Abstract
OBJECTIVES:
Common variable immunodeficiency (CVID) is associated with a spectrum of autoimmune complications. We studied the prevalence of gastrointestinal (GI) manifestations and infections in patients with CVID.
METHODS:
Complete clinical data of 132 Finnish patients with CVID (106 probable and 26 possible CVID) followed up between 2007 and 2016 were collected to a structured database. Data on endoscopies, histology, and laboratory studies were retrieved from patient files.
RESULTS:
Most common referral indications were diarrhea and/or weight loss (47%–67%). Patients with probable CVID had higher fecal calprotectin and α1-antitrypsin and lower blood vitamin B12 than patients with possible CVID. Gastroscopy and colonoscopy were done to 71 (67%) and 63 (59%) patients with probable CVID, respectively. Endoscopies showed that 15% of them had chronic active gastritis and 17% atrophic gastritis and 3% had gastric adenocarcinoma. A celiac sprue-like condition was found in 7 patients (10%), of whom 3 responded to a gluten-free diet. Colonoscopies demonstrated unspecific colitis (14%), ulcerative colitis (8%), microscopic colitis (10%), and Crohn's disease (2%). Colonic polyps were noted in 30% of patients, and 3% had lower GI malignancies. Thirty-five patients with CVID had bacterial or parasitic gastroenteritis; chronic norovirus was detected in 4 patients with probable CVID. Patients with GI inflammation had higher levels of fecal calprotectin and blood CD8+ T lymphocytes but lower counts of CD19+CD27+ memory B cells and/or CD19+ B cells. Immunophenotype with low B-cell counts was associated with higher fecal calprotectin levels.
DISCUSSION:
Patients with CVID had a high prevalence of GI manifestations and infections of the GI tract. GI inflammation was associated with a distinct immunophenotype and elevated fecal calprotectin.
INTRODUCTION
Common variable immunodeficiency (CVID) is the most common severely symptomatic primary immunodeficiency. Its prevalence is 2–4 in 100,000 white population (1), whereas in southern Finland, the CVID prevalence reaches 7.7 in 100,000 (2). CVID is a heterogeneous immunodeficiency disorder characterized by a loss of B-cell function and impaired antibody production (3). B-cell defects in CVID are frequently accompanied by various immunologic changes, such as autoimmune cytopenias, abnormal T-cell function, and polyclonal lymphoproliferation (3). Patients with CVID are treated with immunoglobulin (Ig) G infusions, which effectively reduce the risk of infections (4) but have only a limited effect on additional complications (3). Although newer criteria have been proposed, CVID has traditionally been diagnosed using the European Society for Immunodeficiency/Pan-American Group for Immunodeficiency criteria (2,5). These criteria define categories of probable and possible CVID. Generally, in probable CVID, IgG and IgA/IgM are decreased, whereas in possible CVID, only IgG is decreased. Both diagnoses require impaired vaccine responses and exclusion of secondary causes (5).
Previous studies have suggested a 9%–20% frequency of gastrointestinal (GI) disease in patients with CVID (6–8). Patients with CVID typically have recurrent infections, but in addition, they may develop a wide range of autoimmune and autoinflammatory GI diseases (6). Importantly, the presence of GI manifestations is a major risk factor for patients with CVID, with a 2.7- to 4-fold increased mortality (6,7).
Therefore, to comprehensively analyze the GI phenotypes associated with CVID and their prevalence, we assessed a Finnish cohort of 132 patients with CVID who were followed up between 2007 and 2016. Our cohort was systematically screened for GI-associated symptoms and findings by treating physicians. We used readily available laboratory parameters to identify potential screening tools for GI manifestations in this patient cohort and assessed whether any B-cell immunophenotypes are associated with gut inflammation.
MATERIALS AND METHODS
Patients
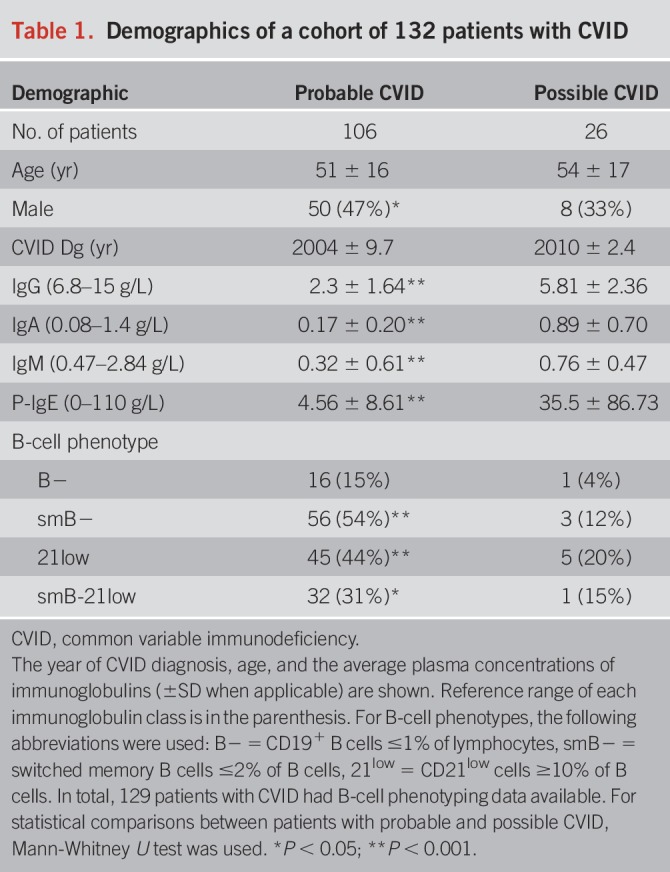
The study received local ethics consent (138/13/03/00/2013). All adult patients receiving immunoglobulin replacement therapy during the years 2007–2016 who fulfilled the European Society for Immunodeficiency/Pan-American Group for Immunodeficiency criteria for primary CVID and the predetermined cutoff criteria for impaired pneumococcal polysaccharide responses were recruited (Table 1). Diagnostic criteria, immunophenotypes, extragastrointestinal manifestations, and demographics of this patient cohort have been described earlier in detail (2).
Table 1.
Demographics of a cohort of 132 patients with CVID
Laboratory parameters and histology
Laboratory tests were analyzed by the accredited HUSLAB (Hospital District of Helsinki and Uusimaa Laboratory). Tests were ordered by the outpatient clinics of the Adult Immunodeficiency Unit or the Department of Gastroenterology as a part of routine follow-up. In addition, most of the 132 patients had additionally been screened by the treating physicians for GI phenotype and malabsorption by measuring calprotectin (92%) and fecal α1-antitrypsin (83%) as well as blood counts (100%) and vitamin B12 levels (79%). Results assessed in this study include blood counts, serum vitamin B12 and/or B12-TC2 (active B12 bound to transcobalamin II), flow cytometry of lymphocytes (2), fecal markers (calprotectin and α1-antitrypsin), and GI pathogens (see Supplementary Table 1, Supplemental Digital Content 1, http://links.lww.com/AJG/A57).
Endoscopies and histology
Patients were referred for endoscopy mainly due to their symptoms and/or laboratory abnormalities (upper GI 92%, lower GI 94%; Figure 1). We analyzed all endoscopies performed for this patient cohort (155 gastroscopies and 163 colonoscopies). Overlap was noted, and altogether 71 patients had both endoscopies (56 of them with probable CVID). Due to the lack of biopsies or endoscopy reports, 6 gastroscopies and 8 colonoscopies were excluded. Given the complexity of CVID-associated GI manifestations, abnormal findings were reviewed by ≥2 expert pathologists and in a weekly joint clinical gastroenterology and pathology meeting. Furthermore, upper GI histology of patients with atrophic gastritis and/or duodenal pathology were reanalyzed by an expert pathologist for Operative Link to Gastritis Assessment (OLGA) classification or Corazza-Villanacci classification, respectively (see Supplementary Tables 2a and 2b, Supplemental Digital Content 1, http://links.lww.com/AJG/A57). All patients with mucosal inflammation had at least two separate endoscopy examinations.
Figure 1.
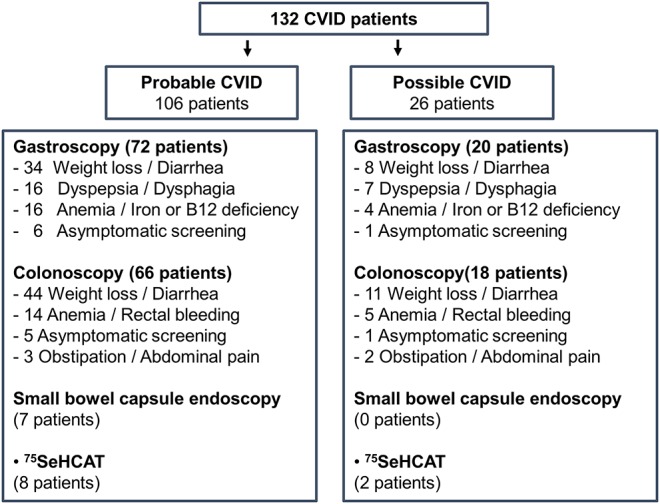
Flowchart of patients with CVID. CVID, common variable immunodeficiency; 75SeHCAT, selenium75-labeled taurine salt absorption measurement for bile acid malabsorption.
Bile acid malabsorption
Bile acid malabsorption was assessed by giving 10 μCi (370 kBq) of selenium75-labeled taurine salt capsule (SeHCAT-test) with a lactose-free meal. Measurements were done by γ-camera 3 hours and 7 days after ingestion of the capsule. For normal bile acid absorption, over 10% of activity was needed to be detectable still at day 7; activity below 10% of the baseline was considered as bile acid malabsorption.
Data collection and analysis
A comprehensive and structured computer database (Microsoft Access; Microsoft Corporation, Redmond, WA) was generated for the input of the medical history, immunophenotype, endoscopy, histology, and retrospective patient data (2). Microsoft Excel (Microsoft Corporation, Redmond, WA) and SPSS Statistics 22.0 (IBM, Armonk, NY) and Prism 7 (GraphPad, San Diego, CA) were used for the analyses. Any frequencies, including GI diseases and aberrant laboratory parameters, were studied using descriptive statistics and crosstabs functions. For comparisons of variables between groups, Mann-Whitney U-test or Fisher's exact test was used, as appropriate. P < 0.05 was considered significant.
RESULTS
Patient population
The patient cohort consisted of 106 patients with probable CVID and 26 with possible CVID (Table 1). There were more male patients among patients with probable CVID than among those with possible CVID (47% vs 33%; P < 0.05). Patients with probable CVID had been diagnosed earlier (average year: 2004 vs 2010; P < 0.001). Chronic anemia was detected in 47 patients (35.6%), of whom 39 (83%) had undergone gastroscopy and 35 (74%) colonoscopy. In comparisons between patients with probable and possible CVID, more patients with probable CVID had decreased vitamin B12 levels (28.6% vs 5.3%; P < 0.05); fecal calprotectin and α1-antitrypsin levels were significantly higher in patients with probable CVID (Figure 2). The defect of B-cell function in patients with CVID makes detection and interpretation of autoantibodies difficult: We did not find IgA autoantibodies in patients with IgA deficiency, and barely detectable levels of IgG autoantibodies may be artificially from gammaglobulin supplementation.
Figure 2.
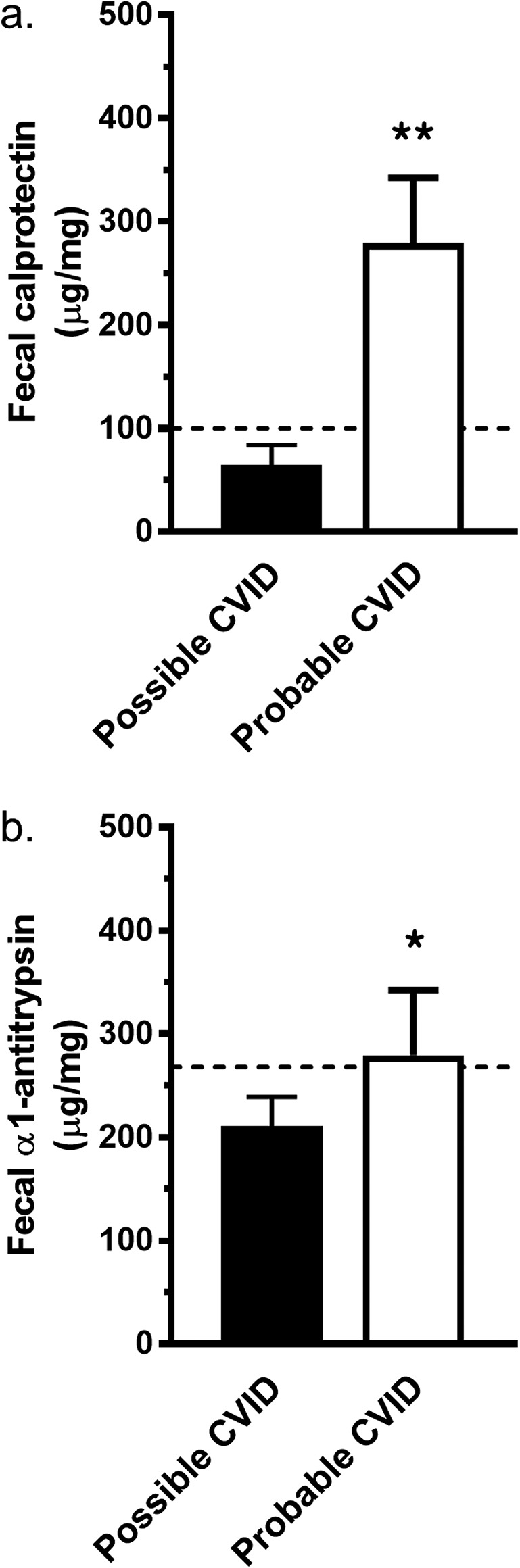
Fecal markers of patients with possible and probable CVID. (a) Fecal calprotectin levels in patients with probable CVID (n = 96, white bar) and in patients with possible CVID (n = 25, black bar). (b) Fecal α1-antitrypsin levels in patients with probable CVID (n = 96, white bar) and in patients with possible CVID (n = 19, black bar). Upper limits of laboratory reference ranges are marked with a dotted line: for fecal calprotectin 100 μg/g and for fecal α1-antitrypsin 268 μg/g. Results are shown for each bar (a and b) as average ± SEM. For statistical comparison between patients with possible and probable CVID, Mann-Whitney U test was used. **P < 0.0001; *P < 0.05. CVID, common variable immunodeficiency.
GI endoscopy and histology
Upper GI.
Gastroscopy findings are presented in Table 2 (probable and possible CVID in separate columns). The findings are presented as a proportion of all 90 patients with CVID (mainly reflecting findings from patients with probable CVID).
Table 2.
GI findings of patients with CVID
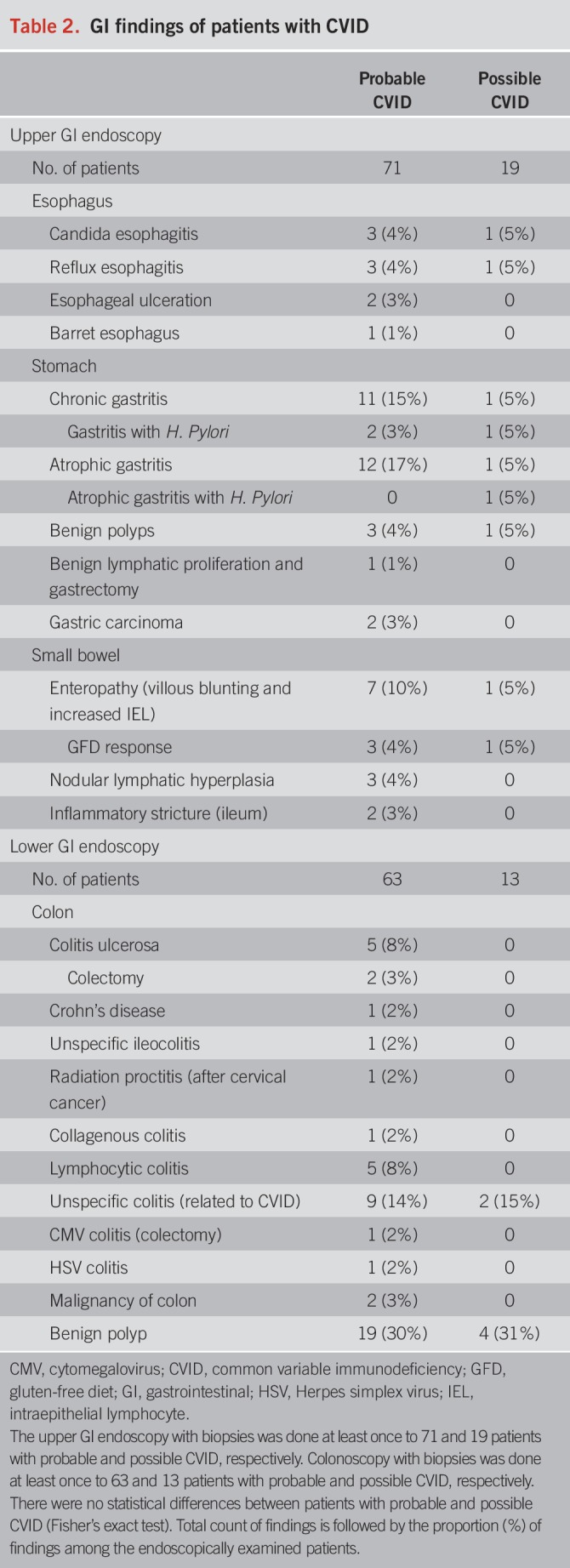
Esophagus.
Typical endoscopic and histologic findings of gastroesophageal reflux disease (GERD) or Candida albicans esophagitis were found altogether in 9% of patients (Table 2). One patient had esophageal inflammatory ulcerations due to Crohn's disease. A single Barrett esophagus without dysplasia was found.
Stomach.
Chronic active or atrophic gastritis was the most common upper GI finding, infrequently linked to Helicobacter pylori infection (Table 2). Parietal cell antibodies were not detected even in patients with corpus-restricted atrophic gastritis without H. pylori infection (autoimmune gastritis), and hence biopsies are necessary in patients with CVID. Two patients had a history of Helicobacter-negative chronic gastritis that had progressed to gastric atrophy when gastric adenocarcinoma was found. Classification of atrophic gastritis histology is presented in Supplementary Table 2a (see Supplemental Digital Content 1, http://links.lww.com/AJG/A57).
Duodenum and small bowel.
Duodenal villous blunting and increased intraepithelial lymphocytes (IELs) were found in 9% of patients (Table 2), and histologic classification is presented in Supplementary Table 2b (see Supplemental Digital Content 1, http://links.lww.com/AJG/A57). One patient with possible CVID had IgG autoantibodies against tissue transglutaminase and had the human leukocyte antigen (HLA)-DQ2 haplotype. This patient experienced a complete response to gluten-free diet (GFD). Another patient was on GFD based on celiac disease diagnosis 40 years earlier (before CVID diagnosis) and had no villous atrophy albeit demonstrated increased IEL. The third patient had detectable anti-endomycial IgG antibodies (on gammaglobulin supplementation) and was also positive for HLA-DQ2 haplotype. He was refractory to GFD and was a chronic norovirus carrier (discussed later). Two patients had no celiac autoantibodies but still got a normalization of villous atrophy after GFD while an increase in IEL persisted. Patients who did not respond to GFD (n = 4) neither had anti-enterocyte IgG antibodies and aberrant lymphocyte infiltrates (duodenal biopsy) nor had T-cell receptor rearrangement detected. However, HLA haplotype was not systemically screened, and only 4 patients from the whole cohort were tested. Two patients with GFD unresponsive enteropathy had HLA-DQ8 or -DQ2 haplotype but were also chronic norovirus carriers. The remaining 2 patients not responding to GFD had extensive inflammation also elsewhere in the GI tract. One of them had “graft-vs-host”-like colitis, and the other had esophagitis, colitis, and chronic gastritis progressing to gastric adenocarcinoma. In addition, 3 patients had ileal inflammatory lesions. One patient was operated for an inflammatory (nongranulomatous, and likely of ischemic etiology) stricture of proximal ileum and remained asymptomatic. The other patient had ileocolonic and perianal Crohn's disease, and a third had unspecific luminal ileocolonic inflammation. Whereas terminal ileum was endoscopically investigated and biopsied in 91% of lower GI endoscopies, a small bowel capsule endoscopy was additionally performed on 7 patients, and only 1 patient with probable CVID had an endoscopic finding, an extensive nodular lymphatic hyperplasia throughout the small bowel.
Lower GI.
Of patients with CVID, 76 had a colonoscopy with available biopsies and endoscopy report. Findings were divided into possible and probable CVID groups (Table 2) but presented as a proportion of all patients (mainly reflecting patients with probable CVID). Table 3 describes the histologic inflammatory changes in the lower GI tract.
Table 3.
Lower GI inflammation in patients with CVID
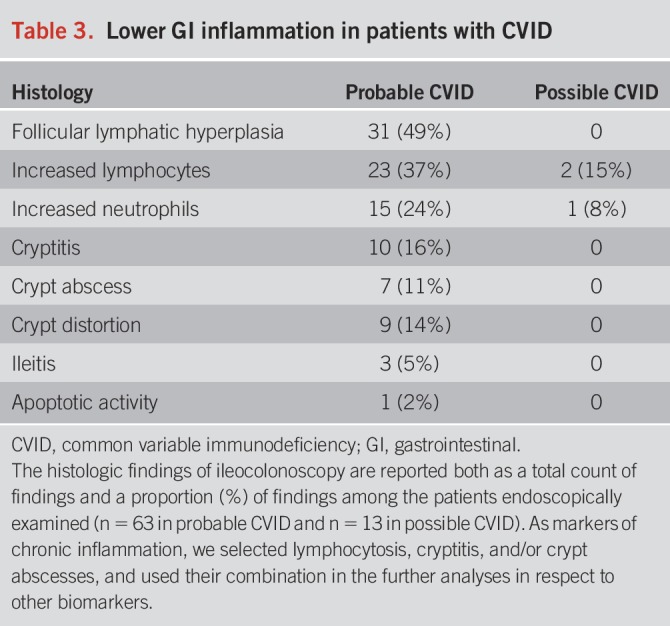
Colon.
Microscopic colitis, i.e., collagenous or lymphocytic colitis, was found in 9% of patients (Table 2). However, an unspecific colitis related to CVID was additionally found in 14% of the patients. Inflammatory bowel disease (IBD) was diagnosed in 8% of patients, consisting predominantly of ulcerative colitis. Two patients with ulcerative colitis were refractory to treatment and underwent colectomy. One patient with unspecific colitis and nodular lymphatic hyperplasia of the gut developed cecal large B-cell lymphoma; another elderly patient was diagnosed with a metastatic colon adenocarcinoma. The most frequent finding was follicular lymphatic hyperplasia (41%), followed by lymphocytosis of the mucosa (30%) and intraepithelial neutrophils (20%) (Table 3). In addition, cryptitis (9%) and/or crypt abscesses (12%) were relatively common. Interestingly, all patients with duodenal inflammation had also colonic inflammation.
Bile acid malabsorption
Ten patients with unexplained diarrhea were tested for bile acid absorption, and malabsorption was found in 4 (40%) of the tested patients, all of them with probable CVID. One of the patients had previous ileocolic and anastomose resections due to stricturing Crohn's disease, whereas the others were type II bile acid malabsorption.
GI infections
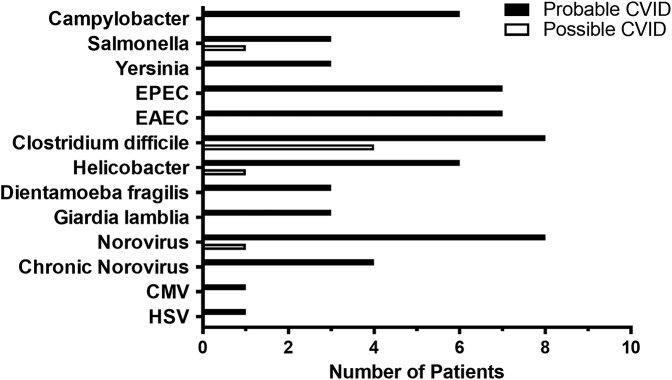
GI infections were mainly found in patients with probable CVID (Figure 3), whereas altogether 35 patients with CVID had at least once a bacterial or parasitic gastroenteritis. Of all patients with CVID, 5% had either Giardia lamblia or Dientamoeba fragilis. H. pylori was found in 8 of the tested 107 patients with CVID. Successful eradication was confirmed by negative fecal Helicobacter antigen or gastric biopsy staining. Seventy-eight patients were tested with fecal bacterial culture and of those 31 additionally with polymerase chain reaction (PCR). In concordance with previous studies (3,9,10), Campylobacter, Salmonella and Yersinia species, and Clostridium difficile were found (concordant results with bacterial culture and PCR). Only by PCR, we detected 2 pathogenic strains of Escherichia coli, enteroaggregative (EAEC) and enteropathogenic (EPEC), in 13% of patients with probable CVID. The most common viral infection was norovirus, which was found in 7% of patients (n = 9). Four patients remained chronic carriers of norovirus. Except for the norovirus, GI infections were not detected concomitantly in patients with colonic inflammation. Patients recovered from their GI infections and had no endoscopy at the time.
Figure 3.
Gastrointestinal infections in patients with CVID. Numbers of patients with GI infection and causing pathogens are shown with bars. We only show the number of patients who have had one or more infections by a pathogen, whereas the numbers of recurrent infections are not shown. Black bars mark probable CVID and white bars mark possible CVID. CVID, common variable immunodeficiency; CMV, cytomegalovirus; EAEC, enteroaggregative E. coli; EPEC, enteropathogenic E. coli; HSV, Herpes simplex virus.
Risk factors of GI inflammation
Fecal markers.
Fecal calprotectin and α1-antritrypsin were used as screening tools before GI endoscopy, and in fact, the patients who had no lower GI endoscopy performed (n = 50) had also low fecal markers (fecal calprotectin 58 ± 68 μg/g and fecal α1-antritrypsin 227 ± 148 μg/g [average ± SD; n = 43 and n = 40, respectively]). To avoid bias in the analyses, we excluded patients without endoscopic examinations. We combined histologic signs of chronic lower GI inflammation (lymphocytosis, cryptitis, and/or crypt abscesses). Patients with inflammatory GI histology had elevated (>95% confidence interval) fecal calprotectin levels (μg/g)—212.6–994.4 with inflammation (n = 26) vs 69.1–312.0 without inflammation (n = 50) (P < 0.05; Figure 4a)—but not of α1-antitrypsin (Figure 4b).
Figure 4.
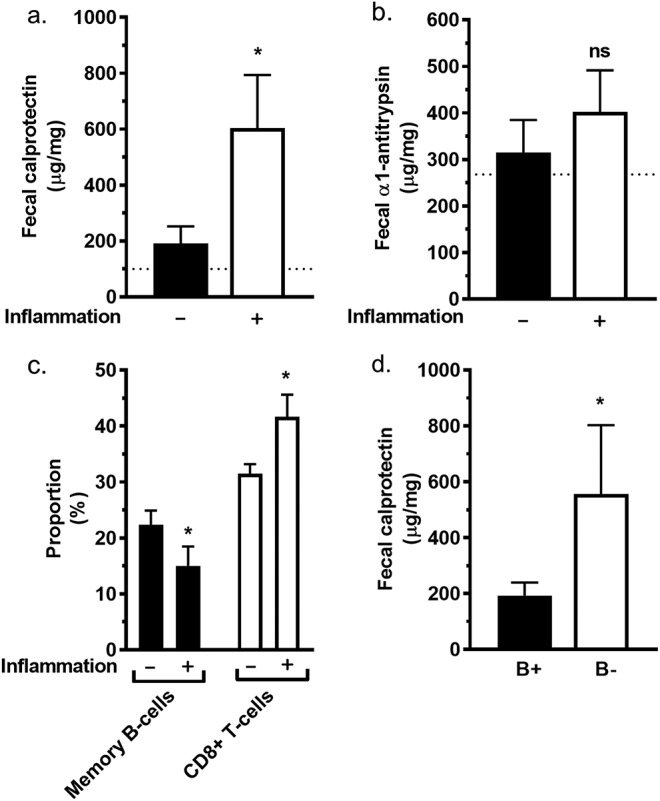
Inflammation of the colon, fecal markers, and immunophenotype. (a) Patients without histologic inflammation in colon biopsies (n = 50) had lower fecal calprotectin levels than patients with inflammation (n = 24). (b) Patients without histologic inflammation in the colon (n = 45) had no difference in fecal α1-antitrypsin levels compared with patients with histologic inflammation (n = 23). (c) Peripheral blood proportions of CD19+CD27+ memory B cells of CD19+ B cells (black bars) and CD3+CD8+ T lymphocytes of CD3+ T cells (white bars) in patients with or without inflammation in the colon. Histologic inflammation is indicated in the graphs with − (n = 54) and + (n = 25) signs below x axis. (d) Fecal calprotectin was higher in the subgroup of patients with a very low amount of CD19+ B lymphocytes (<2% of all lymphocytes; B−; n = 14) than in the rest of patients with CVID (B+; n = 107). The results are shown for each bar (a–d) as average ± SEM. For statistical comparison between patients with possible and probable CVID, Mann-Whitney U test was used. *P < 0.05.
Immunophenotype.
We investigated the flow cytometry findings of peripheral blood lymphocytes in patients with CVID in relation to lower GI inflammation. In patients with lower GI inflammation, the proportion of CD19+ B cells of all lymphocytes was lower, but the proportion of CD3+ T cells of all lymphocytes was higher (Figure 4c). In particular, CD19+CD27+ memory B cells were reduced among CD19+ B cells and CD8+ T lymphocytes were higher among CD3+ T cells (Figure 4c). Furthermore, whereas we compared bowel inflammation markers and B-cell counts among all patients of the cohort (independent of endoscopies), we found that the subgroup of patients having a very low amount of CD19+ B lymphocytes (<2% of all lymphocytes; B−) had elevated fecal calprotectin levels (Figure 4d) but not of fecal α1-antitrypsin (data not shown).
DISCUSSION
We describe here that in systematically evaluated patients with CVID, various inflammatory and infectious GI diseases are frequent. In total, 70% and 64% of our patients with CVID underwent upper and lower GI endoscopy, respectively. In addition, 92% were screened for fecal calprotectin and 87% for fecal antitrypsin. Among patients without a colonoscopy, 86% and 80% were screened with fecal calprotectin and α1-antritrypsin, respectively. Moreover, endoscopic and/or histologic abnormalities were detected in 58% and 68% of upper and lower GI endoscopy, respectively. To the best of our knowledge, this CVID cohort has the highest proportion of patients evaluated with endoscopy and markers for enteric inflammation in the available literature. We also describe bile acid malabsorption and infections by enteropathogenic strains of E. coli and D. fragilis as frequent causes of treatable diarrhea in patients with CVID. However, this study may have inherent sources of bias given the observational setting. Bias might arise from a patient's preference to bring samples or undergo endoscopy as well as the clinician's selection of investigations. Contrarily, if the total patient cohort was limited only to symptomatic patients with CVID with both endoscopies done, this might further bias the detected prevalence.
Endoscopy-positive GERD was found in 4% of patients. This prevalence is less than that in the general Finnish population, where endoscopy-positive GERD was found in 9.8% of patients (11). A plausible explanation may be that atrophic gastritis is often found in patients with CVID. This may have a protective effect against GERD (11). The prevalence of esophageal manifestations in patients with CVID has previously been largely unknown, whereas our study suggests for the first time that endoscopy-positive GERD may be less common in patients with CVID than in the general population.
In a previous retrospective case series, 26 of 30 upper GI endoscopies demonstrated chronic gastritis in biopsies, and H. pylori was found in 8 (27%) (9). In the present study, H. pylori was screened from 81% (107) of patients. H. pylori was relatively uncommon (6%), and the incidence of chronic active gastritis was also lower (12%). Similarly, in a recently published Norwegian CVID cohort, only 3 (6%) H. pylori infections were found in 50 upper GI endoscopies. However, in the present study, atrophic gastritis was found similarly or more frequently than in other CVID cohorts (6,12–14). We found atrophic gastritis in 17% of upper GI endoscopies, whereas the prevalence of atrophic gastritis has varied between 3% and 9% in the large cohorts of patients with CVID (6,12); but these may underestimate its prevalence because in smaller cohorts atrophic gastritis was found in 10%–20% of gastric biopsies of patients with CVID (13,14). In the present cohort, 2 gastric cancers were in 106 patients with probable CVID during 10 years; this 1.8% prevalence of gastric carcinoma is twice as high as that altogether 4 gastric cancers in 472 patients (0.8%) in published large cohorts (15,16). Thus, patients with CVID are at higher risk of stomach cancer, and therefore, a screening gastroscopy is justified and likely a follow-up endoscopy if chronic active gastritis or atrophic gastritis is detected.
CVID enteropathy is associated with higher mortality (6). CVID enteropathy is histologically like that of celiac disease with villous atrophy, crypt hyperplasia, and increased IEL. Distinctive features often include a paucity of plasma cells and unresponsiveness to GFD (17,18). However, we found that half of the patients with CVID enteropathy responded to GFD. Notably, the patients who did not respond to GFD were either carriers of norovirus or had extensive inflammation in the GI tract. CVID enteropathy has been linked to chronic norovirus carriage, and its clearance was associated with the resolution of symptoms and histologic recovery of villous atrophy (19). In the present study, 2 patients with chronic norovirus had GFD refractory enteropathy and malnutrition due to protein-losing diarrhea. In contrast, 2 other chronic norovirus carriers had only occasional GI symptoms, without any duodenal inflammation. In addition to norovirus carriage, other pathogens including Salmonella and EAEC were detected in these patients, suggesting that they may be more susceptible to GI infections in general. Therefore, whether these persistent GI infections are the cause or effect in the context of CVID enteropathy requires further study. Moreover, future investigations on genetic factors predisposing patients with CVID to GI inflammation are warranted.
We found that GI inflammation in patients with CVID is more common in the lower GI tract than in the upper GI tract. Microscopic colitis and unspecific colitis linked to CVID were common findings and found in altogether 23% of colonoscopies. IBD was diagnosed in 11% of patients who were endoscopically examined. The 5% IBD prevalence in the whole cohort was over 5-fold higher than in the Finnish general population (20). Fecal calprotectin was elevated in patients with colonic inflammation. Consequently, we suggest that fecal calprotectin may serve as a screening tool for GI inflammation in patients with CVID, before endoscopy. Importantly, we report here for the first time that patients with CVID with unexplained diarrhea had a high frequency (40%) of bile acid malabsorption. Bile acid malabsorption should be excluded in the workup of chronic diarrhea of a patient with CVID.
Historical reports suggest G. lamblia as the most common cause of gastroenteritis in patients with CVID (21). However, we found altogether 20 patients with bacterial GI infections caused by Salmonella, Campylobacter, Yersinia, or C. difficile, and only 6 parasitic infections caused by G. lamblia or D. fragilis (Figure 3). In addition, a total of 14 positive samples for nucleic acids of EAEC or EPEC were found in patients with gastroenteritis, whereas 10 patients had viral pathogens including norovirus, cytomegalovirus, or Herpes simplex virus. In Iranian patients with CVID, 86 of 115 patients had GI infections during 30 years (22). Furthermore, of 248 patients with CVID from the United States, 6 had bacterial GI infections (Salmonella, Campylobacter) and 8 G. lamblia infections (16). Giardiasis was thus found less frequently in Finnish patients with CVID, whereas Salmonella and Campylobacter infections were similarly common. In addition, the prevalence of D. fragilis and of pathogenic strains of E. coli was similar or higher than that of previously described pathogens in patients with CVID. Given the high incidence of GI infections in them, screening of fecal pathogens is warranted in the initial evaluation of persistent diarrhea and whenever there is an exacerbation of chronic GI symptoms.
In line with a French cohort of 50 patients with CVID (9), we found that patients with CVID with a higher proportion of peripheral blood CD8+ lymphocytes had also more intestinal inflammation. In this previous study (9), intestinal lymphocytosis was largely due to the expansion of CD8+ lymphocytes. In addition, we found that peripheral blood B-cell and memory B-cell proportions were lower in patients with intestinal inflammation. A subgroup of patients with CVID with a very low B-cell count had elevated fecal calprotectin levels, suggesting that very low B-cell counts may be a risk factor for intestinal inflammation. This corroborates previous findings that higher levels of peripheral blood B cells may protect against noninfectious complications of CVID (8).
In conclusion, patients with CVID frequently have GI comorbidity. Infection or inflammation of the GI tract is the main cause. In total, more than 50% of all gastroscopies lead to a diagnosis, ranging from chronic inflammation to gastric cancer. The high incidence of GI infections in patients with CVID warrants the screening of pathogens before endoscopic examinations. Colonic inflammation appears to be more prevalent than upper GI inflammation, yet when present, duodenal inflammation predicts also lower GI inflammation. Fecal calprotectin may help in the screening of these patients for GI inflammation, like in IBD. Gut inflammation was associated with a distinct immunophenotype with lower blood counts of B cells and memory B cells but increased counts of CD8+ T lymphocytes. Therefore, readily available laboratory parameters such as fecal calprotectin and distinct immunophenotype may help to stratify individual risk of GI manifestations in patients with CVID.
CONFLICTS OF INTEREST
Guarantor of the article: Martti Färkkilä, MD, PhD.
Specific author contributions: M.F. and M.S. designed the study, coordinated the project, and participated in writing the article; T.M. and S.P. collected the data and participated in statistical analyses and writing; A.R. reanalyzed and scored histology of atrophic gastritis and duodenal inflammation; S.S. performed laboratory analyses and participated in writing the article; T.M., M.S., S.P., and M.F. provided clinical care for the patients. All authors have read and approved the final manuscript.
Financial support: M.S. and T.M. have received honoraria from the CSL Behring, but this work was independent of the honoraria. The remaining authors declare no competing financial interests. This work was supported by the Finnish Medical Foundation, the Helsinki University Hospital Research Funds, the Sigrid Jusélius Foundation, and the Mary and Georg C. Ehrnrooth Foundation.
Potential competing interests: None.
Study Highlights.
WHAT IS KNOWN
✓ CVID associates with a wide spectrum of autoimmune diseases.
✓ The frequency of GI infections and inflammatory diseases is poorly documented in patients with CVID.
WHAT IS NEW HERE
✓ CVID associates with a high prevalence of GI infections, chronic inflammation of the GI tract, and malignancies.
✓ GI inflammation increases fecal calprotectin and occurs more frequently in patients with CVID with clearly low B-cell counts.
ACKNOWLEDGMENTS
We thank the study nurses Eira Leinonen, Pirkko Tuukkala, and Virpi Pelkonen, and the nurses of the Adult Immunodeficiency Unit Tiina Puttonen, Johanna Heino, and Petra Iwaola, for their excellent help.
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/AJG/A57
REFERENCES
- 1.Al-Herz W, Bousfiha A, Casanova JL, et al. Primary immunodeficiency diseases: An update on the classification from the international union of immunological societies expert committee for primary immunodeficiency. Front Immunol 2011;2:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Selenius JS, Martelius T, Pikkarainen S, et al. Unexpectedly high prevalence of common variable immunodeficiency in Finland. Front Immunol 2017;8:1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Uzzan M, Ko HM, Mehandru S, Cunningham-Rundles C. Gastrointestinal disorders associated with common variable immune deficiency (CVID) and chronic granulomatous disease (CGD). Curr Gastroenterol Rep 2016;18:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quinti I, Soresina A, Guerra A, et al. Effectiveness of immunoglobulin replacement therapy on clinical outcome in patients with primary antibody deficiencies: Results from a multicenter prospective cohort study. J Clin Immunol 2011;31:315–22. [DOI] [PubMed] [Google Scholar]
- 5.Conley ME, Notarangelo LD, Etzioni A. Diagnostic criteria for primary immunodeficiencies. Representing PAGID (Pan-American Group for Immunodeficiency) and ESID (European Society for Immunodeficiencies). Clin Immunol 1999;93:190–7. [DOI] [PubMed] [Google Scholar]
- 6.Chapel H, Lucas M, Lee M, et al. Common variable immunodeficiency disorders: Division into distinct clinical phenotypes. Blood 2008;112:277–86. [DOI] [PubMed] [Google Scholar]
- 7.Chapel H, Lucas M, Patel S, et al. Confirmation and improvement of criteria for clinical phenotyping in common variable immunodeficiency disorders in replicate cohorts. J Allergy Clin Immunol 2012;130:1198.e9. [DOI] [PubMed] [Google Scholar]
- 8.Resnick ES, Moshier EL, Godbold JH, Cunningham-Rundles C. Morbidity and mortality in common variable immune deficiency over 4 decades. Blood 2012;119:1650–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malamut G, Verkarre V, Suarez F, et al. The enteropathy associated with common variable immunodeficiency: The delineated frontiers with celiac disease. Am J Gastroenterol 2010;105:2262–75. [DOI] [PubMed] [Google Scholar]
- 10.Oksenhendler E, Gerard L, Fieschi C, et al. Infections in 252 patients with common variable immunodeficiency. Clin Infect Dis 2008;46:1547–54. [DOI] [PubMed] [Google Scholar]
- 11.Voutilainen M, Sipponen P, Mecklin JP, Juhola M, Farkkila M. Gastroesophageal reflux disease: Prevalence, clinical, endoscopic and histopathological findings in 1,128 consecutive patients referred for endoscopy due to dyspeptic and reflux symptoms. Digestion 2000;61:6–13. [DOI] [PubMed] [Google Scholar]
- 12.Cunningham-Rundles C. Autoimmunity in primary immune deficiency: Taking lessons from our patients. Clin Exp Immunol 2011;164(Suppl 2):6–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jorgensen SF, Reims HM, Frydenlund D, et al. A cross-sectional study of the prevalence of gastrointestinal symptoms and pathology in patients with common variable immunodeficiency. Am J Gastroenterol 2016;111:1467–75. [DOI] [PubMed] [Google Scholar]
- 14.Maarschalk-Ellerbroek LJ, Oldenburg B, Mombers IM, et al. Outcome of screening endoscopy in common variable immunodeficiency disorder and X-linked agammaglobulinemia. Endoscopy 2013;45:320–3. [DOI] [PubMed] [Google Scholar]
- 15.Quinti I, Soresina A, Spadaro G, et al. Long-term follow-up and outcome of a large cohort of patients with common variable immunodeficiency. J Clin Immunol 2007;27:308–16. [DOI] [PubMed] [Google Scholar]
- 16.Cunningham-Rundles C, Bodian C. Common variable immunodeficiency: Clinical and immunological features of 248 patients. Clin Immunol 1999;92:34–48. [DOI] [PubMed] [Google Scholar]
- 17.Agarwal S, Mayer L. Diagnosis and treatment of gastrointestinal disorders in patients with primary immunodeficiency. Clin Gastroenterol Hepatol 2013;11:1050–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Washington K, Stenzel TT, Buckley RH, Gottfried MR. Gastrointestinal pathology in patients with common variable immunodeficiency and X-linked agammaglobulinemia. Am J Surg Pathol 1996;20:1240–52. [DOI] [PubMed] [Google Scholar]
- 19.Woodward JM, Gkrania-Klotsas E, Cordero-Ng AY, et al. The role of chronic norovirus infection in the enteropathy associated with common variable immunodeficiency. Am J Gastroenterol 2015;110:320–7. [DOI] [PubMed] [Google Scholar]
- 20.Jussila A, Virta LJ, Salomaa V, Maki J, Jula A, Farkkila MA. High and increasing prevalence of inflammatory bowel disease in Finland with a clear North-South difference. J Crohns Colitis 2013;7:256. [DOI] [PubMed] [Google Scholar]
- 21.Salzer U, Warnatz K, Peter HH. Common variable immunodeficiency: An update. Arthritis Res Ther 2012;14:223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mohammadinejad P, Pourhamdi S, Abolhassani H, et al. Primary antibody deficiency in a tertiary referral hospital: A 30-year experiment. J Investig Allergol Clin Immunol 2015;25:416–25. [PubMed] [Google Scholar]