Supplemental Digital Content is Available in the Text.
Keywords: Seizure-related protein 6, CSF, Inflammatory pain, Chronic pain, BACE1
Abstract
Introduction:
Seizure-related protein 6 (Sez6) contributes to chronic pain development as sez6 knockout mice show attenuated pain behaviours after peripheral nerve injury, compared with control mice. The type I transmembrane isoform of Sez6 is cleaved by the β-amyloid precursor protein cleavage enzyme 1 (BACE1), resulting in Sez6 extracellular domain shedding from the neuron surface.
Objectives:
To determine whether this BACE1-shed form of Sez6 can be detected in the cerebrospinal fluid (CSF) and whether Sez6 levels in the CSF are altered in neuropathic pain or chronic inflammatory pain (IP).
Methods:
We analysed the CSF samples collected during surgery from patients with chronic neuropathic pain (n = 8) or IP (n = 33), comparing them to the CSF samples from patients with suspected subarachnoid haemorrhage that was subsequently excluded (nonsurgical group, n = 5). Western blots were used to determine the relative Sez6 levels in the CSF from the different patient and nonsurgical comparison groups.
Results:
The results show that BACE1-shed Sez6 can be readily detected in the CSF by Western blot and that the levels of Sez6 are significantly higher in the IP group than in the nonsurgical comparison group.
Conclusion:
The association between elevated Sez6 levels in the CSF and IP is further evidence for persistent alterations in central nervous system activity in chronic IP conditions.
1. Introduction
Neuropathic and other forms of chronic pain affect 7% and 13% of the adult population, respectively.17 Neuropathic pain (NP) arises from a nervous system injury that results in abnormal or spontaneous firing of afferent nociceptive and mechanosensory neurons, a lowered activation threshold of second-order neurons in the spinal cord dorsal horn, and hypersensitisation to stimuli such as heat and touch.2,4
We showed previously that Seizure-related protein 6 (Sez6) is important for the development of neuronal dendrites and synapses.5,15 New findings from our laboratory, using a mixed pain model (chronic constriction injury) in mice, have revealed that Sez6 contributes to the development of chronic hyperalgesia and neuroinflammation after nerve injury. In neurons, Sez6 is almost exclusively cleaved by β-amyloid precursor protein cleaving enzyme 1, also known as β-secretase 1 or BACE1.12,25 After cleavage of the transmembrane isoform of Sez6 by BACE1, the shed extracellular domain of the protein is released into the cerebrospinal fluid (CSF).20
Because the development of NP and chronic inflammatory pain (IP) involves increased excitatory drive into the spinal cord and into the brain through ascending pathways,7,16,26,27 we aimed to test the hypothesis that higher levels of shed Sez6 in the CSF are associated with clinically diagnosed NP or IP. Specifically, this study addressed whether levels of Sez6 in the CSF are significantly changed in surgical patients with (1) chronic NP or (2) chronic IP, compared with a nonsurgical comparison group.
2. Materials and Methods
2.1. Cerebrospinal fluid samples
Cerebrospinal fluid samples from patients undergoing surgery for painful conditions, categorised into NP or IP groups, were obtained from The Alfred Hospital, Melbourne, Victoria, Australia (Alfred Ethics Committee Project No: 247/13), with informed consent. Details of diagnosis and/or surgery are described in Appendix, Table 1 (available at http://links.lww.com/PR9/A41). Prescribed medications (see Appendix, Table 1, available at http://links.lww.com/PR9/A41) were also taken on the day of CSF collection. Pain severity and intensity scores on the modified Brief Pain Inventory (mBPI) scale were recorded. Cerebrospinal fluid was procured through lumbar puncture before any administration of anaesthetics or analgesics intrathecally, centrifuged to remove any contaminating blood cells, and stored at −80°C. Patient age and sex data are summarised in Appendix, Table 2 (available at http://links.lww.com/PR9/A41).
Cerebrospinal fluid samples in the “nonsurgical” comparison group were collected from patients presenting to the emergency department at the Royal Melbourne Hospital, Parkville, Victoria, Australia (RMH Ethics Committee Project ID: HREC 2012-050), with informed consent. Patients in this group presented with a history of sudden-onset headache and, as part of the diagnostic workup, received computed tomography brain scans (that showed no evidence for a subarachnoid haemorrhage) followed by a lumbar puncture, which definitively excluded subarachnoid haemorrhage in all patients in this group. The headaches in these individuals resolved and the cause was not further investigated but presumed to be migraine/tension headache. As indicated in the Appendix, one of these patients had taken Panadol before admission.
2.2. Protein assay and Western blot
Sample protein concentration was measured with the DC Protein Assay (Bio-Rad, Hercules, CA). For Western blots, CSF samples (100 μL) were prepared as follows: 100% trichloroacetic acid was added (10% vol/vol), the samples were then incubated at room temperature for 5 to 10 minutes, and centrifuged (4°C, 20,000g) for 5 minutes. Precipitated protein pellets were dissolved in 100 µL 1x Laemmli buffer, and the pH was adjusted to >pH 4.6 with ammonia vapour. For each sample, 2 wells of a Mini-Protean 7.5% TGX precast polyacrylamide gel (Bio-Rad) were loaded (with 5 or 10 μL). Gels were transferred onto nitrocellulose membranes using the Trans-Blot Turbo semidry blotter (Bio-Rad). Membranes were blocked with skim milk powder (5% wt/vol) in 1x tris-buffered saline with 0.05% Tween-20 and incubated with a primary monoclonal antibody (rabbit anti-Sez6, 1/1000 in blocking solution) as described previously,5 followed by a goat anti-rabbit HRP-conjugated secondary antibody (Upstate, ID: 12-348), diluted 1/10,000 in blocking solution. Sez6 bands were detected using enhanced chemiluminescence (Clarity ECL, Bio-Rad). The Molecular Image Chemidoc MP System and Image Lab software (Bio-Rad) were used to create a multichannel image. Total protein load was normalised from the Stain-Free blot image, and the specific Sez6 protein signal in each lane was measured from the Chemi Hi-Resolution image (exposure time 90 seconds). An average integrated density value for the Sez6 signal was calculated for each CSF sample. Fold differences were calculated relative to the normalization standard or nonsurgical comparison sample run on the same gel and then the relevant adjustment factor was applied to compare all values with the mean value of all the nonsurgical group samples (see Appendix, Tables 3 and 4 for integrated density values and calculations, available at http://links.lww.com/PR9/A41). Fold-difference ratios were log10-transformed to normalize the distribution.
2.3. Statistical analysis
Statistical significance was tested using one-way analysis of variance on log10-transformed data, followed by the Dunnett multiple comparisons test. The strength of the correlation between patients' pain severity scores and relative Sez6 levels in the CSF was tested (Pearson correlation).
3. Results
3.1. Sez6 is elevated in the cerebrospinal fluid of patients with inflammatory pain
Sez6 was readily detected in the CSF by Western blot (Fig. 1), and relative Sez6 levels in each CSF sample are plotted in Figure 2. Levels of shed Sez6 were significantly higher in the CSF of patients with IP relative to the mean of all control samples (mean ± SEM fold difference = 3.09 ± 0.47 (IP), 1.00 ± 0.32 (controls); P = 0.038, n = 33 (IP), n = 5 (controls); Fig. 2). No significant difference in shed Sez6 levels in the CSF of patients with NP was observed, relative to controls (mean ± SEM fold difference = 1.17 ± 0.53 (NP), 1.00 ± 0.32 (controls); P > 0.05, n = 8 (NP), n = 5 (controls); Fig. 2).
Figure 1.
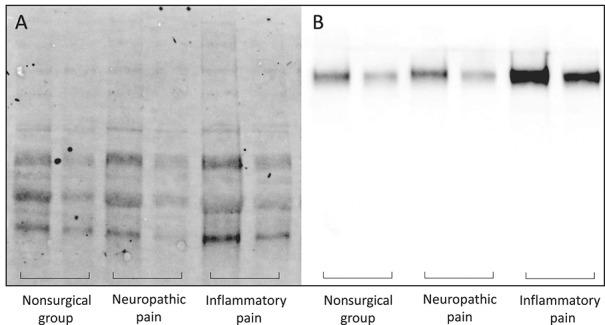
Relative Sez6 levels in the CSF by condition. Example of (A) stain-free and (B) Chemi Hi-Resolution Western blots used for analysis of total protein and Sez6 protein levels, respectively. For each sample, 10 or 5 μL was loaded (left or right lanes of each pair, respectively). CSF, cerebrospinal fluid.
Figure 2.
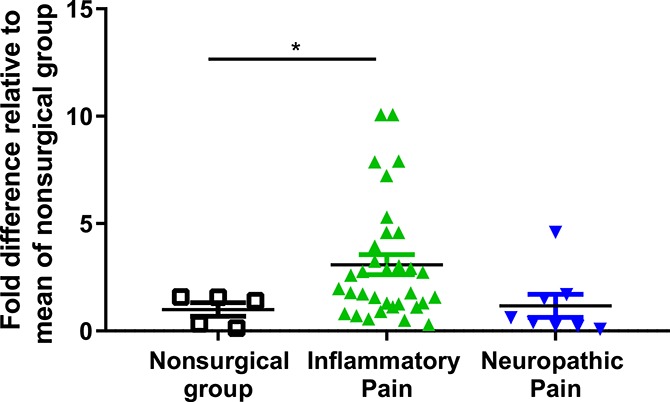
Cerebrospinal fluid Sez6 levels are significantly elevated in inflammatory, but not neuropathic, pain patients compared with samples from the nonsurgical comparison group. A significant increase in shed Sez6 levels in patients with inflammatory pain (n = 33, P < 0.05) was detected compared to samples from the nonsurgical comparison group (n = 5). No significant difference in patients with neuropathic pain (n = 8, p > 0.05) was observed compared to the control samples. CSF, cerebrospinal fluid.
3.2. No correlation between Sez6 levels and modified Brief Pain Inventory pain severity scores
Tukey boxplots of the Sez6 levels in the CSF from IP and NP groups revealed 2 outliers and 1 outlier, respectively (Appendix, Figure 1, available at http://links.lww.com/PR9/A41). All outliers had an mBPI pain severity score of 5.0 or higher (on a scale of 0–10). Heat maps and linear regression plots of mBPI scores against relative CSF Sez6 levels are shown in Figure 3. Severity scores were not correlated with Sez6 CSF levels in patients with IP (Pearson r = 0.13, R2 = 0.0169; P > 0.05) or NP (r = −0.10, R2 = 0.010; P > 0.05).
Figure 3.
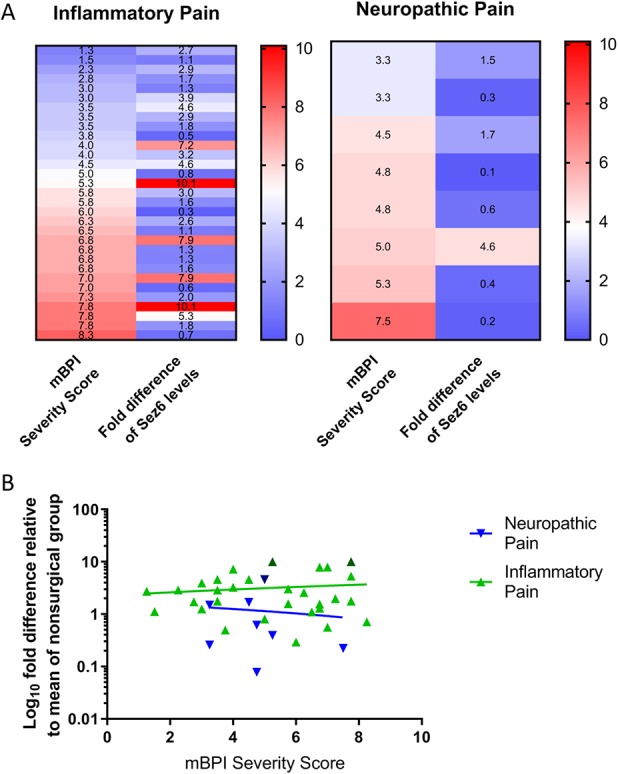
Relative CSF Sez6 levels are not correlated with pain severity scores. (A) Heat maps of modified Brief Pain Inventory (mBPI) pain severity scores and relative Sez6 levels. (B) Linear regression of mBPI pain severity score in patients with neuropathic (y = −0.1115x + 1.703) and inflammatory (y = 0.1118x + 2.484) pain. Dark triangles represent patient outliers (1.5 × interquartile range) for relative Sez6 levels. Pain severity scores were not significantly correlated with CSF Sez6 levels (P > 0.05). CSF, cerebrospinal fluid.
4. Discussion
Patients with inflammatory, but not neuropathic, pain showed significantly elevated levels of shed Sez6 in the CSF compared with the control patients. In neither group were the CSF levels of Sez6 significantly correlated with reported pain scores. The lack of a strong correlation between various measures of inflammation and subjective measures of pain intensity has been previously reported19 and is likely attributable to the multifactorial nature of pain.1
Osteoarthritis (the most common diagnosis of the patients with IP) involves release of inflammatory mediators11 that are capable of sensitising peripheral nociceptors,3 resulting in a lower activation threshold and increased firing of centrally projecting afferent axons in the spinal cord, even with normally innocuous stimuli.10 Increased excitatory drive and local neuroinflammation, in turn, lead to central nervous system sensitisation,9,21 including exaggerated and persistent synaptic long-term potentiation.8 The known roles for Sez6 in the development and maintenance of excitatory synapses,18 and the upregulation of Sez6 mRNA levels during long-term potentiation induction,6 suggest that Sez6 may be involved in the activity-dependent chronification of pain and might explain the observed association between elevated Sez6 levels in the CSF and IP conditions.
Sez6 levels in IP samples seem to be segregating into 2 clusters. No obvious commonalities could be identified amongst patients with the highest Sez6 levels, although clearer patterns may emerge if the sample size were increased and/or serial samples were available. If medically indicated, analysing serial samples from individual patients would be preferable to single sample analysis, provided the protocol for repeated CSF collection was standardised. An important consideration, particularly for interpretation of the results presented here, is that levels of Aβ, itself a product of BACE1 activity, are known to vary diurnally as well as increasing with draw frequency.13
Medical biomarkers are important tools for identifying susceptibility to disease, predicting treatment success, and facilitating objective diagnoses.24 A quantitative proteomics study indicated that Sez6 levels in the CSF are elevated in myalgic encephalomyelitis/chronic fatigue syndrome23 and Sez6 is also implicated in psychiatric disorders, forming part of a CSF biomarker signature for schizophrenia, bipolar disorder, and major depressive disorder.14 Although all 3 Sez6 family protein members are found in the CSF, only one (Sez6L2) has been identified in blood plasma.22 With the enhanced sensitivity (compared with Western blot) of ELISA-based assays, currently under development for shed Sez6 proteins, detection of Sez6 in serum may soon become feasible.
Because Sez6 is almost exclusively cleaved by BACE1,25 levels of shed Sez6 in the CSF can be used as a direct indicator of BACE1 activity. It will be important to determine whether Sez6 shedding contributes to IP and whether BACE1 inhibitors (currently in clinical trials for the treatment of Alzheimer disease) might be useful for treating conditions associated with elevated CSF Sez6 levels, such as chronic IP.
Disclosures
The authors have no conflict of interest to declare.
This project was supported by NHMRC Project Grant GNT1099930 to J.M. Gunnersen, NHMRC GNT1091636 to L.E. Edgington-Mitchell, and by grants from the NIH (NS102722, DE026806, and DK118971) and the Department of Defense (W81XWH1810431) to N.W. Bunnett.
Acknowledgements
The authors thank Sophie Wallace, Research Manager of the Department of Anaesthesia and Perioperative Medicine at the Alfred Hospital, for provision of deidentified patient data.
Appendix A. Supplemental digital content
Supplemental digital content associated with this article can be found online at http://links.lww.com/PR9/A41.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.painrpts.com).
References
- [1].Boyden SD, Hossain IN, Wohlfahrt A, Lee YC. Non-inflammatory causes of pain in patients with rheumatoid arthritis. Curr Rheumatol Rep 2016;18:30–8. [DOI] [PubMed] [Google Scholar]
- [2].Campbell JN, Meyer RA. Mechanisms of neuropathic pain. Neuron 2006;52:77–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Dray A, Perkins M. Bradykinin and inflammatory pain. Trends Neurosci 1993;16:99–104. [DOI] [PubMed] [Google Scholar]
- [4].Dworkin RH, Backonja M, Rowbotham MC, Allen RR, Argoff CR, Bennett GJ, Bushnell MC, Farrar JT, Galer BS, Haythornthwaite JA, Hewitt DJ, Loeser JD, Max MB, Saltarelli M, Schmader KE, Stein C, Thompson D, Turk DC, Wallace MS, Watkins LR, Weinstein SM. Advances in neuropathic pain: diagnosis, mechanisms, and treatment recommendations. Arch Neurol 2003;60:1524–34. [DOI] [PubMed] [Google Scholar]
- [5].Gunnersen JM, Kim MH, Fuller SJ, De Silva M, Britto JM, Hammond VE, Davies PJ, Petrou S, Faber ES, Sah P, Tan SS. Sez-6 proteins affect dendritic arborization patterns and excitability of cortical pyramidal neurons. Neuron 2007;56:621–39. [DOI] [PubMed] [Google Scholar]
- [6].Håvik B, Røkke H, Dagyte G, Stavrum AK, Bramham CR, Steen VM. Synaptic activity-induced global gene expression patterns in the dentate gyrus of adult behaving rats: induction of immunity-linked genes. Neuroscience 2007;148:925–36. [DOI] [PubMed] [Google Scholar]
- [7].Ivanavicius SP, Ball AD, Heapy CG, Westwood FR, Murray F, Read SJ. Structural pathology in a rodent model of osteoarthritis is associated with neuropathic pain: increased expression of ATF-3 and pharmacological characterisation. PAIN 2007;128:272–82. [DOI] [PubMed] [Google Scholar]
- [8].Ji RR, Kohno T, Moore KA, Woolf CJ. Central sensitization and LTP: do pain and memory share similar mechanisms? Trends Neurosci 2003;26:696–705. [DOI] [PubMed] [Google Scholar]
- [9].Ji RR, Xu ZZ, Gao YJ. Emerging targets in neuroinflammation-driven chronic pain. Nat Rev Drug Discov 2014;13:533–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet 2006;367:1618–25. [DOI] [PubMed] [Google Scholar]
- [11].Kidd B. Mechanisms of pain in osteoarthritis. HSS J 2012;8:26–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kuhn PH, Koroniak K, Hogl S, Colombo A, Zeitschel U, Willem M, Volbracht C, Schepers U, Imhof A, Hoffmeister A, Haass C, Roßner S, Bräse S, Lichtenthaler SF. Secretome protein enrichment identifies physiological BACE1 protease substrates in neurons. EMBO J 2012;31:3157–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Lucey BP, Gonzales C, Das U, Li J, Siemers ER, Slemmon JR, Bateman RJ, Huang Y, Fox GB, Claassen JA, Slats D, Verbeek MM, Tong G, Soares H, Savage MJ, Kennedy M, Forman M, Sjogren M, Margolin R, Chen X, Farlow MR, Dean RA, Waring JF. An integrated multi-study analysis of intra-subject variability in cerebrospinal fluid amyloid-beta concentrations collected by lumbar puncture and indwelling lumbar catheter. Alzheimers Res Ther 2015;7:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Maccarrone G, Ditzen C, Yassouridis A, Rewerts C, Uhr M, Uhlen M, Holsboer F, Turck CW. Psychiatric patient stratification using biosignatures based on cerebrospinal fluid protein expression clusters. J Psychiatr Res 2013;47:1572–80. [DOI] [PubMed] [Google Scholar]
- [15].Miyazaki T, Hashimoto K, Uda A, Sakagami H, Nakamura Y, Saito SY, Nishi M, Kume H, Tohgo A, Kaneko I, Kondo H, Fukunaga K, Kano M, Watanabe M, Takeshima H. Disturbance of cerebellar synaptic maturation in mutant mice lacking BSRPs, a novel brain-specific receptor-like protein family. FEBS Lett 2006;580:4057–64. [DOI] [PubMed] [Google Scholar]
- [16].Moalem G, Tracey DJ. Immune and inflammatory mechanisms in neuropathic pain. Brain Res Rev 2006;51:240–64. [DOI] [PubMed] [Google Scholar]
- [17].Moore RA, Derry S, Taylor RS, Straube S, Phillips CJ. The costs and consequences of adequately managed chronic non-cancer pain and chronic neuropathic pain. Pain Pract 2014;14:79–94. [DOI] [PubMed] [Google Scholar]
- [18].Nakayama M, Hama C. Modulation of neurotransmitter receptors and synaptic differentiation by proteins containing complement-related domains. Neurosci Res 2011;69:87–92. [DOI] [PubMed] [Google Scholar]
- [19].Neogi T, Frey-Law L, Scholz J, Niu J, Arendt-Nielsen L, Woolf C, Nevitt M, Bradley L, Felson DT; Multicenter Osteoarthritis (MOST) Study. Sensitivity and sensitisation in relation to pain severity in knee osteoarthritis: trait or state?. Ann Rheum Dis 2015;74:682–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Pigoni M, Wanngren J, Kuhn PH, Munro KM, Gunnersen JM, Takeshima H, Feederle R, Voytyuk I, De Strooper B, Levasseur MD, Hrupka BJ, Müller SA, Lichtenthaler SF. Seizure protein 6 and its homolog seizure 6-like protein are physiological substrates of BACE1 in neurons. Mol Neurodegener 2016;11:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Raghavendra V, Tanga FY, DeLeo JA. Complete Freunds adjuvant-induced peripheral inflammation evokes glial activation and proinflammatory cytokine expression in the CNS. Eur J Neurosci 2004;20:467–73. [DOI] [PubMed] [Google Scholar]
- [22].Schutzer SE, Liu T, Natelson BH, Angel TE, Schepmoes AA, Purvine SO, Hixson KK, Lipton MS, Camp DG, Coyle PK, Smith RD, Bergquist J. Establishing the proteome of normal human cerebrospinal fluid. PLoS One 2010;5:e10980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Schutzer SE, Angel TE, Liu T, Schepmoes AA, Clauss TR, Adkins JN, Camp DG, Holland BK, Bergquist J, Coyle PK, Smith RD, Fallon BA, Natelson BH. Distinct cerebrospinal fluid proteomes differentiate post-treatment lyme disease from chronic fatigue syndrome. PLoS One 2011;6:e17287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Schwarz E, Bahn S. The utility of biomarker discovery approaches for the detection of disease mechanisms in psychiatric disorders. Br J Pharmacol 2008;153(suppl 1):S133–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Vassar R. BACE1 inhibitor drugs in clinical trials for Alzheimer's disease. Alzheimers Res Ther 2014;6:89–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. PAIN 2011;152(3 suppl):S2–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Zhang RX, Ren K, Dubner R. Osteoarthritis pain mechanisms: basic studies in animal models. Osteoarthr Cartil 2013;21:1308–15. [DOI] [PMC free article] [PubMed] [Google Scholar]


