Abstract
Purpose of review
Rheumatoid arthritis (RA) is associated with negative changes in mental health. This is generally attributed to symptoms of inflammation and the adverse impact of RA on quality of life and functioning. Until recently, causal pathways in the opposite direction have not been fully appreciated. This review examines the recent literature on the risk of RA associated with depression.
Recent findings
Current literature links depression with an increased risk of RA and with a more detrimental disease course. These effects are likely to be partially mediated by negative effects of depression on coping with RA and on factors such as medication adherence, both of which lead to poorer disease outcomes. Growing evidence also suggests that inflammation is central both to depression and RA and may account for some of the complex interplay between these conditions.
Summary
Awareness of a bidirectional relationship between depression and RA through a biopsychosocial framework may assist clinicians in maintaining an appropriate index of suspicion about the co-occurrence of these conditions. This review also suggests an important need for integration of rheumatologic and mental health services and generates hypotheses for future research towards a better understanding of both depression and RA.
Keywords: depression, inflammation, rheumatoid arthritis
INTRODUCTION
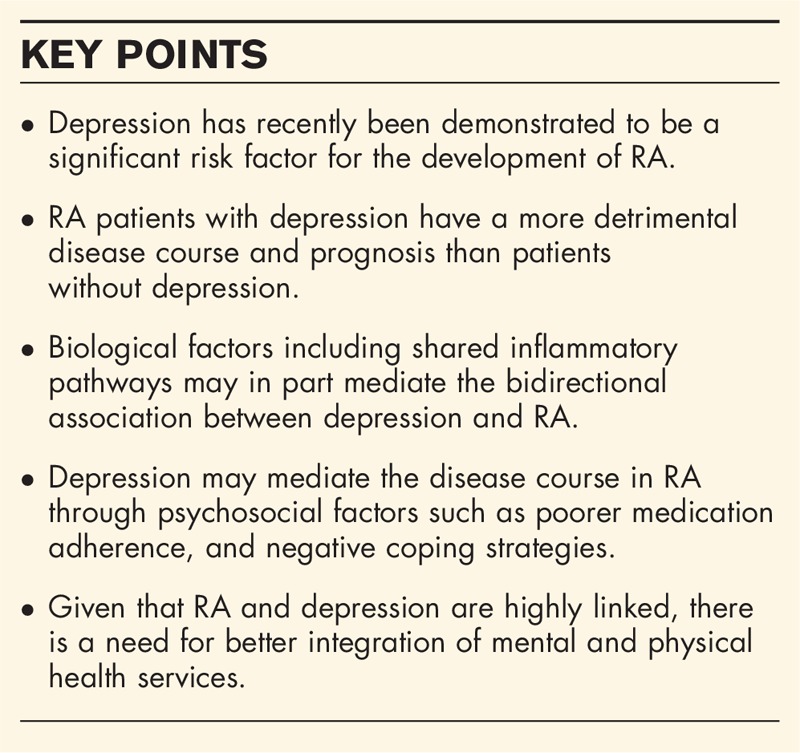
Depression, which is a major contributor to the global burden of disease in the general population [1], has long been regarded in the rheumatology community as a ‘comorbidity’ of rheumatoid arthritis (RA). In a recent meta-analysis, the prevalence of major depressive disorder (MDD), commonly known as ‘depression’, among RA patients using Diagnostic and Statistical Manual of Mental Disorders diagnostic criteria was estimated at 17% [2], and in a population-based cohort in Canada, the incidence rate ratio (IRR) for depression was significantly increased in persons with incident RA compared to the general population [IRR 1.46, 95% confidence interval (CI) 1.35–1.58], after adjustment for covariates [3].
Several explanations for the high frequency of depression in RA patients exist, including the impact of a diagnosis of a chronic disease with no cure, loss of social roles, and employment due to disease and disability, and even medication side effects. The negative impact of depression on functioning has important implications for patients as RA places considerable demands on the coping resources of those afflicted. The loss of energy, pessimism and negative cognitive style that characterizes depression diminishes the depressed person's ability to cope with challenges. Recent advances in medicine and research have facilitated the development of models which offer an inflammatory hypothesis to explain the relationship between the systemic inflammation of RA, and subsequent effect on the brain and its function, summarized in several recent reviews [4▪▪,5▪,6▪,7,8]. In brief, pro-inflammatory cytokines, including tumor necrosis factor (TNF)α, interleukin (IL)-6, and IL-1β, are circulating systemically in the RA inflammatory response. Increased peripheral cytokines directly activate three known pathways to affect brain structures: first, the neural pathway, via afferent nerves, thereby having an effect on hypothalamic brain nuclei; second, the humoral pathway, via direct contact with the choroid plexus and circumventricular organs where there is no blood–brain barrier; and third, via activation of cerebral endothelial cells, which result in microglial activation and subsequent secretion of pro-inflammatory cytokines, proteases and chemokines, which recruit monocytes into areas of the brain associated with behavior [4▪▪]. Once pro-inflammatory cytokines are in contact with central brain structures, they can then affect areas of the brain that are known to have altered function in depression, such as the medial prefrontal cortex, hippocampus, anterior cingulate cortex, and basal ganglia, in conjunction with effects on neuroendocrine function and neurotransmitter metabolism.
More recently, it has been posited that a bidirectional association may exist, namely that depression could also predispose to the development of RA. Furthermore, recent studies have examined if depression is implicated in altering the disease course of RA. If so, there may be a role for specific therapeutics to be used to positively impact depression, enhancing RA outcomes. The purpose of this review is to provide a summary of new literature exploring depression and the risk of RA. Specifically, this review will focus on inflammatory pathways, epidemiologic evidence for the increased risk of RA in patients with MDD, and implications of MDD on RA disease course and prognosis, along with reviewing preliminary effects of targeted therapy for RA that influence depression outcomes.
Box 1.
no caption available
RATIONALE FOR DEPRESSION AS A RISK FACTOR FOR RHEUMATOID ARTHRITIS
It is now accepted that inflammation is a feature of depression, as increased levels of pro-inflammatory cytokines are observed in the absence of autoimmune disease. Interestingly, the variation in patient response to pharmacotherapy for depression is postulated to be related to alterations in the balance of pro-inflammatory and non-inflammatory cytokines in an individual [5▪]. Furthermore, a systematic review of 32 studies reporting cytokine levels prior to and after treatment with antidepressants identified that IL-4, IL-6, and IL-10 in peripheral blood significantly decreased with pharmacological antidepressant treatment, and IL-1 decreased when the selective serotonin reuptake inhibitor class of antidepressants were used [7].
The mechanisms by which central inflammation in depression can induce systemic inflammatory responses, and ultimately result in autoimmune diseases such as RA, are still under study [9▪▪] (Fig. 1). Immune alterations, such as a reduction in the frequency of classical monocytes, and an increase in nonclassical monocytes [10], are hypothesized to be a result of neurotoxic processes occurring in response to stress in morphologically altered brains, mediated by dysregulation of the hypothalamic-pituitary-adrenal axis and a positive inflammation feedback loop [9▪▪]. Cell-mediated immune cytokines are increased, activating indoleamine 2,3-dioxygenase. Indoleamine 2,3-dioxygenase activity enhances tryptophan catabolism, resulting in serotonin depletion and the production of kynurenine. Neurotoxic substances 3-hydroxykynurenine and quinolinic acid are produced via conversion of kynurenine, leading to increased glutamate release and oxidative stress, and reduced GABAergic inhibitory control, which has been implicated in MDD [9▪▪].
FIGURE 1.
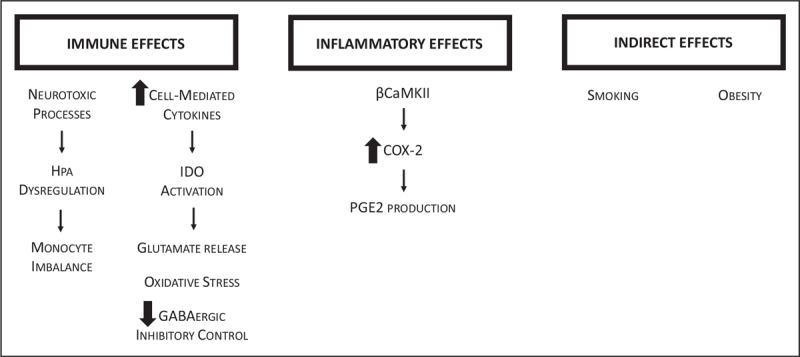
Proposed mechanisms for rheumatoid arthritis resulting from immune, inflammatory and indirect effects of depression. HPA, hypothalamic-pituitary-adrenal axis; IDO, indoleamine 2,3-dioxygenase; βCaMKII, calcium/calmodulin-dependent protein kinases type II; COX-2, cyclo-oxygenase-2; PGE2, prostaglandin E2.
Calcium/calmodulin-dependent protein kinases type II (βCaMKII) may also play an important role. These kinases facilitate cyclo-oxygenase (COX-2) transcription and expression, and enzymatic hyperactivity of COX-2 can result in production of prostaglandin E2 (PGE2), which is pro-inflammatory [11]. In rat models, βCaMKII is upregulated under stress situations in the hippocampal CA1 region, with resulting increased COX-2 levels, and increased expression of PGE2. In these models, beneficial effects have been demonstrated from COX-2 inhibition with celecoxib, in both PGE2 levels and displayed depression behaviors.
An interesting association to consider is the relationship between oxidative stress and depression, obesity, and inflammation. A relationship between these factors and sleep was examined in the United States National Health and Nutritional Examination Survey (2005–2008) using the Patient Health Questionnaire-9 to determine depressive symptoms, measurement of the BMI, and laboratory measurements of C-reactive protein for inflammation, and γ-glutamyltransferase as a marker for oxidative stress [12]. BMI moderated the relationship between oxidative stress and depressive symptoms, and a higher BMI increased the odds of depressive symptoms in women in a dose–response fashion [12]. Concurrently, obesity is associated with higher RA risk [13]. Another important association to consider is exposure to smoking, which increases oxidative stress and also has epigenetic and immune system alteration effects [14]. Smoking is an accepted RA risk factor, and it is also a risk factor for the development of depressive disorders [15]. Strengthening the argument, smoking cessation is associated with improvements in depression [16] and continued smoking negatively impacts RA outcomes [17].
EPIDEMIOLOGIC EVIDENCE OF DEPRESSION AS A RISK FACTOR FOR RHEUMATOID ARTHRITIS
The first investigation of the risk of RA in depression used claim data from the National Health Insurance of Taiwan [18]. The crude incidence of RA in the cohort of persons with depression was increased compared to the rate in those without depression (2.07 vs. 1.21 per 1000 person-years), and in multivariate Cox models the adjusted risk was significantly elevated by 65% (hazard ratio 1.65, 95% CI 1.41–1.77) [18]. This risk did not vary by sex, but the risk of RA was accentuated in persons less than 40 years of age. An effect of the duration of depression was seen, with a higher incidence rate for RA observed in the 3–5 and more than 5 year periods after onset of depression. Postpartum depression and subsequent risk of RA has also been studied in this dataset, with an elevated risk also found (adjusted hazard ratio 2.62, 95% CI 1.28–5.39) [19].
A population-based cohort of British patients from The Health Improvement Network was analyzed to estimate the risk of incident RA in those with MDD compared to a general population cohort without MDD. A total of 2192 patients in the MDD cohort developed incident RA (incidence rate 85 cases per 100 000 person-years) compared to 24 012 patients in the general population cohort (incidence rate 53 per 100 000 person-years). After adjustment for age, sex, smoking, BMI, comorbidities and antidepressant use, persons in the MDD cohort were found to have a 38% increased risk of developing RA (hazard ratio 1.38, 95% CI 1.31–1.46) [20▪▪]. This study also demonstrated that the risk of developing RA was lower among those with MDD who used antidepressants, compared to those with MDD who did not use antidepressants (HR 0.74, 95% CI 0.71–0.76).
The biological effects of stressful life events may be partially mediated by MDD, and studies that have examined RA risk following these events are of relevance. In the Nurses’ Health Study, the risk for incident RA increased by 76% for those with at least four posttraumatic stress disorder (PTSD) symptoms (hazard ratio 1.76, 95% CI 1.16–2.67) after adjustment for age, year, race, and parental education, and a dose–response based on the number of PTSD symptoms was evident [21]. A case–control study from Scandinavia determined there was an increased incidence of RA for those determined to have had a stressful life event within the past year compared to osteoarthritis controls, although the retrospective nature of this study is a methodological limitation [22]. It has been demonstrated recently that mental disorders such as depression can increase the risk of another inflammatory arthropathy, psoriatic arthritis [23], as well as other autoimmune diseases including inflammatory bowel disease [24], alopecia areata [25], and vitiligo [26] among others [27], thus further supporting the temporal nature of these inflammatory relationships.
DEPRESSION AND THE IMPACT ON RHEUMATOID ARTHRITIS DISEASE COURSE
It is increasingly recognized that RA outcomes are affected by depression. Depression is known to impact treatment adherence [28], providing an indirect mechanism through which depression may alter RA outcomes, although other explanations may exist. An analysis of data from the British Society for Rheumatology Biologics Register for Rheumatoid Arthritis (BSRBR-RA) reported that the odds of having a good treatment response (assessed using the European League Against Rheumatism or EULAR response) at 1 year were reduced for those with depression at baseline (OR 0.80, 95% CI 0.69–0.92) after covariate adjustment [29▪]. In subanalysis, this was attributable to persistently higher tender and swollen joint counts, patient global evaluation scores, and inflammatory marker levels. This finding was supported in analyses from the Leiden Early Arthritis Clinic where the combined outcome of depression/anxiety was associated with a significantly lower odds of achieving DAS44 remission after 1 year [OR 0.21 95% CI 0.09–0.46 for the Short Form Health Survey (SF-36) Mental Component Summary (MCS) and OR 0.24 95% CI 0.11–0.51 for SF3 Mental Health (MH) subscale] [30]. In the NOR-DMARD registry, a variety of scales to assess depression/anxiety at inception to the cohort and a variety of remission definitions at 3 and 6 months were applied, with a consistent finding of depression/anxiety negatively predicting remission. However, in contrast to the Leiden data, this was assessed to be related to subjective components of the outcome measures and not swollen joint count or inflammatory marker levels [31▪]. Moreover, Kuriya et al. [32] demonstrated a significant association between high continuous disease activity, measured by the Clinical Disease Activity Index, and persistent depression.
RA flare may also be related to depression. In a study investigating factors associated with disease flare, the majority (86%) of respondents endorsed the main perceived reason being psychological stress and mood disorder [33]. A posthoc analysis of the OPtimising Treatment with Tumour necrosis factor (TNF) Inhibitors in Rheumatoid Arthritis (OPTTIRA) randomized open-label trial investigated risk factors for flare among RA patients tapering anti-TNF therapy. Adjusted analyses revealed that the SF-36 MH score was the only statistically significant predictor of flare [34▪].
The ultimate impact of RA is mortality. In a study using administrative data from Manitoba, Canada, depression was associated with increased mortality (attributable proportion 6.9%) [35▪]. Suicide rates and attempts were considered in this analysis; however, RA-specific estimates with depression and suicide were not statistically significant.
EFFECTS OF TARGETED THERAPY FOR RHEUMATOID ARTHRITIS ON DEPRESSION
With evidence for pro-inflammatory cytokines being elevated in depression, it is of no surprise that targeted anticytokine therapy is of interest in the primary management of MDD. Clinicaltrials.gov lists an active study for tocilizumab (IL-6 inhibitor) in treatment-refractory MDD, and the trial protocol for the insight study, which will assess the effect of a single intravenous infusion of tocilizumab on somatic symptoms score, has been published [36]. JAK/STAT modulation is also of theoretical benefit, as this pathway is involved in neurogenesis, synaptic plasticity, gliogenesis and microglial activation, and also with some antidepressants having a mechanism of action mediated by JAK/STAT-dependent mechanisms [5▪]. Other randomized trials are in progress looking at the effects of anti-TNFα therapy in anhedonia [37].
RA outcomes in patients with depression may be improved through targeted biologic therapies. A review by Choy and Calabrese presents a summary of changes in SF-36 MCS scores in phase 3 clinical trials of anti-IL6 therapy, and present a strong impression of benefit [6▪]. This was formally assessed in a systematic review and meta-analysis of RA randomized controlled trials, where there was a small but significant effect of biologic disease-modifying antirheumatic drugs on the SF-36 MCS, with anti-IL6 therapy being the most effective treatment for improved MCS outcomes [38]. A cohort of RA patients from Portugal was also studied, with evidence that tocilizumab and sulfasalazine were associated with reduced odds of depressive symptoms (OR 0.70, 95% CI 0.52–0.95, and OR 0.58, 95% CI 0.37–0.87, respectively) [39].
CONCLUSION
Evidence is accumulating to suggest that depression may have a greater role on physical health than previously thought. As the biological origins of depression are progressively clarified, the traditional distinction between psychosocial and biological perspectives is becoming progressively blurred. While it is well known that patients with RA have a greater predisposition to developing depression, the reverse association whereby depression also increases the risk of developing RA has only been demonstrated more recently. The inflammatory hypothesis of depression highlights a possible mechanism by which depression increases the risk of RA. We have summarized the evidence that depression can significantly influence the disease course of RA, by increasing the risk of disease flares and decreasing rates of remission. Since depression increases the risk of RA, it is also likely that people who develop RA have a larger burden of depression, which could interfere with the effectiveness of their coping strategies, clinical management (e.g., medication compliance) and quality of life. Since quality of life is a subjective assessment, and because depression causes negative cognitive biases, depression may sometimes be a more important determinant of quality of life in RA than the disease course itself, as has been reported in multiple sclerosis, another immune-mediated disease [40]. Clinicians may need to have an index of suspicion about RA in depressed patients, which is a clinical challenge since depressed patients may have increased somatic distress (such as generalized pain) and clinicians may be more willing to assume that their complaints are less likely to indicate RA, an issue that has been described as diagnostic overshadowing [41]. Recognizing that a bidirectional relationship exists between depression and RA may at least be a step in the right direction toward preventing poorer prognoses in each of these patient groups.
There are direct implications of these findings for clinical care – potentially since it may channel selection of therapies, but more importantly that improved outcomes of depression in RA patients may lead to better clinical outcomes overall. Ultimately, clinicians involved in the care of patients with RA or depression should help to refer to respective specialist care when needed. Moreover, this bidirectional relationship between depression and RA has implications on policy and service administration as it highlights the need for better integration of physical and mental health services. This review also generates hypotheses that could lead to a better understanding both of RA and depression with possible preventive opportunities. Philosophically, this review underscores the salience of a biopsychosocial perspective to research and practice.
Acknowledgements
None.
Financial support and sponsorship
None.
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Global Burden of Disease. 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017; 390:1211–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matcham F, Rayner L, Steer S, Hotopf M. The prevalence of depression in rheumatoid arthritis: a systematic review and meta-analysis. Rheumatology (Oxford) 2013; 52:2136–2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marrie RA, Hitchon CA, Walld R, et al. Increased burden of psychiatric disorders in rheumatoid arthritis. Arthritis Care Res (Hoboken) 2018; 70:970–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4▪▪.Nerurkar L, Siebert S, McInnes IB, Cavanagh J. Rheumatoid arthritis and depression: an inflammatory perspective. Lancet Psychiatry 2018; 6:164–173. [DOI] [PubMed] [Google Scholar]; This article provides a comprehensive review of the evidence for shared innate immune and molecular responses to inflammation between depression and RA.
- 5▪.Shariq AS, Brietzke E, Rosenblat JD, et al. Targeting cytokines in reduction of depressive symptoms: a comprehensive review. Prog Neuropsychopharmacol Biol Psychiatry 2018; 83:86–91. [DOI] [PubMed] [Google Scholar]; This review article highlights the clinical outcomes associated with depression from blocking cytokines in inflammatory diseases.
- 6▪.Choy EHS, Calabrese LH. Neuroendocrine and neurophysiological effects of interleukin 6 in rheumatoid arthritis. Rheumatology (Oxford) 2018; 57:1885–1895. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article describes the role of IL-6 in mood disorders, pain and fatigue associated with RA.
- 7.Wiedlocha M, Marcinowicz P, Krupa R, et al. Effect of antidepressant treatment on peripheral inflammation markers: a meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry 2018; 80 (Pt C):217–226. [DOI] [PubMed] [Google Scholar]
- 8.Hashioka S, Inoue K, Hayashida M, et al. Implications of systemic inflammation and periodontitis for major depression. Front Neurosci 2018; 12:483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9▪▪.Belleau EL, Treadway MT, Pizzagalli DA. The impact of stress and major depressive disorder on hippocampal and medial prefrontal cortex morphology. Biol Psychiatry 2018; pii: S0006-3223(18)31930-9. doi: 10.1016/j.biopsych.2018.09.031. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article details the neurobiological pathways leading to brain changes induced by inflammation through stress and depression. As such, this article links brain structures commonly associated with depression to inflammatory changes as a result of depression.
- 10.Hasselmann H, Gamradt S, Taenzer A, et al. Pro-inflammatory monocyte phenotype and cell-specific steroid signaling alterations in unmedicated patients with major depressive disorder. Front Immunol 2018; 9:2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song Q, Fan C, Wang P, et al. Hippocampal CA1 betaCaMKII mediates neuroinflammatory responses via COX-2/PGE2 signaling pathways in depression. J Neuroinflammation 2018; 15:338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rigobon AV, Kanagasabai T, Taylor VH. Obesity moderates the complex relationships between inflammation, oxidative stress, sleep quality and depressive symptoms. BMC Obes 2018; 5:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qin B, Yang M, Fu H, et al. Body mass index and the risk of rheumatoid arthritis: a systematic review and dose-response meta-analysis. Arthritis Res Ther 2015; 17:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hussain MS, Tripathi V. Smoking under hypoxic conditions: a potent environmental risk factor for inflammatory and autoimmune diseases. Mil Med Res 2018; 5:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khaled SM, Bulloch AG, Williams JV, et al. Persistent heavy smoking as risk factor for major depression (MD) incidence–evidence from a longitudinal Canadian cohort of the National Population Health Survey. J Psychiatr Res 2012; 46:436–443. [DOI] [PubMed] [Google Scholar]
- 16.Patten SB, Williams JVA, Lavorato DH, et al. Major depression and nonspecific distress following smoking cessation in the Canadian general population. J Affect Disord 2017; 218:182–187. [DOI] [PubMed] [Google Scholar]
- 17.Sokolove J, Wagner CA, Lahey LJ, et al. Increased inflammation and disease activity among current cigarette smokers with rheumatoid arthritis: a cross-sectional analysis of US veterans. Rheumatology (Oxford) 2016; 55:1969–1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu M-C, Gu H-R, Lin M-C, et al. Bidirectional associations between rheumatoid arthritis and depression: a nationwide longitudinal study. Sci Rep 2016; 6:20467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin CY, Li CK, Liu JM, et al. Postpartum depression and subsequent autoimmune diseases in Taiwan. Int J Environ Res Public Health 2018; 15:pii: E1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20▪▪.Vallerand IA, Lewinson RT, Frolkis AD, et al. Depression as a risk factor for the development of rheumatoid arthritis: a population-based cohort study. RMD Open 2018; 4:e000670. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is the largest study to date demonstrating a significantly increased risk of developing RA among patients with depression. This study also demonstrated a protective effect of antidepressant use for those with depression on the risk of RA.
- 21.Lee YC, Agnew-Blais J, Malspeis S, et al. Post-traumatic stress disorder and risk for incident rheumatoid arthritis. Arthritis Care Res (Hoboken) 2016; 68:292–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gross J, Oubaya N, Eymard F, et al. Stressful life events as a trigger for rheumatoid arthritis onset within a year: a case-control study. Scand J Rheumatol 2017; 46:507–508. [DOI] [PubMed] [Google Scholar]
- 23.Lewinson RT, Vallerand I, Lowerison M, et al. Depression is associated with an increased risk of psoriatic arthritis among patients with psoriasis: a population-based study. J Invest Dermatol 2017; 137:828–835. [DOI] [PubMed] [Google Scholar]
- 24.Frolkis AD, Vallerand IA, Shaheen AA, et al. Depression increases the risk of inflammatory bowel disease, which may be mitigated by the use of antidepressants in the treatment of depression. Gut 2018; pii: gutjnl-2018-317182. doi: 10.1136/gutjnl-2018-317182. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 25.Vallerand IA, Lewinson RT, Parsons LM, et al. Assessment of a bidirectional association between major depressive disorder and alopecia areata. JAMA Dermatol 2019; doi: 10.1001/jamadermatol.2018.4398. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vallerand IA, Lewinson RT, Parsons LM, et al. ‘Vitiligo and major depressive disorder: a bidirectional population-based cohort study’. J Am Acad Dermatol 2018; pii: S0190-9622(18)32982-7. doi: 10.1016/j.jaad.2018.11.047. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 27.Song H, Fang F, Tomasson G, et al. Association of stress-related disorders with subsequent autoimmune disease. JAMA 2018; 319:2388–2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baeza-Velasco C, Olie E, Beziat S, et al. Determinants of suboptimal medication adherence in patients with a major depressive episode. Depress Anxiety 2018; doi: 10.1002/da.22852. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 29▪.Matcham F, Davies R, Hotopf M, et al. The relationship between depression and biologic treatment response in rheumatoid arthritis: an analysis of the British Society for Rheumatology Biologics Register. Rheumatology (Oxford) 2018; 57:835–843. [DOI] [PubMed] [Google Scholar]; This study reports evidence of poorer prognosis in RA patients with depression as it demonstrates reduced odds of achieving good treatment responses among patients with depression at biologic treatment initiation.
- 30.Boer AC, Huizinga TWJ, van der Helm-van Mil AHM. Depression and anxiety associate with less remission after 1 year in rheumatoid arthritis. Ann Rheum Dis 2018; 78:e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31▪.Michelsen B, Kristianslund EK, Sexton J, et al. Do depression and anxiety reduce the likelihood of remission in rheumatoid arthritis and psoriatic arthritis? Data from the prospective multicentre NOR-DMARD study. Ann Rheum Dis 2017; 76:1906–1910. [DOI] [PubMed] [Google Scholar]; This study demonstrated a reduced likelihood of remission in RA patients with concurrent depression or anxiety, using several validated disease activity scales.
- 32.Kuriya B, Joshi R, Movahedi M, et al. High disease activity is associated with self-reported depression and predicts persistent depression in early rheumatoid arthritis: results from the ontario best practices research initiative. J Rheumatol 2018; 45:1101–1108. [DOI] [PubMed] [Google Scholar]
- 33.Yilmaz V, Umay E, Gundogdu I, et al. Rheumatoid arthritis: are psychological factors effective in disease flare? Eur J Rheumatol 2017; 4:127–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34▪.Bechman K, Sin FE, Ibrahim F, et al. Mental health, fatigue and function are associated with increased risk of disease flare following TNF inhibitor tapering in patients with rheumatoid arthritis: an exploratory analysis of data from the Optimizing TNF Tapering in RA (OPTTIRA) trial. RMD Open 2018; 4:e000676. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article demonstrated that mental health status is independently associated with disease flare in RA patients tapering their anti-TNF therapy. Thus, this article suggests that mental health should be considered as an important factor when making decisions on changes in treatment.
- 35▪.Marrie RA, Walld R, Bolton JM, et al. Psychiatric comorbidity increases mortality in immune-mediated inflammatory diseases. Gen Hosp Psychiatry 2018; 53:65–72. [DOI] [PubMed] [Google Scholar]; This article reported that patients with immune-mediated diseases and comorbid depression have an excess risk of the most severe adverse outcome: mortality.
- 36.Khandaker GM, Oltean BP, Kaser M, et al. Protocol for the insight study: a randomised controlled trial of single-dose tocilizumab in patients with depression and low-grade inflammation. BMJ Open 2018; 8:e025333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee Y, Subramaniapillai M, Brietzke E, et al. Anticytokine agents for anhedonia: targeting inflammation and the immune system to treat dimensional disturbances in depression. Ther Adv Psychopharmacol 2018; 8:337–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matcham F, Galloway J, Hotopf M, et al. The impact of targeted rheumatoid arthritis pharmacologic treatment on mental health: a systematic review and network meta-analysis. Arthritis Rheumatol 2018; 70:1377–1391. [DOI] [PubMed] [Google Scholar]
- 39.Figueiredo-Braga M, Cornaby C, Cortez A, et al. Influence of biological therapeutics, cytokines, and disease activity on depression in rheumatoid arthritis. J Immunol Res 2018; 2018:5954897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.D’Alisa S, Miscio G, Baudo S, et al. Depression is the main determinant of quality of life in multiple sclerosis: a classification-regression (CART) study. Disabil Rehabil 2006; 28:307–314. [DOI] [PubMed] [Google Scholar]
- 41.Jones S, Howard L, Thornicroft G. ‘Diagnostic overshadowing’: worse physical healthcare for people with mental illness. Acta Psychiatr Scand 2008; 118:169–171. [DOI] [PubMed] [Google Scholar]


