Supplemental Digital Content is Available in the Text.
Key Words: meibomian glands, meibography, red filter, infrared, reproducibility of results
Abstract
Purpose:
To evaluate the validity and reliability of alternative methods for evaluating meibomian gland (MG) dropout without using an infrared light system: red-filtered or isolated red-channel images (RCIs) of the everted eyelid.
Methods:
We evaluated MG dropout in the everted upper and lower eyelids of 125 eyes of 64 patients with good-quality infrared meibography images (IMIs) and color digital photographs with and without a red filter. Red-filtered images (RFIs) were converted to black and white and adjusted for contrast/brightness [adjusted red-filtered images (aRFIs)]. RCIs were computationally isolated from color digital photographs obtained without a red filter. After randomization, the total meiboscore (0–6) was evaluated by 2 independent evaluators (interobserver reliability) masked to the image origin, and again after a 30-day interval (intraobserver reliability).
Results:
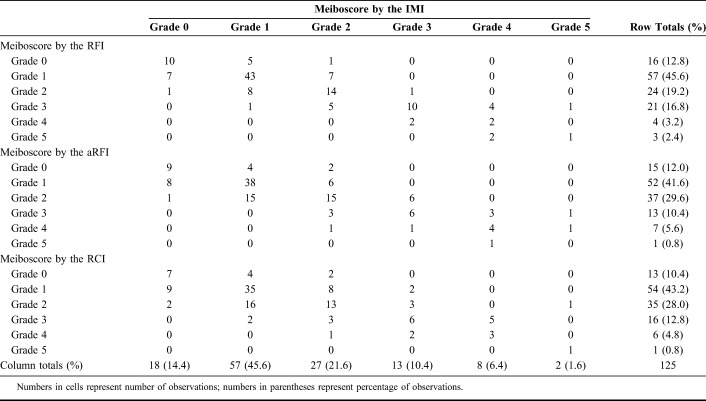
The meiboscores evaluated using the RFI, aRFI, and RCI were strongly positively correlated with those evaluated using the IMI (RFI: ρ = 0.788; aRFI: ρ = 0.735; RCI: ρ = 0.630; all P < 0.001, Spearman correlation analysis). Linear-weighted κ-values (κw) showed substantial agreement between the RFI and IMI (κw = 0.676, 95% CI = 0.594–0.759). The RFI had substantial intraobserver reliability (κw = 0.735, 95% CI = 0.685–0.785) and moderate interobserver reliability (κw = 0.467, 95% CI = 0.371–0.563). Computational adjustment of RFIs did not enhance the validity or reliability, and RCIs had limitations in some cases.
Conclusions:
MGs were successfully visualized using a red filter on a slit lamp and showed substantial agreement with visualization using the standard infrared method. Although interobserver reliability was only moderate, this alternative technique may be useful for evaluating MG dropout when an infrared meibography device is not available.
Dry eye disease can be classified into 2 main categories depending on the pathogenesis—aqueous-deficient type and evaporative type.1 Meibomian gland (MG) dysfunction, which causes the evaporative type, is believed to be the most prevalent cause of dry eye disease, especially in older populations.2 Meibography was developed to evaluate the morphologic changes of the MG. At first, the silhouette of the MG was observed by transilluminating infrared light through an everted eyelid.3,4 This observation method is uncomfortable for patients, however, and was therefore replaced by noncontact infrared meibography, which causes less discomfort to patients.5 Several commercial noncontact meibography devices are currently available. With these devices, infrared light is projected onto the conjunctiva of the everted eyelid, and the reflected infrared light is selectively passed through an infrared-transmitting filter and focused on an infrared-sensitive sensor [charge-coupled device (CCD) or complementary metal-oxide semiconductor], which allows for visualization of the underlying MG.5,6 Interestingly, although the same infrared light is used, the MG appears dark with the transillumination technique, but bright with noncontact meibography.3,5 To our knowledge, however, there is no reported explanation of the visibility of the MG by noncontact infrared meibography.5,7,8 We hypothesized that the visibility of the MG by noncontact infrared meibography could be partly explained by the absence of the shadow of overlying conjunctival vessels, and thus, alternative methods that can hide the conjunctival vessels could make the MG visible without requiring an infrared light system.
To test this hypothesis, we evaluated the validity and reliability of 3 alternative methods for assessing MG dropout without using an infrared light system: a red filter in a trial lens set with or without image adjustment, and isolation of the red-channel of digital color images, which removes the conjunctival vascular shadows.
MATERIALS AND METHODS
Patients
The study was conducted in accordance with the tenets of the Declaration of Helsinki, and the study protocol and modification of a conventional medical device were approved by the Institutional Review Board of Hallym University College of Medicine (#2017-12). A total of 125 eyes of 64 patients who visited a single clinic (Chuncheon Sacred Heart Hospital) for screening and evaluation of dry eye disease were included in the study. Patients with severe systemic illness, recent ocular surgery, or severe conjunctival inflammation were excluded from the study. Furthermore, we excluded eyes that were difficult to evert or did not produce good-quality infrared meibography images (IMIs).
Acquiring MG Images
All the eyes underwent slit-lamp examination of the anterior segment, and images of the tarsal conjunctiva and underlying tarsus including the MG were acquired by an experienced ophthalmologist (H.S.H.) using a CCD camera (Guppy, Allied Vision, Exton, PA) attached to a slit lamp (Slit Lamp BQ 900; Haag-Streit, Köniz, Switzerland). After everting the eyelid, standard anterior segment color digital photographs (1024 × 768 pixels) were obtained first, and then, the photographs were taken again with a red filter from the trial lens set (TLS-FD; Topcon, Tokyo, Japan) positioned in front of one of the 2 objective lenses in the slit lamp, which was connected to the camera (Fig. 1; also see Supplemental Figure 1, Supplemental Digital Content 1, http://links.lww.com/ICO/A753). Direct observation of the MG using the red filter was also possible (see Supplemental Video 1, Supplemental Digital Content 2, http://links.lww.com/ICO/A754). The IMIs were acquired in another room using a slit-lamp microscope equipped with an infrared-sensitive CCD camera (XC-EI50; Sony, Tokyo, Japan) and an infrared-transmitting filter (R72; Hoya, Tokyo, Japan).9 R72 filter is a 720-nm long-pass filter. The BG-4M meibography system (Topcon) uses an 830-nm long-pass filter (IR83; Hoya), and the Keratograph 5M (Oculus, Arlington, WA) uses an infrared light emitting diode (LED) with 840 nm. All the photographs (magnification: ×6.3) were taken at the central portion of the upper and lower tarsus.
FIGURE 1.
Anterior segment color digital photographs of the eyelids with or without the red filter. In the upper (A and B) and lower (C and D) eyelids of the right eye of a 23-year-old female patient, the MGs can be visualized by simply inserting a red filter in front of the objective lens of the slit lamp (B and D). In the lower (E and F) eyelid of the right eye of a 53-year-old male patient, the MGs showed dropout in the nasal half.
Processing MG Images
The images obtained with the red filter were converted to black and white. The contrast and brightness of the photographs were then adjusted to maximize the contrast of the MG because the original image was a little saturated in red color (see Supplemental Fig. 2, Supplemental Digital Content 3, http://links.lww.com/ICO/A755). As another method that would not require a red filter, a red-channel image (RCI) was obtained by isolating the red-channel from a color photograph taken without a red filter (see Supplemental Fig. 3, Supplemental Digital Content 4, http://links.lww.com/ICO/A756). All the images were processed by one of the authors (D.C.) using the public domain Java-based image-processing program ImageJ (Version 1.49p; National Institutes of Health, Bethesda, MD).
Analysis of the Meiboscore and Methods of Masking
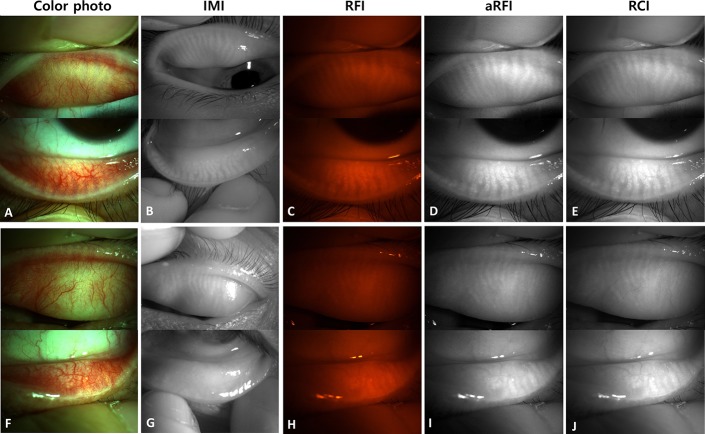
Four sets of images were produced for each upper and lower eyelid (Fig. 2): 1) an IMI acquired with noncontact infrared meibography; 2) a red-filtered image (RFI) acquired as a color digital photograph of the tarsal conjunctiva using a red filter; 3) an adjusted red-filtered image (aRFI) that was produced by converting the RFI to black and white and then adjusting the contrast and brightness of the image to maximize the contrast of the MG; and 4) an RCI acquired by isolating the red-channel of the color digital photographs taken without a red filter. Both aRFI and RCI required postimaging processing.
FIGURE 2.
Four sets of images of the tarsal conjunctiva and the underlying tarsus containing MGs. A–E, Images of the right eye of a 26-year-old female patient show intact MGs. F–J, Images of the left eye of a 56-year-old male patient show impaired MGs. A and F, Images of color photographs. B and G, Images of infrared meibography. C and H, Images acquired through a red filter. D and I, Brightness/contrast-adjusted images acquired through a red filter. E and J, Red-channel images of color photographs.
Each set of images was randomized, masked by 1 author (Y.H.G.), and sent separately to 2 corneal experts (S.-M.L. and H.S.H.). To prevent comparison of the meiboscore according to the evaluation methods from the same eye, the 4 sets of the images were differently numbered in a randomized sequence and sent to the evaluators. For each set of images, the morphology of the MG was scored on a scale from 0 to 3 (meiboscore) for both upper and lower eyelids, as described previously by Arita et al.5 The meiboscores from the upper and lower eyelids were summed to obtain the total meiboscore (0–6) for further analysis. To evaluate intraobserver reliability, meiboscores from the 4 sets of images were evaluated twice by the same evaluators (S.-M.L. and H.S.H.) with a 30-day interval between each evaluation. To assess interobserver reliability, meiboscores from the first evaluation were compared between the 2 evaluators.
Statistical Analysis
Spearman rank correlation coefficient rho (ρ), Kendall rank correlation coefficient tau b (τ), and kappa (κ) with linear weighting were used to evaluate the agreement between the meiboscores evaluated by the IMI, as the standard method, and those by the RFI, aRFI, and RCI. Linear-weighted kappa (κw) with 95% confidence intervals (CI) was also used to evaluate the intraobserver and interobserver reliability of the meiboscore obtained by the 4 methods. Linear-weighted kappa (κw) was used instead of unweighted kappa because the variable, meiboscore in this study, was ordinal with a total of 7 grades (0–6) that semiquantitatively reflect the area of MG dropout. The guidelines used for interpretation of κ values were as follows: <0.01 indicated poor agreement; 0.01 to 0.20, slight; 0.21 to 0.40, fair; 0.41 to 0.60, moderate; 0.61 to 0.80, substantial; and 0.81 to 1.00, almost perfect as suggested previously.10,11 Statistical analyses were performed using SPSS 24.0 (IBM, Chicago, IL) software and the web site for statistical computation (http://vassarstats.net/) for weighted κ.
RESULTS
Of the 64 patients enrolled in this study, 46 patients were women, and 18 patients were men. The mean patient age was 48.1 ± 14.8 years (range: 15–75 years). The diagnoses of the enrolled eyes included dry eye disease (92 eyes), glaucoma (8 eyes), episcleritis (2 eyes), allergic conjunctivitis (2 eyes), superior limbic keratoconjunctivitis (2 eyes), glaucomatocyclitic crisis (1 eye), corneal opacity (1 eye), eyelid mass (1 eye), and punctate keratopathy (1 eye), and 15 eyes were normal.
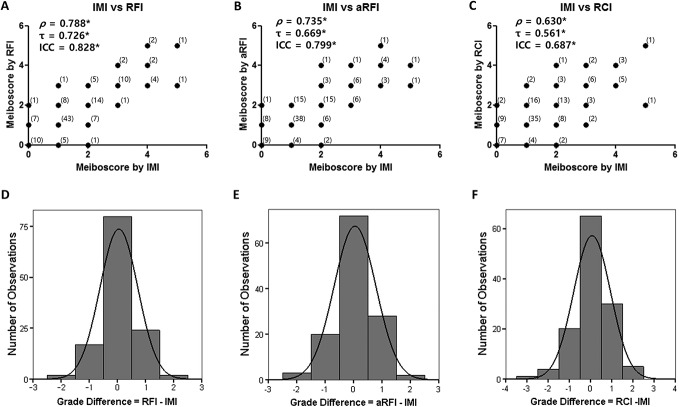
The meiboscores determined using the RFI, aRFI, and RCI had significant strong positive correlations with the meiboscores obtained using the IMI (RFI: ρ = 0.788,τ = 0.726, Fig. 3A; aRFI: ρ = 0.735, τ = 0.669, Fig. 3B; RCI: ρ = 0.630, τ = 0.561, Fig. 3C; all P < 0.001, Spearman rank correlation analysis, Kendall rank correlation analysis). The weighted κ values showed substantial agreement between meiboscores obtained by the RFI and those by the IMI (κw = 0.676, 95% CI = 0.594–0.759) and meiboscores obtained by the aRFI and those by the IMI (κw = 0.603, 95% CI = 0.511–0.696) (Table 1). The weighted κ value showed moderate agreement between meiboscores obtained by the RCI and those by the IMI (κw = 0.511, 95% CI = 0.401–0.621, Table 1). The grade differences of meiboscores obtained by the RFI, aRFI, and RCI compared with those by the IMI showed a normal distribution, and the mean difference ± SD of the total meiboscores was 0.06 ± 0.68 (RFI, Fig. 3D), 0.05 ± 0.74 (aRFI, Fig. 3E), and 0.07 ± 0.87 (RCI, Fig. 3F). The positive mean difference value indicates that the new methods provided a higher meiboscore (indicating more gland dropouts) than the standard method.
FIGURE 3.
Correlation plots of the meiboscores and histograms for the grade differences. A–C, Correlation plots of the meiboscores evaluated by the new methods compared with the standard infrared method. D–F, Histograms for the grade differences between the new methods and the standard infrared method. A and D, Comparison between images acquired through a red filter and the standard method. B and E, Comparison between brightness/contrast-adjusted images acquired through a red filter and the standard method. C and F, Comparison between the red-channel of the color photograph and the standard method. Numbers in parentheses represent the number of observations; ρ, Spearman rank correlation coefficient rho; τ, Kendall rank correlation coefficient tau b; *P < 0.001.
TABLE 1.
Comparison of the Meiboscore Evaluated by the IMI Versus RFI, aRFI, and RCI
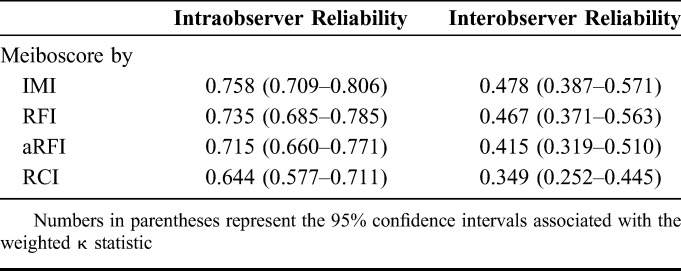
The intraobserver and interobserver reliability of the meiboscores obtained by each method is summarized in Table 2. The intraobserver reliability of the first and the second meiboscores evaluated by the 2 evaluators with a 30-day interval showed substantial agreement (IMI: κw = 0.758, 95% CI = 0.709–0.806, Supplemental Table 1; RFI: κw = 0.735, 95% CI = 0.685–0.785, Supplemental Table 2; aRFI: κw = 0.715, 95% CI = 0.660–0.771, Supplemental Table 3; and RCI: κw = 0.644, 95% CI = 0.577–0.711, Supplemental Table 4; see Supplemental Tables 1–8 at Supplemental Digital Content 4, http://links.lww.com/ICO/A757). The interobserver reliability showed moderate to fair agreement for each evaluation method—moderate agreement for the IMI, RFI, and aRFI (IMI: κw = 0.478, 95% CI = 0.387–0.571, Supplemental Table 5; RFI: κw = 0.467, 95% CI = 0.371–0.563, Supplemental Table 6; and aRFI: κw = 0.415, 95% CI = 0.319–0.510, Supplemental Table 7) and fair agreement for the RCI (κw = 0.349, 95% CI = 0.252–0.445, Supplemental Table 8) (see Supplemental Tables 1–8, Supplemental Digital Content 5, http://links.lww.com/ICO/A757).
TABLE 2.
Weighted κ Statistics (κw) Associated With the Intraobserver and Interobserver Reliability of Meiboscore Evaluation
DISCUSSION
Meibography was introduced 35 years ago to evaluate MG morphology.3 Because of the recent development of the noncontact method, which decreases patient discomfort, several commercial meibography devices are currently available, including the BG-4M, Meiboviewer (Visual Optics, Chuncheon, Korea), and LipiView (TearScience Inc, Morrisville, NC), and some topographic systems, including the Keratograph 5M and the Galilei (Ziemer Ophthalmic Systems AG, Port, Switzerland).5 Noncontact meibography is still not popular in ophthalmology clinics, however, because it requires a new instrument equipped with infrared light. With the increasing information regarding the pathogenesis of dry eye disease and MG dysfunction, accurate evaluation of the MG has become more important for proper diagnosis, classification of dry eye, and development of a treatment strategy.8 For more widespread use of meibography as a basic method of evaluating MG dysfunction and dry eye, we applied several quick and simple screening methods to evaluate MG morphology without using an infrared light system and assessed the advantages and limitations of these alternative methods compared with standard infrared meibography. On the basis of the hypothesis that enhanced visibility of the MG can be achieved by removing the shadow of the conjunctival vessels that may obscure the contour of the MG, we placed a red filter in front of the objective lens of the slit lamp to remove the shadow of the conjunctival vessels (RFI). We also evaluated 2 additional methods (aRFI and RCI) using computational image adjustment—aRFIs adjusted from RFIs and RCIs adjusted from color anterior segment photography of the everted eyelid.
Evaluation of MG dropout by the RFI had substantial agreement with that by standard noncontact infrared meibography (Fig. 3A and Table 1). This method also had substantial intraobserver reliability (κw = 0.735) and moderate interobserver reliability (κw = 0.467) (Table 2). The interobserver reliability using the RFI was slightly lower than its intraobserver reliability but similar to the interobserver reliability of the IMI (κw = 0.478, moderate). The higher discrepancy between the 2 evaluators compared with the intraevaluator discrepancy was similar to that in a previous report [interobserver reliability: κw = 0.57 (moderate), 95% CI = 0.47–0.68; intraobserver reliability: κw = 0.91 (almost perfect), 95% CI = 0.88–0.95].12
To improve the validity and reliability, we attempted a computational adjustment of the images (aRFIs) because the original images obtained with a red filter had lower contrast because of red color saturation. This modification did not enhance the validity and intraobserver and interobserver reliability results (Fig. 3B, Tables 1 and 2). Therefore, we concluded that postimaging processing for the RFI is not helpful for enhancing the reliability of the meiboscores.
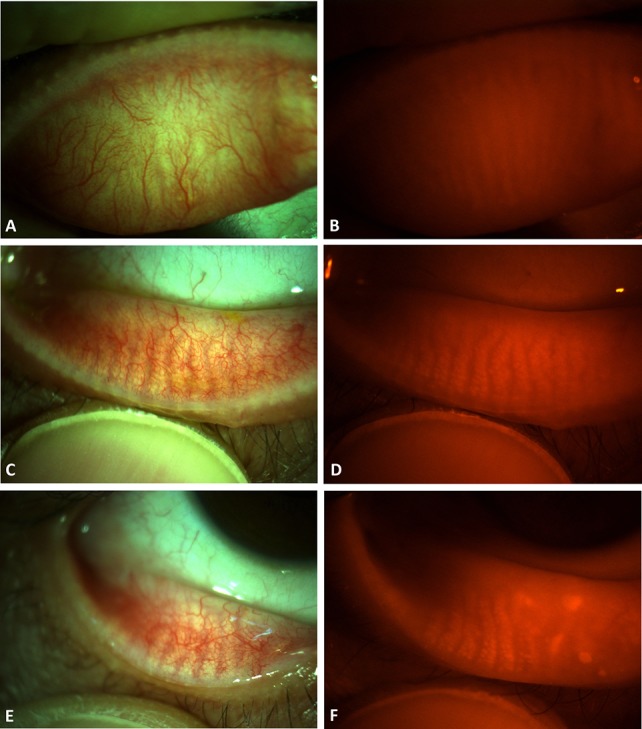
The other method we tried was to simply isolate the red-channel of the anterior segment photographs. The RCI nicely revealed the MG in most of the photographs. The red conjunctival vessels were observed as faint shadows in the red-channel compared with the prominent and darker shadows in the background of the brighter MG in both blue and green channels (see Supplemental Fig. 3, Supplemental Digital Content 4, http://links.lww.com/ICO/A756). However, in several cases, the MGs were hardly distinguishable from the intervening spaces in the RCI with a bright reflection because of saturation of the camera sensor by the strong light, regardless of the color of the underlying structures (Figs. 4A, C). In contrast, RFIs were relatively dark because of the red filter, which prevented saturation of the camera sensor by the bright reflection (Figs. 4B, D). Saturation of the camera sensor by the bright reflection could be reduced by adjusting the brightness and direction of the light source in anterior segment photography. As expected, the meiboscore with the RCI had a weaker correlation with the standard method (Fig. 3C and Table 1) and lower intraobserver and interobserver reliability compared with the other methods (Table 2). In summary, identifying MG morphology by isolating a specific color channel had some limitations, although it can be improved by more skillful photographic techniques to prevent bright reflections.
FIGURE 4.
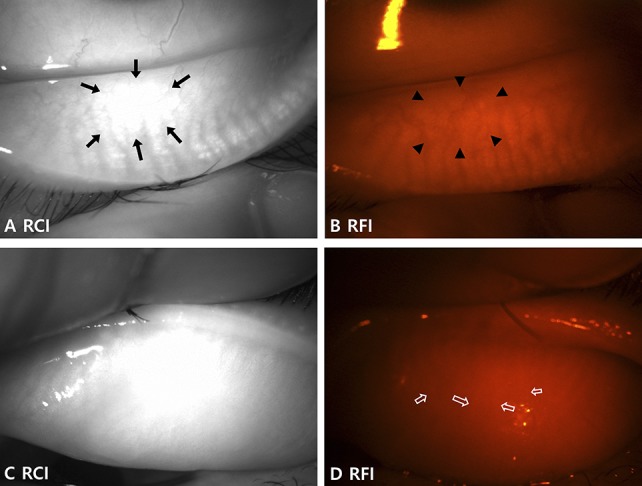
Limitation of the RCIs in some cases. A and B, Images of the lower eyelid of the left eye of a 40-year-old female patient show (A) intact MGs with an indistinguishable area due to the bright reflection in the RCI (arrows). B, The outline of the MGs is more distinguishable in the same area of the RFI (arrowheads). C, The MGs are not quite distinguishable from the RCI of the upper eyelid of the left eye in a 75-year-old female patient. D, Although difficult, the MGs can be distinguished in the RFI of the same eyelid with careful evaluation (empty arrows) and may be easier when viewed live. Evaluation is usually more difficult in the upper eyelid of older patients.
The reliability of the meiboscore by the RFI in advanced gland dropouts may be a concern. Additional comparison of the complete agreement ratio of the meiboscores between the 2 groups was performed after stratifying the eyes into 2 groups based on the meiboscore, normal (meiboscore 0∼2) and abnormal (meiboscore 3∼6), as suggested by Arita et al.6 If the eyes were grouped based on the meiboscore obtained by the IMI, the complete agreement ratio of the meiboscore between the IMI and RFI was 65.7% in the normal group and 56.5% in the abnormal group. Although the complete agreement ratio of the meiboscore in the abnormal group was slightly lower, it was not significantly different from that in the normal group (P = 0.41, n = 125, Pearson χ2 test). For the interobserver reliability test of the RFI, which showed more discrepancy, the complete agreement ratio of the meiboscore between the 2 evaluators was 36.1% in the normal group and 35.7% in the abnormal group if the eyes were stratified based on the meiboscore of the first evaluator and were not significantly different (P = 1, n = 125, Pearson χ2 test). These results indicate that the reliability of the RFI did not decrease significantly in cases with abnormal gland dropouts.
In this study, we applied 3 alternative methods for evaluating MG dropout without using an infrared light system. Of the 3 methods, the results obtained by simply applying the red filter in front of the objective lens of the slit lamp best correlated with results using the standard method, and there was substantial intraobserver reliability. The finding that MG dropout can be evaluated with a red filter in the trial lens set has 2 important implications. Clinically, eye doctors can directly observe the MG using a red filter on a slit lamp without an infrared light system, although some training is needed to reliably evaluate the meiboscore. This simple technique can be called “red-filter meibography.” Optically, blockade of the MG by the conjunctival vessels can be an important reason for the poor visibility of the MG in a visible light system compared with an infrared light system.
The greatest advantage of red-filter meibography is that it enables direct evaluation of the MG morphology in real time (see Supplemental Video 1, Supplemental Digital Content 2, http://links.lww.com/ICO/A754) with only a red filter from the trial lens set on a slit lamp, a commonly available instrument in general eye clinics. Eye doctors require no specialized equipment to perform this technique. Red-filter meibography also has some disadvantages. Although the MGs visualized using a red filter were clearer than those visualized without it, they had lower contrast in varying degrees than those imaged with standard infrared meibography, which requires a higher degree of concentration and more expertise for interpretation. This blurry visualization was more common in the upper eyelid than in the lower eyelid and may be caused by low penetration of the red light compared with the infrared light through the possibly thicker tarsal conjunctiva or denser tarsus of the upper eyelid compared with those of the lower eyelid (Fig. 4D). The second disadvantage is that the examiner must use both hands—everting the eyelid with one hand and holding the red filter in the other hand, which can be resolved by using a holder for the red filter in front of the objective lens of the slit lamp.
In summary, we evaluated MG dropout using a red filter on a slit lamp by removing the shadows of the overlying red conjunctival vessels. The meiboscores evaluated with the red-filter meibography correlated relatively well with those evaluated by infrared meibography and had substantial intraobserver reliability. Although the relatively lower interobserver reliability (moderate) is a limitation of this method, we expect that application of this alternative technique in some situations can improve clinical practice and clinical studies for dry eye disease, especially when an infrared meibography device is not available.
Supplementary Material
Footnotes
Supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education (NRF-2017R1A1A2A10000681 and NRF-2017R1D1A1B03031577), Hallym University Research Fund (HURF-2016-05), and a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI17C0659).
The authors have no conflicts of interest to disclose.
S. Lee and I. Park contributed equally to this study and should therefore be regarded as equivalent authors.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.corneajrnl.com).
REFERENCES
- 1.Craig JP, Nichols KK, Akpek EK, et al. TFOS DEWS II definition and classification report. Ocul Surf. 2017;15:276–283. [DOI] [PubMed] [Google Scholar]
- 2.Schaumberg DA, Nichols JJ, Papas EB, et al. The international workshop on meibomian gland dysfunction: report of the subcommittee on the epidemiology of, and associated risk factors for, MGD. Invest Ophthalmol Vis Sci. 2011;52:1994–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jester JV, Rife L, Nii D, et al. In vivo biomicroscopy and photography of meibomian glands in a rabbit model of meibomian gland dysfunction. Invest Ophthalmol Vis Sci. 1982;22:660–667. [PubMed] [Google Scholar]
- 4.Mathers WD, Daley T, Verdick R. Video imaging of the meibomian gland. Arch Ophthalmol. 1994;112:448–449. [DOI] [PubMed] [Google Scholar]
- 5.Arita R, Itoh K, Inoue K, et al. Noncontact infrared meibography to document age-related changes of the meibomian glands in a normal population. Ophthalmology. 2008;115:911–915. [DOI] [PubMed] [Google Scholar]
- 6.Wolffsohn JS, Arita R, Chalmers R, et al. TFOS DEWS II diagnostic methodology report. Ocul Surf. 2017;15:539–574. [DOI] [PubMed] [Google Scholar]
- 7.Arita R, Itoh K, Maeda S, et al. Proposed diagnostic criteria for obstructive meibomian gland dysfunction. Ophthalmology. 2009;116:2058–2063. [DOI] [PubMed] [Google Scholar]
- 8.Tomlinson A, Bron AJ, Korb DR, et al. The international workshop on meibomian gland dysfunction: report of the diagnosis subcommittee. Invest Ophthalmol Vis Sci. 2011;52:2006–2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hwang HS, Park CW, Joo CK. Novel noncontact meibography with anterior segment optical coherence tomography: Hosik meibography. Cornea. 2013;32:40–43. [DOI] [PubMed] [Google Scholar]
- 10.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 11.Powell DR, Nichols JJ, Nichols KK. Inter-examiner reliability in meibomian gland dysfunction assessment. Invest Ophthalmol Vis Sci. 2012;53:3120–3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nichols JJ, Berntsen DA, Mitchell GL, et al. An assessment of grading scales for meibography images. Cornea. 2005;24:382–388. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.