37 |
| Fasting HDL cholesterol, mg/dL | 67.1 (64.0, 70.1) | 66.5 (63.3, 69.7) | 67.4 (64.4, 70.3) | 68.2 (64.9, 71.4) | 0.60 | 0.88 | 0.41 |
| Fasting LDL cholesterol, mg/dL | 94.3 (87.6, 101.0) | 126.1 (118.8, 133.3) | 94.5 (88.0, 101.1) | 123.5 (116.1, 131.0) | 0.77 | 0.0001 | 0.55 |
| Fasting triglycerides, mg/dL | 136.1 (123.5, 148.6) | 253.0 (239.6, 266.4) | 139.0 (126.8, 151.2) | 236.8 (222.9, 250.6) | 0.37 | 0.0001 | 0.03 |
| Systolic blood pressure, mm Hg | 107.5 (105.4, 109.5) | 111.5 (109.4, 113.6) | 105.30 (103.4, 107.3) | 106.5 (104.4, 108.6) | 0.002 | 0.0006 | 0.06 |
| Diastolic blood pressure, mm Hg | 63.23 (61.6, 64.8) | 67.72 (66.1, 69.4) | 61.84 (60.3, 63.4) | 64.98 (63.31, 66.7) | 0.025 | 0.0001 | 0.24 |
| Leptin, μg/L | 46.9 (44.0, 49.9) | 55.01 (51.8, 58.3) | 50.10 (47.2, 52.9) | 54.2 (51.0, 57.5) | 0.52 | 0.0001 | 0.06 |
| C-peptide, ng/mL | 2.16 (1.95, 2.38) | 3.51 (2.27, 3.74) | 2.14 (1.93, 2.35) | 3.29 (3.04, 3.54) | 0.33 | 0.0001 | 0.28 |
1The mean group values were estimated by using least-square means with covariates in the model (weeks of gestation at study entry, age, income, ethnicity, parity, BMI category, site) and are presented by group along with the corresponding 95% CIs and P values.
2Linear mixed-effects models included group, time, weeks of gestation at study entry, age, income, ethnicity, parity, BMI category, site, and the time × group interaction. The following numbers of outliers were removed from specific analyses: C-peptide, n = 1; leptin, n = 15; insulin, n = 1.
3HOMA-IR was calculated based on the method by Matthews et al. (41).
TABLE 5.
Effect of treatment group on pregnancy complications1
| Enhanced usual | Intervention | OR | ||
|---|---|---|---|---|
| care (n = 128) | (n = 129) | (95% CI)2 | P 2 | |
| Preterm delivery (<36 wk) | 5 (3.9) | 4 (3.2) | 0.76 (0.18, 2.98) | 0.69 |
| Cesarean delivery | 40 (31.2) | 46 (36.8) | 1.24 (0.73, 2.13) | 0.43 |
| Preeclampsia | 8 (6.4) | 10 (8.0) | 1.29 (0.48, 3.58) | 0.62 |
| Maternal hypertension | 7 (5.6) | 5 (4.0) | 0.67 (0.18, 2.28) | 0.52 |
| Gestational diabetes3 | 24 (18.8) | 23 (17.8) | 0.90 (0.46, 1.96) | 0.77 |
| Low birth weight (<2500 g)4 | 7 (6.1) | 9 (8.3) | 1.84 (0.62, 5.83) | 0.27 |
| Macrosomia (>4000 g)4 | 9 (7.8) | 8 (7.4) | 0.95 (0.34, 2.63) | 0.92 |
1Values are n (%) unless otherwise indicated.
2Logistic regression models were adjusted for total weeks of gestation at study entry, age, income, ethnicity, parity, study entry BMI category, and site.
3At 24–27 wk of gestation, a 2-h 75-g oral-glucose-tolerance test was performed by research staff, and gestational diabetes was confirmed by using the International Association of Diabetes and Pregnancy Study Groups criteria (42). If a study-measured oral-glucose-tolerance test was not obtained (n = 96), clinic measures of gestational diabetes were included and based on the American College of Obstetricians and Gynecologists–endorsed, 2-step approach (43) criteria, a 1-h 50-g value ≥200 mg/dL, or a clinical chart indication of “diabetes” (we note that some clinics diagnosed and treated patients with gestational diabetes on the basis of glycated hemoglobin values ≤5.9).
4Based on measured weights that were available for 115 of 128 (89.8%) enhanced–usual care and 108 of 129 (83.7%) intervention infants.
DISCUSSION
A behavioral lifestyle intervention with partial meal replacement reduced weekly GWG rates from 0.39 to 0.33 kg and lowered by 43% the odds of exceeding IOM recommendations for total GWG. To our knowledge, this is the first randomized controlled trial during pregnancy of a multicomponent lifestyle intervention with partial meal replacement and is among the first studies to show a positive effect of an intervention to reduce GWG in ethnically diverse women with overweight or obesity.
The intervention's effect on reducing weekly GWG rate (by 0.07 kg/wk) may appear modest but should be interpreted in the context of overall pregnancy weight gain in women with overweight or obesity. The intervention reduced total GWG from 11.2 to 9.4 kg (a 16% reduction) and lowered the proportion of women who exceeded IOM recommendations for total GWG from 69% to 53% (a 23% reduction). Previous intervention studies, including our own, failed to reduce GWG or increase adherence to IOM guidelines in women with overweight or obesity (3–9). In addition, the intervention was effective in reducing GWG before 24–26 wk of gestation, a time frame strongly linked to later maternal postpartum weight retention (44) and childhood obesity (45).
A key component of the behavioral lifestyle intervention was the provision of meal replacement products. Although both groups used very few meal replacements at baseline, at 35 wk of gestation the intervention group had increased to an average of 0.75 products/d, and 42% of women reported the use of ≥1 meal replacement product/d. The increase in meal replacement intake was significantly related to reduced GWG rate in the intervention group. Moreover, adjustment for the use of meal replacement products or the use of the behavioral weight-loss strategies removed the significant effect of the lifestyle intervention on weekly GWG, suggesting that these aspects of the intervention were important in its success.
Although the intervention had no significant effects on overall calorie intake, the difference (70 kcal/d) between the intervention and enhanced usual care at 35 wk, if achieved throughout the intervention period (24 wk on average) would be sufficient to explain the difference in GWG between the 2 groups. The lack of differences between groups on other measures of dietary intake and activity may reflect the fact that these measures were not collected at midpregnancy, when the intervention appeared most effective in reducing GWG rates. The ASA-24 dietary recall instrument has been validated (34) but not in Hispanic populations, who made up 42% of our sample. In addition, self-reported dietary intake has been consistently underreported among women with obesity (46). The intervention had greater emphasis on dietary intake than on physical activity, because intake is likely the stronger predictor of GWG (47), so the lack of difference between the intervention and enhanced-usual-care groups on physical activity is not surprising. Although there is currently no consensus on the best method to process high-frequency wrist accelerometer data, we used the random forest method, which has been independently validated (48), and also processed the data by using a linear threshold-based method and found similar results. Future research should examine other strategies to decrease intake and increase physical activity during pregnancy and include more frequent measures of these behaviors.
The intervention significantly reduced triglycerides during pregnancy by ∼13 mg/dL relative to enhanced usual care and resulted in trend improvements in leptin, fasting glucose, and systolic blood pressure. The reduction in triglycerides could theoretically translate into a reduced risk of maternal and fetal complications and long-term maternal cardiovascular disease (49), but larger samples sizes will be needed to determine those relations.
The intervention had no significant effect on the incidence of preterm delivery or low birth weight, gestational diabetes, preeclampsia, cesarean delivery, hypertension, or macrosomia, but these outcomes occur relatively infrequently, necessitating larger-scale trials. Although the incidence of inadequate GWG was also not significant, the OR (95% CI) of 1.54 (0.87, 2.79) suggested a potential association for examination in future research. The current study is part of the LIFE-Moms consortium (25), which will allow a larger sample size to further investigate the effects of GWG lifestyle interventions on maternal and neonatal complications.
The success of this intervention raises the important next question of whether it could be disseminated through usual prenatal care. Pregnant women with obesity increasingly receive advice about GWG from prenatal care nurses, dietitians, public health aides, WIC counselors, and other providers (50); these members of the health care team may be in a position provide the content of the lifestyle intervention to these women. In addition, the contact schedule used in the intervention was similar to that in enhanced usual care. Meal replacement programs have been effectively implemented in busy health care settings (51). Interestingly, in our study, the use of meal replacements—not frequency of attendance—was related to reduced GWG rate, suggesting that providing meal replacements with minimal counseling may be sufficient to reduce GWG. In our study, the meal replacements were provided free of charge but would have cost the participant an estimated $420 in total (assuming 1 replacement/d at $2.50 each over 24 wk). Future trials are needed to adapt and test the intervention as part of usual prenatal care and to evaluate its cost-effectiveness.
Strengths of this study include the randomized blinded design and the novel intervention strategy that may have practical relevance during prenatal care. The study also included a diverse sample, and all but one participant completed the study. Limitations are that the enhanced–usual care group was not matched with the intervention group on the number of contacts or other intervention components, so it remains unknown if the observed weight differences were attributable to increased contact with the interventionist or other components of the behavioral intervention package. Moreover, the enhanced–usual care group received one face-to-face educational visit and newsletters, which could have reduced the typical GWG rate. Group assignment and covariates accounted for 19% of the variance in GWG rate, suggesting other unmeasured factors. Participants were not blinded to treatment assignment, which could have biased responses to meal replacement intake and other self-reported measures. Women enrolled in the study at 13 wk of gestation on average, which may be too late to curb excessive GWG in some women (3). Future analysis will examine the long-term effects of this intervention on postpartum outcomes.
In sum, this study showed that a behavioral lifestyle intervention with partial meal replacement reduced the rate of GWG and incidence of excessive GWG in Hispanic and non-Hispanic women with overweight or obesity. The intervention offers an alternative to current guidelines, which recommend general healthy eating and activity for pregnant women yet have little evidence for reducing GWG in women with obesity (1). Given the significant beneficial effects of this intervention on GWG in women with overweight or obesity, future research is needed to examine the effectiveness of this program as part of routine prenatal care.
Supplementary Material
Acknowledgements
We acknowledge our unpaid recruitment sites, including the following—California: Pacific Central Coast Health Centers, including Santa Maria Women's Health Center, Bishop's Peak Women's Health Center, and Templeton Women's Health Center and the Community Health Centers in Templeton, San Luis Obispo, Santa Maria, and Nipomo; Creating Harmony Women's Healthcare, French Hospital Medical Center; Marian Regional Medical Center; and San Luis Obispo and Santa Barbara WIC clinics; Rhode Island: Women's Primary Care Center at Women and Infants Hospital; Center for Obstetrics-Gynecology (OB-GYN); Women's Care, Inc., in Providence, Pawtucket, and East Greenwich; Dr. Beitle, Bayside OB-GYN; Broadway OB/GYN; and Rhode Island WIC clinics. We thank the paid research team members in California, including Adilene Quintana-Diaz, Noemi Alarcon, Martha La Spina, Maria Legato, Samantha Lalush, Adrian Mercado, Nick Katsantones, Megan Ershov, Natali Valdez, Ana Stewart, Vanessa Rodriguez, Jill Jacoby, and Hannah Feldman; and in Rhode Island, including Juliana Duszlak (Luciani), Erica Ferguson-Robichaud, Denise Fernandes-Pierre, Kristen DeLayo, Kathryn Story, Stephanie Guerra, Patricia Sandoval, Zeely Sylvia-Denmat, Isabella Cassell, Julie Krol, Sarah Morris, Briana Borgolini, Molly La Rue, Kaitlyn Dahlborg, Genevieve Ramos, Whitney Howie, and Leah Sabatino. We thank the LIFE-Moms consortium members for their paid contributions to the development and oversight of the common measures and procedures shared across the trials.
The authors’ responsibilities were as follows—SP, TAH, AS, EJ, CNH, BA, TOS, KM-C, MGP, and RRW: designed the research; SP, AB, AM, TAH, EJ, CNH, BA, TOS, KM-C, EY, MGP, and RRW: conducted the research; SP, AS, and SK: analyzed the data; SP, AS, and RRW: wrote the manuscript; SP: had primary responsibility for the final content; and all authors: read and approved the final manuscript. The authors had no potential conflicts of interest to declare.
Notes
Supported by the NIH National Heart, Lung, and Blood Institute (NHLBI; HL114377). LIFE-Moms is supported by the NIH through the National Institute of Diabetes and Digestive and Kidney Diseases [NIDDK; U01 DK094418, U01 DK094463, U01 DK094416, and 5U01 DK094466 (Research Coordinating Unit)]; the NHLBI (U01 HL114344 and U01 HL114377); the Eunice Kennedy Shriver National Institute of Child Health and Human Development (U01 HD072834); the National Center for Complementary and Integrative Health; the NIH Office of Research in Women's Health; the Office of Behavioral and Social Science Research; the Indian Health Service; and the Intramural Research Program of the NIDDK. Orgain and PureFit provided product purchase discounts in support of this study.
Supplemental Table 1 is available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used:
- ASA-24
National Cancer Institute Automated Self-Administered 24-h recall
- GWG
gestational weight gain
- IOM
Institute of Medicine
- IRB
institutional review board
- LIFE-Moms
Lifestyle Interventions for Expectant Moms.
REFERENCES
- 1. Rasmussen KM, Yaktine AL; Institute of Medicine Committee to Reexamine IOM Pregnancy Weight Guidelines. Weight gain during pregnancy: reexamining the guidelines. Washington (DC): National Academies Press; 2009. [PubMed] [Google Scholar]
- 2. Chu SY, Callaghan WM, Bish CL, D'Angelo D. Gestational weight gain by body mass index among US women delivering live births, 2004–2005: fueling future obesity. Am J Obstet Gynecol 2009;200:271, e1–7. [DOI] [PubMed] [Google Scholar]
- 3. Phelan S, Phipps MG, Abrams B, Darroch F, Schaffner A, Wing RR. Randomized trial of a behavioral intervention to prevent excessive gestational weight gain: the Fit for Delivery Study. Am J Clin Nutr 2009;93:772–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Guelinckx I, Devlieger R, Mullie P, Vansant G. Effect of lifestyle intervention on dietary habits, physical activity, and gestational weight gain in obese pregnant women: a randomized controlled trial. Am J Clin Nutr 2010;91:373–80. [DOI] [PubMed] [Google Scholar]
- 5. Skouteris H, McPhie S, Hill B, McCabe M, Milgrom J, Kent B, Bruce L, Herring S, Gale J, Mihalopoulos C, et al. Health coaching to prevent excessive gestational weight gain: a randomized-controlled trial. Br J Health Psychol 2016;21:31–51. [DOI] [PubMed] [Google Scholar]
- 6. Dodd JM, Kannieappan LM, Grivell RM, Deussen AR, Moran LJ, Yelland LN, Owens JA. Effects of an antenatal dietary intervention on maternal anthropometric measures in pregnant women with obesity. Obesity (Silver Spring) 2015;23:1555–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jeffries K, Shub A, Walker SP, Hiscock R, Permezel M. Reducing excessive weight gain in pregnancy: a randomised controlled trial. Med J Aust 2009;191:429–33. [DOI] [PubMed] [Google Scholar]
- 8. Rhodes ET, Pawlak DB, Takoudes TC, Ebbeling CB, Feldman HA, Lovesky MM, Cooke EA, Leidig MM, Ludwig DS. Effects of a low-glycemic load diet in overweight and obese pregnant women: a pilot randomized controlled trial. Am J Clin Nutr 2010;92:1306–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hui AL, Back L, Ludwig S, Gardiner P, Sevenhuysen G, Dean HJ, Sellers E, McGavock J, Morris M, Jiang D, et al. Effects of lifestyle intervention on dietary intake, physical activity level, and gestational weight gain in pregnant women with different pre-pregnancy body mass index in a randomized control trial. BMC Pregnancy Childbirth 2014;14:331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Thornton YS. Preventing excessive weight gain during pregnancy through dietary and lifestyle counseling: a randomized controlled trial. Obstet Gynecol 2009;114:173; author reply: 4. [DOI] [PubMed] [Google Scholar]
- 11. Shirazian T, Monteith S, Friedman F, Rebarber A. Lifestyle modification program decreases pregnancy weight gain in obese women. Am J Perinatol 2009;27:411–4. [DOI] [PubMed] [Google Scholar]
- 12. Olson CM, Strawderman MS, Reed RG. Efficacy of an intervention to prevent excessive gestational weight gain. Am J Obstet Gynecol 2004;191:530–6. [DOI] [PubMed] [Google Scholar]
- 13. Vesco KK, Karanja N, King JC, Gillman MW, Leo MC, Perrin N, McEvoy CT, Eckhardt CL, Smith KS, Stevens VJ. Efficacy of a group-based dietary intervention for limiting gestational weight gain among obese women: a randomized trial. Obesity (Silver Spring) 2014;22:1989–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vinter CA, Jorgensen JS, Ovesen P, Beck-Nielsen H, Skytthe A, Jensen DM. Metabolic effects of lifestyle intervention in obese pregnant women: results from the randomized controlled trial “Lifestyle in Pregnancy” (LiP). Diabet Med 2014;31:1323–30. [DOI] [PubMed] [Google Scholar]
- 15. Wolff S, Legarth J, Vangsgaard K, Toubro S, Astrup A. A randomized trial of the effects of dietary counseling on gestational weight gain and glucose metabolism in obese pregnant women. Int J Obes (Lond) 2008;32:495–501. [DOI] [PubMed] [Google Scholar]
- 16. Bogaerts AF, Devlieger R, Nuyts E, Witters I, Gyselaers W, Van den Bergh BR. Effects of lifestyle intervention in obese pregnant women on gestational weight gain and mental health: a randomized controlled trial. Int J Obes (Lond) 2013;37:814–21. [DOI] [PubMed] [Google Scholar]
- 17. Quinlivan JA, Lam LT, Fisher J. A randomised trial of a four-step multidisciplinary approach to the antenatal care of obese pregnant women. Aust N Z J Obstet Gynaecol 2011;51:141–6. [DOI] [PubMed] [Google Scholar]
- 18. Horan MK, McGowan CA, Gibney ER, Donnelly JM, McAuliffe FM. Maternal diet and weight at 3 months postpartum following a pregnancy intervention with a low glycaemic index diet: results from the ROLO randomised control trial. Nutrients 2014;6:2946–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Poston L, Bell R, Croker H, Flynn AC, Godfrey KM, Goff L, Hayes L, Khazaezadeh N, Nelson SM, Oteng-Ntim E, et al. Effect of a behavioural intervention in obese pregnant women (the UPBEAT study): a multicentre, randomised controlled trial. Lancet Diab Endocrin 2015;3:767–77. [DOI] [PubMed] [Google Scholar]
- 20. US Census Bureau [cited 2017 Oct 31]. The Hispanic Population 2010; 2010 Census Briefs. Available from: https://www.census.gov/prod/cen2010/briefs/c2010br-04.pdf. [Google Scholar]
- 21. Martin JA, Hamilton BE, Sutton PD, Ventura SJ, Menacker F, Kirmeyer S. Births: final data for 2004. Natl Vital Stat Rep 2006;55:1–101. [PubMed] [Google Scholar]
- 22. Heymsfield SB. Meal replacements and energy balance. Physiol Behav 2010;100:90–4. [DOI] [PubMed] [Google Scholar]
- 23. Makola D, Ash DM, Tatala SR, Latham MC, Ndossi G, Mehansho H. A micronutrient-fortified beverage prevents iron deficiency, reduces anemia and improves the hemoglobin concentration of pregnant Tanzanian women. J Nutr 2003;133:1339–46. [DOI] [PubMed] [Google Scholar]
- 24. Mardones-Santander F, Rosso P, Stekel A, Ahumada E, Llaguno S, Pizarro F, Salinas J, Vial I, Walter T. Effect of a milk-based food supplement on maternal nutritional status and fetal growth in underweight Chilean women. Am J Clin Nutr 1988;47:413–9. [DOI] [PubMed] [Google Scholar]
- 25. Clifton RG, Evans M, Cahill AG, Franks PW, Gallagher D, Phelan S, Pomeroy J, Redman LM, Van Horn L; LIFE-Moms Research Group Design of lifestyle intervention trials to prevent excessive gestational weight gain in women with overweight or obesity. Obesity (Silver Spring) 2016;24:305–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Conner P, Bartlett S, Mendelson M, Condon K, Sutcliffe C, editors. WIC participant and program characteristics 2008, WIC-08-PC. Alexandria (VA): USDA, Food and Nutrition Service, Office of Research and Analysis; 2010. [Google Scholar]
- 27. Bandura A. Social learning theory. Englewood Cliffs (NJ): Prentice-Hall; 1977. [Google Scholar]
- 28. Phelan S. Pregnancy: a “teachable moment” for weight control and obesity prevention. Am J Obstet Gynecol 2010;202:135, e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wing RR, Jeffery RW, Burton LR, Thorson C, Nissinoff KS, Baxter JE. Food provision vs structured meal plans in the behavioral treatment of obesity. Int J Obes Relat Metab Disord 1996;20:56–62. [PubMed] [Google Scholar]
- 30. Artal R, Catanzaro RB, Gavard JA, Mostello DJ, Friganza JC. A lifestyle intervention of weight-gain restriction: diet and exercise in obese women with gestational diabetes mellitus. Appl Physiol Nutr Metab 2007;32:596–601. [DOI] [PubMed] [Google Scholar]
- 31. Otten JJ, Hellwig JP, Meyers LD. DRI, Dietary Reference Intakes: the essential guide to nutrient requirements. Washington (DC): National Academies Press; 2006. [Google Scholar]
- 32. Committee on Obstetric Practice Exercise during pregnancy and the postpartum period. American College of Obstetricians and Gynecologists Committee Opinion. No. 267. Int J Gynaecol Obstet 2002;77:79–81. [DOI] [PubMed] [Google Scholar]
- 33. Headen I, Cohen AK, Mujahid M, Abrams B. The accuracy of self-reported pregnancy-related weight: a systematic review. Obes Rev 2017;18:350–69. [DOI] [PubMed] [Google Scholar]
- 34. Kirkpatrick SI, Subar AF, Douglass D, Zimmerman TP, Thompson FE, Kahle LL, George SM, Dodd KW, Potischman N. Performance of the Automated Self-Administered 24-hour Recall relative to a measure of true intakes and to an interviewer-administered 24-h recall. Am J Clin Nutr 2014;100:233–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Subar AF, Kirkpatrick SI, Mittl B, Zimmerman TP, Thompson FE, Bingley C, Willis G, Islam NG, Baranowski T, McNutt S, et al. The Automated Self-Administered 24-hour dietary recall (ASA24): a resource for researchers, clinicians, and educators from the National Cancer Institute. J Acad Nutr Diet 2012;112:1134–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sasaki JE, John D, Freedson PS. Validation and comparison of ActiGraph activity monitors. J Sci Med Sport 2011;14:411–6. [DOI] [PubMed] [Google Scholar]
- 37. van Hees VT, Sabia S, Anderson KN, Denton SJ, Oliver J, Catt M, Abell JG, Kivimaki M, Trenell MI, Singh-Manoux A. A novel, open access method to assess sleep duration using a wrist-worn accelerometer. PLoS One 2015;10:e0142533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Staudenmayer J, He S, Hickey A, Sasaki J, Freedson P. Methods to estimate aspects of physical activity and sedentary behavior from high-frequency wrist accelerometer measurements. J Appl Physiol (1985) 2015;119:396–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pinto AM, Fava JL, Raynor HA, LaRose JG, Wing RR. Development and validation of the Weight Control Strategies Scale. Obesity (Silver Spring) 2013;21:2429–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Stunkard AJ, Messick S. The Three-Factor Eating Questionnaire to measure dietary restraint, disinhibition and hunger. J Psychosom Res 1985;29:71–83. [DOI] [PubMed] [Google Scholar]
- 41. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–9. [DOI] [PubMed] [Google Scholar]
- 42. Sacks DA, Hadden DR, Maresh M, Deerochanawong C, Dyer AR, Metzger BE, Lowe LP, Coustan DR, Hod M, Oats JJ, et al. Frequency of gestational diabetes mellitus at collaborating centers based on IADPSG consensus panel-recommended criteria: the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study. Diabetes Care 2012;35:526–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Committee on Practice Bulletins-Obstetrics Practice bulletin no. 180: gestational diabetes mellitus. Obstet Gynecol 2017;130:17–37. [DOI] [PubMed] [Google Scholar]
- 44. Walter JR, Perng W, Kleinman KP, Rifas-Shiman SL, Rich-Edwards JW, Oken E. Associations of trimester-specific gestational weight gain with maternal adiposity and systolic blood pressure at 3 and 7 years postpartum. Am J Obstet Gynecol 2015;212:499.e1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hivert MF, Rifas-Shiman SL, Gillman MW, Oken E. Greater early and mid-pregnancy gestational weight gains are associated with excess adiposity in mid-childhood. Obesity (Silver Spring) 2016;24:1546–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Trabulsi J, Schoeller DA. Evaluation of dietary assessment instruments against doubly labeled water, a biomarker of habitual energy intake. Am J Physiol Endocrinol Metab 2001;281:E891–9. [DOI] [PubMed] [Google Scholar]
- 47. Phelan S, Jankovitz K, Hagobian T, Abrams B. Reducing excessive gestational weight gain: lessons from the weight control literature and avenues for future research. Womens Health (Lond) 2011;7:641–61. [DOI] [PubMed] [Google Scholar]
- 48. Ellingson LD, Hibbing PR, Kim Y, Frey-Law LA, Saint-Maurice PF, Welk GJ. Lab-based validation of different data processing methods for wrist-worn ActiGraph accelerometers in young adults. Physiol Meas 2017;38:1045–60. [DOI] [PubMed] [Google Scholar]
- 49. Ghio A, Bertolotto A, Resi V, Volpe L, Di Cianni G. Triglyceride metabolism in pregnancy. Adv Clin Chem 2011;55:133–53. [DOI] [PubMed] [Google Scholar]
- 50. Mercado A, Marquez B, Abrams B, Phipps MG, Wing RR, Phelan S. Where do women get advice about weight, eating, and physical activity during pregnancy? J Womens Health (Larchmt) 2017;26:951–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ashley JM, St Jeor ST, Schrage JP, Perumean-Chaney SE, Gilbertson MC, McCall NL, Bovee V. Weight control in the physician's office. Arch Intern Med 2001;161:1599–604. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.