Abstract
Background
Sepsis may contribute to more than 200 000 annual deaths in the USA. Little is known about the regional patterns of sepsis mortality and the community characteristics that explain this relationship. We aimed to determine the influence of community characteristics upon regional variations in sepsis incidence and case fatality.
Methods
We performed a retrospective analysis of data from the REasons for Geographic and Racial Differences in Stroke (REGARDS) cohort. Using US sepsis mortality data, we used two strategies for defining geographic regions: (i) Sepsis ‘Belt’ vs Non-Belt and (ii) Sepsis ‘Cluster’ vs Non-Cluster. We determined sepsis incidence and case fatality among REGARDS participants in each region, adjusting for participant characteristics. We examined the mediating effect of community characteristics upon regional variations in sepsis incidence and case fatality.
Results
Among 29 680 participants, 16 493 (55.6%) resided in the Sepsis Belt and 2958 (10.0%) resided in a Sepsis Cluster. Sepsis incidence was higher for Sepsis Belt than Non-Belt participants [adjusted hazard ratio (HR) = 1.14; 95% confidence interval (CI) = 1.02–1.24] and higher for Sepsis Cluster than Non-Cluster participants (adjusted HR = 1.18; 95% CI = 1.01–1.39). Sepsis case fatality was similar between Sepsis Belt and Non-Belt participants, as well as between Cluster and Non-Cluster participants. Community poverty mediated the regional differences in sepsis incidence.
Conclusions
Regional variations in sepsis incidence may be partly explained by community poverty. Other community characteristics do not explain regional variations in sepsis incidence or case fatality.
Keywords: sepsis, epidemiology, poverty, region, mediation analysis
Key Messages
Limited research has investigated regional patterns of sepsis incidence and case fatality among community-dwelling participants and the community characteristics that mediate this association.
Our study provides evidence that persons living in ‘Sepsis-Belt’ and ‘Sepsis Cluster’ communities may be likely to develop sepsis due to higher community-level poverty.
Community poverty may be a proxy for access to healthcare, healthcare utilization and healthcare literacy, therefore hindering treatment for community-acquired sepsis.
Introduction
Sepsis is defined as life-threatening organ dysfunction resulting from a dysregulated host response to infection. In the USA, sepsis is a significant public health concern, associated with a high mortality rate, and may contribute to over 200 000 annual deaths.1–4 Whereas prior studies have highlighted differences in regional sepsis mortality rates, few studies have examined these differences among participants in a large national prospective cohort.3,5 Therefore, little is known about the regional patterns of sepsis mortality and the community characteristics that mediate this relationship.
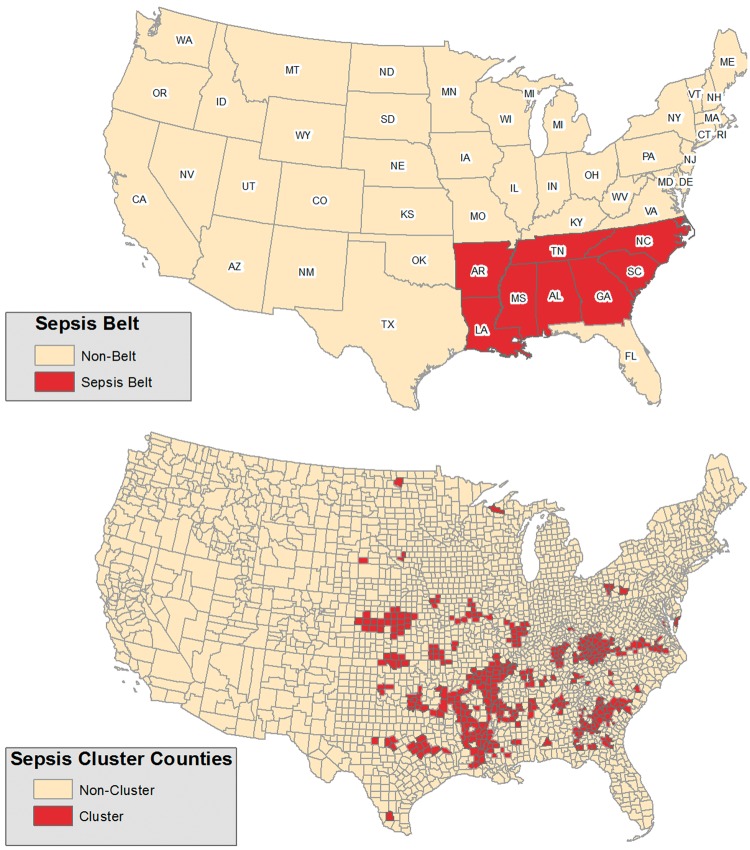
The study of geographic patterns of disease is important because clustering in a specific area or region may represent associations with localized population or environmental characteristics. For example, individuals living in the south-eastern USA are more likely to be obese and experience an increased risk of stroke.6 Research also suggests that individuals living in communities with higher poverty, a proxy for lower socio-economic status, are at increased risk of morbidity, mortality and bloodstream infections.7–10 Previous studies have reported that sepsis mortality in the USA may be characterized by two distinct patterns of regional variation: (i) a state-level ‘belt’ encompassing the states of Alabama, Arkansas, Georgia, Louisiana, Mississippi, North Carolina, South Carolina and Tennessee and (ii) county-level ‘clusters’ located in the Mississippi Valley, Central Appalachia and Middle Georgia.3,5
The underlying reasons for regional variations in sepsis mortality are unknown, but these patterns may be explained by differences in individual resident characteristics or characteristics related to their places of residence. The REasons for Geographic and Racial Differences in Stroke (REGARDS) cohort is one of the nation’s largest longitudinal population-based cohorts. In this study, we sought to determine the mediating effect of community characteristics upon regional variations in sepsis incidence and case fatality in the REGARDS cohort.
Methods
Ethics statement
The REGARDS study was approved by the institutional review boards of participating institutions, and all participants provided verbal consent before the telephone interview and written informed consent before the in-home study visit.
Study design and data source
We performed a retrospective analysis of prospectively collected data using REGARDS, a cohort of community-dwelling adults in the USA.11 Designed to evaluate the origins for racial and geographic differences in stroke mortality, REGARDS includes 30 239 participants aged ≥45 years at baseline. The cohort is 45% male, 41% Black race and 69% >60 years old. REGARDS recruited participants between January 2003 and October 2007. At 6-month intervals until 31 December 2012, REGARDS contacted the participants by telephone to identify any hospitalizations experienced by the participant in the previous 6 months. Further details related to REGARDS study methods are described elsewhere.11 Whereas the objective of REGARDS was to identify and characterize stroke events, the population of REGARDS included community-dwelling adults at healthy baseline.
Identification of sepsis incidence and fatality
The primary outcomes of this study were (i) first incident sepsis events and (ii) fatality after the sepsis event (case fatality). We included hospitalization events reported from 1 January 2003 through 31 December 2012. Using the taxonomy of Angus et al. (2001), we identified all hospitalizations (Emergency Department visits and/or hospital admission) attributed by participants to a serious infection (i.e. all hospitalizations with a bacterial, fungal or viral infectious process).1 We defined a sepsis event as a hospital admission for serious infection with the presence of at least two Systemic Inflammatory Response Syndrome (SIRS) criteria, including heart rate >90 beats/minute, fever (temperature >38.3°C or <36°C), tachypnea (>20 breaths/min) or PCO2 < 32 mmHg and leukocytosis (white blood cells >12 000 or <4000 cells/mm3 or >10% band forms).1 Based on medical records and/or death certificates, we defined sepsis case fatality as an either in-hospital death attributed to sepsis or death within 30 days after hospital discharge of a physician-adjudicated sepsis event.
We assessed vital signs and laboratory findings within the first 28 hours of hospitalization to include Emergency Department care and up to 1 full day of inpatient care. Our analysis focused on community-acquired sepsis, so we did not assess vital signs, laboratory findings or development of sepsis at later time points. Initial review of 1329 hospital records indicated excellent inter-rater consensus for the presence of serious infection (kappa = 0.92) and the presence of sepsis (kappa = 0.90) at the time of hospital presentation.
International consensus conferences (‘Sepsis-3’) have proposed new definitions for sepsis.12 Because of its common use in prior sepsis epidemiology studies, we used the SIRS-based sepsis definition as the primary analysis. However, in a sensitivity analysis, we repeated the analysis using the Sepsis-3 definition of sepsis as the presence of a serious infection plus a sequential organ failure assessment (SOFA) score ≥2.12
Definition of sepsis geographic regions
We previously described two strategies for characterizing regional sepsis variations using 2003–12 US national mortality data.3,5 In short, we defined sepsis deaths as all deaths attributed to an infection.3,13 We identified age- and sex-adjusted sepsis mortality rates for each US county.3 We observed that sepsis mortality was higher along a contiguous group of states encompassing the south-eastern USA (Alabama, Arkansas, Georgia, Louisiana, Mississippi, North Carolina, South Carolina and Tennessee); we defined this region as the ‘Sepsis Belt’. We categorized all REGARDS participants as residing in ‘Sepsis Belt’ or ‘Non-Belt’ regions (Figure 1).
Figure 1.
Sepsis exposure regions.
In a subsequent analysis, we identified sepsis county ‘hot spots’ of increased sepsis mortality using three geospatial autocorrelation measures [Empirical Bayes (EB) smoothed sepsis mortality rates, Local Indicators of Spatial Association (LISA)14 and the Getis-Ord Gi statistic15]; these regions were localized to the Mississippi Valley, Central Appalachia and Middle Georgia. From this prior analysis, US counties were designated as ‘Sepsis Clusters’ if they were identified as counties of high sepsis mortality based on at least two of the three geospatial methods.5 In this study, we categorized REGARDS participants as residing in ‘Sepsis Cluster’ or ‘Non-Cluster’ counties.
Participant characteristics
REGARDS participant demographics included self-reported age, race, sex, income, education and geographic location. Health behaviours included tobacco and alcohol use. Smoking status included current, past and never. We defined alcohol use as moderate (one drink per day for women or two drinks per day for men) and heavy alcohol use (more than drink per day for women and more than two drinks per day for men), per the National Institute on Alcohol Abuse and Alcoholism classification.16 Baseline medical conditions included atrial fibrillation, chronic lung disease, coronary artery disease, deep vein thrombosis, diabetes, dyslipidemia, hypertension, myocardial infarction, obesity, peripheral artery disease and stroke. We have provided detailed information regarding participant characteristics in Appendix A (available as Supplementary Data at IJE online).
Community characteristics
We determined the characteristics of REGARDS participant communities using 2010 American Community Survey (ACS) data available from the National Historical Geographic Information System (NHGIS).13,17 The ACS 2010 provides demographic information for each county for 2006–10. We determined community characteristics for this study based on publicly available variables that characterize county-level socio-economic status and healthcare availability, including median household income, percentage of the population completing college, percentage of the population below the poverty line, percentage of population without medical insurance coverage, unemployment rate, percentage of urban population and number of active medical doctors per 100 000 persons.5,18
Statistical analysis
We compared differences in participant and community characteristics between ‘Sepsis Belt’ vs ‘Non-Belt’ and ‘Sepsis Cluster’ vs ‘Non-Cluster’ participants. We used a chi-square test for categorical characteristics, analysis of variance (ANOVA) for normally distributed continuous characteristics and Kruskal-Wallis test for non-parametric continuous variables.
The objective of our analysis were to test for the mediating effect of community characteristics and not potential for effect modification. Therefore, we examined the mediating effect of community characteristics upon regional differences in sepsis incidence and case fatality. Mediators (or intervening/intermediary variables) are variables that are causally located between exposure and outcome variables, and that explain, in part, the effect of exposure on outcome.19–23 In a mediation model, the indirect (or mediation) effect represents the pathway in which an exposure affects an outcome indirectly through the mediator(s).19–23 In addition, the exposure can have a direct association with the outcome of interest, independently of the mediator variable.20–22
We determined the mediating effect of community characteristics on the association between region (Sepsis Cluster and Sepsis Belt) and the odds of sepsis and case fatality using PROCESS—a statistical macro developed by Hayes (2013) for mediation analysis.20–22 As all community characteristics may serve as equivalent mediators,24 we chose to fit four parallel logistic regression mediation models with the seven community characteristics. The first model examined the mediating effect of community characteristics upon differences in sepsis incidence between the Sepsis Belt and Non-Belt participants. We then repeated the analysis comparing Sepsis Cluster with Non-Cluster participants. We repeated these models using sepsis case fatality as the primary outcome.
We adjusted the estimates for participant socio-demographics, health behaviours and chronic medical conditions significant in univariate analysis (p-value ≤ 0.05). Our outcomes were binary, so we presented the indirect (mediation) effects and mediator-to-outcome associations (path b) as odds ratios (ORs) and their associated 95% confidence intervals (CIs), determined using a bootstrapping technique with 1000 resamples and with replacement.20–22,25 As our mediators were normalized continuous variables, we presented the exposure to mediator associations as parameter estimates (β estimates) and their associated standard errors (SEs), estimated from linear regression. We used SAS version 9.4, Stata version 13 and GeoDa version 1.6.7.9 for all statistical analyses. We used ArcGIS version 10.4 for all maps.
Results
Baseline participant characteristics
Among 30 239 REGARDS participants, there were a total of 1526 sepsis events, with the majority being attributed to pneumonia (38.2%), urinary tract infections (16.9%) and abdominal (15.5%) infections (Table 1). We excluded 559 participants from the analysis due to incomplete follow-up time, missing geocode data or missing county information. Among the 29 680 remaining in the analysis, 16 493 (55.6%) resided in the Sepsis Belt and 13 187 (44.4%) resided in Non-Belt areas (Table 2). Non-Belt participants were older and more likely to have Black race and male gender. Sepsis Belt participants reported lower education and income (p < 0.001). Current tobacco use was more common among Sepsis Belt participants, whereas Non-Belt participants were more likely to have moderate to heavy alcohol consumption. Sepsis Belt participants were more likely to have deep vein thrombosis, diabetes, dyslipidemia and hypertension (p < 0.05). There was a similar distribution of all other baseline medical conditions.
Table 1.
Infection characteristics of 1526 sepsis events
| N (%) | |
|---|---|
| Infection type | |
| Pneumonia | 583 (38.2) |
| Urinary tract infections | 258 (16.9) |
| Abdominal | 236 (15.5) |
| Bronchitis | 142 (9.3) |
| Skin | 138 (9.0) |
| Sepsis | 95 (6.2) |
| Fever of unknown origin | 29 (1.9) |
| Catheter/other | 45 (2.9) |
| Sequential organ failure assessment score | |
| 0 | 432 (28.3) |
| 1 | 375 (24.6) |
| ≥2 | 719 (47.1) |
Table 2.
REGARDS participant characteristics stratified by geographic region
| Geographic region |
||||||
|---|---|---|---|---|---|---|
| Sepsis Belta (N = 16 493) | Non-Belt (N = 13 187) | p-value* | Sepsis Clusterb (N = 2958) | Non-Cluster (N = 26 722) | p-value* | |
| Age† | 64.3 (9.3) | 65.6 (9.5) | <0.01 | 64.1 (9.4) | 65.0 (9.4) | <0.01 |
| Race (%) | ||||||
| Black | 6258 (37.9) | 5956 (45.2) | <0.01 | 1029 (35.5) | 11 165 (41.8) | <0.01 |
| White | 10 235 (62.1) | 7231 (54.8) | 1909 (64.5) | 15 557 (58.2) | ||
| Sex (%) | ||||||
| Male | 7040 (42.7) | 6289 (47.7) | <0.01 | 1268 (42.9) | 12 061 (45.1) | 0.02 |
| Female | 9453 (57.3) | 6898 (52.3) | 1690 (57.1) | 14 661 (54.9) | ||
| Education (%) | ||||||
| ≤High school | 2307 (14.0) | 1400 (10.6) | <0.01 | 510 (17.3) | 3197 (12.0) | <0.01 |
| High-school graduate | 4434 (26.9) | 3231 (24.5) | 880 (29.8) | 6785 (25.4) | ||
| Some college | 4331 (26.3) | 3616 (27.5) | 733 (24.8) | 7214 (27.0) | ||
| ≥College graduate | 5413 (32.8) | 4925 (37.4) | 833 (28.2) | 9505 (35.6) | ||
| Income (%) | ||||||
| ≤$20 000 | 3204 (19.4) | 2137 (16.2) | <0.01 | 643 (21.7) | 4698 (17.6) | <0.01 |
| $20 000–$34 000 | 3974 (24.1) | 3199 (24.3) | 732 (24.8) | 6441 (24.1) | ||
| $35 000–$74 000 | 4823 (29.2) | 3981 (30.2) | 837 (28.3) | 7967 (29.8) | ||
| ≥$75 000 | 2390 (14.5) | 2306 (17.5) | 370 (12.5) | 4326 (16.2) | ||
| Refused | 2102 (12.7) | 1564 (11.9) | 376 (12.7) | 3290 (12.3) | ||
| Tobacco use (%) | ||||||
| Never | 7577 (46.1) | 5802 (44.2) | <0.01 | 1394 (47.2) | 11 985 (45.0) | <0.01 |
| Past | 6416 (39.1) | 5489 (41.8) | 1106 (37.5) | 10 799 (40.6) | ||
| Current | 2436 (14.8) | 1846 (14.1) | 451 (15.3) | 3831 (14.4) | ||
| Alcohol use (%) | ||||||
| None | 10 861 (67.0) | 7377 (57.3) | <0.01 | 2105 (72.3) | 16 133 (61.6) | <0.01 |
| Moderate | 4739 (29.2) | 4948 (38.4) | 717 (24.6) | 8970 (34.3) | ||
| Heavy | 615 (3.8) | 560 (4.4) | 89 (3.1) | 1086 (4.2) | ||
| Chronic medical conditions (%) | ||||||
| Atrial fibrillation | 1454 (9.0) | 1094 (8.5) | 0.10 | 273 (9.4) | 2275 (8.7) | 0.19 |
| History of cancer | 1534 (9.3) | 1425 (10.8) | <0.01 | 269 (9.1) | 2690 (10.1) | 0.09 |
| Chronic lung disease | 1540 (9.3) | 1191 (9.0) | 0.37 | 267 (9.0) | 2464 (9.2) | 0.73 |
| Coronary artery disease | 2902 (17.9) | 2326 (18.0) | 0.93 | 584 (20.1) | 4644 (17.7) | <0.01 |
| Deep vein thrombosis | 916 (5.6) | 639 (4.9) | <0.01 | 187 (6.4) | 1368 (5.1) | <0.01 |
| Diabetes | 3872 (23.6) | 2826 (21.5) | <0.01 | 755 (25.6) | 5943 (22.3) | <0.01 |
| Dyslipidemia | 9559 (60.1) | 7393 (58.4) | <0.01 | 1807 (63.0) | 15 145 (58.9) | <0.01 |
| Hypertension | 9902 (60.2) | 7637 (58.1) | <0.01 | 1846 (62.6) | 15 693 (58.9) | <0.01 |
| Myocardial infarction | 2045 (12.6) | 1672 (12.9) | 0.47 | 416 (14.3) | 3301 (12.6) | 0.01 |
| Obesity | 8858 (53.8) | 7002 (53.2) | 0.27 | 1644 (55.7) | 14 216 (53.3) | 0.01 |
| Peripheral artery disease | 360 (2.2) | 303 (2.3) | 0.51 | 68 (2.3) | 595 (2.2) | 0.80 |
| Stroke | 1023 (6.2) | 873 (6.6) | 0.15 | 199 (6.8) | 1697 (6.4) | 0.43 |
†Mean (Standard deviation).
*Significance determined using ANOVA or chi-square test. (%) Denotes column percentages.
aStates with increased sepsis mortality (Alabama, Arkansas, Georgia, Louisiana, Mississippi, North Carolina, South Carolina and Tennessee).
cCounties with high sepsis mortality (fulfilling two of three criteria for geographic clustering).
Among the 29 680 REGARDS participants, 2958 (10%) resided in a Sepsis Cluster and 26 722 (90%) resided in a Non-Cluster community (Table 2). Sepsis Cluster participants were younger than Non-Cluster participants. Sepsis Cluster participants had a larger proportion of women and participants with White race than Non-Cluster participants. Non-Cluster participants reported higher education and income. Sepsis Cluster participants reported slightly higher current tobacco use (15.3% vs 14.4%) than Non-Cluster participants. Non-Cluster participants were more likely to be moderate to heavy alcohol users than Sepsis Cluster participants (p < 0.001). Sepsis Cluster participants were more likely to have a history of coronary artery disease, deep vein thrombosis, diabetes, dyslipidemia, hypertension, myocardial infarction and obesity (p < 0.05).
Community characteristics
Sepsis Belt participants lived in communities with lower median household income, value of housing units and a lower proportion of population that completed college when compared with Non-Belt participants (p < 0.001) (Table 3). Non-Belt participants lived in communities with lower poverty rates, greater population density and percentage urban (p < 0.001). Sepsis Belt participants lived in communities with a greater number of medical resources, characterized by larger proportions of primary care physicians, hospitals, beds and medical doctors per 100 000 persons (p < 0.001). Non-Belt participants resided in communities with lower uninsured population and lower unemployment rates (<0.001).
Table 3.
REGARDS participant community characteristics stratified by geographic region
| Geographical region |
||||||
|---|---|---|---|---|---|---|
| Sepsis Belta (N = 16 493) | Non-Beltb (N = 13 187) | p-value | Sepsis Clusterb (N = 2958) | Non-Cluster (N = 26 722) | p-value | |
| Community characteristic | ||||||
| Household Income† | 39 048 (8771) | 47 245 (12 656) | <0.01 | 39 400 (9090) | 43 055 (11 597) | <0.01 |
| % Completed college† | 17.1 (7.8) | 20.2 (8.9) | <0.01 | 16.3 (6.4) | 18.7 (8.6) | <0.01 |
| % Below poverty line† | 19.5 (6.6) | 13.7 (4.7) | <0.01 | 19.3 (6.9) | 16.7 (6.4) | <0.01 |
| % Urban† | 42.1 (29.1) | 52.5 (28.3) | <0.01 | 45.3 (27.5) | 46.8 (29.4) | <0.01 |
| Medical doctors۠ | 40.2 (163.7) | 22.5 (153.2) | <0.01 | 65.8 (252.7) | 28.6 (144.9) | <0.01 |
| % Without insurance coverage† | 20.4 (3.1) | 17.0 (5.4) | <0.01 | 20.9 (2.8) | 18.7 (4.7) | <0.01 |
| Unemployment rate† | 0.06 (0.02) | 0.05 (0.02) | <0.01 | 0.06 (0.02) | 0.05 (0.02) | <0.01 |
Community determined by participant residence.
†Mean (Standard deviation). €Ratio per 100 000 persons.
aStates with increased sepsis mortality (Alabama, Arkansas, Georgia, Louisiana, Mississippi, North Carolina, South Carolina and Tennessee). bCounties with high sepsis mortality (fulfilling two of three criteria for geographic clustering). Significance determined using ANOVA or Wilcoxon test.
Sepsis Cluster participants resided in communities with lower median household income, value of housing units and a lower proportion of population that completed college compared with Non-Cluster participants (p < 0.001). Sepsis Cluster participants lived in communities with higher poverty rates, lower population density and percentage urban (p < 0.001). Non-Cluster participants lived in communities with a lower number of medical resources, characterized by smaller proportions of primary care physicians, hospitals, beds and medical doctors per 100 000 persons (p < 0.001) when compared with Sepsis Cluster participants. Non-Cluster participants resided in communities with lower uninsured population and lower unemployment rates (p < 0.001).
Sepsis incidence and case fatality
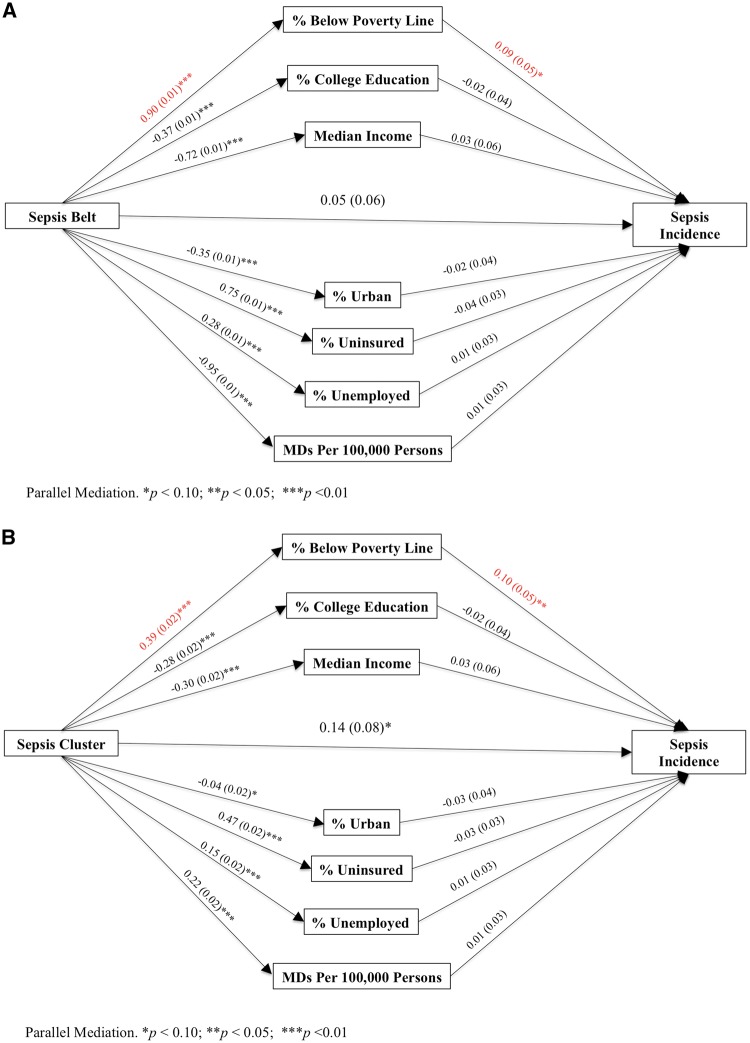
The odds of sepsis were higher among Sepsis Belt than Non-Belt participants after adjustment for age, sex, race, education, income, health behaviours, cancer, deep vein thrombosis, diabetes, dyslipidemia and hypertension (adjusted total effect OR = 1.14; 95% CI = 1.02–1.24, p-value = 0.02). The mediation effect of poverty on the association between Sepsis Belt residents and sepsis incidence did not reach significance (indirect effect OR = 1.08; 95% CI = 1.00–1.18; p-value = 0.06). Further, the mediation pathway explained that Sepsis Belt residents were more likely to live below the poverty line (β = 0.90; SE = 0.01; p-value < 0.01); however, living below the poverty line was non-significantly associated (mediator-to-outcome OR = 1.09; 95% CI = 0.99–1.19; p-value = 0.06) with higher odds of sepsis (Figure 2A and Appendix B, available as Supplementary Data at IJE online).
Figure 2.
Parallel mediation models for the associations between Sepsis Region, potential community mediators and sepsis incidence. Pathways represent the association between variables. For example, in Figure 2A, the Sepsis Belt has an effect of β = 0.90 on the percentage of population below poverty line, and poverty has an effect of β = 0.09 on sepsis incidence. Pathways are presented as β estimates (Standard Error) from logistic regression.
The odds of sepsis were also higher for Sepsis Cluster than Non-Cluster participants after adjustment for age, sex, race, education, income, health behaviours, CAD, deep vein thrombosis, diabetes, dyslipidemia, hypertension, MI and obesity (adjusted total effect OR = 1.18; 95% CI = 1.01–1.39, p-value = 0.046). In addition, the mediation pathway explained that Sepsis Cluster residents were more likely to live below the poverty line (β = 0.39; SE = 0.02; p-value < 0.01) and living below line was associated (mediator-to-outcome OR = 1.11; 95% CI = 1.01–1.21; p-value = 0.04) with higher odds of sepsis (Figure 2B and Appendix C, available as Supplementary Data at IJE online). No other community characteristics exhibited mediating effects upon the relationship between region and odds of sepsis.
Among REGARDS participants, there were a total of 197 sepsis deaths among participants. Sepsis case fatality was similar between Sepsis Belt and Non-Belt participants (adjusted total effect OR = 1.21; 95% CI = 0.87–1.66). Sepsis Case fatality was similar between Sepsis Cluster and Non-Cluster participants (adjusted total effect OR = 1.48; 95% CI = 0.96–2.30). Community characteristics did not mediate the associations between geographic region and sepsis case fatality (Appendices D and E, available as Supplementary Data at IJE online).
In sensitivity analysis, community characteristics did not mediate associations between region with sepsis or sepsis case fatality when sepsis was defined as infection plus a SOFA score ≥ 2 (Appendices F and G, available as Supplementary Data at IJE online).
Discussion
The objectives of this study were to determine the mediating effect of community characteristics upon regional variations in sepsis incidence and sepsis case fatality among the REGARDS cohort. In this study, we found that the association between region and sepsis was explained mostly by individual level factors as the regional differences in sepsis attenuated after adjustment for participant characteristics. Among community characteristics, community poverty explained the remaining variation in sepsis incidence, corresponding to the greatest mediating factor for both Sepsis Belt and Sepsis Clusters. We did not observe regional differences in risk of sepsis case fatality, and thus community-level mediating factors were not associated.
The findings of this study suggest that community impoverishment may partly explain the association between geographic region and sepsis risk. Impoverishment may impact health through access to personal health care providers, quality of care, healthcare literacy and availability of effective medical treatments.18 We found that counties in Sepsis Cluster or Sepsis Belt regions had larger numbers of medical physicians per 100 000 persons, but lower education and income. Our study delineates that persons living in regions with otherwise higher ‘access to care’ are still at greater odds for sepsis.5 As explained by Gulliford et al. (2002), ‘access’ to healthcare is a complex concept that involves multiple components and the process in which a person actually ‘gains access’ depends on financial, organizational, social and cultural barriers that limit the utilization of services.26
Our results highlight that residents of the Sepsis Cluster counties live in areas that are commonly affected by poorer health outcomes due to increased poverty.11,27–36 Areas of greater unemployment and lower SES are subsequently victim to greater health disparities that alternatively perpetuate cultural patterns which further reduce access to healthcare and opportunities for sustainable disease prevention.37 A possible explanation of regional differences in sepsis risk is that individuals from socio-economically disadvantaged communities are more likely adopt unhealthy diets.38 As explained by Gutierrez et al. (2015), an unhealthy ‘Southern’ diet consisting of greater consumption of energy intake derived from fats at the expense of protein and carbohydrates was associated with a 40% increased risk of sepsis.39 Neighbourhood poverty and lower personal income are associated with greater adherence to poorer dietary patterns due to the higher cost of healthy foods and availability of healthy, nutrient rich, affordable food within poorer neighbourhoods (i.e. food deserts).38 Therefore, it is important that US policy focuses on increasing employment opportunities in effort to remedy the disparities in health outcomes among those living in underserved communities. Interventions specifically focused on reducing sepsis risk may include targeting factors at both the community (e.g. cultural competence and customer service skills of medical professionals) and personal (e.g. health literacy and education) levels.40
Investigating the mediating role of community characteristics in the risk of sepsis allows for the possibility of intervening with health policy and implementation of needed healthcare resources. In the USA, socio-economic status remains an important determinant of health and mortality.7,41,42 Whereas geographic and socio-economic differences in diseases such as cancer and cardiovascular disease have been extensively researched,11,18,27,28,43–45 very few studies have attempted to uncover the community characteristics that predict sepsis risk. We found that community poverty was a weak mediator of the association between region and odds of sepsis, and similarly other studies have indicated that neighbourhood poverty and income are associated with bloodstream and bacterial infections.8,42,46 For example, Mendu et al. (2012) observed that, among critically ill patients, those living in communities with higher neighbourhood poverty rates were at a 49% increased risk of developing acute bloodstream infections. Possible explanations for the increased risk of bacterial infections among individuals from poorer communities is that individuals from lower SES environments are less likely to vaccinate and may have weakened immune function as a result of their environment.47–49 Another explanation for increased sepsis incidence among individuals of socio-economically disadvantaged communities is that these patients are likely to present with more complicated infections.50 Earlier treatment with intravenous fluid resuscitation and antibiotic administration is pivotal for reducing risk of sepsis complications.51,52 However, patients from historically underserved populations and impoverished environments are less likely to seek and obtain healthcare resources and have a lower level of trust in medical professionals.53 Whereas timing is key in the treatment of septic patients,51,52,54 impetus for individuals of lower SES communities receiving timely healthcare may be promoted through an affordable/universal healthcare system.55
Limitations
Whereas the REGARDS sepsis cohort provided a unique opportunity to explore the odds of sepsis and case fatality within a longitudinal cohort of community-dwelling adults, our study has several important limitations. REGARDS is a longitudinal study intended to investigate stroke among a racially dichotomous cohort of Black and White participants, and not sepsis outcomes. The current analysis is not a surveillance study and thus we may not have achieved complete ascertainment of all sepsis events. In addition, we did not observe any regional differences in sepsis case fatality, although these estimates were likely underpowered due to small number of sepsis deaths. We did not have information regarding the hospitals that REGARDS sepsis patients admitted to, and therefore were unable to address hospital sepsis quality and antibiotic resistance. A future longitudinal study dedicated to the surveillance and identification of sepsis among a nationally representative cohort could further delineate socio-demographic differences in risk of sepsis. In addition, we used county-level characteristics to approximate state-level community characteristics. Further, the mediation effect of poverty on the association between Sepsis Belt region and sepsis incidence did not reach statistical significance (p = 0.06) and should be interpreted accordingly. Nevertheless, the current study provides a foundational observation of the existing regional differences in sepsis incidence.
Conclusion
Using definitions of the ‘Sepsis Belt’ and ‘Sepsis Clusters’ previously defined using US mortality data, we found that, in the REGARDS cohort, participants living in the Sepsis Belt and Sepsis Cluster counties were at increased risk of sepsis but not at increased risk of case fatality following sepsis. The most significant mediating community characteristic on the relationship between region and sepsis incidence was the proportion of the population living below the poverty level. Future efforts should focus on sepsis prevention for participants residing in socio-economically disadvantaged communities.
Supplementary Data
Supplementary data are available at IJE online.
Supplementary Material
Acknowledgements
J.X.M., J.P.D., R.G., M.M.S., G.H., J.B. and H.E.W. conceived of the study. H.E.W., R.G., M.M.S., G.H. and J.B. oversaw data collection. J.X.M., J.P.D., H.E.W. conducted the analysis. J.X.M. drafted the manuscript and all authors contributed to its critical review. H.E.W. assumes overall responsibility for the paper. Representatives of the funding agencies have been involved in the review of the manuscript but not directly involved in the collection, management, analysis or interpretation of the data. The authors thank the other investigators, the staff and the participants of the REGARDS study for their valuable contributions. A full list of participating REGARDS investigators and institutions can be found at http://www.regardsstudy.org and http://www.regardssepsis.org.
Funding
This work was supported by award grant number R01-NR012726 from the National Institute for Nursing Research, grant number UL1-RR025777 from the National Center for Research Resources, as well as by grants from the Center for Clinical and Translational Science and the Lister Hill Center for Health Policy of the University of Alabama at Birmingham. The parent REGARDS study was supported by cooperative agreement (grant number U01-NS041588) from the National Institute of Neurological Disorders and Stroke, National Institutes of Health, Department of Health and Human Service. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies. Mr Donnelly received support from the Agency for Healthcare Research and Quality, Rockville, MD (grant number T32-HS013852) and National Institutes of General Medical Sciences, NIH (grant number F31-GM122180). Mr Moore received grant support from the Cancer Prevention and Control Training Program (grant R25 CA47888), funded by the National Cancer Institute, National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Conflict of interest: Dr Safford reports the following potential conflicts of interest: Amgen—salary support to study patterns of statin use in Medicare and other large databases; diaDexus—salary support for a research grant on lipids and CHD outcomes; diaDexus—consulting to help with FDA application; NIH, AHRQ—salary support for research grants. Mr Moore, Mr Donnelly and Drs Griffin, Wang, Howard and Baddley do not report any related conflicts of interest.
References
- 1. Angus DC, Linde-Zwirble WT, Lidicker J. et al. Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome, and associated costs of care. Crit Care Med 2001;29:1303–10. [DOI] [PubMed] [Google Scholar]
- 2. Wang HE, Shapiro NI, Angus DC. et al. National estimates of severe sepsis in United States emergency departments. Crit Care Med 2007;35:1928–36. [DOI] [PubMed] [Google Scholar]
- 3. Wang HE, Devereaux RS, Yealy DM. et al. National variation in United States sepsis mortality: a descriptive study. Int J Health Geogr 2010;9:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Angus DC. The lingering consequences of sepsis: a hidden public health disaster? JAMA 2010;304:1833–4. [DOI] [PubMed] [Google Scholar]
- 5. Moore JX, Donnelly JP, Griffin R. et al. Defining sepsis mortality clusters in the United States. Crit Care Med 2016; doi: 10.1097/CCM.0000000000001665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Howard G. Why do we have a stroke belt in the southeastern United States? A review of unlikely and uninvestigated potential causes. Am J Med Sci 1999;317:160–7. [DOI] [PubMed] [Google Scholar]
- 7. Isaacs SL, Schroeder SA. Class—the ignored determinant of the nation’s health. N Engl J Med;351:1137–42. [DOI] [PubMed] [Google Scholar]
- 8. Mendu ML, Zager S, Gibbons FK. et al. Relationship between neighborhood poverty rate and bloodstream infections in the critically ill. Crit Care Med 2012;40:1427–36. [DOI] [PubMed] [Google Scholar]
- 9. Miday RK, Wilson ER. Toxic shock syndrome: incidence and geographic distribution from a hospital medical records reporting system. Am J Public Health 1988;78:578–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Morrison C, Woodward M, Leslie W. et al. Effect of socioeconomic group on incidence of, management of, and survival after myocardial infarction and coronary death: analysis of community coronary event register. BMJ 1997;314:541–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Howard VJ, Cushman M, Pulley L. et al. The reasons for geographic and racial differences in stroke study: objectives and design. Neuroepidemiology 2005;25:135–43. [DOI] [PubMed] [Google Scholar]
- 12. Singer M, Deutschman CS, Seymour CW. et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA: J Am Med Assoc 2016;315:801–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wide-Ranging Online Data for Epidemiologic Research (CDC-WONDER). 2015. http://wonder.cdc.gov (30 March 2015, date last accessed).
- 14. Hollisaaz MT, Lorgard-Dezfuli-Nezad M, Assari S. et al. Medical comorbidities after renal transplantation. Transplant P 2007;39:1048–50. [DOI] [PubMed] [Google Scholar]
- 15. Nassel AF, Root ED, Haukoos JS. et al. Multiple cluster analysis for the identification of high-risk census tracts for out-of-hospital cardiac arrest (OHCA) in Denver, Colorado. Resuscitation 2014;85:1667–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Willenbring ML, Massey SH, Gardner MB. Helping patients who drink too much: an evidence-based guide for primary care clinicians. Am Fam Physician 2009;80:44–50. [PubMed] [Google Scholar]
- 17. Center MP. National Historical Geographic Information System: Version 2.0. In: 2011 UoM, editor. Minneapolis, MN2015. [Google Scholar]
- 18. Akinyemiju T, Waterbor JW, Pisu M. et al. Availability of healthcare resources and colorectal cancer outcomes among non-Hispanic White and non-Hispanic Black adults. J Commun Health 2015, 7 October, PubMed PMID: 26446012. [DOI] [PubMed] [Google Scholar]
- 19. Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol 1986;51:1173–82. [DOI] [PubMed] [Google Scholar]
- 20. Hayes AF, Scharkow M. The relative trustworthiness of inferential tests of the indirect effect in statistical mediation analysis: does method really matter? Psychol Sci 2013;24:1918–27. [DOI] [PubMed] [Google Scholar]
- 21. Hayes AF. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach. New York: Guilford Press, 2013. [Google Scholar]
- 22. Hayes AF, Preacher KJ. Statistical mediation analysis with a multicategorical independent variable. The British Journal of Mathematical and Statistical Psychology 2014;67:451–70. [DOI] [PubMed] [Google Scholar]
- 23. Vanderweele TJ, Vansteelandt S. Odds ratios for mediation analysis for a dichotomous outcome. Am J Epidemiol 2010;172:1339–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jones CL, Jensen JD, Scherr CL. et al. The Health Belief Model as an explanatory framework in communication research: exploring parallel, serial, and moderated mediation. Health Commun 2015;30:566–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods 2008;40:879–91. [DOI] [PubMed] [Google Scholar]
- 26. Gulliford M, Figueroa-Munoz J, Morgan M. et al. What does ‘access to health care’ mean? J Health Serv Res Po 2002;7:186–8. [DOI] [PubMed] [Google Scholar]
- 27. Howard G, Evans GW, Pearce K. et al. Is the stroke belt disappearing? An analysis of racial, temporal, and age effects. Stroke 1995;26:1153–8. [DOI] [PubMed] [Google Scholar]
- 28. Howard G. Why do we have a stroke belt in the southeastern United States? A review of unlikely and uninvestigated potential causes. Am J Med Sci 1999;317:160–7. [DOI] [PubMed] [Google Scholar]
- 29. Le A, Judd SE, Allison DB. et al. The geographic distribution of obesity in the US and the potential regional differences in misreporting of obesity. Obesity (Silver Spring) 2014;22:300–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pickle LW, Mungiole M, Gillum RF. Geographic variation in stroke mortality in blacks and whites in the United States. Stroke 1997;28:1639–47. [DOI] [PubMed] [Google Scholar]
- 31. Pickle LW, Gillum RF. Geographic variation in cardiovascular disease mortality in US blacks and whites. J Natl Med Assoc 1999;91:545–56. [PMC free article] [PubMed] [Google Scholar]
- 32. Lanska DJ, Kuller LH. The geography of stroke mortality in the United States and the concept of a stroke belt. Stroke 1995;26:1145–9. [DOI] [PubMed] [Google Scholar]
- 33. Gillum RF, Ingram DD. Relation between residence in the southeast region of the United States and stroke incidence: the NHANES I epidemiologic followup study. Am J Epidemiol 1996;144:665–73. [DOI] [PubMed] [Google Scholar]
- 34. Casper ML, Wing S, Anda RF. et al. The shifting stroke belt: changes in the geographic pattern of stroke mortality in the United States, 1962 to 1988. Stroke 1995;26:755–60. [DOI] [PubMed] [Google Scholar]
- 35. Lanska DJ, Peterson PM. Geographic variation in the decline of stroke mortality in the United States. Stroke 1995;26:1159–65. [DOI] [PubMed] [Google Scholar]
- 36. Howard G, Anderson R, Johnson NJ. et al. Evaluation of social status as a contributing factor to the stroke belt region of the United States. Stroke 1997;28:936–40. [DOI] [PubMed] [Google Scholar]
- 37. Thomas TL, DiClemente R, Snell S. Overcoming the triad of rural health disparities: how local culture, lack of economic opportunity, and geographic location instigate health disparities. Health Educ J 2014;73:285–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Darmon N, Drewnowski A. Contribution of food prices and diet cost to socioeconomic disparities in diet quality and health: a systematic review and analysis. Nutr Rev 2015;73:643–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gutierrez OM, Judd SE, Voeks JH. et al. Diet patterns and risk of sepsis in community-dwelling adults: a cohort study. BMC Infect Dis 2015;15:231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tajeu GS, Cherrington AL, Andreae L. et al. ‘We’ll get to you when we get to you’: exploring potential contributions of health care staff behaviors to patient perceptions of discrimination and satisfaction. Am J Public Health 2015;105:2076–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Smith GD, Hart C, Blane D. et al. Lifetime socioeconomic position and mortality: prospective observational study. BMJ 1997;314:547–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zager S, Mendu ML, Chang D. et al. Neighborhood poverty rate and mortality in patients receiving critical care in the academic medical center setting. Chest 2011;139:1368–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Brooks GA, Li L, Sharma DB. et al. Regional variation in spending and survival for older adults with advanced cancer. J Natl Cancer Inst 2013;105:634–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Perdue DG, Haverkamp D, Perkins C. et al. Geographic variation in colorectal cancer incidence and mortality, age of onset, and stage at diagnosis among American Indian and Alaska Native people, 1990–2009. Am J Public Health 2014;104(Suppl 3):S404–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kaplan GA, Keil JE. Socioeconomic factors and cardiovascular disease: a review of the literature. Circulation 1993;88:1973–98. [DOI] [PubMed] [Google Scholar]
- 46. Flory JH, Joffe M, Fishman NO. et al. Socioeconomic risk factors for bacteraemic pneumococcal pneumonia in adults. Epidemiol Infect 2009;137:717–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cohen S, Alper CM, Doyle WJ. et al. Objective and subjective socioeconomic status and susceptibility to the common cold. Health Psychol 2008;27:268–74. [DOI] [PubMed] [Google Scholar]
- 48. Cohen S, Doyle WJ, Turner RB. et al. Childhood socioeconomic status and host resistance to infectious illness in adulthood. Psychosom Med 2004;66:553–8. [DOI] [PubMed] [Google Scholar]
- 49. Luman ET, McCauley MM, Shefer A. et al. Maternal characteristics associated with vaccination of young children. Pediatrics 2003;111:1215–18. [PubMed] [Google Scholar]
- 50. Goodwin AJ, Nadig NR, McElligott JT. et al. Where you live matters: the impact of place of residence on severe sepsis incidence and mortality. Chest 2016;150:829–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kumar A, Roberts D, Wood KE. et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med 2006;34:1589–96. [DOI] [PubMed] [Google Scholar]
- 52. Gaieski DF, Mikkelsen ME, Band RA. et al. Impact of time to antibiotics on survival in patients with severe sepsis or septic shock in whom early goal-directed therapy was initiated in the emergency department. Crit Care Med 2010;38:1045–53. [DOI] [PubMed] [Google Scholar]
- 53. Richardson A, Allen JA, Xiao H. et al. Effects of race/ethnicity and socioeconomic status on health information-seeking, confidence, and trust. J Health Care Poor U 2012;23:1477–93. [DOI] [PubMed] [Google Scholar]
- 54. Zubert S, Funk DJ, Kumar A. Antibiotics in sepsis and septic shock: like everything else in life, timing is everything. Crit Care Med 2010;38:1211–12. [DOI] [PubMed] [Google Scholar]
- 55. Akinyemiju T, Jha M, Moore JX. et al. Disparities in the prevalence of comorbidities among US adults by state Medicaid expansion status. Prev Med 2016;88:196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.