Abstract
Background
Pediatric cancer incidence has been steadily increasing over the last several decades with the largest increases reported in infants. Few evaluations have looked at international pediatric cancer incidence trends in the youngest children. The aim of this analysis was to evaluate trends in cancer incidence in children under 5 years of age, overall and by type, using data from Cancer Incidence in 5 Continents (CI5) from 1988 to 2012 (CI5 volumes VII–XI).
Methods
Rates of cancers in children ages 0–4 years were extracted from registries available in CI5 from 1988 to 2012. To overcome small numbers in individual registries, numerators and denominators were aggregated within regions corresponding to the United Nations’ geoscheme. Average annual percent change (AAPC) was estimated using Poisson regression. Robust standard errors were used in all models to correct for overdispersion in some regions, and 95% Wald confidence intervals and P values were reported. The top five cancers by increasing AAPC were ranked within each region.
Results
Overall, in children under 5 years, increasing incidence was seen in multiple regions for acute lymphoblastic leukemia, acute myeloid leukemia, ependymal tumors, neuroblastoma, and hepatoblastoma. Hepatoblastoma had the largest AAPC in 11 out of 15 regions and showed an increase in all regions except southern Asia. Astrocytic tumors were the only cancer that decreased over the time period.
Conclusions
We evaluated 25 years of cancer incidence in children ages 0–4 years and observed increases in incidence for hepatoblastoma, leukemia, neuroblastoma, and ependymal tumors. Further etiologic evaluation will be required to explain these increases in incidence.
To date, few international studies have been conducted to compare trends in pediatric cancer incidence. When conducted, international comparisons have shown differences in overall childhood cancer incidence and trends by geographic region but have been less successful in identifying etiologic insight than similar studies of adult cancers (1,2). Furthermore, studies have shown that pediatric cancer incidence rates have been steadily increasing (1,3). Steliarova-Foucher et al. showed that overall cancer in 0- to 14-year-olds increased from 124.0 per million person-years in the 1980s to 140.6 per million person-years in 2001–2010 (3). Estimates using data from registries in Europe and North America suggest that infants are the age group with the strongest increase in incidence (1).
Most of the current literature evaluates pediatric cancer incidence in the 0–14 or 0–19 years age range. Although currently known risk factors account for a small percentage of the overall disease burden, it is plausible that known risk factors such as birthweight, parental age, congenital abnormalities, chronic infections, and genetic syndromes may differ in infants and young children compared to adolescents (4–12). Moreover, concentration on the 0- to 4-year-old age range captures the peak of the acute lymphoblastic leukemia (ALL) and embryonal tumor incidence curves. Although it is recognized that pediatric cancers should be classified by histology rather than site (13), some previous analyses have reported incidence rates by site because of limitations in available registry data. Lastly, current literature often focuses on one region or country and is difficult to compare as manuscripts often report incidence trends for disparate time periods.
The Cancer Incidence in Five Continents (CI5) series presents the opportunity to look at global trends in childhood cancer incidence in children under 5 years old using peer-reviewed data from population-based cancer registries worldwide (14). The series also adheres to stringent criteria on quality for registry inclusion (15). Starting with volume VII (1988–1992), the CI5 data were expanded to include cancer by histology rather than just primary site, which permits the evaluation of trends in childhood cancer incidence using the more appropriate histologic classification. The aim of this analysis was to evaluate pediatric cancer incidence trends in children under 5 years old in each geographic region from 1988 to 2012.
Methods
Incident cases and population data were extracted from registries with data available during 1988–1992, 1993–1997, 1998–2002, 2003–2007, and 2008–2012 (16). The analysis was limited to these volumes because histological subtypes were available. Incidence rates were aggregated into regions using the regional and subregional categories provided by the United Nations geoscheme system, devised by the United Nations Statistics Division (UNSD), as a way to divide countries into macro-geographical and regional and subregional groups for statistical analysis (17). This aggregation was implemented to overcome small numbers in individual registries. Aggregation was also performed by human development index (HDI) category and change in HDI from 1990 to 2012. Countries were categorized as low, medium, high, or very high HDI according to their 2012 HDI scores and changes were categorized as movement from low to medium, low or medium to high, and medium to very high HDI during 1990 to 2012. Aggregation of the data into regions and HDI categories was conducted by pooling the cases and population from each registry within a region. Incidence rates for each time period were calculated by dividing pooled case numbers by pooled population denominators (18). As our analysis was limited to the 0- to 4-year-old age category, incidence rates did not require age adjustment. Because of lower sample size and variation in the registries available over time, Africa was split into northern and sub-Saharan Africa rather than the five UNSD subregions. Oceania was largely comprised of Australia and New Zealand and, therefore, was not broken down into further regions. Of note, Costa Rica was the only country with registry data in Central America, and although South America comprises a continent, the UNSD does not break them down further.
Central Asia was excluded because it did not have registry data for two or more time periods. There was variability in the countries and number of registries included in each region over time. Generally, more registries and countries were eligible for inclusion in later volumes (see Supplementary Table 1, available online, for registries included in each volume). To avoid dropping cancers entirely from analysis, we did not impose a cutoff for the number of cases needed to include a disease or registry in the dataset. Inclusion criteria for CI5 registries has been described in detail elsewhere (16).
Childhood cancer was classified using International Childhood Cancer Classification – 3 (ICCC-3) categories (13). We included specific subtypes of childhood cancer that comprised more than 2% of the cancers in children less than 5 years old in the United States (9) with the exception of rhabdomyosarcoma (9). Kidney tumors were not classified histologically but were presumed to be overwhelmingly nephroblastoma. We included 11 cancers: acute lymphoblastic leukemia (ALL), acute myeloid leukemia (AML), non-Hodgkin lymphoma (NHL), astrocytic tumors (AST), ependymal tumors (EPN), medulloblastoma (MB), neuroblastoma (NB), retinoblastoma (RB), kidney tumors (KT), hepatoblastoma (HB), and testicular germ cell tumors (TGCT).
Statistical Analysis
Total and sex-specific incidence was calculated and expressed as per one million children for cancers diagnosed at ages 0–4 years. Incidence was not calculated when there were fewer than five cases. For trends in incidence, the crude average annual percent change (AAPC) was estimated using Poisson regression to model counts as previously described (19). We controlled for population size heterogeneity by using the log population size as an offset term in the model. Robust standard errors were used in all models to correct for overdispersion in some regions or cancers. All analyses were conducted using SAS software version 9.4 (Cary, NC).
Results
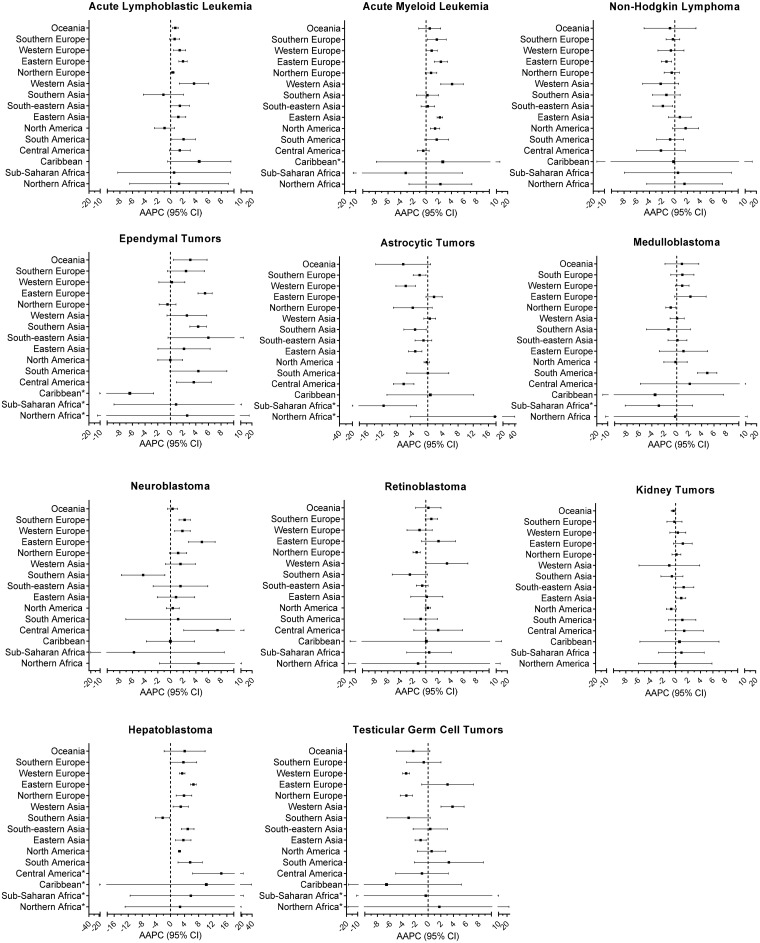
We compared estimated AAPC and 95% confidence interval (CI) for each cancer by region (Figure 1). Notable results for each major cancer grouping are described below. An analysis was also completed by HDI category (Table 1) and HDI change from 1990 to 2012 (Supplementary Table 2, available online). Additionally, we include detailed information with the estimated AAPC, 95% confidence interval, incidence, and sex-specific incidence for all regions in Supplementary Tables 3–13 (available online).
Figure 1.
Estimated annual average percent change (AAPC) and 95% confidence intervals (CI) by region for each cancer. *Three or more time periods had less than five cases. AAPC = average annual percent change; CI = confidence interval.
Table 1.
Estimated average annual percent change (AAPC) and incidence (per million) of pediatric cancer by human development index (HDI) category and year of diagnosis
| Cancer | HDI level† | Year of diagnosis |
AAPC (95% CI) | ||||
|---|---|---|---|---|---|---|---|
| 1988–1992 | 1993–1997 | 1998–2002 | 2003–2007 | 2008–2012 | |||
| Acute lymphoblastic leukemia | Low | * | 3.3 | 2.2 | 1.8 | 5.9 | 4.04 (−2.96 to 11.55) |
| Medium | 23.5 | 25.2 | 28.3 | 23.9 | 25.6 | 0.10 (−0.92 to 1.13) | |
| High | 29.9 | 33.5 | 43.7 | 41.5 | 44.7 | 1.72 (0.70 to 2.76) | |
| Very high | 49.6 | 51.6 | 54.1 | 57.7 | 53.3 | 0.44 (−0.20 to 1.08) | |
| Acute myeloid leukemia | Low | * | * | 3.1 | 1.8 | * | 1.55 (−10.86 to 15.68) |
| Medium | 6.1 | 6.4 | 4.3 | 6.4 | 5.8 | −0.14 (−2.46 to 2.23) | |
| High | 8.1 | 6.9 | 9.3 | 7.8 | 9.3 | 0.89 (−0.61 to 2.41) | |
| Very high | 8.2 | 8.8 | 9.4 | 10.2 | 10.9 | 1.47 (1.42 to 1.53) | |
| Non-Hodgkin lymphoma | Low | 11.4 | 23.0 | 14.5 | 49.2 | 21.0 | 3.88 (−4.04 to 12.46) |
| Medium | 5.9 | 6.9 | 7.9 | 6.6 | 4.5 | −1.50 (−4.07 to 1.13) | |
| High | 10.6 | 10.3 | 12.4 | 10.4 | 8.8 | −1.10 (−2.56 to 0.39) | |
| Very high | 10.2 | 9.7 | 8.4 | 8.2 | 10.8 | 0.16 (−1.68 to 2.04) | |
| Ependymal tumors | Low | * | * | * | * | * | ‡ |
| Medium | 0.5 | 0.5 | 0.7 | 1.0 | 1.0 | 3.92 (1.76 to 6.13) | |
| High | 0.7 | 1.0 | 3.0 | 2.0 | 2.7 | 4.74 (−0.36 to 10.10) | |
| Very high | 3.4 | 3.5 | 3.7 | 3.9 | 4.3 | 1.19 (0.91 to 1.48) | |
| Astrocytic tumors | Low | * | * | * | * | * | ‡ |
| Medium | 2.4 | 2.6 | 2.6 | 1.8 | 1.6 | −2.67 (−4.38 to -0.92) | |
| High | 2.7 | 3.9 | 4.4 | 5.0 | 3.9 | 0.98 (−1.60 to 3.63) | |
| Very high | 8.8 | 11.0 | 6.7 | 6.8 | 6.6 | −2.19 (−4.36 to 0.04) | |
| Medulloblastoma | Low | * | * | * | * | * | ‡ |
| Medium | 3.1 | 2.0 | 2.7 | 2.8 | 2.1 | −0.50 (−3.17 to 2.24) | |
| High | 1.8 | 2.8 | 2.7 | 3.4 | 4.2 | 3.70 (2.59 to 4.82) | |
| Very high | 4.7 | 5.4 | 5.2 | 5.2 | 5.2 | 0.27 (−0.29 to 0.83) | |
| Neuroblastoma | Low | * | 2.2 | * | * | * | ‡ |
| Medium | 3.8 | 5.3 | 4.4 | 3.8 | 2.1 | −3.42 (−6.65 to -0.07) | |
| High | 1.9 | 4.7 | 11.0 | 6.5 | 6.6 | 2.10 (−3.90 to 8.48) | |
| Very high | 10.3 | 11.7 | 13.0 | 13.6 | 14.2 | 1.51 (1.04 to 1.98) | |
| Retinoblastoma | Low | 12.6 | 14.8 | 12.3 | 25.0 | 12.8 | 1.52 (−3.34 to 6.62) |
| Medium | 10.2 | 11.8 | 8.5 | 10.8 | 7.6 | −1.56 (−3.70 to 0.63) | |
| High | 6.8 | 6.8 | 11.8 | 9.0 | 8.6 | 0.65 (−2.06 to 3.43) | |
| Very high | 8.5 | 8.8 | 8.4 | 8.6 | 8.6 | 0.02 (−0.24 to 0.28) | |
| Kidney tumors | Low | 20.8 | 15.6 | 18.9 | 33.9 | 24.6 | 2.80 (−1.10 to 6.86) |
| Medium | 8.6 | 8.8 | 10.3 | 9.9 | 9.6 | 0.60 (−0.27 to 1.48) | |
| High | 8.5 | 10.3 | 14.9 | 15.5 | 15.2 | 2.38 (0.58 to 4.22) | |
| Very high | 17.6 | 17.2 | 17.0 | 17.3 | 17.3 | −0.04 (−0.21 to 0.13) | |
| Hepatoblastoma | Low | * | * | * | * | * | ‡ |
| Medium | 2.7 | 1.7 | 1.9 | 2.5 | 2.6 | 1.37 (−1.44 to 4.26) | |
| High | 0.8 | 1.8 | 2.2 | 2.8 | 4.2 | 7.19 (5.55 to 8.86) | |
| Very high | 0.8 | 3.2 | 4.3 | 4.5 | 5.6 | 3.43 (2.73 to 4.13) | |
| Testicular germ cell tumors | Low | * | * | * | * | * | ‡ |
| Medium | 2.4 | 2.4 | 2.2 | 2.6 | 1.9 | −0.73 (−2.34 to 0.90) | |
| High | 3.7 | 2.9 | 2.1 | 2.8 | 3.9 | 1.17 (−2.11 to 4.56) | |
| Very high | 3.2 | 3.1 | 3.1 | 2.5 | 2.7 | −1.17 (−2.12 to -0.20) | |
Rates were not calculated for time periods with less than five cases. CI = confidence interval.
There were 5 countries with low HDI, 7 countries with medium HDI, 21 countries with high HDI, and 53 countries with very high HDI.
AAPCs were not calculated when greater than two time periods had zero cases.
Leukemias
ALL showed increasing incidence in most regions, with the exception of southern Asia, North America, northern Europe, or Africa, although the AAPC did not reach statistical significance in all regions (Figure 1; Supplementary Table 3, available online). When stratified by HDI, there was a statistically significant increase in ALL within high HDI countries (Table 1). There was evidence of increased incidence of AML in Europe, eastern and western Asia, and South and North America (Figure 1; Supplementary Table 4, available online). HDI stratified results showed that the AML increase was statistically significant and precise in very high HDI countries (AAPC = 1.47%, 95% CI = 1.42 to 1.53) (Table 1).
Lymphoma
NHL incidence decreased in eastern Europe and southeastern Asia (Figure 1; Supplementary Table 5, available online). The AAPC estimates for Central America, western Asia, and southern Asia suggested a decrease but were imprecise and did not reach statistical significance. There were no statistically significant changes in NHL when countries were stratified by HDI (Table 1).
Central Nervous System (CNS) Tumors
Incidence for EPN increased statistically significantly in four regions (Figure 1). Southern Asia, eastern Europe, and Oceania showed the sharpest and statistically significant increases at 4.37%, 5.46%, and 3.13%, respectively (Supplementary Table 6, available online). When AAPCs were estimated by HDI category (Table 1), EPN had increases in medium, high, and very high categories. In contrast to EPN, incidence of AST decreased or remained constant in all areas but northern Africa (Figure 1). The estimate was imprecise in northern Africa (95% CI = -3.88 to 40.7), but the magnitude of the effect (AAPC = 16.3) was large (Supplementary Table 7, available online). HDI stratified results also suggested decreases in AST (Table 1; Supplementary Table 2, available online). MB incidence increased in South America and decreased in northern Europe over this time period, but there were no other statistically significant changes (Figure 1; Supplementary Table 9, available online).
Non-CNS Embryonal Tumors
Overall, NB trended toward increasing incidence (Figure 1). Statistically significant increases were observed in Europe and Central America, whereas nonsignificant increases were observed in six additional regions (Supplementary Table 8, available online). In contrast to other regions, NB incidence statistically significantly decreased in Southern Asia. NB increases appear to be driven by increased incidence in very high HDI countries (Table 1). RB decreased in northern Europe and southern Asia, but no other changes in incidence were observed by subregion or HDI category (Figure 1; Supplementary Table 10, available online; Table 1). There were no statistically significant changes in KT incidence over the time period in any subregion or HDI category (Figure 1; Supplementary Table 11, available online; Table 1). HB incidence increased in all areas except southern Asia, although the 95% confidence interval for the AAPC overlapped the null in several regions, particularly those with imprecise estimates (Figure 1; Supplementary Table 12, available online). In contrast to the rest of the regions, the AAPC in southern Asia indicated decreasing incidence. When we stratified countries by HDI, the strongest increases were seen in high and very high HDI countries at 7.19% (95% CI = 5.55 to 8.86) and 3.43% (95% CI = 2.73 to 4.13), respectively.
Germ Cell Tumors
TGCTs decreased in western Europe, northern Europe, and eastern Asia, and increased in western Asia and potentially in eastern Europe and South America (Figure 1; Supplementary Table 13, available online). No changes in incidence were seen when stratifying countries by HDI (Table 1).
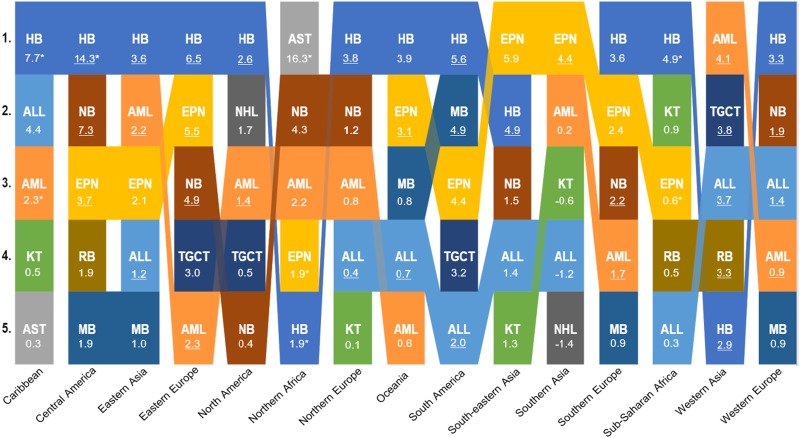
Trends in Incidence Rate Changes
To better visualize the childhood cancers with the fastest rising incidence, we ranked the five largest AAPC estimates for each region (Figure 2). Notably, the AAPC was the highest for HB in 11 of 15 regions, with AAPC estimates ranging from 2.6% in North America to 14.3% in Central America. In addition, the AAPC for HB ranked in the top five for southeastern Asia (4.9%), northern Africa (1.9%), and western Asia (2.9%). Southern Asia was the only region in which HB did not show an increase (-2.2%). ALL, AML, NB, and EPN were also common in the five largest AAPC estimates.
Figure 2.
Top five ranked estimated average annual percent change (AAPC) for each region. *Three or more time periods had less than five cases. Underlined AAPCs are statistically significant. ALL = acute lymphoblastic leukemia; AML = acute myeloid leukemia, AST = astrocytic tumor; EPN = ependymal tumor; HB = hepatoblastoma = KT = kidney tumor; NB = neuroblastoma; NHL = non-Hodgkin lymphoma; MB = medulloblastoma; RB = retinoblastoma; TGCT = testicular germ cell tumor.
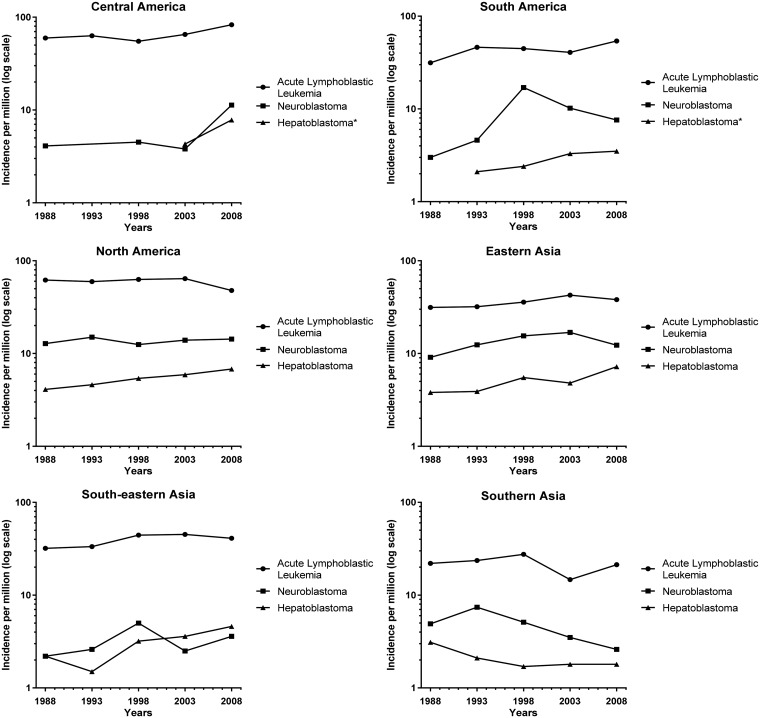
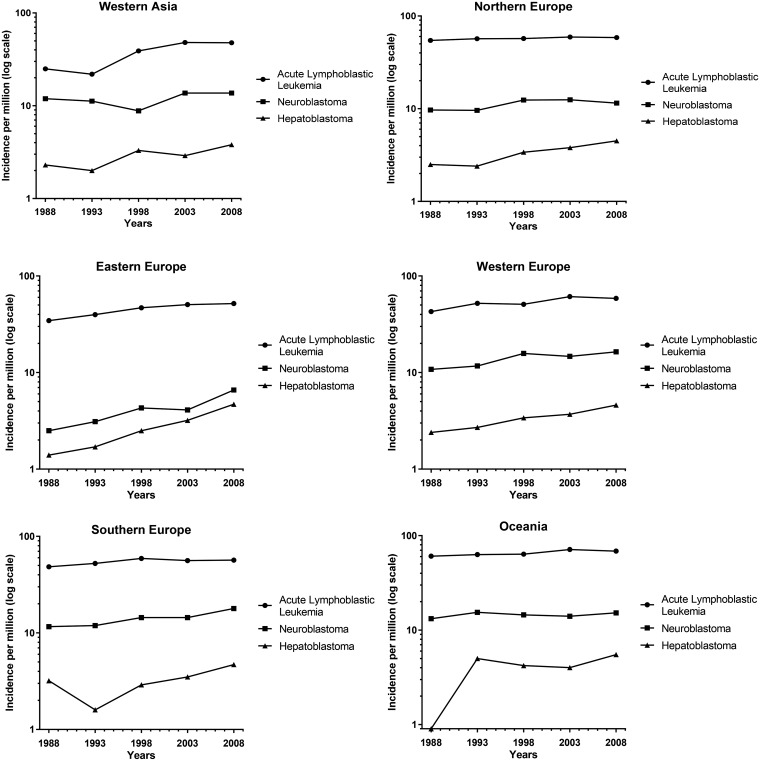
We compared the increased incidence in HB to NB, the most commonly diagnosed cancer in infants, and ALL, the most common cancer in 1- to 4-year-olds, by plotting the log of the incidence of HB, ALL, and NB for each region over time (Figure 3) (1). Rates were not calculated, or plotted, for time periods where there were fewer than five cases. Although the incidence of HB is smaller than the incidence of NB and ALL, the slope is greater in most regions. Our results indicate that HB is the fastest rising cancer globally in children younger than 5 years of age.
Figure 3.
Log incidence (per million) for acute lymphoblastic leukemia, neuroblastoma, and hepatoblastoma. *Rates were not calculated or plotted for time periods with less than five cases. Regions were not included if they did not have at least two time periods plotted.
Figure 3.
Continued.
Discussion
Using the CI5 data, we compared incidence and estimated average annual percent change for the most common types of childhood cancer in children aged 0–4 years from 1988 to 2012. Generally, we observed increasing incidence in HB, AML, ALL, NB, and EPN and decreasing incidence for AST. We recognize, particularly in comparisons of international rates, that not all population‐based registries are created equal (2). However, these results show consistency in the trends in childhood cancer incidence in many regions.
A major limitation of these findings are the sparse data available in some regions. Generally, the patterns we recognized represent those in high and very high HDI countries, which comprised most of the data. Sub-Saharan Africa and South America are large and ethnically diverse regions; however, because of the limitations of the available data, we were unable to analyze these regions in any further subregional breakdowns. There was also a great deal of heterogeneity in the registries included in each time point. We also must acknowledge that Costa Rica is the only country that provided data for Central America. Data is missing for some regions entirely, like Central Asia. The results of this analysis are limited by the data availability and should be interpreted with those limitations in mind.
Previously, increases in leukemias were reported in Europe, Beijing, Canada, Australia, and the United States (19–23). Although diagnostic differences could be partially responsible for the increase in incidence, ALL is less susceptible than other cancers to underdiagnosis (24). The increases in ALL incidence were generally 1%–2% per year, but much greater increases were seen in western Asia (3.68%) and the Caribbean (4.4%). Future studies should evaluate what proportion of the increase is due to diagnostic or screening changes in these regions. As previously reported (25) Costa Rica had the highest incidence of ALL, with a reported incidence rate of 95.4 per million in males in the latest time period (Supplementary Table 3, available online). In the United States, Hispanic children have the highest leukemia rates (3,23). The share of the US population that was Hispanic or Latino grew over time, which may explain some of the increase seen in North America.
As noted earlier, leukemias overall have been reported to be increasing in several regions, but little attention has been given to AML specifically. In our analysis, AML incidence is increasing, with the largest increases noted in eastern Europe (AAPC = 2.34%) and western Asia (AAPC = 4.09%), but these countries still report lower incidence at 7.8 and 8.8 per million, respectively. AML incidence has been reported to increase in highly developed settings (24). In support of this, our HDI stratified results showed the AML increase was only statistically significantly increasing in very high HDI countries (Table 1) with a particularly notable increase in countries that changed from medium to very high HDI during the time period (Supplementary Table 2, available online). The increases in eastern Europe and western Asia may thus be explained in part because of these regions becoming more developed (26).
Particular attention should be given to the changes in incidence rates for CNS tumors. EPN incidence is often evaluated together with AST as combined CNS tumors. We report that EPN incidence is increasing, and AST incidence is decreasing. This trade-off could suggest that different etiologic factors are involved in these subtypes, and they should be evaluated separately for better understanding. However, these tumors have been reported to have overlapping imaging features, and preoperative diagnostic differentiation has often posed challenges (27,28). So, it may be possible that better diagnostic distinction has resulted in an artificial increase in EPN and decrease in AST.
Although these other changes in incidence are noteworthy, the most striking change in incidence occurred for HB. HB is a rare childhood cancer that comprises most cases of liver cancer in children 0–5 years (9,29). Increases in HB incidence have been reported in the United States, Germany, and Taiwan (30–32). Other regions have also reported an increase in hepatic tumors that may be driven by HB (19–22). However, international incidence reports have yet to evaluate HB trends globally, and reports from individual countries looked at different time periods and are not directly comparable. Furthermore, few investigations have compared HB trends to other cancers predominant in children under 5 years of age. Our data suggest that HB has the largest increase in incidence during the time period investigated.
The etiologic risk factors for HB are still poorly understood, thus it is difficult to determine what is responsible for the increase in incidence over the past three decades. An alternative explanation for the increase in HB incidence is the increase in the ability to differentiate HB from other liver cancers (31). If this were the case, we would expect to see a decrease in liver cancers of unspecified morphology over the same time period. However, when we evaluated the trend in unspecified liver cancer tumors during this time period, their decrease was not present in all countries with rising HB incidence (data not shown). The correlation between the estimated AAPC for unspecified liver tumors and HB was weak (0.26) and not statistically significant (P = .08). We also recalculated our estimated AAPC with the conservative assumption that all unspecified liver tumors were HB, and our conclusions did not change for any region. HB was still the fastest rising cancer in all regions reported in Figure 2. Although the ability to differentiate between HB and other liver cancers may not explain the difference, there is still a possibility that changes in registration, reporting, or health service delivery account for the observed increases.
There are a number of strengths to this analysis, including the high-quality and consistent standards used to gather the registry data, the availability of a geographically diverse dataset, and the classification of cancers by histology rather than site. However, a number of limitations should also be considered. First, although robust standard errors were used in the model, the model assumes that the incidence rates are constant within five-year periods and that the new cancer cases follow a Poisson distribution. Second, cancer in this age group is very rare. Small numerators can give rise to unstable incidence rates, and this factor is especially important when considering sex-specific estimates (2). Likewise, 95% confidence intervals serve to provide context for descriptive analysis but are not statistically rigorous. Third, it is possible that changes in rates reflect changes in reporting, surveillance, diagnostics, and health-care delivery over this time period. Our analyses of rates in countries with increasing HDI suggest that this is not likely to explain the increases in incidence observed here. Although HDI may not be a sufficient indicator of health system improvements, our analysis suggests that the observed changes are not purely a result of health system strengthening. Furthermore, it is unlikely that these changes would affect one cancer to the point that it would display a higher rate in most regions. Finally, caution should be applied to comparing incidence rates between countries because the wide variation in childhood cancer incidence may be in part due to underdiagnosis, underreporting, care abandonment, or comorbid conditions, particularly in low-income countries (3,33). As stated previously, the data in low- and medium-income countries are sparse and do not permit us to draw definitive conclusions regarding trends in incidence rates.
In our evaluation of international trends in pediatric cancers over 25 years, we found evidence that HB, ALL, AML, NB, and EPN are increasing in children under 5 years of age. Analyses stratified by HDI and HDI change show the increases in incidence we observed are mainly driven by increases in high and very high HDI countries. Further exploration should be devoted to determining which increases cannot be explained by changes in health systems over the time period. Etiologic exploration should be devoted to those cancers with unexplained increases in incidence.
Funding
This work was supported by the National Institutes of Health (T32 CA099936 to AKH) and the Children’s Cancer Research Fund, Minneapolis, MN.
Notes
Affiliations of authors: Division of Epidemiology and Clinical Research, Department of Pediatrics, University of Minnesota, Minneapolis, MN (AKH, LGS, ELM, JNP); Masonic Cancer Center, University of Minnesota, Minneapolis, MN (LGS, ELM, JNP); Department of Collective Health, Faculdade de Ciências Médicas da Santa Casa de São Paulo, São Paulo, Brazil (GF).
The authors do not have any conflicts of interest to disclose.
Supplementary Material
References
- 1. Kaatsch P. Epidemiology of childhood cancer. Cancer Treat Rev. 2010;36(4):277–285. [DOI] [PubMed] [Google Scholar]
- 2. Robison LL, Shu X-O.. Assessing temporal trends in pediatric cancer incidence. Pediatr Blood Cancer. 2010;54(7):868–869. [DOI] [PubMed] [Google Scholar]
- 3. Steliarova-Foucher E, Colombet M, Ries LAG, et al. International incidence of childhood cancer, 2001–10: a population-based registry study. Lancet Oncol. 2017;18(6):719–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kenney LB, Miller BA, Ries LAG, Nicholson HS, Byrne J, Reaman G.. Increased incidence of cancer in infants in the U.S.: 1980-1990. Cancer. 1998;82(7):1396–1400. https://onlinelibrary.wiley.com/doi/pdf/10.1002/(SICI)1097-0142(19980401)82:7%3C1396::AID-CNCR25%3E3.0.CO;2-0. Accessed June 19, 2018. [DOI] [PubMed] [Google Scholar]
- 5. Ansell P, Mitchell CD, Roman E, Simpson J, Birch JM, Eden TOB.. Relationships between perinatal and maternal characteristics and hepatoblastoma: a report from the UKCCS. Eur J Cancer. 2005;41(5):741–748. [DOI] [PubMed] [Google Scholar]
- 6. Spector LG, Puumala SE, Carozza SE, et al. Cancer risk among children with very low birth weights. Pediatrics. 2009;124(1):96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Spector LG, Pankratz N, Marcotte EL.. Genetic and nongenetic risk factors for childhood cancer. Pediatr Clin North Am. 2015;62(1):11–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Johnson KJ, Carozza SE, Chow EJ, et al. Parental age and risk of childhood cancer. Epidemiology. 2009;20(4):475–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ries LAG, Smith MA, Gurney JG, et al. Cancer incidence and survival among children and adolescents: United States SEER Program 1975-1995. Bethesda, MD: National Institutes of Health; 1999. NIH Pub No 99-4649.
- 10. Maucort-Boulch D, de Martel C, Franceschi S, Plummer M.. Fraction and incidence of liver cancer attributable to hepatitis B and C viruses worldwide. Int J Cancer. 2018;142(12):2471–2477. [DOI] [PubMed] [Google Scholar]
- 11. Stiller CA. Epidemiology and genetics of childhood cancer. Oncogene. 2004;23(38):6429–6444. [DOI] [PubMed] [Google Scholar]
- 12. Kehm RD, Osypuk TL, Poynter JN, Vock DM, Spector LG.. Do pregnancy characteristics contribute to rising childhood cancer incidence rates in the United States? Pediatr Blood Cancer. 2018;65(3):e26888.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Steliarova-Foucher E, Stiller C, Lacour B, Kaatsch P.. International classification of childhood cancer, third edition. Cancer. 2005;103(7):1457–1467. [DOI] [PubMed] [Google Scholar]
- 14. Parkin DM, Ferlay J, Curado M-P, et al. Fifty years of cancer incidence: CI5 I-IX. Int J Cancer. 2010;127(12):2918–2927. [DOI] [PubMed] [Google Scholar]
- 15. Bray F, Parkin DM.. Evaluation of data quality in the cancer registry: principles and methods. Part I: comparability, validity and timeliness. Eur J Cancer. 2009;45(5):747–755. [DOI] [PubMed] [Google Scholar]
- 16. Ferlay J, Bray F, Steliarova-Foucher E, Forman D. Cancer incidence in five continents, CI5plus. IARC CancerBase No. 9. Lyon: International Agency for Research on Cancer; 2017. http://ci5.iarc.fr. Accessed July 30, 2018.
- 17.United Nations Statistics Division. UNSD—Methodology. Series M, No. 49. https://unstats.un.org/unsd/methodology/m49/. Accessed February 25, 2018.
- 18. Jensen OM, Parkin DM, MacLennan R, Muir CS, Skeet RRS, eds. Cancer Registration: Principles and Methods. No. 95. Lyon: International Agency for Research on Cancer; 1991. http://ci5.iarc.fr/Default.aspx.
- 19. Steliarova-Foucher E, Stiller C, Kaatsch P, et al. Geographical patterns and time trends of cancer incidence and survival among children and adolescents in Europe since the 1970s (the ACCIS project): an epidemiological study. Lancet. 2004;364(9451):2097–2105. [DOI] [PubMed] [Google Scholar]
- 20. Yang L, Yuan Y, Sun T, Li H, Wang N.. Characteristics and trends in incidence of childhood cancer in Beijing, China, 2000-2009. Chin J Cancer Res. 2014;26(3):285–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Public Health Agency of Canada, Mitra D, Shaw AK, Hutchings K.. Trends in incidence of childhood cancer in Canada, 1992-2006. Chronic Dis Inj Canada. 2012;32(3):1–11. https://www.canada.ca/en/public-health/services/reports-publications/health-promotion-chronic-disease-prevention-canada-research-policy-practice/vol-32-no-3-2012/trends-incidence-childhood-cancer-canada-1992-2006.html. Accessed May 3, 2018. [PubMed] [Google Scholar]
- 22. Baade PD, Youlden DR, Valery PC, et al. Trends in incidence of childhood cancer in Australia, 1983-2006. Br J Cancer. 2010;102(3):620–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Linabery AM, Ross JA.. Trends in childhood cancer incidence in the U.S. (1992–2004). Cancer. 2008;112(2):416–432. [DOI] [PubMed] [Google Scholar]
- 24. Miranda-Filho A, Piñeros M, Ferlay J, Soerjomataram I, Monnereau A, Bray F.. Epidemiological patterns of leukaemia in 184 countries: a population-based study. Lancet Haematol. 2018;5(1):e14–e24. [DOI] [PubMed] [Google Scholar]
- 25. Erdmann F, Li T, Luta G, et al. Incidence of childhood cancer in Costa Rica, 2000–2014: an international perspective. Cancer Epidemiol. 2018;56:21–30. [DOI] [PubMed] [Google Scholar]
- 26.United Nations Development Programme. Human Development Indices and Indicators 2018 Statistical Update. http://hdr.undp.org/sites/default/files/2018_human_development_statistical_update.pdf. Accessed January 3, 2019.
- 27. Kim DH, Kim J-H, Choi SH, et al. Differentiation between intramedullary spinal ependymoma and astrocytoma: comparative MRI analysis. Clin Radiol. 2014;69(1):29–35. [DOI] [PubMed] [Google Scholar]
- 28. Koeller KK, Rosenblum RS, Morrison AL.. Neoplasms of the spinal cord and filum terminale: radiologic-pathologic correlation. RadioGraphics. 2000;20(6):1721–1749. [DOI] [PubMed] [Google Scholar]
- 29. Allan BJ, Parikh PP, Diaz S, Perez EA, Neville HL, Sola JE.. Predictors of survival and incidence of hepatoblastoma in the paediatric population. HPB. 2013;15(10):741–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Spector LG, Birch J.. The epidemiology of hepatoblastoma. Pediatr Blood Cancer. 2012;59(5):776–779. [DOI] [PubMed] [Google Scholar]
- 31. Tulla M, Berthold F, Graf N, et al. Incidence, trends, and survival of children with embryonal tumors. Pediatrics. 2015;136(3):e623–e632. [DOI] [PubMed] [Google Scholar]
- 32. Hung G-Y, Lin L-Y, Yu T-Y, Lee C-Y, Yen H-J, Horng J-L.. Hepatoblastoma incidence in Taiwan: a population-based study. J Chin Med Assoc. 2018;81(6):541–547. [DOI] [PubMed] [Google Scholar]
- 33. Magrath I, Steliarova-Foucher E, Epelman S, et al. Paediatric cancer in low-income and middle-income countries. Lancet Oncol. 2013;14(3):e104–e116. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.