Abstract
Rationale:
Aromatase inhibitors (AIs) are a class of drugs widely used in the treatment of estrogen sensitive breast and ovarian cancer which convert testosterone to estradiol and androstenedione to estrogen. The AIs of third generation, including anastrazole, letrozole and exemestane, have actually become the standard of care of estrogen-receptor-positive breast cancer in menopausal women and are recommended as adjuvant treatment after surgery in place of/or following tamoxifen. Their main side-effects include reduction in bone mineral density, occurrence of menopausal manifestations and development of musculoskeletal symptoms which are, usually, transient, but sometimes evolve into a typical form of arthritis, such as rheumatoid arthritis (RA). Recently, a pathogenic linkage with other autoimmunity diseases, such as Sjogren syndrome (SjS), anti-synthetase antibody syndrome (ASAS), systemic sclerosis (SS) and subacute cutaneous lupus erythematosus (SCLE), was also described.
Patient concerns:
Here, we report the first case of a patient with primary antiphospholipid syndrome (APS) developed during treatment with anastrazole.
Diagnosis:
The patient developed a sudden onset of speech disturbance and disorientation, due to ischemic lesions, after 6 months of AIs therapy and the laboratory examination showed the positivity of anti-Cardiolipin antibodies, anti-β2 Glycoprotein 1 antibodies and Lupus Anticoagulant, so a certain diagnosis of APS was achieved.
Interventions:
The patient was treated with warfarin associated to hydroxychloroquine and monthly cycles of low doses intravenous immunoglobulins.
Outcomes:
A good control of the disease was obtained despite the continuation of anastrazole; the patient's clinical and laboratory situation remained not modified after AIs withdrawal.
Lessons:
We discussed the possible role of anastrazole treatment in inducing APS in our patient, reporting the available literature data about the association between AIs treatment and autoimmune diseases. Furthermore, we analyzed the mechanism of action of estrogens in the pathophysiology of autoimmune rheumatic disorders.
Keywords: anastrazole, antiphospholipid syndrome, aromatase inhibitors, autoimmune diseases, autoimmunity, case report, estrogens
1. Introduction
Aromatase inhibitors (AIs) are a class of drugs widely used in the treatment of estrogen sensitive breast and ovarian cancer. Their mechanism of action consists in inhibiting the aromatase enzyme which is responsible for the conversion of testosterone to estradiol and androstenedione to estrogen.[1] The AIs of third generation, including anastrazole, letrozole and exemestane, have actually become the standard of care of estrogen-receptor-positive breast cancer in menopausal women and are recommended as adjuvant treatment after surgery in place of/or following tamoxifen, considered for decades the cornerstone of endocrine therapy in this field.[2] AIs insure more efficacy and induce less life-threatening adverse events than tamoxifen.[3] The main side-effects of AIs therapy include reduction in bone mineral density (BMD), occurrence of menopausal manifestations and development of musculoskeletal symptoms, such as arthralgia, morning stiffness, tenosynovitis, trigger finger, and carpal tunnel syndrome.[4,5] Joint pain is usually transient and tends to disappear after therapy discontinuation, but growing evidence has demonstrated that AIs can sometimes induce or exacerbate a typical arthritis, as rheumatoid arthritis (RA)[6–10] or some subsets of spondyloarthropaty (SpA).[11] A pathogenic linkage between treatment with AIs and other autoimmunity diseases, such as Sjogren syndrome (SjS), anti-synthetase antibody syndrome (ASAS), systemic sclerosis (SS) and subacute cutaneous lupus erythematosus (SCLE), was also reported.[12–19] AIs reduce the synthesis of estrogens, which are known to affect the immune response, exerting opposite immunostimolant or immunodepressive effects, according to the dose and the duration of exposure.[20–23] Furthermore, the increase of aromatase activity in normal tissue occurs during aging and is responsible for the production of the main amount of peripheral estrogens which modulate the immune reactivity and cell proliferation.[22]
Here, we describe the first case of antiphospholipid syndrome (APS), a systemic autoimmune disease defined by the occurrence of venous and arterial thromboses and recurrent fetal losses, in the presence of antiphospholipid antibodies (aPL), which developed during treatment with anastrazole.[24]
2. Case presentation
In October 2012, a 56 years old female patient was admitted to the Neurology Unit for sudden onset of speech disturbance and disorientation. She had a history of right breast cancer (ductal carcinoma estrogen-receptor positive; T1N0M0) diagnosed in March 2010 and treated with upper-outer quadrantectomy and adjuvant radiotherapy in the months of August and September 2010. At the end of the radiotherapy cycle, she received treatment with tamoxifen (20 mg/day). In February 2012, she experienced a superficial venous thrombophlebitis of the left femoral vein; at this time, the patient was not taking any pharmacological therapy, including glucocorticoids, except for tamoxifen. Laboratory analysis showed an increase of D-dimer (1.2 ng/ml, range <500 ng/ml), fibrinogen (590 mg/dl, range 200–400) and C reactive protein (CRP) (1.2 mg/dl, vn <0.5 mg/dl), while the thrombophilia tests, including protein C, protein S, antithrombin III, homocysteine resulted within normal limits. Furthermore, the aPL, anti-Cardiolipin antibodies (aCL), anti-β2 Glycoprotein 1 antibodies (aGP1), Lupus Anticoagulant (LAC) and anti-nuclear antibodies (ANA), were negative. The thrombophlebitis was managed with low molecular weight heparin (LMWH) (fondaparinux 7,5 mg/day tapered to 2,5 mg/day in June 2012), switched to ticlopidine 500 mg/day a month later. The occurrence of thrombophlebitis was supposed to be due to tamoxifen, considering the known risk of this drug of inducing thrombotic events;[25,26] so, tamoxifen was switched to anastrazole (1 mg/day). In August 2012, after 6 months of anastrazole therapy, despite the ticlopidine treatment, she developed a transient motor aphasia and she was admitted to Stroke Unit. Brain magnetic resonance imaging (MRI) showed multiple lacunar infarcts. Unfortunately, no evaluation for autoimmunity or thrombophilia markers was performed. Anticoagulant therapy with warfarin was introduced during the hospitalization. In September 2012, she went to the emergency department complaining of a band-like abdominal pain; a computed tomoghraphy (CT) scan of the abdomen showed the enlargement of left adrenal gland with evidence of recent haemorrage. Warfarin was stopped and LMWH treatment (enoxaparin 6000 UI/day) was started.
In October 2012, she was first time referred to the Neurology Unit. At the admission, she was not oriented to person, place, and time and she spoke hesitantly and non-fluently (motor aphasia). Neurological examination also showed moderate right facial and limb hemi-paresis and no sensory deficit. The blood pressure was severely reduced (max value 55 mmHg) while cardiac examination was normal. The neck and skin examinations were normal, showing no lymphadenopathy, malar rash, acrocyanosis, or livedo reticularis. No significant abnormalities were found during lung auscultation; the abdomen was soft, not-tender, and not-distended with no hepatosplenomegaly. No signs of edema or thrombosis were pointed out.
Laboratory examinations revealed mild thrombocytopenia (120,000/mmc, range 150,000–400,000), mild erythrocyte sedimentation rate (ESR) increase (47 mm/h, range 0–35 mm/h), D-dimer and fibrinogen elevation, prolonged activated partial thromboplastin time (APTT) and normal prothrombin time (PT), International normalized ratio (INR), CRP, thrombophilia screening, kidney and liver function, hemoglobin, electrolytes, glycemia, immunoglobulins, C3, C4 complement factors and urinalysis (Table 1). Consistently with a sub-acute adrenal insufficiency, basal serum concentrations of cortisol were reduced and adrenocorticotropic hormone (ACTH) increased. The serum levels of dehydroepiandrosterone sulfate (DHEAS), testosterone and androstenedione resulted extremely low, while the concentrations of estradiol (E2), progesterone and 17-OH progesterone were within normal range according to the age of the patient (Table 1). Immunological tests showed positive ANA, (1:640, homogeneous pattern), high titer of aCL antibodies IgG and IgM (94.3 GPLU/ml, range <10; 16.4 MPLU/ml, range <7, respectively), aGP1 antibodies IgG, IgM and IgA (81.3 U/ml, range <5; 18.1 U/ml, range <5; 28.2 U/ml, range <5, respectively). The test for the detection of LAC was positive, as well as antiphosphatydilserine antibodies IgG and IgM (106 GPLU/ml, range <10; 18 MPLU/ml, range <10). Anti-dsDNA, extractable nuclear antigens (ENA), anti-cyclic citrullinated peptide antibodies (anti-CCP) and rheumatoid factor (RF) were negative.
Table 1.
Coagulation tests and endocrine screening results in the patient described in the text at the time of admission at Neurology Unit.
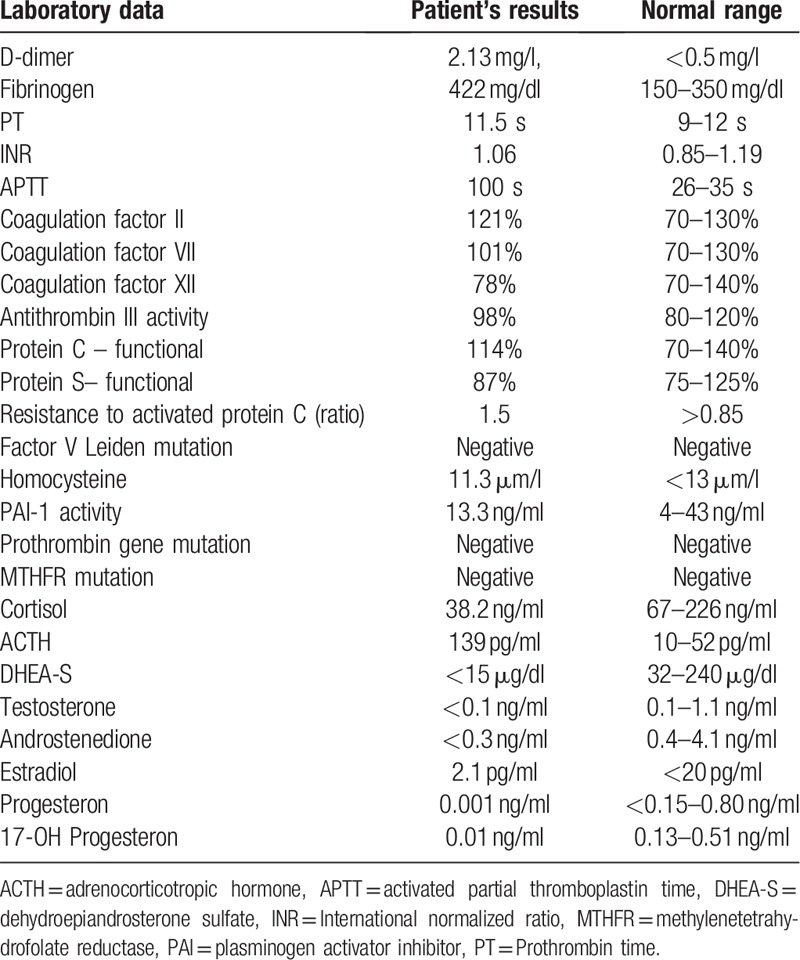
The brain Diffusion-Weighted MRI revealed lesions with restricted diffusion in the left occipital and temporo-parietal lobe, and in the left insular cortex not detectable in the previous examination and consistent with subacute ischemia. The brain CT- angiography showed occlusion of the first tract of left-sided middle cerebral artery (MCA1). The other performed instrumental examinations (chest radiography, abdomen ultrasonography, electrocardiogram, echocardiogram, arterial and venous lower limb Doppler ultrasonography) did not show anything significant except for a mild-moderate mitral insufficiency.
Considering the multiple episodes of cerebral ischemia and the high titer positivity of all aPL subtypes, a diagnosis of primary APS, according to the 2006 updated criteria,[24] was made.
The patient was treated with enoxaparin 6000 UI for 2 times a day and then a bridging therapy with warfarin was introduced until the achievement of an optimal INR value (3–4). Furthermore, she started a replacement treatment for adrenal insufficiency with hydrocortone acetate (intravenous flebocortid at the dosage of 50 mg every 6 hours for 48 hours), followed by oral cortone acetate at the dosage of 25 mg at 8 hours and of 6.5 mg at 13 hours and at 18 hours. Before the discharge, a cycle of intravenous human immunoglobulins (Flebogamma) at a dosage of 400 mg/kg/day for 5 consecutive days was administered and hydroxychloroquine (400 mg/day) was added.
Considering the high risk of cancer recurrence when AIs therapy lasts less than 5 years, the patient continued anastrazole therapy.[27] As the time of writing, the patient is still on therapy with warfarin, hydroxychloroquine (400 mg/day) and monthly cycles of low-dose intravenous immunoglobulins (400 mg/kg/day in a single monthly infusion), according to the dose protocol previously experienced in systemic lupus erythematosus (SLE), discoid lupus erythematosus (DLE) and primary and secondary APS.[28–30] Her clinical conditions appeared stable over time and no more ischemic or thrombotic events occurred. The patient discontinued anastrazole after the recommended 5 years without any changes in her clinical history.
Informed consent was obtained from the patient for publication of this case report.
3. Discussion and review of the literature
We presented the case of a patient with primary APS occurred after 6 months of anastrazole treatment. The diagnosis was made after recurrent episodes of cerebral ischemia and the detection of a triple positivity for aPL (LAC+ aCL antibodies+ aGP1 antibodies). We think that the APS occurred and has been caused by 6 months of anastrazole treatment. Conversely, one could argue that the onset of APS following AIs therapy is just coincidence rather than a causal relationship.
It is actually known that one of the major side effects associated to AIs therapy is represented by arthralgias.[31–33] Although being usually mild or moderate, these musculoskeletal symptoms may cause an important impact on quality of life and significantly reduce the compliance to the AIs therapy.[34] The first description of the frequent occurrence of arthralgia during AIs therapy came back to 2001, when Donnellan et al[35] reported that the 16% of 77 patients treated with AIs for breast cancer developed joint pain. Subsequently, more studies explored the frequency of musculoskeletal complaints during AIs therapy, observing a much higher prevalence (about 40%).[36,37] Furthermore, the term AIs-induced musculoskeletal symptoms (AIMSS) has been introduced to indicate the association of arthralgia, symmetric morning stiffness which improves with activity, a feeling of abrupt aging and soft tissue thickening.[31,32] The incidence of AIMSS in woman treated with AIs widely varies from 5% to 47%, due to the lacking of formal diagnostic criteria for this syndrome.[37] The exact mechanism responsible for AIMSS is not fully understood, but a major role is played by estrogens deprivation.[38] Indeed, it was demonstrated that estrogens have anti-nociceptive and pain modulating properties.[39]
More recently, a pathogenic linkage between AIs therapy and well established autoimmune diseases has been hypothesized.
We conducted a search of the literature concerning clinical reports on the development of autoimmune diseases during AIs therapy in September 2018 (the period examined was September 2007–August 2018). The strategy to select the literature data consisted in a detailed search in scientific databases PubMed, Scopus, Cochrane Library and EMBASE. A MEDLINE search was conducted using the MeSH terms “aromatase inhibitors”, “anastrazole”, “letrozole”, “exemestane” in combination with “autoimmune diseases" "autoimmunity" and “rheumatological disorders”. Studies were considered eligible if they met the following criteria:
-
1.
patients with a diagnosis of autoimmune and rheumatic diseases,
-
2.
therapy with anastrazole, letrozole or exemestane,
-
3.
studies published from 2007 to the present, totally written in English language.
The studies which were excluded from the review were the following:
-
1.
those analyzing the effects of AIs therapy on the development of musculoskeletal symptoms, as arthralgia, not conclusive for the diagnosis of autoimmune rheumatic diseases,
-
2.
review articles,
-
3.
anti-estrogen drugs other than AIs.
In total, 46 potential studies were found; no additional papers were found by hand searching of references. Of these, 2 were excluded because they were written in a language other than English. Based on the title and the abstract content, 21 of these articles were not included in our review. The full texts of the remaining 23 studies were read, and a further 7 studies were excluded, because the patients analyzed were not diagnosed with autoimmune rheumatic diseases. Finally, we identified 16 assessable articles reporting the development of rheumatic diseases during AIs therapy (Table 2).
Table 2.
Summary of literature data about rheumatic diseases associated to AIs therapy for breast cancer in post-menopausal women.
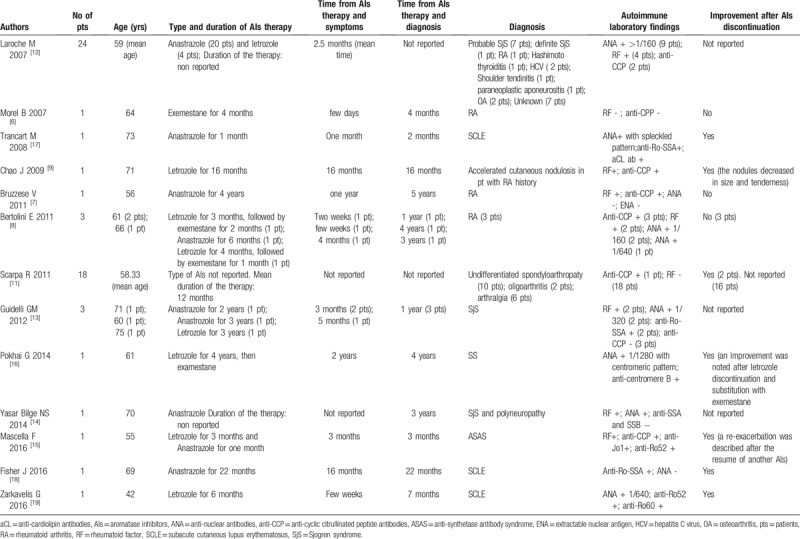
Four reports showed a potential association between AIs and RA (Table 2).[6–9] The first paper was published in 2007 and described the case of a woman with a history of advanced right breast cancer treated with exemestane who developed, after few days from the beginning of the therapy, joint pain of her hips, shoulders, knees, wrists and hands associated to prolonged morning stiffness. After 4 weeks, she presented a symmetric and active arthritis with wrists, metacarpophalangeal (MCF) and proximal interphalangeal (PIP) swellings which did not improve after exemestane discontinuation. ESR and CRP resulted increased and hands radiographs and MRI showed typical erosions, so the diagnosis of RA was made.[6] Another similar report was described by Bruzzese et al.[7] Also in this case, after 1 year from the starting of anastrazole, the patient firstly experienced widespread arthralgias evolving 3 years later in high activity RA with positivity of RF and anti-CCP. In the same year, Bertolini et al[8] presented 3 cases of RA occurred after AIs therapy (anastrazole in 1 patient and letrozole followed by exemestane in the other 2 patients). The symptoms characterized mainly by hands arthralgias developed after few weeks from the AIs initiation, while the diagnosis of RA was obtained after a mean period of 33 months. A correlation between AIs therapy and cutaneous nodulosis, a particular RA manifestation, was also reported. Indeed, a woman with a pre-existent long-standing RA, who took letrozole for breast cancer, developed, after 16 months, multiple small subcutaneous nodules on the fingers of both hands diagnosed as "accelerated cutaneous nodulosis". After the drug discontinuation, a slow decrease in size and tenderness of the nodules was observed.[9]
Not only RA, but also other definite types of arthritis in the field of SpA, were found to be associated to AIs treatment (Table 2). Indeed, Scarpa et al[11] carefully evaluated 18 post-menopausal women in treatment with AIs for breast cancer and, according to the European Spondyloarhropathy Study Group,[40] diagnosed 10 subjects with undifferentiated spondyloarhropathy, 2 with oligoarthritis and simple arthralgia the other 6 patients.
Interestingly, Laroche et al[12] referred 24 women treated with AIs and experiencing pain greater than 5/10 on a visual analog scale, to rheumatologic consultation to find out if any established condition could cause these complaints (Table 2). Apart from 5 patients with osteoarthritis, shoulder tendinitis or paraneoplastic aponeurositis, Authors classified 10 patients as having sicca syndrome of the eyes and mouth, of whom 7 with probable SjS and 1 definite SjS, according to San Diego criteria.[41] Furthermore, the number of patients with autoantibodies resulted higher than they expected. A relationship between AIs and SjS was suggested also in a more recent case-series study which describes the development of primary SjS, according to the 2002 European criteria,[42] in 3 women during the first year of adjuvant therapy (anastrazole in 2 patients and letrozole in 1 patient) for breast cancer (Table 2).[13] These findings confirm the protective role of estrogens against apoptosis of the exocrine secretory glands, previously suggested.[43] Others presented the case of a patient who developed SjS with neuropathy of both legs, after anastrazole therapy for breast cancer (Table 2). The Authors, after excluding other possible causes of neuropathy, such as chemotherapy side effects, paraneoplastic manifestation or cryoglobulinemia-related vasculitis, concluded that there was a causal relationship between AIs and SjS.[14]
Also a case of ASAS, after treatment with AIs, in a patient with a previous diagnosis of RA was described (Table 2).[15] The woman developed dyspnea related to a severe bilateral interstitial lung disease and necrotizing myopathy associated to a creatine kinase (CK) increase and positivity of anti-Jo1 and antiRo52 antibodies, after 3 months of therapy with letrozole. After the drug withdrawal and the establishment of immunosuppressive treatment, the myositis and the interstitial disease significantly improved, but a re-exacerbation was reported, after the introduction of a second AIs agent (anastrazole). The temporal correlation between AIs administration and the onset of the clinical manifestations and the rapid recurrence induced by the resume of the therapy, strongly supported an etiological association between these drugs and ASAS.
Among the wide range of connective tissue disorders, also SS, in a very early phase, has been described as associated to AIs therapy (Table 2). However, in this case the patient started to present hand joint pain and stiffness after two years of letrozole therapy and 2 years later was diagnosed as having early SS; letrozole was switched to exemestane and an improvement of the articular pain was noted.[16]
Particularly interesting are the reports of SCLE induced by AIs, because the role of estrogens in lupus disease activity have been the subject of debate for many years (Table 2).[17–19] Indeed, opposite to what observed during AIs treatment, reports in women with lupus showed an increase of disease flares due to estrogen-containing hormone replacement therapy and animal model studies demonstrated a significant clinical improvement of SLE, after anti-estrogen therapy.[44,45]
Finally, there are some reports supporting the hypothesis of a role of AIs in triggering also autoimmune hepatitis (AIH), probably affecting immune regulation.[46–48]
In the current case, it seems that the APS is not occurred by chance, since the AIs therapy have induced some pathogenetic changes of the immune system which persisted over time, also after anastrazole discontinuation. There are several factors known to trigger the development of thrombosis in APS including infection, malignancies, trauma, surgery, withdrawal of oral anticoagulation and drugs.[49,50] At this regard, several pharmacological agents, including anti-epileptic, anti-hypertensive, anti-arrhythmic drugs and antibiotics, have been linked to the occurrence of aPL antibodies, especially LA and rarely to the development of clinical manifestations of APS.[51] More recently, the detection of aPL antibodies was demonstrated also to be associated to immunotherapy (interferon-α and Interleukin-2) and tumor necrosis factor inhibitors.[52] It was hypothesized that drugs could insult cell membranes leading to the formation of neo-antigens or increasing the density of antigens bound to the cell surface; alternately, they could induce the production of circulating antibodies.[52] Although such evidence, there are no previous published data about estrogens antagonists and APS. It was actually demonstrated that estrogens play a pivotal role in the pathophysiology of autoimmune rheumatic diseases, considering for instance the prevalence of such disorders in female gender or their ability to influence the diseases flares.[22] However, various in vitro and in vivo studies showed that the estrogens may exert opposite functions on immune system, as inhibiting T cell autoimmunity and stimulating antibodies production from B cells. These multifaced effects of estrogens on autoimmunity could depend on their different concentrations, on the phase of the disease in which they act and on ability to generate various types of metabolites which are effectively active hormones.[53,54] The blockade of aromatase, the key enzyme for the conversion of androgens to estrogens, has been associated to the development of a wide range of autoimmune diseases, as described above. It has been suggested that estrogens may play an anti-inflammatory, protective role before the autoimmunity occurrence, while AIs, reducing the estrogens levels, could acts as triggering factors.[54] At this regard, some Authors investigated the involvement of aromatase blockade in the development of SjS. They used female aromatase gene knockout (ArK0) mice as a model of estrogen deficiency, observing that ArK0 mice presented SjS-like autoimmune lesions in lacrimal and salivary glands, due to B-cell proliferation and production of autoantibodies. Furthermore, they reported a significant amount of adiposity of the ArK0 mice salivary glands with an increased number of macrophages and consequent cytokines production, compared to wild type mice. Finally, the autoimmune lesions were exacerbated by administration of an AI, hypothesizing a key role of estrogens deficiency in the pathogenesis of autoimmunity.[55]
Actually, the role of AIs in APS remains totally unexplored, despite a correlation between the estradiol serum levels with aCL antibodies and ischemic attacks was previously reported.[56]
Our case report and review of the literature highlight that AIs therapy for breast cancer can trigger the onset of autoimmune changes resulting in various rheumatic diseases, which persisted over time, such as APS. These considerations may be useful in the clinical practice, because we think it should be very important to screen the patients starting AIs therapy for rheumatological remarks. However, the pathogenetic mechanism behind the development of APS, after AIs treatment, still remains unexplored. So preclinical studies are needed to investigate the pathophysiology of APS induced by this class of drugs and large clinical studies are mandatory to confirm our experience.
Author contributions
Author Resources: Fabio Giannini, Antonella Fioravanti.
Conceptualization: Fabio Giannini, Antonella Fioravanti.
Formal analysis: Sara Tenti.
Resources: Fabio Giannini, Antonella Fioravanti.
Validation: Nicola Giordano.
Writing – Original Draft: Sara Tenti, Fabio Giannini, Antonella Fioravanti.
Writing – Review & Editing: Nicola Giordano, Maurizio Cutolo.
Footnotes
Abbreviations: aCL = anti-cardiolipin antibodies, ACTH = adrenocorticotropic hormone, aGP1 = anti-β2 Glycoprotein 1 antibodies, AIH = autoimmune hepatitis, AIMSS = aromatase inhibitors-induced musculoskeletal symptoms, AIs = aromatase inhibitors, ANA = anti-nuclear antibodies, anti-CCP = anti-cyclic citrullinated peptide antibodies, aPL = antiphospholipid antibodies, APS = antiphospholipid syndrome, APTT = prolonged activated partial thromboplastin time, ASAS = anti-synthetase antibody syndrome, BMD = bone mineral density, CK = creatine kinase, CRP = C reactive protein, CT = computed tomoghraphy, DHEAS = dehydroepiandrosterone sulfate, DLE = discoid lupus erythematosus, E2 = estradiol, ENA = extractable nuclear antigens, ESR = erythrocyte sedimentation rate, INR = International normalized ratio, LAC = lupus anticoagulant, LMWH = low molecular weight heparin, MCA = middle cerebral artery, MCF = metacarpophalangeal, MRI = magnetic resonance imaging, PIP = proximal interphalangeal, PT = prothrombin time, RA = rheumatoid arthritis, RF = rheumatoid factor, SCLE = subacute cutaneous lupus erythematosus, SjS = Sjogren syndrome, SLE = systemic lupus erythematosus, SpA = spondyloarthropaty, SS = systemic sclerosis.
FG and AF contributed equally to this work.
Informed written consent was obtained from the patient for publication of this case report and accompanying images.
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
References
- [1].Winer EP, Hudis C, Burstein HJ, et al. American Society of Clinical Oncology technology assessment on the use of aromatase inhibitors as adjuvant therapy for postmenopausal women with hormone receptor-positive breast cancer: status report 2004. J Clin Oncol 2005;23:619–29. [DOI] [PubMed] [Google Scholar]
- [2].Burstein HJ, Prestrud AA, Seidenfeld J, et al. American Society of Clinical Oncology clinical practice practice guideline: Update on adjuvant endocrine therapy for women with hormone receptor-positive breast cancer. J Clin Oncol 2010;28:3784–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Buzdar A, Howell A, Cuzick J, et al. Comprehensive side-effect profile of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: long-term safety analysis of the ATAC trial. Lancet Oncol 2006;7:633–43. [DOI] [PubMed] [Google Scholar]
- [4].Henry NL, Giles JT, Stearns V. Aromatase inhibitor-associated musculoskeletal symptoms: etiology and strategies for management. Oncology (Williston Park) 2008;22:1401–8. [PubMed] [Google Scholar]
- [5].Amir E, Seruga B, Niraula S, et al. Toxicity of adjuvant endocrine therapy in postmenopausal breast cancer patients: a systematic review and meta-analysis. J Natl Cancer Inst 2011;103:1299–309. [DOI] [PubMed] [Google Scholar]
- [6].Morel B, Marotte H, Miossec P. Will steroidal aromatase inhibitors induce rheumatoid arthritis? Ann Rheum Dis 2007;66:557–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Bruzzese V, Hassan C, Zullo A, et al. Rheumatoid arthritis: a complication of aromatase inhibitor therapy? Int J Immunopathol Pharmacol 2011;24:1099–101. [DOI] [PubMed] [Google Scholar]
- [8].Bertolini E, Letho-Gyselinck H, Prati C, et al. Rheumathoid arthritis and aromatase inhibitors. Joint Bone Spine 2011;78:62–4. [DOI] [PubMed] [Google Scholar]
- [9].Chao J, Parker BA, Zvaifler NJ. Accelerated cutaneous nodulosis associated with aromatase inhibitor therapy in a patient with rheumatoid arthritis. J Rheumatol 2009;36:1087–8. [DOI] [PubMed] [Google Scholar]
- [10].Chen JY, Ballou SP. The effect of antiestrogen agents on risk of autoimmune disorders in patients with breast cancer. J Rheumatol 2015;42:55–9. [DOI] [PubMed] [Google Scholar]
- [11].Scarpa R, Atteno M, Peluso R, et al. Rheumatic complaints in women taking aromatase inhibitors for treatment of hormone-dependent breast cancer. J Clin Rheumatol 2011;17:169–72. [DOI] [PubMed] [Google Scholar]
- [12].Laroche M, Borg S, Lassoued S, et al. Joint pain with aromatase inhibitors: abnormal frequency of Sjögren's syndrome. J Rheumatol 2007;34:2259–563. [PubMed] [Google Scholar]
- [13].Guidelli GM, Martellucci I, Galeazzi M, et al. Sjögren's syndrome and aromatase inhibitors treatment: is there a link? Clin Exp Rheumatol 2013;31:653–4. [PubMed] [Google Scholar]
- [14].Yasar Bilge NS, Korkmaz C. Does Aromatase Inhibitors Cause Sjogren's Syndrome and Polyneuropathy? World J Oncol 2014;5:181–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Mascella F, Gianni L, Affatato A, et al. Aromatase inhibitors and anti-synthetase syndrome. Int J Immunopathol Pharmacol 2016;29:494–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Pokhai G, Buzzola R, Abrudescu A. Letrozole-induced very early systemic sclerosis in a patient with breast cancer: a case report. Arch Rheumatol 2014;29:126–9. [Google Scholar]
- [17].Trancart M, Cavailhes A, Balme B, et al. Anastrozole-induced subacute cutaneous lupus erythematosus. Br J Dermatol 2008;158:628–9. [DOI] [PubMed] [Google Scholar]
- [18].Fisher J, Patel M, Miller M, et al. Anastrozole-induced subacute cutaneous lupus erythematosus. Cutis 2016;98:E22–26. [PubMed] [Google Scholar]
- [19].Zarkavelis G, Kollas A, Kampletsas E, et al. Aromatase inhibitors induced autoimmune disorders in patients with breast cancer: a review. J Adv Res 2016;7:719–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Castagnetta L, Granata OM, Traina A, et al. A role for sex steroids in autoimmune diseases: a working hypothesis and supporting data. Ann N Y Acad Sci 2002;966:193–203. [DOI] [PubMed] [Google Scholar]
- [21].Cutolo M, Capellino S, Montagna P, et al. New roles for estrogens in rheumatoid arthritis. Clin Exp Rheumatol 2003;21:687–90. [PubMed] [Google Scholar]
- [22].Cutolo M, Sulli A, Straub RH. Estrogen metabolism and autoimmunity. Autoimmun Rev 2012;11:A460–464. [DOI] [PubMed] [Google Scholar]
- [23].Chighizola C, Meroni PL. The role of environmental estrogens and autoimmunity. Autoimmun Rev 2012;11:A493–501. [DOI] [PubMed] [Google Scholar]
- [24].Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost 2006;4:295–306. [DOI] [PubMed] [Google Scholar]
- [25].Cosman F, Baz-Hecht M, Cushman M, et al. Short-term effects of estrogen, tamoxifen and raloxifene on hemostasis: a randomized-controlled study and review of the literature. Thromb Res 2005;116:1–3. [DOI] [PubMed] [Google Scholar]
- [26].Takayanagi H, Hayami R, Tsuneizumi M, et al. Thrombophlebitis in an Elderly Japanese Woman treated with tamoxifen for breast cancer. Gan To Kagaku Ryoho 2015;42:1203–5. [PubMed] [Google Scholar]
- [27].Forbes JF, Cuzick J, Buzdar A, et al. Arimidex, Tamoxifen, Alone or in Combination (ATAC) Trialists’ Group. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 100-month analysis of the ATAC trial. Lancet Oncol 2008;9:45–53. [DOI] [PubMed] [Google Scholar]
- [28].Sherer Y, Kuechler S, Jose Scali J, et al. Low dose intravenous immunoglobulin in systemic lupus erythematosus: analysis of 62 cases. Isr Med Assoc J 2008;10:55–7. [PubMed] [Google Scholar]
- [29].Tenti S, Fabbroni M, Mancini V, et al. Intravenous Immunoglobulins as a new opportunity to treat discoid lupus erythematosus: a case report and review of the literature. Autoimmun Rev 2018;17:791–5. [DOI] [PubMed] [Google Scholar]
- [30].Tenti S, Guidelli GM, Bellisai F, et al. Long-term treatment of antiphospholipid syndrome with intravenous immunoglobulin in addition to conventional therapy. Clin Exp Rheumatol 2013;31:877–82. [PubMed] [Google Scholar]
- [31].Burstein HJ. Aromatase inhibitor-associated arthralgia syndrome. Breast 2007;16:223–34. [DOI] [PubMed] [Google Scholar]
- [32].Burstein HJ, Winer EP. Aromatase inhibitors and arthralgias: a new frontier in symptom management for breast cancer survivors. J Clin Oncol 2007;25:3797–9. [DOI] [PubMed] [Google Scholar]
- [33].Coleman RE, Bolten WW, Lansdown M, et al. Aromatase inhibitor-induced arthralgia: clinical experience and treatment recommendations. Cancer Treat Rev 2008;34:275–82. [DOI] [PubMed] [Google Scholar]
- [34].Garreau JR, Delamelena T, Walts D, et al. Side effects of aromatase inhibitors versus tamoxifen: the patients’ perspective. Am J Surg 2006;192:496–8. [DOI] [PubMed] [Google Scholar]
- [35].Donnellan PP, Douglas SL, Cameron DA, et al. Aromatase inhibitors and arthralgia. J Clin Oncol 2001;19:2767. [PubMed] [Google Scholar]
- [36].Moxley G. Rheumatic disorders and functional disability with aromatase inhibitor therapy. Clin Breast Cancer 2010;10:144–7. [DOI] [PubMed] [Google Scholar]
- [37].Shanmugam VK, McCloskey J, Elston B, et al. The CIRAS study: a case control study to define the clinical, immunologic, and radiographic features of aromatase inhibitor-induced musculoskeletal symptoms. Breast Cancer Res Treat 2012;131:699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Din OS, Dodwell D, Wakefield RJ, et al. Aromatase inhibitor-induced arthralgia in early breast cancer: what do we know and how can we find out more? Breast Cancer Res Treat 2010;120:525–38. [DOI] [PubMed] [Google Scholar]
- [39].Dawson-Basoa M, Gintzler AR. Involvement of spinal cord delta opiate receptors in the antinociception of gestation and its hormonal simulation. Brain Res 1997;757:37–42. [DOI] [PubMed] [Google Scholar]
- [40].Dougados M, van der Linden S, Juhlin R, et al. The European Spondylarthropathy Study Group preliminary criteria for the classification of spondylarthropathy. Arthritis Rheum 1991;34:1218–27. [DOI] [PubMed] [Google Scholar]
- [41].Fox RI, Howell FV, Bone RC, et al. Primary Sjogren syndrome: clinical and immunopathologic features. Semin Arthritis Rheum 1984;14:77–105. [DOI] [PubMed] [Google Scholar]
- [42].Vitali C, Bombardieri S, Jonsson R, et al. European Study Group on Classification Criteria for Sjögren's Syndrome. Classification criteria for Sjögren's syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis 2002;61:554–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Konttinen YT, Fuellen G, Bing Y, et al. Sex steroids in Sjögren's syndrome. J Autoimmun 2012;39:49–56. [DOI] [PubMed] [Google Scholar]
- [44].Lateef A, Petri M. Hormone replacement and contraceptive therapy in autoimmune diseases. J Autoimmun 2012;38:J170–176. [DOI] [PubMed] [Google Scholar]
- [45].Sthoeger ZM, Zinger H, Mozes E. Beneficial effects of the anti-oestrogen tamoxifen on systemic lupus erythematosus of (NZBxNZW)F1 female mice are associated with specific reduction of IgG3 autoantibodies. Ann Rheum Dis 2003;62:341–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Inno A, Basso M, Vecchio FM, et al. Anastrozole-related acute hepatitis with autoimmune features: a case report. BMC Gastroenterol 2011;11:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Islam MS, Wright G, Tanner P, et al. A case of anastrazole-related drug-induced autoimmune hepatitis. Clin J Gastroenterol 2014;7:414–7. [DOI] [PubMed] [Google Scholar]
- [48].Klapko O, Ghoulam E, Jakate S, et al. Anastrozole-induced autoimmune hepatitis: a rare complication of breast cancer therapy. Anticancer Res 2017;37:4173–6. [DOI] [PubMed] [Google Scholar]
- [49].Schreiber K, Sciascia S, de Groot PG, et al. Antiphospholipid syndrome. Nat Rev Dis Primers 2018;4:18005. [DOI] [PubMed] [Google Scholar]
- [50].Gómez-Puerta JA, Cervera R, Espinosa G, et al. Antiphospholipid antibodies associated with malignancies: clinical and pathological characteristics of 120 patients. Semin Arthritis Rheum 2006;35:322–32. [DOI] [PubMed] [Google Scholar]
- [51].Sherer Y, Blank M, Shoenfeld Y. Antiphospholipid syndrome (APS): where does it come from? Best Pract Res Clin Rheumatol 2007;21:1071–8. [DOI] [PubMed] [Google Scholar]
- [52].Dlott JS, Roubey RA. Drug-induced lupus anticoagulants and antiphospholipid antibodies. Curr Rheumatol Rep 2012;14:71–8. [DOI] [PubMed] [Google Scholar]
- [53].Zandman-Goddard G, Peeva E, Shoenfeld Y. Gender and autoimmunity. Autoimmun Rev 2007;6:366–72. [DOI] [PubMed] [Google Scholar]
- [54].Capellino S, Straub RH, Cutolo M. Aromatase and regulation of the estrogen-to-androgen ratio in synovial tissue inflammation: common pathway in both sexes. Ann N Y Acad Sci 2014;1317:24–31. [DOI] [PubMed] [Google Scholar]
- [55].Iwasa A, Arakaki R, Honma N, et al. Aromatase controls Sjögren syndrome-like lesions through monocyte chemotactic protein-1 in target organ and adipose tissue-associated macrophages. Am J Pathol 2015;185:151–61. [DOI] [PubMed] [Google Scholar]
- [56].Kaliterna DM, Radić M, Ljutić D. Does estrogen stimulate the pathogenic sort of anticardiolipin antibodies? Isr Med Assoc J 2014;16:197–8. [PubMed] [Google Scholar]


