Abstract
BACKGROUND
Reported declines in sperm counts remain controversial today and recent trends are unknown. A definitive meta-analysis is critical given the predictive value of sperm count for fertility, morbidity and mortality.
OBJECTIVE AND RATIONALE
To provide a systematic review and meta-regression analysis of recent trends in sperm counts as measured by sperm concentration (SC) and total sperm count (TSC), and their modification by fertility and geographic group.
SEARCH METHODS
PubMed/MEDLINE and EMBASE were searched for English language studies of human SC published in 1981–2013. Following a predefined protocol 7518 abstracts were screened and 2510 full articles reporting primary data on SC were reviewed. A total of 244 estimates of SC and TSC from 185 studies of 42 935 men who provided semen samples in 1973–2011 were extracted for meta-regression analysis, as well as information on years of sample collection and covariates [fertility group (‘Unselected by fertility’ versus ‘Fertile’), geographic group (‘Western’, including North America, Europe Australia and New Zealand versus ‘Other’, including South America, Asia and Africa), age, ejaculation abstinence time, semen collection method, method of measuring SC and semen volume, exclusion criteria and indicators of completeness of covariate data]. The slopes of SC and TSC were estimated as functions of sample collection year using both simple linear regression and weighted meta-regression models and the latter were adjusted for pre-determined covariates and modification by fertility and geographic group. Assumptions were examined using multiple sensitivity analyses and nonlinear models.
OUTCOMES
SC declined significantly between 1973 and 2011 (slope in unadjusted simple regression models −0.70 million/ml/year; 95% CI: −0.72 to −0.69; P < 0.001; slope in adjusted meta-regression models = −0.64; −1.06 to −0.22; P = 0.003). The slopes in the meta-regression model were modified by fertility (P for interaction = 0.064) and geographic group (P for interaction = 0.027). There was a significant decline in SC between 1973 and 2011 among Unselected Western (−1.38; −2.02 to −0.74; P < 0.001) and among Fertile Western (−0.68; −1.31 to −0.05; P = 0.033), while no significant trends were seen among Unselected Other and Fertile Other. Among Unselected Western studies, the mean SC declined, on average, 1.4% per year with an overall decline of 52.4% between 1973 and 2011. Trends for TSC and SC were similar, with a steep decline among Unselected Western (−5.33 million/year, −7.56 to −3.11; P < 0.001), corresponding to an average decline in mean TSC of 1.6% per year and overall decline of 59.3%. Results changed minimally in multiple sensitivity analyses, and there was no statistical support for the use of a nonlinear model. In a model restricted to data post-1995, the slope both for SC and TSC among Unselected Western was similar to that for the entire period (−2.06 million/ml, −3.38 to −0.74; P = 0.004 and −8.12 million, −13.73 to −2.51, P = 0.006, respectively).
WIDER IMPLICATIONS
This comprehensive meta-regression analysis reports a significant decline in sperm counts (as measured by SC and TSC) between 1973 and 2011, driven by a 50–60% decline among men unselected by fertility from North America, Europe, Australia and New Zealand. Because of the significant public health implications of these results, research on the causes of this continuing decline is urgently needed.
Keywords: human reproduction, male infertility, andrology, semen quality, sperm count, semen analysis, environmental effects, epidemiology, systematic review, meta-analysis
Introduction
Have sperm counts declined? This question remains as controversial today as in 1992 when Carlsen et al. (1992) wrote that: ‘There has been a genuine decline in semen quality over the past 50 years’. This controversy has continued unabated both because of the importance of the question and limitations in studies that have attempted to address it (Swan et al., 2000; Safe, 2013; Te Velde and Bonde, 2013).
Sperm count is of considerable public health importance for several reasons. First, sperm count is closely linked to male fecundity and is a crucial component of semen analysis, the first step to identify male factor infertility (World Health Organization, 2010; Wang and Swerdloff, 2014). The economic and societal burden of male infertility is high and increasing (Winters and Walsh, 2014; Hauser et al., 2015; Skakkebaek et al., 2016). Second, reduced sperm count predicts increased all-cause mortality and morbidity (Jensen et al., 2009; Eisenberg et al., 2014b, 2016). Third, reduced sperm count is associated with cryptorchidism, hypospadias and testicular cancer, suggesting a shared prenatal etiology (Skakkebaek et al., 2016). Fourth, sperm count and other semen parameters have been plausibly associated with multiple environmental influences, including endocrine disrupting chemicals (Bloom et al., 2015; Gore et al., 2015), pesticides (Chiu et al., 2016), heat (Zhang et al., 2015) and lifestyle factors, including diet (Afeiche et al., 2013; Jensen et al., 2013), stress (Gollenberg et al., 2010; Nordkap et al., 2016), smoking (Sharma et al., 2016) and BMI (Sermondade et al., 2013; Eisenberg et al., 2014a). Therefore, sperm count may sensitively reflect the impacts of the modern environment on male health throughout the life course (Nordkap et al., 2012).
Given this background, we conducted a rigorous and complete systematic review and meta-regression analysis of recent trends in sperm count as measured by sperm concentration (SC) and total sperm count (TSC), and their modification by fertility and geographic group.
Methods
This systematic review and meta-regression analysis was conducted and the results reported in accordance with MOOSE (Meta-analysis in Observational Studies in Epidemiology) (Stroup et al., 2000) and PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analysis) guidelines (Liberati et al., 2009; Moher et al., 2009) [checklists available upon request—contact corresponding author for access]. Our research team included epidemiologists, andrologists and a qualified medical librarian, with consultation with an expert in meta-analysis. Our predefined protocol, detailed in Supplementary Information, was developed following best practices (Borenstein et al., 2009; Higgins and Green, 2011; Program NT, 2015), and informed by two pilot studies, the first using all 1996 publications and the second all 1981 and 2013 publications.
Systematic review
The goal of the search was to identify all articles that reported primary data on human sperm count. We searched MEDLINE on November 21, 2014 and Embase (Excerpta Medica database) on December 10, 2014 for peer-reviewed, English-language publications. Following the recommendation of the Cochrane Handbook for Systematic Reviews, we searched in title and abstract for both index (MeSH) terms and keywords and filtered out animal-only studies. We used the MeSH term ‘sperm count’, which includes seven additional terms, and to increase sensitivity we added 13 related keywords (e.g. ‘sperm density’ and ‘sperm concentration’). We included all publications between January 1, 1981 (the first full year after the term ‘Sperm Count’ was added to MEDLINE as a MeSH term) and December 31, 2013 (the last full year at the time we began our MEDLINE search).
All studies that reported primary data on human SC were considered eligible for abstract screening. We evaluated the eligibility of all subgroups within a study. For example, in a case-control study, the control group might have been eligible for inclusion even though, based on our exclusion criteria, the case group was not.
We divided eligible studies into two fertility-defined groups: men unselected by fertility status, hereafter ‘Unselected’ (e.g. young men unlikely to be aware of their fertility such as young men screened for military service or college students); and fertile men, hereafter ‘Fertile’ (e.g. men who were known to have conceived a pregnancy, such as fathers or partners of pregnant women regardless of pregnancy outcome).
A study was excluded if study participants were selected based on: infertility or sub-fertility; range of semen parameters (e.g. studies selecting normospermic men); genital abnormalities, other diseases or medication. We also excluded studies limited to men with exposures that may affect fertility such as occupational exposure, post-intervention or smoking. Studies of candidates for vasectomy or semen donation were included only if semen quality was not a criterion for men's study participation. Studies with fewer than 10 men and those that used non-standard methods to collect or count sperm (e.g. methods other than masturbation for collection, or methods other than hemocytometer for counting) were also excluded.
First, based on the title and abstract the publication was either excluded or advanced to full text screening. Any publication without an abstract was automatically referred for full text screening. Second, we reviewed the full text and assigned it to exclusion within a specific category, or data extraction. We then confirmed study eligibility and identified multiple publications from the same study to ensure that estimates from the same population were not used more than once.
Data extraction
We extracted summary statistics on SC and TSC (mean, SD, SE, minimum, maximum, median, geometric mean and percentiles), mean or additional data on semen volume, sample size (for SC and for TSC), sample collection years and covariates: fertility group, country, age, ejaculation abstinence time, methods of semen collection, methods of assessing of SC and semen volume, selection of population and study exclusion criteria as well as number of samples per man. The range of permissible values, both for categorical and numerical variables, and information on data completeness were recorded. Data were extracted on all eligible subgroups separately as well as for the total population, if relevant. We attempted to extract data on additional potential confounders such as BMI, smoking and other lifestyle factors (e.g. alcohol and stress). However, except for smoking (which was examined in sensitivity analysis), data were available for such variables in only a minority of studies so these were not included in meta-regression analyses.
Quality control
The study was conducted following a predefined protocol (Supplementary Information). Screening for this extensive systematic review was conducted by a team of eight reviewers (H.L., N.J., A.M.A., J.M., D.W.D., I.M., J.D.M., S.H.S.). The screening protocol was piloted by screening of 50 abstracts by all reviewers followed by a comparison of results, resolution of any inconsistencies and clarification of the protocol as needed. The same quality control process was followed for full text screening (35 studies reviewed by all reviewers) and data extraction (data extracted from three studies by all reviewers). All data were entered into digital spreadsheets with explicit permissible values (no open-ended entries) to increase consistency. After data extraction, an additional round of data editing and quality control of all studies was conducted by H.L. The process ensured that each study was evaluated by at least two different reviewers.
Statistical analysis
We used point estimates of mean SC or mean TSC from individual studies to model time trends during the study period, as measured by slope of SC or TSC per calendar year. The midpoint of the sample collection period was the independent variable in all analyses. Units were million/ml for SC and million for TSC (defined as SC × sample volume) and all slopes denote unit change per calendar year.
We first used simple linear regression models to estimate SC and TSC as functions of year of sample collection, with each study weighted by sample size. We then used random-effects meta-regression to model both SC and TSC as linear functions of time, weighting studies by the SE. In all meta-regression analyses, we included indicator variables to denote studies with more than one SC estimate. We controlled for a pre-determined set of potential confounders: fertility group, geographic group, age, abstinence time, whether semen collection and counting methods were reported, number of samples per man and indicators for exclusion criteria (Supplementary Table S1).
For several key variables missing values were estimated and a variable was included in meta-regression analyses to denote that the value had been estimated. For example, for studies that reported median (not mean) SC or TSC, we estimated the mean by adding the average difference between the mean and median in studies for which both were reported. For studies that did not report the range or midpoint year of sample collection, the midpoint was estimated by subtracting the average difference between year of publication and midpoint year of sample collection in studies for which both were reported from publication year. When SD but not SE of SC or TSC were reported, the SE was calculated by dividing the SD by the square root of sample size for each estimate. For studies that did not report SD or SE, we estimated SE by dividing the mean SD of studies that reported SD by the square root of sample size for this estimate. If mean TSC was not reported it was calculated by multiplying mean SC by mean semen volume (Supplementary Information).
Our final analyses included two groups of countries. One group (referred to here as ‘Western’) includes studies from North America, Europe, Australia and New Zealand. The second (‘Other’/‘Non-Western’) includes studies from all other countries (from South America, Asia and Africa). We initially examined studies from North America separately from Europe/Australia but combined these because trends were similar and only 16% of estimates were from North America. We assessed modification of slope by fertility group (Unselected versus Fertile) and geographic group (Western versus Other). Because of significant modification by fertility and geography, results of models with interaction terms are presented for four categories: Unselected Western; Fertile Western; Unselected Other; and Fertile Other. Overall percentage declines were calculated by estimating the sperm count (SC or TSC) in the first and last year of data collection, and dividing the difference by the estimate in the first year. The percentage decline per year was calculated by dividing the overall percentage declines by the number of years.
We ran all analyses for TSC weighting by SE of TSC and adjusted for method used to assess semen volume: weighing, read from pipette, read from tube or other.
We conducted several sensitivity analyses; adding cubic and quadratic terms for year of sample collection in meta-regression analyses to assess non-linearity; excluding a specific group for each covariate, such as a group with incomplete information; removing covariates one at a time from the model; removing studies with SEs > 20 million/ml; replacing age group by mean age, excluding studies that did not report mean age; adding covariate for high smoking prevalence (>30%); excluding countries that contributed the greatest number of estimates in order to examine the influence of these countries; restricting analyses to studies with data collected after 1985 and after 1995 to examine recent trends.
All analyses were conducted using STATA version 14.1 (StataCorp, TX, USA). A value of P < 0.05 was considered significant for main effect and P< 0.10 for interaction.
Results
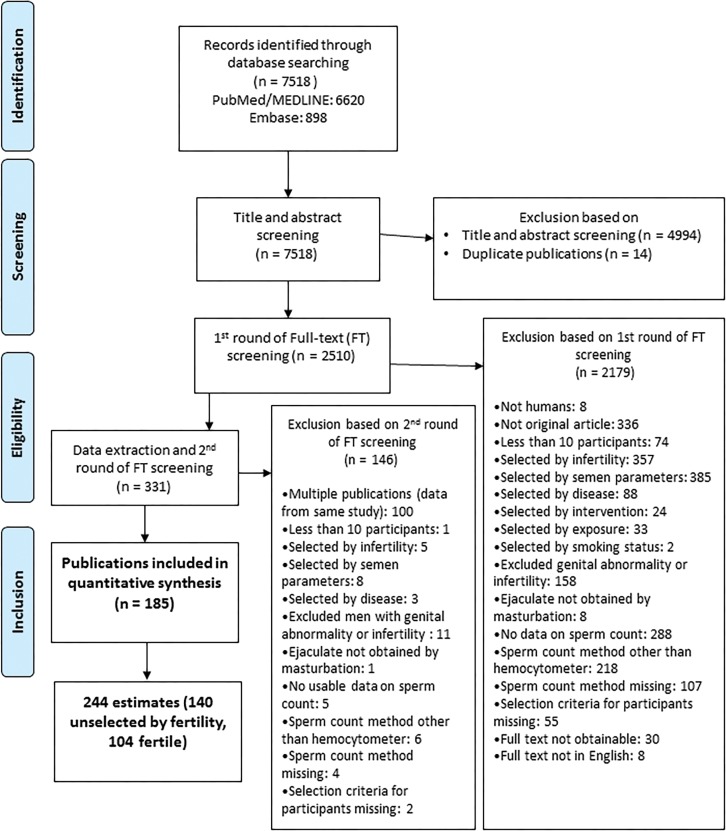
Systematic review and summary statistics
Using PubMed and Embase searches we identified 7518 publications meeting our criteria for abstract screening (Fig. 1). Of these, 14 duplicate records were removed and 4994 were excluded based on title or abstract screening. Full texts of the remaining 2510 articles were reviewed for eligibility and 2179 studies were excluded. Of the remaining 331 articles, 146 were excluded during data extraction and the second round of full text screening (mainly due to multiple publications). The meta-regression analysis is based on the remaining 185 studies, which included 244 unique mean SC estimates based on samples collected between 1973 and 2011 from 42 935 men. Data were available from 6 continents and 50 countries. The mean SC was 81 million/ml, the mean TSC was 260 million and the mean year of data sample collection was 1995. Of the 244 estimates, 110 (45%) were Unselected Western, 65 (27%) Fertile Western, 30 (12%) Unselected Other and 39 (16%) Fertile Other. Data from the 185 publications included in the meta-analysis are available upon request—contact corresponding author for access (Abyholm, 1981; Fariss et al., 1981; Leto and Frensilli, 1981; Wyrobek et al., 1981a,b; Aitken et al., 1982; Nieschlag et al., 1982; Obwaka et al., 1982; Albertsen et al., 1983; Fowler and Mariano, 1983; Sultan Sheriff, 1983; Wickings et al., 1983; Asch et al., 1984; de Castro and Mastrorocco, 1984; Fredricsson and Sennerstam, 1984; Freischem et al., 1984; Ward et al., 1984; Ayers et al., 1985; Heussner et al., 1985; Rosenberg et al., 1985; Aribarg et al., 1986; Comhaire et al., 1987; Kirei, 1987; Giblin et al., 1988; Kjaergaard et al., 1988; Mieusset et al., 1988, 1995; Jockenhovel et al., 1989; Sobowale and Akiwumi, 1989; Svanborg et al., 1989; Zhong et al., 1990; Culasso et al., 1991; Dunphy et al., 1991; Gottlieb et al., 1991; Nnatu et al., 1991; Pangkahila, 1991; Weidner et al., 1991; Levine et al., 1992; Sheriff and Legnain, 1992; Ali et al., 1993; Arce et al., 1993; Bartoov et al., 1993; Fedder et al., 1993; Noack-Fuller et al., 1993; World Health Organization, 1993; Hill et al., 1994; Rehan, 1994; Rendon et al., 1994; Taneja et al., 1994; Vanhoorne et al., 1994; Auger et al., 1995; Cottell and Harrison, 1995; Figa-Talamanca et al., 1996; Fisch et al., 1996; Irvine et al., 1996; Van Waeleghem et al., 1996; Vierula et al., 1996; Vine et al., 1996; Auger and Jouannet, 1997; Jensen et al., 1997; Lemcke et al., 1997; Handelsman, 1997a,b; Chia et al., 1998; Muller et al., 1998; Naz et al., 1998; Gyllenborg et al., 1999; Kolstad et al., 1999; Kuroki et al., 1999; Larsen et al., 1999; Purakayastha et al., 1999; Reddy and Bordekar, 1999; De Celis et al., 2000; Glazier et al., 2000; Mak et al., 2000; Selevan et al., 2000; Wiltshire et al., 2000; Zhang et al., 2000; Foppiani et al., 2001; Guzick et al., 2001; Hammadeh et al., 2001; Jorgensen et al., 2001, 2002, 2011, 2012; Kelleher et al., 2001; Lee and Coughlin, 2001; Patankar et al., 2001; Tambe et al., 2001; Xiao et al., 2001; Costello et al., 2002; Junqing et al., 2002; Kukuvitis et al., 2002; Luetjens et al., 2002; Punab et al., 2002; Richthoff et al., 2002; Danadevi et al., 2003; de Gouveia Brazao et al., 2003; Firman et al., 2003; Liu et al., 2003; Lundwall et al., 2003; Roste et al., 2003; Serra-Majem et al., 2003; Uhler et al., 2003; Xu et al., 2003; Ebesunun et al., 2004; Rintala et al., 2004; Toft et al., 2004, 2005; Bang et al., 2005; Mahmoud et al., 2005; Muthusami and Chinnaswamy, 2005; O'Donovan, 2005; Tsarev et al., 2005, 2009; Durazzo et al., 2006; Fetic et al., 2006; Giagulli and Carbone, 2006; Haugen et al., 2006; Iwamoto et al., 2006, 2013a,b; Pal et al., 2006; Yucra et al., 2006; Aneck-Hahn et al., 2007; Garcia et al., 2007; Multigner et al., 2007; Plastira et al., 2007; Rignell-Hydbom et al., 2007; Wu et al., 2007; Akutsu et al., 2008; Bhattacharya, 2008; Gallegos et al., 2008; Goulis et al., 2008; Jedrzejczak et al., 2008; Kobayashi et al., 2008; Korrovits et al., 2008; Li and Gu, 2008; Lopez-Teijon et al., 2008; Paasch et al., 2008; Peters et al., 2008; Recabarren et al., 2008; Recio-Vega et al., 2008; Saxena et al., 2008; Shine et al., 2008; Andrade-Rocha, 2009; Kumar et al., 2009, 2011; Rylander et al., 2009; Stewart et al., 2009; Vani et al., 2009, 2012; Verit et al., 2009; Engelbertz et al., 2010; Hossain et al., 2010; Ortiz et al., 2010; Rubes et al., 2010; Tirumala Vani et al., 2010; Al Momani et al., 2011; Auger and Eustache, 2011; Axelsson et al., 2011; Brahem et al., 2011; Jacobsen et al., 2011; Khan et al., 2011; Linschooten et al., 2011; Venkatesh et al., 2011; Vested et al., 2011; Absalan et al., 2012; Al-Janabi et al., 2012; Katukam et al., 2012; Mostafa et al., 2012; Nikoobakht et al., 2012; Rabelo-Junior et al., 2012; Splingart et al., 2012; Bujan et al., 2013; Girela et al., 2013; Halling et al., 2013; Ji et al., 2013; Mendiola et al., 2013; Redmon et al., 2013; Thilagavathi et al., 2013; Valsa et al., 2013; Zalata et al., 2013; Zareba et al., 2013; Huang et al., 2014).
Figure 1.
PRISMA Flow chart showing the selection of studies eligible for meta-regression analysis.
Simple linear models
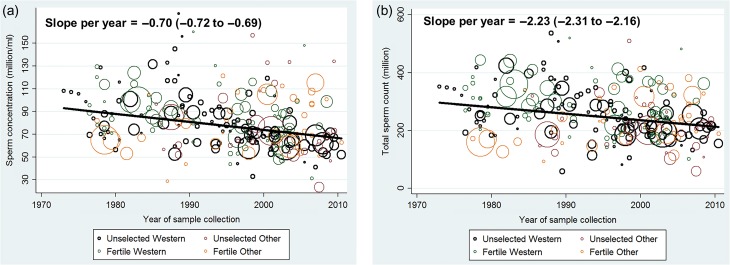
Combining results from all four groups of men SC declined significantly (slope per year −0.70 million/ml; 95% CI: −0.72 to −0.69; P < 0.001) over the study period when using simple linear models (unadjusted, weighted by sample size) (Fig. 2a). SC declined by 0.75% per year (95% CI: 0.73–0.77%) and overall by 28.5% between 1973 and 2011. A similar trend was seen for TSC (slope per year = −2.23 million; 95% CI: −2.31 to −2.16; P < 0.001) (Fig. 2b), corresponding to a decline in TSC of 0.75% per year (95% CI: 0.72–0.78%), and 28.5% overall. Semen volume (156 estimates), did not change significantly over the study period (slope per year = 0.0003 ml; 95% CI: −0.0003 to 0.0008; P = 0.382).
Figure 2.
(a) Mean sperm concentration by year of sample collection in 244 estimates collected in 1973–2011 and simple linear regression. (b) Mean total sperm count by year of sample collection in 244 estimates collected in 1973–2011 and simple linear regression.
Meta-regression models
We ran meta-regression models, unadjusted and adjusted, with and without interaction terms for fertility and geographic groups (Supplementary Table S2). In the simple meta-regression model for SC, in which estimates were weighted by their SE but without covariate adjustment, slopes were similar to those for simple regression, but with wider CIs (SC slope = −0.68; −0.99 to −0.37; P < 0.001). Covariate adjustment did not appreciably alter the slope but widened the CI further (−0.64; −1.06 to −0.22; P = 0.003).
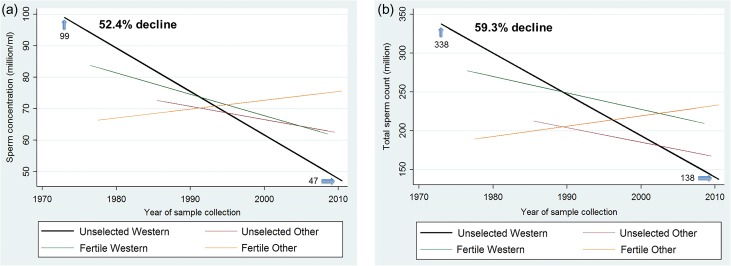
Slopes were significantly modified by the interaction of time with both fertility and geographic group. The three-way interaction term (time × fertility group × geographic group) was not significant (P = 0.57) and was not included in final models. In the final adjusted models for SC, which included two interaction terms [time × fertility group (P = 0.064) and time × geographic group (P = 0.027)], significant declines were seen among both Unselected Western (−1.38 million/ml/year, −2.02 to −0.74; P < 0.001) and Fertile Western (−0.68, −1.31 to −0.05; P = 0.033) (Table I, Fig. 3a), with a steeper slope for Unselected Western. Using estimates from the fully adjusted model of 99.0 million/ml in 1973 to 47.1 million/ml in 2011, SC in the Unselected Western group declined 1.4% per year and overall by 52.4% between 1973 and 2011.
Table I.
Sperm concentration and total sperm count in first and last years of meta-regression analysis with percentage change and slope per year, for all men and by fertility and geographic groupsa.
| Category | N (estimates) | First year | First year SC (million/ml) | Last year | Last year SC (million/ml) | Percentage change/year | Slope (95% CI), million/ml/year |
|---|---|---|---|---|---|---|---|
| All men | 244 | 1973 | 92.8 | 2011 | 66.4 | −0.75 | −0.70 (−0.72 to −0.69) |
| Unselected Western | 110 | 1973 | 99.0 | 2011 | 47.1 | −1.40 | −1.38 (−2.02 to −0.74) |
| Fertile Western | 65 | 1977 | 83.8 | 2009 | 62.0 | −0.81 | −0.68 (−1.31 to −0.05) |
| Unselected Other | 30 | 1986 | 72.7 | 2010 | 62.6 | −0.58 | −0.42 (−1.24 to 0.40) |
| Fertile Other | 39 | 1978 | 66.4 | 2011 | 75.7 | 0.42 | 0.28 (−0.44 to 1.00) |
| Category | N (estimates) | First Year | First year TSC (million) | Last year | Last year TSC (million) | Percentage change/year | Slope (95% CI), million/year |
| All men | 244 | 1973 | 295.7 | 2011 | 212.0 | −0.75 | −2.23 (−2.31 to −2.16) |
| Unselected Western | 110 | 1973 | 337.5 | 2011 | 137.5 | −1.58 | −5.33 (−7.56 to −3.11) |
| Fertile Western | 65 | 1977 | 277.4 | 2009 | 209.5 | −0.76 | −2.12 (−4.31 to 0.07) |
| Unselected Other | 30 | 1986 | 212.4 | 2010 | 167.3 | −0.88 | −1.88 (−4.77 to 1.01) |
| Fertile Other | 39 | 1978 | 189.2 | 2011 | 233.2 | 0.70 | 1.33 (−1.20 to 3.86) |
aFor all men: simple linear regression weighted by sample size. For all other categories: Meta-regression model weighted by sperm concentration (SC) SE, adjusted for fertility group, time x fertility group interaction, geographic group, time × geographic group interaction, age, abstinence time, semen collection method reported, counting method reported, having more than one sample per men, indicators for study selection of population and exclusion criteria (some vasectomy candidates, some semen donor candidates, exclusion of men with chronic diseases, exclusion by other reasons not related to fertility, selection by occupation not related to fertility), whether year of collection was estimated, whether arithmetic mean of SC was estimated, whether SE of SC was estimated and indicator variable to denote studies with more than one estimate. Total sperm count (TSC) meta-regression models weighted by TSC SE, adjusted for similar covariates and method used to assess semen volume.
Figure 3.
(a) Meta-regression model for mean sperm concentration by fertility and geographic groups, adjusted for potential confounders. (b) Meta-regression model for mean total sperm count by fertility and geographic groups, adjusted for potential confounders. Meta-regression model weighted by sperm concentration (SC) SE, adjusted for fertility group, time × fertility group interaction, geographic group, time × geographic group interaction, age, abstinence time, semen collection method reported, counting method reported, having more than one sample per men, indicators for study selection of population and exclusion criteria (some vasectomy candidates, some semen donor candidates, exclusion of men with chronic diseases, exclusion by other reasons not related to fertility, selection by occupation not related to fertility), whether year of collection was estimated, whether arithmetic mean of SC was estimated, whether SE of SC was estimated and indicator variable to denote studies with more than one estimate. Total sperm count (TSC) meta-regression models weighted by TSC SE, adjusted for similar covariates and method used to assess semen volume.
In the final adjusted models for TSC (Table I), which included time × fertility group (P = 0.014) and time × geographic group (P = 0.021), in Western studies a steeper slope in TSC was seen among Unselected (−5.33 million/year, −7.56 to −3.11; P < 0.001) versus Fertile (−2.12, −4.31 to 0.07; P = 0.057) (Table I, Fig. 3b). Using estimates from the fully adjusted model of 337.5 million in 1973 to 137.5 million in 2011, TSC in the Unselected Western group declined 1.6% per year and overall by 59.3% between 1973 and 2011.
No significant trends in SC or TSC were seen in Other countries overall, or for Unselected or Fertile men separately.
Sensitivity analyses
We performed multiple analyses to examine the sensitivity of results to assumptions about our model, influence of covariates, estimation of missing data, trends in SEs and study period. For the sake of brevity, results from sensitivity analyses are presented here for slope of SC in Unselected Western group. In all sensitivity analyses there was a significant (P < 0.01) and strong (>1.0 million/ml/year) decline for Unselected Western group.
– Adding a quadratic or cubic function of year to meta-regression models did not substantially change the shape of the trend or improve model fit (as adjusted R-square declined), overall or within any of the geographic or fertility groups (coefficient for the quadratic term: 0.0009; 95% CI: −0.04 to 0.05, P = 0.969; for the cubic term −0.0003; 95% CI: −0.0007 to 0.0007, P = 0.942).
– Results of sensitivity analyses excluding a specific group for each covariate, or removing each covariate at a time from the model are in Supplementary Table S3.
– After excluding nine estimates with a SE of SC > 20 million/ml, the slope for Unselected Western was −1.31 million/ml (−1.96 to −0.66; P = 0.001).
– Excluding 85 studies with no data on mean age and adjusting for mean age instead of age group, yielded a slope of −1.68 million/ml (−2.35 to −1.01; P < 0.001).
– The proportion of smokers was reported in only 25% of studies. To examine this variable a sensitivity analysis including a covariate for high proportion of smokers (>30%) was performed, and slopes changed only slightly (−1.39 million/ml, −2.03 to −0.75; P < 0.001).
– The slope for Unselected Western did not change appreciably after excluding each country/region with more than 10 estimates at a time. Excluding 28 estimates from Australia and New Zealand, the slope for studies of unselected men from North America/Europe was −1.13 million/ml (−1.79 to −0.47; P = 0.001). Excluding estimates from the USA (n = 39) or Denmark (n = 19) the slopes were −1.46 million/ml (−2.25 to −0.67; P < 0.001) and −1.57 million/ml (−2.26 to −0.87; P < 0.001), respectively.
– Restricting the analysis to data from recent years (196 estimates collected post-1985) the slope (−1.57 million/ml, −2.51 to −0.62; P = 0.001) was similar to that for the full model. Restricting the analysis to data post-1995 (model restricted to 53 estimates of Unselected Western due to insufficient observations for interaction terms) the slope (−2.06 million/ml, −3.38 to −0.74; P = 0.004) was somewhat steeper.
Results for TSC slope were also robust in all sensitivity analyses. Restricting the analysis to data post-1995 the slope (−8.12 million, −13.73 to −2.51; P = 0.006) was somewhat steeper.
Discussion
Key findings
In this first systematic review and meta-regression analysis of temporal trends in sperm counts we report a significant overall decline in both SC and TSC in samples collected between 1973 and 2011. Declines were significant only in studies from North America, Europe, Australia (and New Zealand), where they were most pronounced among men unselected by fertility. In this latter group, SC declined 52.4% (−1.4% per year) and TSC 59.3% (−1.6% per year) over the study period. These slopes remained substantially unchanged after controlling for multiple preselected covariates (age, abstinence time, method of semen collection, method of counting sperm, selection of population and study exclusion criteria, number of samples per man and completeness of data) and in multiple sensitivity analyses. Thus, these data provide robust indication for a decline in SC and TSC in North America, Europe, Australia and New Zealand over the last 4 decades. There was no sign of ‘leveling off’ of the decline, when analyses were restricted to studies with sample collection in 1996–2011.
Comparison to previous studies
The overall decline in SC reported here (−0.70 million/ml/year) was consistent with, but not as steep as (−0.93 and −0.94 million/ml/year), previously reported for an earlier period (Carlsen et al., 1992; Swan et al., 1997, 2000) (Table II). The annual percentage change in SC reported here was −0.75% million/ml, comparable to −0.83% reported by Carlsen et al. (1992). As in prior analyses (Swan et al., 1997, 2000), we saw no significant declines for studies from South America, Asia and Africa, which may, in part be accounted for by limited statistical power and an absence of studies in unselected men from these countries prior to 1985. However, we note that the modification of the slope by geographic group was significant. Thus, based on the results presented here, while it is not possible to rule out a trend in non-Western countries, these data do not support a decline as steep as that observed in Western countries. In the current analysis, declines in North America and Europe/Australia were similar, unlike prior analyses which included a higher proportion of studies from North America (Swan et al., 1997, 2000).
Table II.
Characteristics and results of fitting a simple linear regression model (without adjustment, weighted by sample size) for trends of sperm concentration in the current study, in Carlsen et al. (1992), and in Swan et al. (2000).
| Study | Levine et al. (2017, current study) | Carlsen et al. (1992) | Swan et al. (2000) |
|---|---|---|---|
| Publication years | 1981–2013 | 1938–1990 | 1934–1996 |
| Number of studies | 185 (244 estimates) | 61 | 101 |
| Number of countries | 50 | 20 | 28 |
| Fertility group: N (%) | |||
| Fertile | 104 (43%) | 39 (64%) | 51 (50%)a |
| Unselected | 140 (57%) | 22 (36%) | 50 (50%) |
| Geographic group: N (%) | |||
| Westernb | 175 (72%) | 45 (74%) | 78 (77%) |
| Other | 69 (28%) | 16 (26%) | 23 (23%) |
| Slope | −0.70 | −0.93 | −0.94 |
| P-value | <0.001 | <0.001 | <0.001 |
aWife pregnant or post-partum or at least 90% of men with proven fertility.
bWestern includes studies from North America, Europe and Australia (and New Zealand). Other includes studies from all other countries.
Owing to the completeness of our search, our considerable sample size across the entire study period and use of meta-regression methods, this analysis avoids many of the limitations of previous studies. The study of Carlsen et al. (1992), which weighted studies by sample size, was criticized for having one study that included 30% of all subjects and for the paucity of data in the first 30 years of the analysis (Olsen et al., 1995). The largest study in the current meta-regression analysis included only 5% of all subjects, sensitivity analyses demonstrated that no one country drove the overall trend, and studies were well distributed over the 39 years of the study period and among 50 different countries. Furthermore, the meta-regression methods utilized in the current study addressed the issue of heterogeneity in the reliability of study estimates by weighting of estimates by their SE. This conservative method inflates the CI and is appropriate when the number of studies is sufficiently large, as it was in our analysis (Baker and Jackson, 2010). In addition, we adjusted for a pre-determined set of covariates, as well as variables indicating data completeness and study exclusion criteria, thus avoiding the main pitfall in reaching reliable conclusions from meta-regression analyses (Thompson and Higgins, 2002).
Our statistical power enabled us to assess modification by fertility and geographic group. Modification by fertility group is especially important since fertile men represent a selected population, while unselected men are more likely to be representative of the general population.
Some researchers have criticized the use of sperm count estimates from the past arguing that greater measurement error would be expected in historical studies. This is an unlikely explanation for the trend we report here for several reasons. First, unlike earlier analyses that included studies in which samples were collected as far back as 1931, our analysis includes studies with samples collected only since 1973. Even if measurements were less reliable in the past, this greater imprecision would produce greater uncertainty in earlier studies but not a change in slope. Further, since we weighted estimates by their SE, we avoided this hypothetical limitation. In addition, results were robust in sensitivity analyses that excluded studies in which SE was estimated, or very large.
Chance is an unlikely explanation for our results, which were significant even in the more conservative meta-regression models. We used written protocols and extensive quality control procedures to minimize potential information and selection bias in all steps of the study.
Limitations
There are several possible limitations to this systematic review and meta-regression analysis. It is possible that failure to include non-English publications may have limited our analyses of non-Western countries. It has been claimed that men who are willing to provide semen sample may differ from the rest of the population leading to potential selection bias, but current evidence does not support this claim (Cooper et al., 2010).
We analyzed sperm counts (both by SC and TSC) but not sperm motility and morphology because information regarding motility and morphology were seldom available in older studies. Moreover, the recommended methods and criteria for motility and morphology assessments have changed significantly over time making across-time comparisons difficult. In contrast, the assessment of SC by hemocytometer, first described in 1902 (Benedict, 1902), has been the method recommended by the World Health Organization since 1980 (World Health Organization, 2010), and there is no evidence that this method has varied systematically over time. For these reasons SC is considered to be the most reliable endpoint for epidemiological analysis (Le Moal et al., 2016). Because of this stability and the variability of other counting methods over time we only included studies in which counting was done (or likely done) by hemocytometer and excluded studies that used alternative counting chambers (e.g. Makler, Coulter and Microcell) or non-manual methods (i.e. computer assisted sperm analysis or flow cytometry). Even though we followed detailed protocol, this study was not preregistered in Prospero.
Analysing trends by birth cohorts instead of year of sample collection may aid in assessing the causes of the decline (prenatal or in adult life) but was not feasible owing to lack of information.
Wider implications
This rigorous and comprehensive analysis finds that SC declined 52.4% between 1973 and 2011 among unselected men from Western countries, with no evidence of a ‘leveling off’ in recent years. Declining mean SC implies that an increasing proportion of men have sperm counts below any given threshold for sub-fertility or infertility. The high proportion of men from western countries with concentration below 40 million/ml is particularly concerning given the evidence that SC below this threshold is associated with a decreased monthly probability of conception (Bonde et al., 1998).
Declines in sperm count have implications beyond fertility and reproduction. The decline we report here is consistent with reported trends in other male reproductive health indicators, such as testicular germ cell tumors, cryptorchidism, onset of male puberty and total testosterone levels (Skakkebaek et al., 2016). The public health implications are even wider. Recent studies have shown that poor sperm count is associated with overall morbidity and mortality (Jensen et al., 2009; Eisenberg et al., 2014b, 2016; Latif et al., 2017). While the current study is not designed to provide direct information on the causes of the observed declines, sperm count has been plausibly associated with multiple environmental and lifestyle influences, both prenatally and in adult life. In particular, endocrine disruption from chemical exposures or maternal smoking during critical windows of male reproductive development may play a role in prenatal life, while lifestyle changes and exposure to pesticides may play a role in adult life. Thus, a decline in sperm count might be considered as a ‘canary in the coal mine’ for male health across the lifespan. Our report of a continuing and robust decline should, therefore, trigger research into its causes, aiming for prevention.
Conclusion
In this comprehensive meta-analysis, sperm counts whether measured by SC or TSC declined significantly among men from North America, Europe and Australia during 1973–2011, with a 50–60% decline among men unselected by fertility, with no evidence of a ‘leveling off’ in recent years. These findings strongly suggest a significant decline in male reproductive health, which has serious implications beyond fertility concerns. Research on causes and implications of this decline is urgently needed.
Supplementary Material
Acknowledgments
We thank Charles Poole, ScD, University of North Carolina School of Public Health, Chapel Hill, USA, for his contribution to protocol development and meta-regression analysis planning. We thank John D. Meyer, MD, MPH, Icahn School of Medicine at Mount Sinai, USA, for his contribution to abstract screening. We thank Haim Ricas, MSc, Tashtit Scientific Consultants, Israel, for his assistance with preparation of figures.
Supplementary data
Supplementary data are available at Human Reproduction Update online.
Authors' roles
H.L. had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: H.L. and S.H.S. Search strategy design and execution: H.L., R.P. Acquisition, analysis, or interpretation of data: H.L., N.J., A.M.A., J.M., D.W.D., I.M., R.P. and S.H.S. Drafting of the article: H.L. and S.H.S. Critical revision of the article for important intellectual content: H.L., N.J., A.M.A., J.M., D.W.D., I.M., R.P. and S.H.S. Statistical analysis: HL. Administrative, technical or material support: H.L., R.P. and S.H.S. Study supervision: H.L. and S.H.S.
Funding
Environment and Health Fund (EHF), Jerusalem, Israel, and supplementary support from American Healthcare Professionals and Friends for Medicine in Israel (APF) and Israel Medical Association (IMA) to H.L. Research Fund of Rigshospitalet (Grant no. R42-A1326) to N.J. The Brazilian National Council for Scientific and Technological Development (CNPq 249184/2013-3) to A.M.A. The Mount Sinai Transdisciplinary Center on Early Environmental Exposures (NIH P30ES023515) to S.S. The EHF, APF, IMA, Research Fund of Rigshospitalet, CNPq and The Mount Sinai Transdisciplinary Center had no role in the design and conduct of the study; collection, management, analysis and interpretation of the data; preparations, review or approval of the article; and decision to submit the article for publication.
Conflict of interest
All authors declare no conflict of interest.
References
- Absalan F, Ghannadi A, Kazerooni M, Parifar R, Jamalzadeh F, Amiri S. Value of sperm chromatin dispersion test in couples with unexplained recurrent abortion. J Assist Reprod Genet 2012;29:11–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abyholm T. An andrological study of 51 fertile men. Int J Androl 1981;4:646–656. [DOI] [PubMed] [Google Scholar]
- Afeiche M, Williams PL, Mendiola J, Gaskins AJ, Jorgensen N, Swan SH, Chavarro JE. Dairy food intake in relation to semen quality and reproductive hormone levels among physically active young men. Hum Reprod 2013;28:2265–2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aitken RJ, Best FS, Richardson DW, Djahanbakhch O, Lees MM. The correlates of fertilizing capacity in normal fertile men. Fertil Steril 1982;38:68–76. [DOI] [PubMed] [Google Scholar]
- Akutsu K, Takatori S, Nozawa S, Yoshiike M, Nakazawa H, Hayakawa K, Makino T, Iwamoto T. Polybrominated diphenyl ethers in human serum and sperm quality. Bull Environ Contam Toxicol 2008;80:345–350. [DOI] [PubMed] [Google Scholar]
- Al Momani W, Abu Shaqra QM, Wahed AA. Relationship between the recovery of aerobic bacteria from semen and male infertility in Jordan. Jordan Med J 2011;45:62–69. [Google Scholar]
- Albertsen PC, Chang TS, Vindivich D, Robinson JC, Smyth JW. A critical method of evaluating tests for male infertility. J Urol 1983;130:467–475. [DOI] [PubMed] [Google Scholar]
- Ali ST, Shaikh RN, Siddiqi NA, Siddiqi PQ. Semen analysis in insulin-dependent/non-insulin-dependent diabetic men with/without neuropathy. Arch Androl 1993;30:47–54. [DOI] [PubMed] [Google Scholar]
- Al-Janabi AS, Al-Mehdawi FA, Al-Lami MQ. Relationship of seminal biochemical parameters and serum reproductive hormones with sperm function tests in asthenospermic patients. Jordan Med J 2012;46:97–107. [Google Scholar]
- Andrade-Rocha FT. Colonization of Gardnerella vaginalis in semen of infertile men: prevalence, influence on sperm characteristics, relationship with leukocyte concentration and clinical significance. Gynecol Obstet Invest 2009;68:134–136. [DOI] [PubMed] [Google Scholar]
- Aneck-Hahn NH, Schulenburg GW, Bornman MS, Farias P, de Jager C. Impaired semen quality associated with environmental DDT exposure in young men living in a malaria area in the Limpopo Province, South Africa. J Androl 2007;28:423–434. [DOI] [PubMed] [Google Scholar]
- Arce JC, De Souza MJ, Pescatello LS, Luciano AA. Subclinical alterations in hormone and semen profile in athletes. Fertil Steril 1993;59:398–404. [PubMed] [Google Scholar]
- Aribarg A, Kenkeerati W, Vorapaiboonsak V, Leepipatpaiboon S, Farley TM. Testicular volume, semen profile and serum hormone levels in fertile Thai males. Int J Androl 1986;9:170–180. [DOI] [PubMed] [Google Scholar]
- Asch RH, Fernandez EO, Siler-Khodr TM, Pauerstein CJ. Peptide and steroid hormone concentrations in human seminal plasma. Int J Fertil 1984;29:25–32. [PubMed] [Google Scholar]
- Auger J, Eustache F. Second to fourth digit ratios, male genital development and reproductive health: a clinical study among fertile men and testis cancer patients. Int J Androl 2011;34:e49–e58. [DOI] [PubMed] [Google Scholar]
- Auger J, Jouannet P. Evidence for regional differences of semen quality among fertile French men. Federation Francaise des Centres d'Etude et de Conservation des Oeufs et du Sperme humains. Hum Reprod 1997;12:740–745. [DOI] [PubMed] [Google Scholar]
- Auger J, Kunstmann JM, Czyglik F, Jouannet P. Decline in semen quality among fertile men in Paris during the past 20 years. N Engl J Med 1995;332:281–285. [DOI] [PubMed] [Google Scholar]
- Axelsson J, Rylander L, Rignell-Hydbom A, Giwercman A. No secular trend over the last decade in sperm counts among Swedish men from the general population. Hum Reprod 2011;26:1012–1016. [DOI] [PubMed] [Google Scholar]
- Ayers JW, Komesu Y, Romani T, Ansbacher R. Anthropomorphic, hormonal, and psychologic correlates of semen quality in endurance-trained male athletes. Fertil Steril 1985;43:917–921. [DOI] [PubMed] [Google Scholar]
- Baker R, Jackson D. Inference for meta-analysis with a suspected temporal trend. Biom J 2010;52:538–551. [DOI] [PubMed] [Google Scholar]
- Bang AK, Carlsen E, Holm M, Petersen JH, Skakkebaek NE, Jorgensen N. A study of finger lengths, semen quality and sex hormones in 360 young men from the general Danish population. Hum Reprod 2005;20:3109–3113. [DOI] [PubMed] [Google Scholar]
- Bartoov B, Eltes F, Pansky M, Lederman H, Caspi E, Soffer Y. Estimating fertility potential via semen analysis data. Hum Reprod 1993;8:65–70. [DOI] [PubMed] [Google Scholar]
- Benedict AL. The enumeration of spermatozoa. Philad Med J 1902;10:173. [Google Scholar]
- Bhattacharya SM. Association of various sperm parameters with unexplained repeated early pregnancy loss—which is most important? Int Urol Nephrol 2008;40:391–395. [DOI] [PubMed] [Google Scholar]
- Bloom M, Whitcomb B, Chen Z, Ye A, Kannan K, Louis GB. Associations between urinary phthalate concentrations and semen quality parameters in a general population. Hum Reprod 2015;30:2645–2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonde JP, Ernst E, Jensen TK, Hjollund NH, Kolstad H, Henriksen TB, Scheike T, Giwercman A, Olsen J, Skakkebaek NE. Relation between semen quality and fertility: a population-based study of 430 first-pregnancy planners. Lancet 1998;352:1172–1177. [DOI] [PubMed] [Google Scholar]
- Borenstein M, Hedges LV, Higgins J, Rothstein HR. Introduction to Meta-Analysis. Chichester: Wiley, 2009. [Google Scholar]
- Brahem S, Mehdi M, Elghezal H, Saad A. The effects of male aging on semen quality, sperm DNA fragmentation and chromosomal abnormalities in an infertile population. J Assist Reprod Genet 2011;28:425–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bujan L, Walschaerts M, Moinard N, Hennebicq S, Saias J, Brugnon F, Auger J, Berthaut I, Szerman E, Daudin M et al. Impact of chemotherapy and radiotherapy for testicular germ cell tumors on spermatogenesis and sperm DNA: a multicenter prospective study from the CECOS network. Fertil Steril 2013;100:673–680. [DOI] [PubMed] [Google Scholar]
- Carlsen E, Giwercman A, Keiding N, Skakkebaek NE. Evidence for decreasing quality of semen during past 50 years. Br Med J 1992;305:609–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia SE, Tay SK, Lim ST. What constitutes a normal seminal analysis? Semen parameters of 243 fertile men. Hum Reprod 1998;13:3394–3398. [DOI] [PubMed] [Google Scholar]
- Chiu YH, Gaskins AJ, Williams PL, Mendiola J, Jorgensen N, Levine H, Hauser R, Swan SH, Chavarro JE. Intake of fruits and vegetables with low-to-moderate pesticide residues is positively associated with semen-quality parameters among young healthy men. J Nutr 2016;146:1084–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comhaire FH, Vermeulen L, Schoonjans F. Reassessment of the accuracy of traditional sperm characteristics and adenosine triphosphate (ATP) in estimating the fertilizing potential of human semen in vivo. Int J Androl 1987;10:653–662. [DOI] [PubMed] [Google Scholar]
- Cooper TG, Noonan E, von Eckardstein S, Auger J, Baker HW, Behre HM, Haugen TB, Kruger T, Wang C, Mbizvo MT et al. World Health Organization reference values for human semen characteristics. Hum Reprod Update 2010;16:231–245. [DOI] [PubMed] [Google Scholar]
- Costello MF, Sjoblom P, Haddad Y, Steigrad SJ, Bosch EG. No decline in semen quality among potential sperm donors in Sydney, Australia, between 1983 and 2001. J Assist Reprod Genet 2002;19:284–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottell E, Harrison RF. The value of subcellular elemental analysis in the assessment of human spermatozoa. Hum Reprod 1995;10:3186–3189. [DOI] [PubMed] [Google Scholar]
- Culasso F, Lenzi A, Favilli S, Dondero F. Statistical analysis in andrology. Arch Androl 1991;26:163–172. [DOI] [PubMed] [Google Scholar]
- Danadevi K, Rozati R, Reddy PP, Grover P. Semen quality of Indian welders occupationally exposed to nickel and chromium. Reprod Toxicol 2003;17:451–456. [DOI] [PubMed] [Google Scholar]
- de Castro MP, Mastrorocco DA. Reproductive history and semen analysis in prevasectomy fertile men with and without varicocele. J Androl 1984;5:17–20. [DOI] [PubMed] [Google Scholar]
- De Celis R, Feria-Velasco A, Gonzalez-Unzaga M, Torres-Calleja J, Pedron-Nuevo N. Semen quality of workers occupationally exposed to hydrocarbons. Fertil Steril 2000;73:221–228. [DOI] [PubMed] [Google Scholar]
- de Gouveia Brazao CA, Pierik FH, Erenpreiss Y, de Jong FH, Dohle GR, Weber RF. The effect of cryptorchidism on inhibin B in a subfertile population. Clin Endocrinol (Oxf) 2003;59:136–141. [DOI] [PubMed] [Google Scholar]
- Dunphy BC, Barratt CL, Kay R, Thomas EJ, Neal LM, Cooke ID. The importance of employing stringent methods to recruit fertile male controls. Andrologia 1991;23:35–39. [DOI] [PubMed] [Google Scholar]
- Durazzo M, Premoli A, Di Bisceglie C, Bertagna A, Faga E, Biroli G, Manieri C, Bo S, Pagano G. Alterations of seminal and hormonal parameters: an extrahepatic manifestation of HCV infection? World J Gastroenterol 2006;12:3073–3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebesunun MO, Solademi BA, Shittu OB, Anetor JI, Onuegbu JA, Olisekodiaka JM, Agbedana EO, Onyeaghala AA. Plasma and semen ascorbic levels in spermatogenesis. West Afr J Med 2004;23:290–293. [DOI] [PubMed] [Google Scholar]
- Eisenberg ML, Kim S, Chen Z, Sundaram R, Schisterman EF, Buck Louis GM. The relationship between male BMI and waist circumference on semen quality: data from the LIFE study. Hum Reprod 2014. a;29:193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg ML, Li S, Behr B, Cullen MR, Galusha D, Lamb DJ, Lipshultz LI. Semen quality, infertility and mortality in the USA. Hum Reprod 2014. b;29:1567–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg ML, Li S, Cullen MR, Baker LC. Increased risk of incident chronic medical conditions in infertile men: analysis of United States claims data. Fertil Steril 2016;105:629–636. [DOI] [PubMed] [Google Scholar]
- Engelbertz F, Korda JB, Engelmann U, Rothschild M, Banaschak S. Longevity of spermatozoa in the post-ejaculatory urine of fertile men. Forensic Sci Int 2010;194:15–19. [DOI] [PubMed] [Google Scholar]
- Fariss BL, Fenner DK, Plymate SR, Brannen GE, Jacob WH, Thomason AM. Seminal characteristics in the presence of a varicocele as compared with those of expectant fathers and prevasectomy men. Fertil Steril 1981;35:325–327. [DOI] [PubMed] [Google Scholar]
- Fedder J, Askjaer SA, Hjort T. Nonspermatozoal cells in semen: relationship to other semen parameters and fertility status of the couple. Arch Androl 1993;31:95–103. [DOI] [PubMed] [Google Scholar]
- Fetic S, Yeung CH, Sonntag B, Nieschlag E, Cooper TG. Relationship of cytoplasmic droplets to motility, migration in mucus, and volume regulation of human spermatozoa. J Androl 2006;27:294–301. [DOI] [PubMed] [Google Scholar]
- Figa-Talamanca I, Cini C, Varricchio GC, Dondero F, Gandini L, Lenzi A, Lombardo F, Angelucci L, Di Grezia R, Patacchioli FR. Effects of prolonged autovehicle driving on male reproduction function: a study among taxi drivers. Am J Ind Med 1996;30:750–758. [DOI] [PubMed] [Google Scholar]
- Firman RC, Simmons LW, Cummins JM, Matson PL. Are body fluctuating asymmetry and the ratio of 2nd to 4th digit length reliable predictors of semen quality? Hum Reprod 2003;18:808–812. [DOI] [PubMed] [Google Scholar]
- Fisch H, Goluboff ET, Olson JH, Feldshuh J, Broder SJ, Barad DH. Semen analyses in 1,283 men from the United States over a 25-year period: no decline in quality. Fertil Steril 1996;65:1009–1014. [DOI] [PubMed] [Google Scholar]
- Foppiani L, Cavani S, Piredda S, Perroni L, Fazzuoli L, Giusti M. Lack of evidence of a genetic origin in the impaired spermatogenesis of a patient cohort with low-grade varicocele. J Endocrinol Invest 2001;24:217–223. [DOI] [PubMed] [Google Scholar]
- Fowler JE Jr, Mariano M. Immunoglobulin in seminal fluid of fertile, infertile, vasectomy and vasectomy reversal patients. J Urol 1983;129:869–872. [DOI] [PubMed] [Google Scholar]
- Fredricsson B, Sennerstam R. Morphology of live seminal and postcoital cervical spermatozoa and its bearing on human fertility. Acta Obstet Gynecol Scand 1984;63:329–333. [DOI] [PubMed] [Google Scholar]
- Freischem CW, Knuth UA, Langer K, Schneider HP, Nieschlag E. The lack of discriminant seminal and endocrine variables in the partners of fertile and infertile women. Arch Gynecol 1984;236:1–12. [DOI] [PubMed] [Google Scholar]
- Gallegos G, Ramos B, Santiso R, Goyanes V, Gosalvez J, Fernandez JL. Sperm DNA fragmentation in infertile men with genitourinary infection by Chlamydia trachomatis and Mycoplasma. Fertil Steril 2008;90:328–334. [DOI] [PubMed] [Google Scholar]
- Garcia PC, Rubio E, Pereira OCM. Antisperm antibodies in infertile men and their correlation with seminal parameters. Reprod Med Biol 2007;6:33–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giagulli VA, Carbone D. Hormonal control of inhibin B in men. J Endocrinol Invest 2006;29:706–713. [DOI] [PubMed] [Google Scholar]
- Giblin PT, Poland ML, Moghissi KS, Ager JW, Olson JM. Effects of stress and characteristic adaptability on semen quality in healthy men. Fertil Steril 1988;49:127–132. [DOI] [PubMed] [Google Scholar]
- Girela JL, Gil D, Johnsson M, Gomez-Torres MJ, De Juan J. Semen parameters can be predicted from environmental factors and lifestyle using artificial intelligence methods. Biol Reprod 2013;88:99. [DOI] [PubMed] [Google Scholar]
- Glazier DB, Marmar JL, Diamond SM, Gibbs M, Corson SL. A modified acrosome induction test. Arch Androl 2000;44:59–64. [DOI] [PubMed] [Google Scholar]
- Gollenberg AL, Liu F, Brazil C, Drobnis EZ, Guzick D, Overstreet JW, Redmon JB, Sparks A, Wang C, Swan SH. Semen quality in fertile men in relation to psychosocial stress. Fertil Steril 2010;93:1104–1111. [DOI] [PubMed] [Google Scholar]
- Gore AC, Chappell VA, Fenton SE, Flaws JA, Nadal A, Prins GS, Toppari J, Zoeller RT. Executive summary to EDC-2: The Endocrine Society's Second Scientific Statement on endocrine-disrupting chemicals. Endocr Rev 2015;36:593–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb C, Svanborg K, Bygdeman M. Adenosine triphosphate (ATP) in human spermatozoa. Andrologia 1991;23:421–425. [DOI] [PubMed] [Google Scholar]
- Goulis DG, Iliadou PK, Tsametis C, Gerou S, Tarlatzis BC, Bontis IN, Papadimas I. Serum anti-Mullerian hormone levels differentiate control from subfertile men but not men with different causes of subfertility. Gynecol Endocrinol 2008;24:158–160. [DOI] [PubMed] [Google Scholar]
- Guzick DS, Overstreet JW, Factor-Litvak P, Brazil CK, Nakajima ST, Coutifaris C, Carson SA, Cisneros P, Steinkampf MP, Hill JA et al. Sperm morphology, motility, and concentration in fertile and infertile men. N Engl J Med 2001;345:1388–1393. [DOI] [PubMed] [Google Scholar]
- Gyllenborg J, Skakkebaek NE, Nielsen NC, Keiding N, Giwercman A. Secular and seasonal changes in semen quality among young Danish men: a statistical analysis of semen samples from 1927 donor candidates during 1977–1995. Int J Androl 1999;22:28–36. [DOI] [PubMed] [Google Scholar]
- Halling J, Petersen MS, Jorgensen N, Jensen TK, Grandjean P, Weihe P. Semen quality and reproductive hormones in Faroese men: a cross-sectional population-based study of 481 men. BMJ Open 2013;3:e001946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammadeh ME, Greiner S, Rosenbaum P, Schmidt W. Comparison between human sperm preservation medium and TEST-yolk buffer on protecting chromatin and morphology integrity of human spermatozoa in fertile and subfertile men after freeze-thawing procedure. J Androl 2001;22:1012–1018. [DOI] [PubMed] [Google Scholar]
- Handelsman DJ. Estimating familial and genetic contributions to variability in human testicular function: a pilot twin study. Int J Androl 1997. a;20:215–221. [DOI] [PubMed] [Google Scholar]
- Handelsman DJ. Sperm output of healthy men in Australia: magnitude of bias due to self-selected volunteers. Hum Reprod 1997. b;12:2701–2705. [DOI] [PubMed] [Google Scholar]
- Haugen TB, Egeland T, Magnus O. Semen parameters in Norwegian fertile men. J Androl 2006;27:66–71. [DOI] [PubMed] [Google Scholar]
- Hauser R, Skakkebaek NE, Hass U, Toppari J, Juul A, Andersson AM, Kortenkamp A, Heindel JJ, Trasande L. Male reproductive disorders, diseases, and costs of exposure to endocrine-disrupting chemicals in the European Union. J Clin Endocrinol Metab 2015;100:1267–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heussner JC, Ward JB Jr, Legator MS. Genetic monitoring of aluminum workers exposed to coal tar pitch volatiles. Mutat Res 1985;155:143–155. [DOI] [PubMed] [Google Scholar]
- Higgins J, Green S Cochrane handbook for systematic reviews of interventions version 5.1. 0 [updated March 2011]. The Cochrane Collaboration, 2011.
- Hill JA, Abbott AF, Politch JA. Sperm morphology and recurrent abortion. Fertil Steril 1994;61:776–778. [PubMed] [Google Scholar]
- Hossain F, Ali O, D'Souza UJ, Naing DK. Effects of pesticide use on semen quality among farmers in rural areas of Sabah, Malaysia. J Occup Health 2010;52:353–360. [DOI] [PubMed] [Google Scholar]
- Huang LP, Lee CC, Fan JP, Kuo PH, Shih TS, Hsu PC. Urinary metabolites of di(2-ethylhexyl) phthalate relation to sperm motility, reactive oxygen species generation, and apoptosis in polyvinyl chloride workers. Int Arch Occup Environ Health 2014;87:635–646. [DOI] [PubMed] [Google Scholar]
- Irvine S, Cawood E, Richardson D, MacDonald E, Aitken J. Evidence of deteriorating semen quality in the United Kingdom: birth cohort study in 577 men in Scotland over 11 years. Br Med J 1996;312:467–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto T, Nozawa S, Mieno MN, Yamakawa K, Baba K, Yoshiike M, Namiki M, Koh E, Kanaya J, Okuyama A et al. Semen quality of 1559 young men from four cities in Japan: a cross-sectional population-based study. BMJ Open 2013. a;3:e002222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto T, Nozawa S, Yoshiike M, Hoshino T, Baba K, Matsushita T, Tanaka SN, Naka M, Skakkebaek NE, Jorgensen N. Semen quality of 324 fertile Japanese men. Hum Reprod 2006;21:760–765. [DOI] [PubMed] [Google Scholar]
- Iwamoto T, Nozawa S, Yoshiike M, Namiki M, Koh E, Kanaya J, Okuyama A, Matsumiya K, Tsujimura A, Komatsu K et al. Semen quality of fertile Japanese men: a cross-sectional population-based study of 792 men. BMJ Open 2013. b;3:e002223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen K, Ramlau-Hansen CH, Thulstrup AM, Olsen J, Bonde JP. Maternal folic acid supplement intake and semen quality in Danish sons: a follow-up study. Fertil Steril 2011;96:295–298. [DOI] [PubMed] [Google Scholar]
- Jedrzejczak P, Taszarek-Hauke G, Hauke J, Pawelczyk L, Duleba AJ. Prediction of spontaneous conception based on semen parameters. Int J Androl 2008;31:499–507. [DOI] [PubMed] [Google Scholar]
- Jensen TK, Andersson AM, Hjollund NH, Scheike T, Kolstad H, Giwercman A, Henriksen TB, Ernst E, Bonde JP, Olsen J et al. Inhibin B as a serum marker of spermatogenesis: correlation to differences in sperm concentration and follicle-stimulating hormone levels. A study of 349 Danish men. J Clin Endocrinol Metab 1997;82:4059–4063. [DOI] [PubMed] [Google Scholar]
- Jensen TK, Heitmann BL, Blomberg Jensen M, Halldorsson TI, Andersson AM, Skakkebaek NE, Joensen UN, Lauritsen MP, Christiansen P, Dalgard C et al. High dietary intake of saturated fat is associated with reduced semen quality among 701 young Danish men from the general population. Am J Clin Nutr 2013;97:411–418. [DOI] [PubMed] [Google Scholar]
- Jensen TK, Jacobsen R, Christensen K, Nielsen NC, Bostofte E. Good semen quality and life expectancy: a cohort study of 43,277 men. Am J Epidemiol 2009;170:559–565. [DOI] [PubMed] [Google Scholar]
- Ji G, Yan L, Liu W, Huang C, Gu A, Wang X. Polymorphisms in double-strand breaks repair genes are associated with impaired fertility in Chinese population. Reproduction 2013;145:463–470. [DOI] [PubMed] [Google Scholar]
- Jockenhovel F, Khan SA, Nieschlag E. Diagnostic value of bioactive FSH in male infertility. Acta Endocrinol (Copenh) 1989;121:802–810. [DOI] [PubMed] [Google Scholar]
- Jorgensen N, Andersen AG, Eustache F, Irvine DS, Suominen J, Petersen JH, Andersen AN, Auger J, Cawood EH, Horte A et al. Regional differences in semen quality in Europe. Hum Reprod 2001;16:1012–1019. [DOI] [PubMed] [Google Scholar]
- Jorgensen N, Carlsen E, Nermoen I, Punab M, Suominen J, Andersen AG, Andersson AM, Haugen TB, Horte A, Jensen TK et al. East-West gradient in semen quality in the Nordic-Baltic area: a study of men from the general population in Denmark, Norway, Estonia and Finland. Hum Reprod 2002;17:2199–2208. [DOI] [PubMed] [Google Scholar]
- Jorgensen N, Joensen UN, Jensen TK, Jensen MB, Almstrup K, Olesen IA, Juul A, Andersson AM, Carlsen E, Petersen JH et al. Human semen quality in the new millennium: a prospective cross-sectional population-based study of 4867 men. BMJ Open 2012;2:e000990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen N, Vierula M, Jacobsen R, Pukkala E, Perheentupa A, Virtanen HE, Skakkebaek NE, Toppari J. Recent adverse trends in semen quality and testis cancer incidence among Finnish men. Int J Androl 2011;34:e37–e48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junqing W, Qiuying Y, Jianguo T, Wei Y, Liwei B, Yuxian L, Yumei Z, Kangshou Y, Weiqun L, Lu C et al. Reference value of semen quality in Chinese young men. Contraception 2002;65:365–368. [DOI] [PubMed] [Google Scholar]
- Katukam V, Kulakarni M, Syed R, Alharbi K, Naik J. Effect of benzene exposure on fertility of male workers employed in bulk drug industries. Genet Test Mol Biomarkers 2012;16:592–597. [DOI] [PubMed] [Google Scholar]
- Kelleher S, Wishart SM, Liu PY, Turner L, Di Pierro I, Conway AJ, Handelsman DJ. Long-term outcomes of elective human sperm cryostorage. Hum Reprod 2001;16:2632–2639. [DOI] [PubMed] [Google Scholar]
- Khan MS, Deepa F, Ahmed Z, Tahir F, Khan MA. Assessment of male reproductive health by conventional method of semen analysis. J Ayub Med Coll Abbottabad 2011;23:84–88. [PubMed] [Google Scholar]
- Kirei BR. Semen characteristics in 120 fertile Tanzanian men. East Afr Med J 1987;64:453–457. [PubMed] [Google Scholar]
- Kjaergaard N, Kjaergaard B, Lauritsen JG. Prazosin, an adrenergic blocking agent inadequate as male contraceptive pill. Contraception 1988;37:621–629. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Masumori N, Hisasue S, Kato R, Hashimoto K, Itoh N, Tsukamoto T. Inhibition of seminal emission is the main cause of anejaculation induced by a new highly selective alpha1A-blocker in normal volunteers. J Sex Med 2008;5:2185–2190. [DOI] [PubMed] [Google Scholar]
- Kolstad HA, Bonde JP, Spano M, Giwercman A, Zschiesche W, Kaae D, Larsen SB, Roeleveld N. Change in semen quality and sperm chromatin structure following occupational styrene exposure. ASCLEPIOS. Int Arch Occup Environ Health 1999;72:135–141. [PubMed] [Google Scholar]
- Korrovits P, Ausmees K, Mandar R, Punab M. Prevalence of asymptomatic inflammatory (National Institutes of Health Category IV) prostatitis in young men according to semen analysis. Urology 2008;71:1010–1015. [DOI] [PubMed] [Google Scholar]
- Kukuvitis A, Georgiou I, Bouba I, Tsirka A, Giannouli CH, Yapijakis C, Tarlatzis B, Bontis J, Lolis D, Sofikitis N et al. Association of oestrogen receptor alpha polymorphisms and androgen receptor CAG trinucleotide repeats with male infertility: a study in 109 Greek infertile men. Int J Androl 2002;25:149–152. [DOI] [PubMed] [Google Scholar]
- Kumar K, Venkatesh S, Sharma PR, Tiwari PK, Dada R. DAZL 260A > G and MTHFR 677C > T variants in sperm DNA of infertile Indian men. Indian J Biochem Biophys 2011;48:422–426. [PubMed] [Google Scholar]
- Kumar R, Venkatesh S, Kumar M, Tanwar M, Shasmsi MB, Kumar R, Gupta NP, Sharma RK, Talwar P, Dada R. Oxidative stress and sperm mitochondrial DNA mutation in idiopathic oligoasthenozoospermic men. Indian J Biochem Biophys 2009;46:172–177. [PubMed] [Google Scholar]
- Kuroki Y, Iwamoto T, Lee J, Yoshiike M, Nozawa S, Nishida T, Ewis AA, Nakamura H, Toda T, Tokunaga K et al. Spermatogenic ability is different among males in different Y chromosome lineage. J Hum Genet 1999;44:289–292. [DOI] [PubMed] [Google Scholar]
- Larsen SB, Spano M, Giwercman A, Bonde JP. Semen quality and sex hormones among organic and traditional Danish farmers. ASCLEPIOS Study Group. Occup Environ Med 1999;56:139–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latif T, Kold Jensen T, Mehlsen J, Holmboe SA, Brinth L, Pors K, Skouby SO, Jorgensen N, Lindahl-Jacobsen R. Semen quality is a predictor of subsequent morbidity. A Danish cohort study of 4,712 men with long-term follow-up. Am J Epidemiol 2017. 10.1093/aje/kwx067. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Le Moal J, Sharpe RM, Jørgensen N, Levine H, Jurewicz J, Mendiola J, Swan SH, Virtanen H, Christin-Maitre S, Cordier S et al. Toward a multi-country monitoring system of reproductive health in the context of endocrine disrupting chemical exposure. Eur J Public Health 2016;26:76–83. [DOI] [PubMed] [Google Scholar]
- Lee PA, Coughlin MT. Fertility after bilateral cryptorchidism. Evaluation by paternity, hormone, and semen data. Horm Res 2001;55:28–32. [DOI] [PubMed] [Google Scholar]
- Lemcke B, Behre HM, Nieschlag E. Frequently subnormal semen profiles of normal volunteers recruited over 17 years. Int J Androl 1997;20:144–152. [DOI] [PubMed] [Google Scholar]
- Leto S, Frensilli FJ. Changing parameters of donor semen. Fertil Steril 1981;36:766–770. [DOI] [PubMed] [Google Scholar]
- Levine RJ, Brown MH, Bell M, Shue F, Greenberg GN, Bordson BL. Air-conditioned environments do not prevent deterioration of human semen quality during the summer. Fertil Steril 1992;57:1075–1083. [DOI] [PubMed] [Google Scholar]
- Li JW, Gu YQ. Predictors for partial suppression of spermatogenesis of hormonal male contraception. Asian J Androl 2008;10:723–730. [DOI] [PubMed] [Google Scholar]
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. Br Med J 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linschooten JO, Laubenthal J, Cemeli E, Baumgartner A, Anderson D, Sipinen VE, Brunborg G, Haenen GR, Fthenou E, Briede JJ et al. Incomplete protection of genetic integrity of mature spermatozoa against oxidative stress. Reprod Toxicol 2011;32:106–111. [DOI] [PubMed] [Google Scholar]
- Liu DY, Stewart T, Baker HW. Normal range and variation of the zona pellucida-induced acrosome reaction in fertile men. Fertil Steril 2003;80:384–389. [DOI] [PubMed] [Google Scholar]
- Lopez-Teijon M, Elbaile M, Alvarez JG. Geographical differences in semen quality in a population of young healthy volunteers from the different regions of Spain. Andrologia 2008;40:318–328. [DOI] [PubMed] [Google Scholar]
- Luetjens CM, Rolf C, Gassner P, Werny JE, Nieschlag E. Sperm aneuploidy rates in younger and older men. Hum Reprod 2002;17:1826–1832. [DOI] [PubMed] [Google Scholar]
- Lundwall A, Giwercman A, Ruhayel Y, Giwercman Y, Lilja H, Hallden C, Malm J. A frequent allele codes for a truncated variant of semenogelin I, the major protein component of human semen coagulum. Mol Hum Reprod 2003;9:345–350. [DOI] [PubMed] [Google Scholar]
- Mahmoud A, Kiss P, Vanhoorne M, De Bacquer D, Comhaire F. Is inhibin B involved in the toxic effect of lead on male reproduction? Int J Androl 2005;28:150–155. [DOI] [PubMed] [Google Scholar]
- Mak V, Jarvi K, Buckspan M, Freeman M, Hechter S, Zini A. Smoking is associated with the retention of cytoplasm by human spermatozoa. Urology 2000;56:463–466. [DOI] [PubMed] [Google Scholar]
- Mendiola J, Jorgensen N, Minguez-Alarcon L, Sarabia-Cos L, Lopez-Espin JJ, Vivero-Salmeron G, Ruiz-Ruiz KJ, Fernandez MF, Olea N, Swan SH et al. Sperm counts may have declined in young university students in Southern Spain. Andrology 2013;1:408–413. [DOI] [PubMed] [Google Scholar]
- Mieusset R, Bujan L, Mansat A, Pontonnier F, Grandjean H, Chap H. Glycerophosphocholine in seminal plasma of fertile and infertile men. Int J Androl 1988;11:405–413. [DOI] [PubMed] [Google Scholar]
- Mieusset R, Bujan L, Massat G, Mansat A, Pontonnier F. Clinical and biological characteristics of infertile men with a history of cryptorchidism. Hum Reprod 1995;10:613–619. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Br Med J 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostafa T, El-Shahid LH, El Azeem AA, Shaker O, Gomaa H, Abd El Hamid HM. Androgen receptor-CAG repeats in infertile Egyptian men. Andrologia 2012;44:147–151. [DOI] [PubMed] [Google Scholar]
- Muller CH, Coombs RW, Krieger JN. Effects of clinical stage and immunological status on semen analysis results in human immunodeficiency virus type 1-seropositive men. Andrologia 1998;30:15–22. [DOI] [PubMed] [Google Scholar]
- Multigner L, Ben Brik E, Arnaud I, Haguenoer JM, Jouannet P, Auger J, Eustache F. Glycol ethers and semen quality: a cross-sectional study among male workers in the Paris Municipality. Occup Environ Med 2007;64:467–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthusami KR, Chinnaswamy P. Effect of chronic alcoholism on male fertility hormones and semen quality. Fertil Steril 2005;84:919–924. [DOI] [PubMed] [Google Scholar]
- Naz RK, Evans L, Armstrong JS, Sikka SC. Decreased levels of interleukin-12 are not correlated with leukocyte concentration and superoxide dismutase activity in semen of infertile men. Arch Androl 1998;41:91–96. [DOI] [PubMed] [Google Scholar]
- Nieschlag E, Lammers U, Freischem CW, Langer K, Wickings EJ. Reproductive functions in young fathers and grandfathers. J Clin Endocrinol Metab 1982;55:676–681. [DOI] [PubMed] [Google Scholar]
- Nikoobakht MR, Aloosh M, Nikoobakht N, Mehrsay AR, Biniaz F, Karjalian MA. The role of hypothyroidism in male infertility and erectile dysfunction. Urol J 2012;9:405–409. [PubMed] [Google Scholar]
- Nnatu SN, Giwa-Osagie OF, Essien EE. Effect of repeated semen ejaculation on sperm quality. Clin Exp Obstet Gynecol 1991;18:39–42. [PubMed] [Google Scholar]
- Noack-Fuller G, De Beer C, Seibert H. Cadmium, lead, selenium, and zinc in semen of occupationally unexposed men. Andrologia 1993;25:7–12. [DOI] [PubMed] [Google Scholar]
- Nordkap L, Jensen TK, Hansen AM, Lassen TH, Bang AK, Joensen UN, Blomberg Jensen M, Skakkebaek NE, Jorgensen N. Psychological stress and testicular function: a cross-sectional study of 1,215 Danish men. Fertil Steril 2016;105:174–187.e1–e2. [DOI] [PubMed] [Google Scholar]
- Nordkap L, Joensen UN, Blomberg Jensen M, Jorgensen N. Regional differences and temporal trends in male reproductive health disorders: semen quality may be a sensitive marker of environmental exposures. Mol Cell Endocrinol 2012;355:221–230. [DOI] [PubMed] [Google Scholar]
- O'Donovan M. An evaluation of chromatin condensation and DNA integrity in the spermatozoa of men with cancer before and after therapy. Andrologia 2005;37:83–90. [DOI] [PubMed] [Google Scholar]
- Obwaka JM, Mati JK, Lequin RM, Sekadde-kigondu CB, Muitta MN, Nthale JM, Njoroge JK. Baseline studies on semen and hormonal parameters in fertile Black males in Kenya. J Obstet Gynaecol East Cent Africa 1982;1:96–99. [PubMed] [Google Scholar]
- Olsen GW, Bodner KM, Ramlow JM, Ross CE, Lipshultz LI. Have sperm counts been reduced 50% in 50 years? A statistical model revisited. Fertil Steril 1995;63:887–893. [DOI] [PubMed] [Google Scholar]
- Ortiz A, Espino J, Bejarano I, Lozano GM, Monllor F, Garcia JF, Pariente JA, Rodriguez AB. The correlation between urinary 5-hydroxyindoleacetic acid and sperm quality in infertile men and rotating shift workers. Reprod Biol Endocrinol 2010;8:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paasch U, Salzbrunn A, Glander HJ, Plambeck K, Salzbrunn H, Grunewald S, Stucke J, Vierula M, Skakkebaek NE, Jorgensen N. Semen quality in sub-fertile range for a significant proportion of young men from the general German population: a co-ordinated, controlled study of 791 men from Hamburg and Leipzig. Int J Androl 2008;31:93–102. [DOI] [PubMed] [Google Scholar]
- Pal PC, Rajalakshmi M, Manocha M, Sharma RS, Mittal S, Rao DN. Semen quality and sperm functional parameters in fertile Indian men. Andrologia 2006;38:20–25. [DOI] [PubMed] [Google Scholar]
- Pangkahila W. Reversible azoospermia induced by an androgen-progestin combination regimen in Indonesian men. Int J Androl 1991;14:248–256. [DOI] [PubMed] [Google Scholar]
- Patankar SS, Deshkar AM, Sawane MV, Mishra NV, Kale AH, Gosavi GB. The role of hypo-osmotic swelling test in recurrent abortions. Indian J Physiol Pharmacol 2001;45:373–377. [PubMed] [Google Scholar]
- Peters M, Rhodes G, Simmons LW. Does attractiveness in men provide clues to semen quality? J Evol Biol 2008;21:572–579. [DOI] [PubMed] [Google Scholar]
- Plastira K, Msaouel P, Angelopoulou R, Zanioti K, Plastiras A, Pothos A, Bolaris S, Paparisteidis N, Mantas D. The effects of age on DNA fragmentation, chromatin packaging and conventional semen parameters in spermatozoa of oligoasthenoteratozoospermic patients. J Assist Reprod Genet 2007;24:437–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Program NT. Handbook for Conducting a Literature-Based Health Assessment Using OHAT Approach for Systematic Review and Evidence Integration. US Department of Health and Human Services, Editor, 2015. https://ntp.niehs.nih.gov/pubhealth/hat/noms/index-2.html. [Google Scholar]
- Punab M, Zilaitiene B, Jorgensen N, Horte A, Matulevicius V, Peetsalu A, Skakkebaek NE. Regional differences in semen qualities in the Baltic region. Int J Androl 2002;25:243–252. [DOI] [PubMed] [Google Scholar]
- Purakayastha M, Mukhopadhyay SK, Chattopadhyay S. Heterogeneity in antigen distribution on spermatozoa of fertile and infertile subjects. J Indian Med Assoc 1999;97:65–67. [PubMed] [Google Scholar]
- Rabelo-Junior CN, Freire de Carvalho J, Lopes Gallinaro A, Bonfa E, Cocuzza M, Saito O, Silva CA. Primary antiphospholipid syndrome: morphofunctional penile abnormalities with normal sperm analysis. Lupus 2012;21:251–256. [DOI] [PubMed] [Google Scholar]
- Recabarren SE, Sir-Petermann T, Rios R, Maliqueo M, Echiburu B, Smith R, Rojas-Garcia P, Recabarren M, Rey RA. Pituitary and testicular function in sons of women with polycystic ovary syndrome from infancy to adulthood. J Clin Endocrinol Metab 2008;93:3318–3324. [DOI] [PubMed] [Google Scholar]
- Recio-Vega R, Ocampo-Gomez G, Borja-Aburto VH, Moran-Martinez J, Cebrian-Garcia ME. Organophosphorus pesticide exposure decreases sperm quality: association between sperm parameters and urinary pesticide levels. J Appl Toxicol 2008;28:674–680. [DOI] [PubMed] [Google Scholar]
- Reddy KV, Bordekar AD. Spectrophotometric analysis of resazurin reduction test and semen quality in men. Indian J Exp Biol 1999;37:782–786. [PubMed] [Google Scholar]
- Redmon JB, Thomas W, Ma W, Drobnis EZ, Sparks A, Wang C, Brazil C, Overstreet JW, Liu F, Swan SH. Semen parameters in fertile US men: the study for future families. Andrology 2013;1:806–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehan N. Semen characteristics of fertile Pakistani men. J Pak Med Assoc 1994;44:62–64. [PubMed] [Google Scholar]
- Rendon A, Rojas A, Fernandez SI, Pineda I. Increases in chromosome aberrations and in abnormal sperm morphology in rubber factory workers. Mutat Res 1994;323:151–157. [DOI] [PubMed] [Google Scholar]
- Richthoff J, Rylander L, Hagmar L, Malm J, Giwercman A. Higher sperm counts in Southern Sweden compared with Denmark. Hum Reprod 2002;17:2468–2473. [DOI] [PubMed] [Google Scholar]
- Rignell-Hydbom A, Axmon A, Lundh T, Jonsson BA, Tiido T, Spano M. Dietary exposure to methyl mercury and PCB and the associations with semen parameters among Swedish fishermen. Environ Health 2007;6:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rintala MA, Grenman SE, Pollanen PP, Suominen JJ, Syrjanen SM. Detection of high-risk HPV DNA in semen and its association with the quality of semen. Int J STD AIDS 2004;15:740–743. [DOI] [PubMed] [Google Scholar]
- Rosenberg MJ, Wyrobek AJ, Ratcliffe J, Gordon LA, Watchmaker G, Fox SH, Moore DH II, Hornung RW. Sperm as an indicator of reproductive risk among petroleum refinery workers. Br J Ind Med 1985;42:123–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roste LS, Tauboll E, Haugen TB, Bjornenak T, Saetre ER, Gjerstad L. Alterations in semen parameters in men with epilepsy treated with valproate or carbamazepine monotherapy. Eur J Neurol 2003;10:501–506. [DOI] [PubMed] [Google Scholar]
- Rubes J, Rybar R, Prinosilova P, Veznik Z, Chvatalova I, Solansky I, Sram RJ. Genetic polymorphisms influence the susceptibility of men to sperm DNA damage associated with exposure to air pollution. Mutat Res 2010;683:9–15. [DOI] [PubMed] [Google Scholar]
- Rylander L, Wetterstrand B, Haugen TB, Malm G, Malm J, Bjorsvik C, Henrichsen T, Saether T, Giwercman A. Single semen analysis as a predictor of semen quality: clinical and epidemiological implications. Asian J Androl 2009;11:723–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safe S. Endocrine disruptors and falling sperm counts: lessons learned or not. Asian J Androl 2013;15:191–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena P, Misro MM, Chaki SP, Chopra K, Roy S, Nandan D. Is abnormal sperm function an indicator among couples with recurrent pregnancy loss? Fertil Steril 2008;90:1854–1858. [DOI] [PubMed] [Google Scholar]
- Selevan SG, Borkovec L, Slott VL, Zudova Z, Rubes J, Evenson DP, Perreault SD. Semen quality and reproductive health of young Czech men exposed to seasonal air pollution. Environ Health Perspect 2000;108:887–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sermondade N, Faure C, Fezeu L, Shayeb AG, Bonde JP, Jensen TK, Van Wely M, Cao J, Martini AC, Eskandar M et al. BMI in relation to sperm count: an updated systematic review and collaborative meta-analysis. Hum Reprod Update 2013;19:221–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra-Majem L, Bassas L, Garcia-Glosas R, Ribas L, Ingles C, Casals I, Saavedra P, Renwick AG. Cyclamate intake and cyclohexylamine excretion are not related to male fertility in humans. Food Addit Contam 2003;20:1097–1104. [DOI] [PubMed] [Google Scholar]
- Sharma R, Harlev A, Agarwal A, Esteves SC. Cigarette smoking and semen quality: a new meta-analysis examining the effect of the 2010 World Health Organization Laboratory methods for the examination of human semen. Eur Urol 2016;70:635–645. [DOI] [PubMed] [Google Scholar]
- Sheriff DS, Legnain M. Evaluation of semen quality in a local Libyan population. Indian J Physiol Pharmacol 1992;36:83–87. [PubMed] [Google Scholar]
- Shine R, Peek J, Birdsall M. Declining sperm quality in New Zealand over 20 years. NZ Med J 2008;121:50–56. [PubMed] [Google Scholar]
- Skakkebaek NE, Rajpert-De Meyts E, Buck Louis GM, Toppari J, Andersson AM, Eisenberg ML, Jensen TK, Jorgensen N, Swan SH, Sapra KJ et al. Male reproductive disorders and fertility trends: influences of environment and genetic susceptibility. Physiol Rev 2016;96:55–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobowale OB, Akiwumi O. Testicular volume and seminal fluid profile in fertile and infertile males in Ilorin, Nigeria. Int J Gynaecol Obstet 1989;28:155–161. [DOI] [PubMed] [Google Scholar]
- Splingart C, Frapsauce C, Veau S, Barthelemy C, Royere D, Guerif F. Semen variation in a population of fertile donors: evaluation in a French centre over a 34-year period. Int J Androl 2012;35:467–474. [DOI] [PubMed] [Google Scholar]
- Stewart TM, Liu DY, Garrett C, Jorgensen N, Brown EH, Baker HW. Associations between andrological measures, hormones and semen quality in fertile Australian men: inverse relationship between obesity and sperm output. Hum Reprod 2009;24:1561–1568. [DOI] [PubMed] [Google Scholar]
- Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008–2012. [DOI] [PubMed] [Google Scholar]
- Sultan Sheriff D. Setting standards of male fertility I. Semen analyses in 1500 patients—a report. Andrologia 1983;15:687–692. [DOI] [PubMed] [Google Scholar]
- Svanborg K, Gottlieb C, Bendvold E, Bygdeman M. Variation in, and inter-relationship between, prostaglandin levels and other semen parameters in normal men. Int J Androl 1989;12:411–419. [DOI] [PubMed] [Google Scholar]
- Swan SH, Elkin EP, Fenster L. Have sperm densities declined? A reanalysis of global trend data. Environ Health Perspect 1997;105:1228–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan SH, Elkin EP, Fenster L. The question of declining sperm density revisited: an analysis of 101 studies published 1934–1996. Environ Health Perspect 2000;108:961–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tambe AS, Kaore SB, Sawane MV, Gosavi GB. Acrosome intactness and seminal hyaluronidase activity: relationship with conventional seminal parameters. Indian J Med Sci 2001;55:125–132. [PubMed] [Google Scholar]
- Taneja N, Kucheria K, Jain S, Maheshwari MC. Effect of phenytoin on semen. Epilepsia 1994;35:136–140. [DOI] [PubMed] [Google Scholar]
- Te Velde ER, Bonde JP. Misconceptions about falling sperm counts and fertility in Europe. Asian J Androl 2013;15:195–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thilagavathi J, Kumar M, Mishra SS, Venkatesh S, Kumar R, Dada R. Analysis of sperm telomere length in men with idiopathic infertility. Arch Gynecol Obstet 2013;287:803–807. [DOI] [PubMed] [Google Scholar]
- Thompson SG, Higgins JP. How should meta-regression analyses be undertaken and interpreted? Stat Med 2002;21:1559–1573. [DOI] [PubMed] [Google Scholar]
- Tirumala Vani G, Mukesh N, Siva Prasad B, Rama Devi P, Hema Prasad M, Usha Rani P, Pardhanandana Reddy P. Role of glutathione S-transferase Mu-1 (GSTM1) polymorphism in oligospermic infertile males. Andrologia 2010;42:213–217. [DOI] [PubMed] [Google Scholar]
- Toft G, Axmon A, Giwercman A, Thulstrup AM, Rignell-Hydbom A, Pedersen HS, Ludwicki JK, Zvyezday V, Zinchuk A, Spano M et al. Fertility in four regions spanning large contrasts in serum levels of widespread persistent organochlorines: a cross-sectional study. Environ Health 2005;4:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toft G, Pedersen HS, Bonde JP. Semen quality in Greenland. Int J Circumpolar Health 2004;63:174–178. [DOI] [PubMed] [Google Scholar]
- Tsarev I, Bungum M, Giwercman A, Erenpreisa J, Ebessen T, Ernst E, Erenpreiss J. Evaluation of male fertility potential by Toluidine Blue test for sperm chromatin structure assessment. Hum Reprod 2009;24:1569–1574. [DOI] [PubMed] [Google Scholar]
- Tsarev I, Gagonin V, Giwercman A, Erenpreiss J. Sperm concentration in Latvian military conscripts as compared with other countries in the Nordic-Baltic area. Int J Androl 2005;28:208–214. [DOI] [PubMed] [Google Scholar]
- Uhler ML, Zinaman MJ, Brown CC, Clegg ED. Relationship between sperm characteristics and hormonal parameters in normal couples. Fertil Steril 2003;79:1535–1542. [DOI] [PubMed] [Google Scholar]
- Valsa J, Skandhan KP, Gusani PH, Sahab Khan P, Amith S. Quality of 4-hourly ejaculates—levels of calcium and magnesium. Andrologia 2013;45:10–17. [DOI] [PubMed] [Google Scholar]
- Van Waeleghem K, De Clercq N, Vermeulen L, Schoonjans F, Comhaire F. Deterioration of sperm quality in young healthy Belgian men. Hum Reprod 1996;11:325–329. [DOI] [PubMed] [Google Scholar]
- Vanhoorne M, Comhaire F, De Bacquer D. Epidemiological study of the effects of carbon disulfide on male sexuality and reproduction. Arch Environ Health 1994;49:273–278. [DOI] [PubMed] [Google Scholar]
- Vani GT, Mukesh N, Siva Prasad B, Rama Devi P, Hema Prasad M, Usha Rani P, Pardhanandana Reddy P. Association of CYP1A1*2A polymorphism with male infertility in Indian population. Clin Chim Acta 2009;410:43–47. [DOI] [PubMed] [Google Scholar]
- Vani K, Kurakula M, Syed R, Alharbi K. Clinical relevance of vitamin C among lead-exposed infertile men. Genet Test Mol Biomarkers 2012;16:1001–1006. [DOI] [PubMed] [Google Scholar]
- Venkatesh S, Singh A, Shamsi MB, Thilagavathi J, Kumar R, Mitra DK, Dada R. Clinical significance of sperm DNA damage threshold value in the assessment of male infertility. Reprod Sci 2011;18:1005–1013. [DOI] [PubMed] [Google Scholar]
- Verit FF, Verit A, Ciftci H, Erel O, Celik H. Paraoxonase-1 activity in subfertile men and relationship to sperm parameters. J Androl 2009;30:183–189. [DOI] [PubMed] [Google Scholar]
- Vested A, Ramlau-Hansen CH, Bonde JP, Thulstrup AM, Kristensen SL, Toft G. A comparison of conventional and computer-assisted semen analysis (CRISMAS software) using samples from 166 young Danish men. Asian J Androl 2011;13:453–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierula M, Niemi M, Keiski A, Saaranen M, Saarikoski S, Suominen J. High and unchanged sperm counts of Finnish men. Int J Androl 1996;19:11–17. [DOI] [PubMed] [Google Scholar]
- Vine MF, Tse CK, Hu P, Truong KY. Cigarette smoking and semen quality. Fertil Steril 1996;65:835–842. [DOI] [PubMed] [Google Scholar]
- Wang C, Swerdloff RS. Limitations of semen analysis as a test of male fertility and anticipated needs from newer tests. Fertil Steril 2014;102:1502–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward JB Jr, Hokanson JA, Smith ER, Chang LW, Pereira MA, Whorton EB Jr, Legator MS. Sperm count, morphology and fluorescent body frequency in autopsy service workers exposed to formaldehyde. Mutat Res 1984;130:417–424. [DOI] [PubMed] [Google Scholar]
- Weidner W, Jantos C, Schiefer HG, Haidl G, Friedrich HJ. Semen parameters in men with and without proven chronic prostatitis. Arch Androl 1991;26:173–183. [DOI] [PubMed] [Google Scholar]
- Wickings EJ, Freischem CW, Langer K, Nieschlag E. Heterologous ovum penetration test and seminal parameters in fertile and infertile men. J Androl 1983;4:261–271. [DOI] [PubMed] [Google Scholar]
- Wiltshire EJ, Flaherty SP, Couper RT. Hepatocyte growth factor in human semen and its association with semen parameters. Hum Reprod 2000;15:1525–1528. [DOI] [PubMed] [Google Scholar]
- Winters BR, Walsh TJ. The epidemiology of male infertility. Urol Clin North Am 2014;41:195–204. [DOI] [PubMed] [Google Scholar]
- World Health Organization Comparison of two androgens plus depot-medroxyprogesterone acetate for suppression to azoospermia in indonesian men. World Health Organization. Task Force on Methods for the Regulation of Male Fertility. Fertil Steril 1993;60:1062–1068. [PubMed] [Google Scholar]
- World Health Organization WHO laboratory manual for the examination and processing of human semen, 2010.
- Wu B, Lu NX, Xia YK, Gu AH, Lu CC, Wang W, Song L, Wang SL, Shen HB, Wang XR. A frequent Y chromosome b2/b3 subdeletion shows strong association with male infertility in Han-Chinese population. Hum Reprod 2007;22:1107–1113. [DOI] [PubMed] [Google Scholar]
- Wyrobek AJ, Brodsky J, Gordon L, Moore DH II, Watchmaker G, Cohen EN. Sperm studies in anesthesiologists. Anesthesiology 1981. a;55:527–532. [DOI] [PubMed] [Google Scholar]
- Wyrobek AJ, Watchmaker G, Gordon L, Wong K, Moore D II, Whorton D. Sperm shape abnormalities in carbaryl-exposed employees. Environ Health Perspect 1981. b;40:255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao G, Pan C, Cai Y, Lin H, Fu Z. Effect of benzene, toluene, xylene on the semen quality and the function of accessory gonad of exposed workers. Ind Health 2001;39:206–210. [DOI] [PubMed] [Google Scholar]
- Xu DX, Zhu QX, Zheng LK, Wang QN, Shen HM, Deng LX, Ong CN. Exposure to acrylonitrile induced DNA strand breakage and sex chromosome aneuploidy in human spermatozoa. Mutat Res Genet Toxicol Environ Mutagen 2003;537:93–100. [DOI] [PubMed] [Google Scholar]
- Yucra S, Rubio J, Gasco M, Gonzales C, Steenland K, Gonzales GF. Semen quality and reproductive sex hormone levels in Peruvian pesticide sprayers. Int J Occup Environ Health 2006;12:355–361. [DOI] [PubMed] [Google Scholar]
- Zalata A, Atwa A, El-Naser Badawy A, Aziz A, El-Baz R, Elhanbly S, Mostafa T. Tumor necrosis factor-alpha gene polymorphism relationship to seminal variables in infertile men. Urology 2013;81:962–966. [DOI] [PubMed] [Google Scholar]
- Zareba P, Colaci DS, Afeiche M, Gaskins AJ, Jorgensen N, Mendiola J, Swan SH, Chavarro JE. Semen quality in relation to antioxidant intake in a healthy male population. Fertil Steril 2013;100:1572–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JP, Meng QY, Wang Q, Zhang LJ, Mao YL, Sun ZX. Effect of smoking on semen quality of infertile men in Shandong, China. Asian J Androl 2000;2:143–146. [PubMed] [Google Scholar]
- Zhang M-H, Zhang A-D, Shi Z-D, Wang L-G, Qiu Y. Changes in levels of seminal nitric oxide synthase, macrophage migration inhibitory factor, sperm DNA integrity and caspase-3 in fertile men after scrotal heat stress. PLoS One 2015;10:e0141320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong CQ, Lui QL, Tang YJ, Wang Y, Shi FJ, Qian SZ. Study on sperm function in men long after cessation of gossypol treatment. Contraception 1990;41:617–622. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.