Abstract
Introduction:
Increase in excitability of the primary motor cortex (M1) is associated with pain inhibition by analgesics, which is, in turn, associated with the psychophysical antinociceptive pain modulation profile. However, the relationship between neurophysiological M1 excitability and psychophysical pain modulation has not yet been explored.
Objectives:
We aim to study these relationships in healthy subjects.
Methods:
Forty-one young healthy subjects (22 women) underwent a wide battery of psychophysical testing that included conditioned pain modulation (CPM) and pain temporal summation, and a transcranial magnetic stimulation neurophysiological assessment of the motor corticospinal excitability, including resting motor threshold, motor-evoked potentials (MEPs), and cortical silent period.
Results:
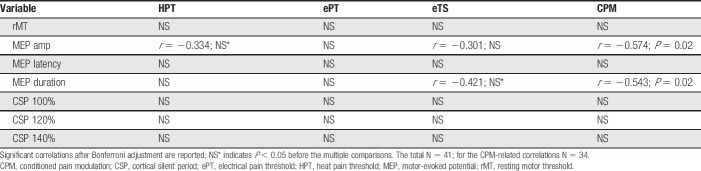
Increased motor corticospinal excitability in 2 parameters was associated with more efficient CPM: (1) higher MEP amplitude (r = −0.574; P_Bonferroni = 0.02) and (2) longer MEP duration (r = −0.543; P_Bonferroni = 0.02). The latter also correlated with the lower temporal summation magnitude (r = −0.421; P = 0.007); however, on multiplicity adjustment, significance was lost.
Conclusions:
Increased corticospinal excitability of the primary motor cortex is associated with more efficient inhibitory pain modulation as assessed by CPM, in healthy subjects. Motor-evoked potential amplitude and duration may be considered as an additional, objective and easy to measure parameter to allow for better individual assessment of pain modulation profile.
Keywords: Pain modulation, Conditioned pain modulation, Temporal summation, Motor cortex, Corticospinal excitability, Motor-evoked potentials, Transcranial magnetic stimulation
1. Introduction
Higher excitability of the primary motor cortex and the efficient inhibitory facet of endogenous pain modulation are associated with analgesic responses and can therefore be considered as neurophysiological and psychophysical correlates of antinociception. Indeed, from the neurophysiological point of view, the stimulation of primary motor cortex (M1) through high-frequency repeated transcranial magnetic stimulation (rTMS) or electrical stimuli, as well as its activation through physical exercises, exerts inhibitory effects on the pain system through activation of limbic, cortical, and subcortical brain areas associated with antinociception.22,43,53 From psychophysical point of view, the activation of the same brain structures can be observed during induction of endogenous analgesia using several experimental methods (ie, offset analgesia37; stress-induced analgesia80; and conditioned pain modulation [CPM] response).6,50,55,63 Furthermore, similar to stimulation-induced M1 activation, various pain-alleviating treatments increase the efficiency of descending pain inhibition, as was reported for CPM.33,51,67,76,77
Complementary to psychophysical assessment of endogenous pain inhibition, activation and measuring of spatial and temporal summation (TS) of pain can be considered psychophysical methods for evaluation of ascending pain facilitatory pathways. In comparison with simple pain threshold and suprathreshold pain estimation, where the contribution of peripheral components can be considered, the mechanisms of pain summation are centrally mediated, reflecting central neuronal sensitization state.4,16,73 The assessment of TS is widely used in clinical studies; enhanced TS magnitude characterizes many chronic pain states3,45,65,69 and was explored as a predictor of acute postoperative pain.71 Therefore, the combination of less-efficient pain inhibition and enhanced pain facilitation presumably characterizes a pronociceptive state of pain modulation.24,78 We addressed such inhibition and facilitation using CPM and TS, which are most widely explored for the assessment of individual pain modulation profile (PMP).
A positive association between higher excitability of M1, efficient CPM/low TS magnitude, and analgesic responses would allow us to suggest that the neurophysiological and psychophysical domains of antinociception are interrelated. In this study, we aimed to test this hypothesis in a group of young healthy subjects, using a battery of tests for psychophysical and neurophysiological assessment. We hypothesized that higher corticospinal excitability would be associated with antinociceptive PMP.
2. Methods
Forty-one young healthy subjects (22 women) underwent comprehensive assessment of corticospinal excitability and pain psychophysics. In addition, all subjects filled a battery of pain-related psychological questionnaires. The questionnaire-related data will be reported separately. The inclusion criteria for the participants were as follows: age range of 18 to 40 years; no chronic or acute pain events; and no self-reported attention deficit. The subjects were asked to have a full night's sleep before the experimental session and to avoid caffeine consumption at least 2 hours before the experimental session. The Investigational Review Board of Rambam Health Care Campus, Haifa, Israel, approved the experimental protocol; the study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki. Informed consent was obtained from all participants.
2.1. Neurophysiological measurements
We measured the resting motor threshold (rMT); the motor-evoked potential (MEP) latency, amplitude, and duration; and the duration of the cortical silent period (CSP) in the left right abductor pollicis brevis (APB) muscle in response to dominant M1 stimulation using a figure-of-eight coil (MCF-B65) with MagPro X100 magnetic stimulator (MagVenture, Inc., Farum, Denmark). The TMS preparations started with supporting the subject's neck using an airplane pillow and putting a tight swimmer's cap over the head to mark coil location and angulation. The exact coil orientation for APB stimulation was adjusted individually. Two surface electrodes in a tendon-belly construction were applied over the nondominant APB muscle for MEP recordings. Subjects were instructed to recline comfortably, keep eyes open, lean head back, and report any pain or discomfort on the head or muscle twitches in the hand. Participants were instructed to keep their hand relaxed throughout the experiment. We used the MEB-9400 electromyography (EMG)/evoked potentials (EP) system (Nihon Kohden, Tokyo, Japan) to record and analyze the waveforms with a bandpass filter at 5 Hz–10 kHz.
The measures of corticospinal excitability were defined as follows:
(1) Resting motor threshold was defined as the lowest stimulus intensity able to elicit MEPs at amplitudes of 50 μV in 5 of 10 consecutive stimuli when the muscle is at rest. The coil was placed above the contralateral M1 to the examined hand with the coil oriented at 45° towards the contralateral forehead. The coil was moved to determine the spot with maximal MEP amplitude.58 The TMS intensity was reported as the percentage of the maximal TMS machine output. Starting at 30% stimulus intensity, the intensity was increased incrementally until every stimulus resulted in a consistent MEP. Stimuli were given at interstimulus interval ±8 seconds to avoid facilitation.
(2) The MEPs were measured at 20% above the individual rMT in 8 to 10 successful (the response ≥50 μV) single trials. The MEPs were quantified as amplitude (mV) of the peak response, the onset of the MEP (MEP latency), and its duration from onset of the response to its return to the baseline (MEP duration). Motor-evoked potential duration was highly correlated with short-interval intracortical inhibition (SICI)68; therefore, we decided to use this measure, although it is not commonly used.
(3) The CSPs were measured in 6 to 8 single trials per stimulation intensity—at 100%, 120%, and 140% of the rMT, the stimuli were delivered while the subject performed a tonic voluntary contraction of nondominant APB muscle at 50% of maximal force. Data were subsequently averaged to provide one mean value per variable per measure or condition. The duration of the CSP was taken from the end of the MEP to the latency at which the EMG activity returned to its mean prestimulus level.
2.2. Psychophysics
(1) Heat pain thresholds were assessed by the method of limits.74 The TSA thermode was attached to the volar aspect of the nondominant forearm. Starting at a baseline temperature of 32°C, the thermode warmed at a rate of 1.5°C/s until pain sensation was perceived. This was repeated 3 times, and results were averaged to obtain a heat pain threshold value.
(2) Conditioned pain modulation was assessed using the parallel paradigm. The test-stimulus was a tonic noxious contact heat stimulus applied to the volar aspect of the dominant forearm using TSA. The intensity of the test-stimulus was predetermined individually based on the psychophysical parameter of Pain60 temperature.23 This method is based on delivery of several triplets of 7-second-long stimuli of various intensities; the closest temperature that induced pain at a level of 60 on a 0 (no pain) to 100 (the most imaginable pain) through the numerical pain score (NPS) was considered as the Pain60 temperature. For each stimulus, the baseline temperature was set at 32°C, which increased at a rate of 2°C/s to the destination temperature. The test-stimulus was applied for 30 seconds and decreased back to baseline at the same rate. Subjects rated the intensity of test-stimulus at 10th, 20th, and 30th second along stimulus duration. The mean pain score served as the pain level of “test-stimulus.” After a 15-minute break, subjects immersed their nondominant hand up to the wrist into a hot water bath at 46.5°C (Heto CBN 8-30 Lab equipment, Heto‐Holten A/S, Allerod, Denmark), for 1 minute (a conditioning stimulus). During the first 30 seconds of immersion, the conditioning stimulus was applied stand alone; subjects rated the pain intensity every 10 seconds; the mean score of the 3 pain ratings served the conditioning stimulus pain level. During the last 30 seconds of the conditioning stimulus, an identical test-stimulus was repeated, and pain of the test-stimulus was rated again every 10 seconds. The CPM effect was calculated as the difference between 2 test-stimuli: one applied under dual-stimulation vs the test-stimulus given stand alone. More negative values indicated more efficient CPM.23,26,75,77
(3) Electrical temporal summation (eTS) was measured by delivering electrical stimuli with a constant current stimulator (Digitimer DS5; Digitimer Ltd, WelWyn Garden City, England); 2 bipolar Ag/AgCl-electrodes were attached to the skin overlying the belly of the nondominant brachioradialis muscle. Square-wave 2-ms-long pulses were given. First, electrical pain threshold (ePT) was determined through the continued increase of stimulation intensity step of 1 mA, starting at 3 mA, until the participant indicates pain sensation. The eTS assessment was performed with stimulation intensity at 30% above the individual ePT. Ten repetitive stimuli were delivered with interstimulus interval of 1 second. The numerical pain score was obtained after first application and after the last of the ten stimuli. eTS magnitude was calculated as absolute difference between last and first pain scores. This stimulation protocol was recently published by our laboratory.36
2.3. Statistical analysis
Data were processed and analyzed by Excel (Microsoft Corp, Redmond, WA) and JMP (SAS Institute, Cary, NC) software.
Resting motor threshold; MEP amplitude, latency, and duration along with the CSP onset latency; and duration data were subjected to correlation analysis with the pain modulation measures (CPM and TS) and pain thresholds. No formal power or sample size analysis was performed before the study. A post hoc power analysis was performed to determine the minimum correlation, which would be regarded as significant based on the number of cases in the analysis, power of 0.80, and alpha = 0.05 Bonferroni-adjusted for 28 comparisons. The range of correlations is reported for the maximum and minimum number of cases actually included in correlation analyses.
All data are presented in mean ± SD unless indicated otherwise.
3. Results
3.1. The description of demographic characteristics, and psychophysical and neurophysiological responses
The mean age of the participants was 26.2 ± 4.2 years. Because we did not detect a significant correlation between subject age and any of psychophysical or neurophysiological variables, nor find any significant sex effect of any of the tested variables, we decided to exclude age and sex from further analyses. Post hoc analysis of the correlation values indicated that the largest analysis of 41 cases would find significance with r of at least ±0.57, and the analysis of 29 cases would find significance for r of at least ±0.66.
Thirty-nine of 41 subjects were right-handed.
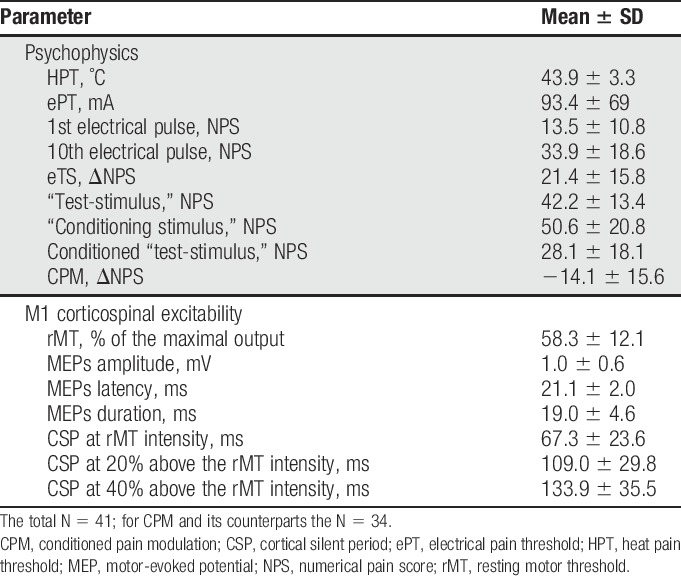
The descriptive values for psychophysical and neurophysiological responses are presented in Table 1. Please note that the data on CPM or its counterparts (“test,” “conditioning,” and “conditioned” stimuli) are based on responses from 34 subjects, as 7 participants had a mean “test” pain score below 20 NPS. Based on previous experience of our and other laboratories, the pain scores <20 are considered as too mild a pain experience.1,23,79 Using this cutoff, we aimed to eliminate the possible floor effect on the test-stimulus pain scores.29
Table 1.
Psychophysical and neurophysiological characteristics of the study group.
3.2. The relationship between corticospinal excitability and psychophysics
Among all psychophysical parameters, after correction for the multiply comparisons, the measures of corticospinal excitability significantly correlated with the extent of CPM (n = 34) (Table 2). More specifically, efficient CPM (negative values) was associated with higher MEP amplitudes and longer duration of MEPs. By contrast, high TS magnitude (positive values) was associated with shorter MEP duration; however, the statistical significance of this correlation was lost after multiplicity adjustment. The correlation plot of MEP duration and CPM/eTS is presented in Figure 1.
Table 2.
Correlations between psychophysical and M1 corticospinal excitability parameters.
Figure 1.
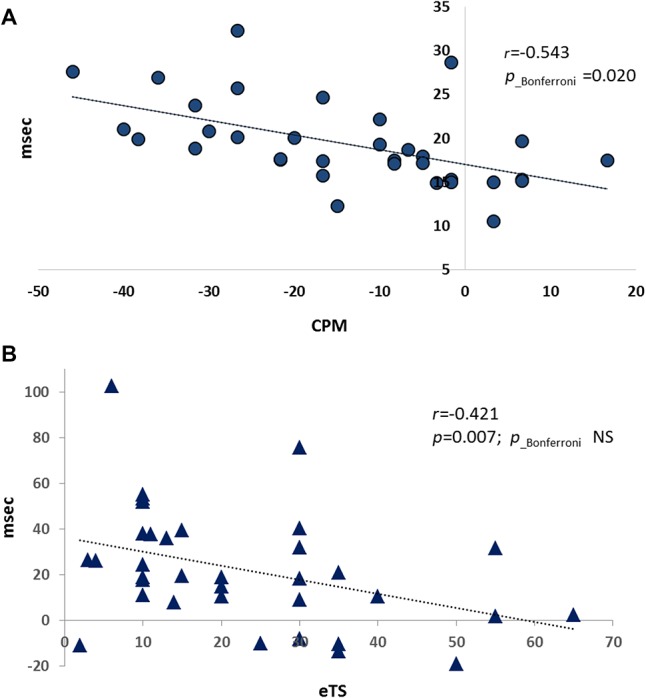
The MEP duration positively correlates with the CPM efficiency (A) and negatively correlates with the TS magnitude (B). CPM, conditioned pain modulation; MEP, motor-evoked potential; TS, temporal summation.
4. Discussion
The results of our study demonstrate association between antinociceptive pattern of pain modulation as reflected by the correlations between efficient CPM and lower TS magnitude, on one hand, and higher excitability at the motor pathways, reflected by higher MEPs amplitude and its longer duration. This association was stronger for the CPM. This is the first study that tested the relationship between corticospinal excitability and parameters of pain modulation in healthy subjects. Our findings thus suggest that the MEP characteristics can serve a neurophysiological counterpart of the inhibitory facet of the individual PMP responses with a potential to be explored in clinical setups.
TMS can excite deep gray matter neurons either directly or indirectly through volleys from superficial neurons.5 Primary motor cortex stimulation evokes indirect excitation of pyramidal neurons through local interneurons with higher probability than direct excitation.2 These activation of the corticospinal pathway can elicit MEPs in the muscles contralateral to the muscle cortical representation in M1.28 Motor-evoked potentials are used routinely in research and under several clinical settings in evaluating motor cortex excitability. This evaluation should however differentiate between the indices of the overall corticospinal excitability and indices specific to the excitability of the motor cortex (cortical excitability). EMG in general and MEPs in particular are affected by a combination of cortical, subcortical, and spinal cord mechanisms, which usually coincide in time, making their separation almost impossible.
It is widely accepted that experimental pain has inhibitory influence on M1 excitability in healthy subjects. Most studies reported reduced MEP amplitudes in response to various pain modalities such as capsaicin cream application,12 noxious heat,19,44 or acute muscle pain.9,38 In line, activation of the motor cortex induced by physical exercise or rTMS/cathodal (inhibitory) transcranial direct current stimulation (tDCS) has inhibitory effect on experimental pain as reflected by increased pain thresholds48,49 and reduced pain perception.20,25 Thus, we can relate the higher level of corticospinal excitability in healthy state to one of the neurophysiological correlates of antinociception.
In chronic pain states, enhanced M1 excitability loses its pain inhibitory functions, probably due to the pain-related cortical reorganization.59 M1 excitability in chronic states and syndromes such as neuropathic pain, myofascial pain syndrome (MPS), and fibromyalgia is characterized by reduced CSP, lower SICI, and enhanced intracortical facilitation (ICF) and MEPs.10,11,54,60–62,66,70 In contrast to the normal state, M1 excitability in pain patients is inversely correlated with inhibitory pain modulation as was demonstrated by higher MEPs observed in those patients with MPS who failed to induce efficient CPM response.7 Increased MEPs in MPS were also observed after exposure to experimental pain further pointing to the disinhibition of corticospinal system.70 Furthermore, the pain-relieving effect of M1-directed rTMS treatment is often parallel with activation of inhibitory mechanisms of homeostatic plasticity (normalization) of the motor cortex excitability.8,14,40,41 Importantly, high-frequency rTMS has different impact on the motor cortex excitability in healthy subjects vs chronic pain patients, probably due to self-limiting hyperexcitability capacity. It increases MEPs in controls but decreased it in migraine patients with aura,8 prolongs SICI in neuropathic pain,40,41 reduces ICF, and enhances MEPs in patients with MPS.15 Harmonizing with abnormal M1 excitability, many chronic pain syndromes are characterized by less-efficient CPM and/or high TS and therefore can be anchored to the pronociceptive edge of the nociception spectrum.42,52,78 We believe therefore that the strong correlation between MEPs and CPM efficiency reported in our study may carefully suggest the additive value of motor corticospinal excitability for comprehensive assessment of individual PMP.
The neurophysiological basis of our findings requires explanation. Beside the expected correlation with the amplitude, CPM efficiency was significantly associated with MEP duration. There are a number of physiological processes which are likely to be involved in the increase in MEP duration, and they are likely to operate at different levels of the neuraxis. Single TMS pulse applied to M1 gives rise to a series of descending corticospinal volleys.18,27 The preferred diagnostic coil is circular; however, we used a figure-of-eight coil, which is more focal and demands exact coil placement over the stimulated motor cortex area of the specific muscle,27 as was performed in this study. At spinal level, these volleys activate motoneurons at slightly different latencies, dependent on their thresholds. This asynchronization results in MEPs with prolonged duration and lower amplitude.57 In case of higher neural excitability at the cortical or spinal level, more motor neurons will exceed the threshold resulting in enhanced MEP amplitudes and longer duration, without a change in stimulus intensity. This implies that the stimulus–response curve, reflecting the relation between stimulus intensity and MEP amplitude and duration, is subject to dynamic changes that relate to the present physiological state of the motor system.56,64 Furthermore, there are reports of a significant inverse relationship between SICI and facilitated MEP duration, suggesting a contribution of cortical processes in prolongation of facilitated MEP duration.68 Correspondingly, the lack of correlation between SICI and the facilitated MEP amplitude is consistent with existing evidence that facilitation of MEP amplitude during a tonic voluntary contraction is primarily spinal.17,30 It is therefore possible that longer MEPs may represent more indirect volleys contribution to the final MEP, which, in turn, could constitute a marker of more effective intrinsic pain inhibitory top-down mechanisms.
On the biochemical level, MEPs represent the net facilitatory effect of a TMS pulses that engage the excitation of the motor cortex mediated by 4 major neuromodulatory neurotransmitter systems; glutamate, acetylcholine, dopamine, and noradrenaline.32,34,35,39,47 We may hypothesize therefore that higher MEPs reflect higher level of corticocortical and corticospinal glutamatergic, cholinergic, dopaminergic, and noradrenergic neurotransmission. In line, the activity of these neuropharmacological systems in the motor cortex may be involved in its connectivity with cortical and subcortical areas associated with pain processing and inhibitory pain modulation. Indeed, the mechanism of the rTMS-evoked analgesia implies rapid and phasic activation in the lateral thalamus, which leads to a cascade of synaptic events influencing activity in the medial thalamus and in the brain structures involved in descending pain inhibition such as perigenual anterior cingulate cortex, orbitofrontal cortex, and periaqueductal gray.21,22,46 In addition to the established involvement of opioid neurotransmission, cellular mechanisms of the rTMS-M1 analgesic effect, at least partially, are mediated by the glutamate13 and may involve other neurotransmitters46 Thus, we can assume that intracortical neuropharmacological systems might indirectly contribute to the efficiency of antinociception.
Among the parameters of the motor corticospinal excitability applied in this study, we explored the CSP—the arrest of motor cortex activity due to single suprathreshold TMS pulse given during muscle contraction. Its length is believed to reflect the activity of inhibitory interneurons,14 mediated by GABA-B receptors72 and cholinergic neurotransmission31,32; its shortening indicates deficient GABA-B–mediated intracortical inhibition reported also in pain patients. Our results did not reveal substantial relationship between the CSP prolongation at any stimulation intensity, and for any pain psychophysics parameter. This may indicate lower relevance of motor corticospinal GABAergic transmission to processing of the experimental pain.
Our study has several limitations. The main limitation is the restriction of evaluation of motor corticospinal excitability. This restricted assessment of motor cortex excitability is related to the fact that the TMS system we used could not perform a paired-pulse stimulation required for the assessment of SICI and ICF—the most commonly applied methods for the neurophysiological assessment of primary motor cortex. The main findings of our study refer to the measures of MEPs; however, beyond intracortical processes, this response magnitude depends also on the corticospinal transmission and, therefore, could be affected by individual functioning of the motor system. We believe that this factor has minimal or no influence on our results, as our participants were young healthy subjects with no suspect of any neurological disease. Another study limitation is that the researcher who performed offline excitability data extraction was not blinded to the results of psychophysical assessment.
To conclude, the results of our study demonstrated a relationship between the neurophysiological and psychophysical tests of pain modulation; higher motor corticospinal excitability as reflected by larger MEP amplitudes and duration was associated with antinociceptive pattern of pain modulation. The described relationship may advance the development of comprehensive neurophysiology-based measures for pain modulation. We believe that objectification of pain modulation assessment will contribute to its use in the clinical setting.
Disclosures
The authors have no conflict of interest to declare.
Acknowledgment
The authors thank the Lester Aronberg Foundation and the Office of the Executive Vice President for Research, Technion, for their support of the performance of this study.
Author's contribution: Y. Granovsky contributed to logistics of this study and the data collection, data analysis, and the manuscript preparation. E. Sprecher contributed to the statistical analysis. A. Sinai contributed to the data collection and the manuscript preparation. All the authors approved the final draft of the manuscript.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
References
- [1].Albu S, Gómez-Soriano J, Avila-Martin G, Taylor J. Deficient conditioned pain modulation after spinal cord injury correlates with clinical spontaneous pain measures. PAIN 2015;156:260–72. [DOI] [PubMed] [Google Scholar]
- [2].Amassian VE, Cracco RQ. Human cerebral cortical responses to contralateral transcranial stimulation. Neurosurgery 1987;20:148–55. [PubMed] [Google Scholar]
- [3].Arendt-Nielsen L. Central sensitization in humans: assessment and pharmacology. Handb Exp Pharmacol 2015;227:79–102. [DOI] [PubMed] [Google Scholar]
- [4].Arendt-Nielsen L, Yarnitsky D. Experimental and clinical applications of quantitative sensory testing applied to skin, muscles and viscera. J Pain 2009;10:556–72. [DOI] [PubMed] [Google Scholar]
- [5].Barker AT, Jalinous R, Freeston IL. Non-invasive magnetic stimulation of human motor cortex. Lancet 1985;1:1106–7. [DOI] [PubMed] [Google Scholar]
- [6].Bogdanov VB, Viganò A, Noirhomme Q, Bogdanova OV, Guy N, Laureys S, Renshaw PF, Dallel R, Phillips C, Schoenen J. Cerebral responses and role of the prefrontal cortex in conditioned pain modulation: an fMRI study in healthy subjects. Behav Brain Res 2015;281:87–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Botelho LM, Morales-Quezada L, Rozisky JR, Brietzke AP, Torres IL, Deitos A, Fregni F, Caumo W. A framework for understanding the relationship between descending pain modulation, motor corticospinal, and neuroplasticity regulation systems in chronic myofascial pain. Front Hum Neurosci 2016;10:308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Brighina F, Cosentino G, Vigneri S, Talamanca S, Palermo A, Giglia G, Fierro B. Abnormal facilitatory mechanisms in motor cortex of migraine with aura. Eur J Pain 2011;15:928–35. [DOI] [PubMed] [Google Scholar]
- [9].Burns E, Chipchase LS, Schabrun SM. Primary sensory and motor cortex function in response to acute muscle pain: a systematic review and meta-analysis. Eur J Pain 2016;20:1203–13. [DOI] [PubMed] [Google Scholar]
- [10].Caumo W, Deitos A, Carvalho S, Leite J, Carvalho F, Dussán-Sarria JA, Lopes Tarragó MdaG, Souza A, Torres IL, Fregni F. Motor cortex excitability and BDNF levels in chronic musculoskeletal pain according to structural pathology. Front Hum Neurosci 2016;10:357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Chen R, Corwell B, Yaseen Z, Hallett M, Cohen LG. Mechanisms of cortical reorganization in lower-limb amputees. J Neurosci 1998;18:3443–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Cheong JY, Yoon TS, Lee SJ. Evaluations of inhibitory effect on the motor cortex by cutaneous pain via application of capsaicin. Electromyogr Clin Neurophysiol 2003;43:203–10. [PubMed] [Google Scholar]
- [13].Ciampi de Andrade D, Mhalla A, Adam F, Texeira MJ, Bouhassira D. Repetitive transcranial magnetic stimulation induced analgesia depends on N-methyl-D-aspartate glutamate receptors. PAIN 2014;155:598–605. [DOI] [PubMed] [Google Scholar]
- [14].Currà A, Modugno N, Inghilleri M, Manfredi M, Hallett M, Berardelli A. Transcranial magnetic stimulation techniques in clinical investigation. Neurology 2002; 59:1851–9. Review. [DOI] [PubMed] [Google Scholar]
- [15].Dall'Agnol L, Medeiros LF, Torres IL, Deitos A, Brietzke A, Laste G, de Souza A, Vieira JL, Fregni F, Caumo W. Repetitive transcranial magnetic stimulation increases the corticospinal inhibition and the brain-derived neurotrophic factor in chronic myofascial pain syndrome: an explanatory double-blinded, randomized, sham-controlled trial. J Pain 2014;15:845–55. [DOI] [PubMed] [Google Scholar]
- [16].Defrin R, Givon R, Raz N, Urca G. Spatial summation and spatial discrimination of pain sensation. PAIN 2006;126:123–31. [DOI] [PubMed] [Google Scholar]
- [17].Di Lazzaro V, Oliviero A, Profice P, Saturno E, Pilato F, Insola A, Mazzone P, Tonali P, Rothwell JC. Comparison of descending volleys evoked by transcranial magnetic and electric stimulation in conscious humans. Electroencephalogr Clin Neurophysiol 1998;109:397–401. [DOI] [PubMed] [Google Scholar]
- [18].Di Lazzaro V, Oliviero A, Pilato F, Saturno E, Dileone M, Mazzone P, Insola A, Tonali PA, Rothwell JC. The physiological basis of transcranial motor cortex stimulation in conscious humans. Clin Neurophysiol 2004;115:255–66. [DOI] [PubMed] [Google Scholar]
- [19].Dubé JA, Mercier C. Effect of pain and pain expectation on primary motor cortex excitability. Clin Neurophysiol 2011;122:2318–23. [DOI] [PubMed] [Google Scholar]
- [20].Fierro B, De Tommaso M, Giglia F, Giglia G, Palermo A, Brighina F. Repetitive transcranial magnetic stimulation (rTMS) of the dorsolateral prefrontal cortex (DLPFC) during capsaicin-induced pain: modulatory effects on motor cortex excitability. Exp Brain Res 2010;203:31–8. [DOI] [PubMed] [Google Scholar]
- [21].García-Larrea L, Peyron R, Mertens P, Gregoire MC, Lavenne F, Le Bars D, Convers P, Mauguière F, Sindou M, Laurent B. Electrical stimulation of motor cortex for pain control: a combined PET-scan and electrophysiological study. PAIN 1999;83:259–73. [DOI] [PubMed] [Google Scholar]
- [22].García-Larrea L, Peyron R. Motor cortex stimulation for neuropathic pain: from phenomenology to mechanisms. Neuroimage 2007;37(suppl 1):S71–79. [DOI] [PubMed] [Google Scholar]
- [23].Granot M, Weissman-Fogel I, Crispel Y, Pud D, Granovsky Y, Sprecher E, Yarnitsky D. Determinants of endogenous analgesia magnitude in a diffuse noxious inhibitory control (DNIC) paradigm: do conditioning stimulus painfulness, gender and personality variables matter? PAIN. 2008;136(1-2):142–9. [DOI] [PubMed] [Google Scholar]
- [24].Granovsky Y, Yarnitsky D. Personalized pain medicine: the clinical value of psychophysical assessment of pain modulation profile. Rambam Maimonides Med J 2013;4:e0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Granovsky Y, Liem KS, Weissman-Fogel I, Yarnitsky D, Chistyakov A, Sinai A. “Virtual lesion” in pain research; a study on magnetic stimulation of the primary motor cortex. Eur J Pain 2016; 20:241–9. [DOI] [PubMed] [Google Scholar]
- [26].Granovsky Y, Shor M, Shifrin A, Sprecher E, Yarnitsky D, Bar-Shalita T. Assessment of responsiveness to everyday non-noxious stimuli in pain-free migraineurs with versus without aura. J Pain 2018;19:943–51. [DOI] [PubMed] [Google Scholar]
- [27].Groppa S, Oliviero A, Eisen A, Quartarone A, Cohen LG, Mall V, Insola A, Ranieri F, Meglio M, Tonali PA, Rothwell JC. A practical guide to diagnostic transcranial magnetic stimulation: report of an IFCN committee. Clin Neurophysiol 2012;123:858–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Hallett M. Transcranial magnetic stimulation and the human brain. Nature 2000;406:147–50. [DOI] [PubMed] [Google Scholar]
- [29].Honigman L, Bar-Bachar O, Yarnitsky D, Sprecher E, Granovsky Y. Nonpainful wide-area compression inhibits experimental pain. PAIN 2016;157:2000–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kaneko K, Kawai S, Fuchigami Y, Shiraishi G, Ito T. Effect of stimulus intensity and voluntary contraction on corticospinal potentials following transcranial magnetic stimulation. J Neurol Sci 1996;139:131–6. [PubMed] [Google Scholar]
- [31].Korchounov A, Ilic TV, Schwinge T, Ziemann U. Modification of motor cortical excitability by an acetylcholinesterase inhibitor. Exp Brain Res 2005;164:399–405. [DOI] [PubMed] [Google Scholar]
- [32].Korchounov A, Ziemann U. Neuromodulatory neurotransmitters influence LTP-like plasticity in human cortex: a pharmaco-TMS study. Neuropsychopharmacology 2011;36:1894–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Kosek E, Ordeberg G. Abnormalities of somatosensory perception in patients with painful osteoarthritis normalize following successful treatment. Eur J Pain 2000;4:229–38. [DOI] [PubMed] [Google Scholar]
- [34].Kuo MF, Grosch J, Fregni F, Paulus W, Nitsche MA. Focusing effect of acetylcholine on neuroplasticity in the human motor cortex. J Neurosci 2007;27:14442–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Kuo MF, Paulus W, Nitsche MA. Boosting focally-induced brain plasticity by dopamine. Cereb Cortex 2008, 18; :648–51. [DOI] [PubMed] [Google Scholar]
- [36].Kuperman P, Granovsky Y, Granot M, Bahouth H, Fadel S, Hyams G, Ben Lulu H, Aspis O, Salame R, Begal J, Hochstein D, Grunner S, Honigman L, Reshef M, Sprecher E, Bosak N, Sterling M, Yarnitsky D. Psychophysic-psychological dichotomy in very early acute mTBI pain: a prospective study. Neurology 2018;91:e931–e38. [DOI] [PubMed] [Google Scholar]
- [37].Kurata J. Neural mechanisms of offset analgesia. Adv Exp Med Biol 2018;1099:141–6. [DOI] [PubMed] [Google Scholar]
- [38].Le Pera D, Graven-Nielsen T, Valeriani M, Oliviero A, Di Lazzaro V, Tonali PA, Arendt-Nielsen L. Inhibition of motor system excitability at cortical and spinal level by tonic muscle pain. Clin Neurophysiol 2001;112:1633–41. [DOI] [PubMed] [Google Scholar]
- [39].Lefaucheur JP. Motor cortex dysfunction revealed by cortical excitability studies in Parkinson's disease: influence of antiparkinsonian treatment and cortical stimulation. Clin Neurophysiol 2005;116:244–53. [DOI] [PubMed] [Google Scholar]
- [40].Lefaucheur JP, Drouot X, Ménard-Lefaucheur I, Keravel Y, Nguyen JP. Motor cortex rTMS restores defective intracortical inhibition in chronic neuropathic pain. Neurology 2006;67:1568–74. [DOI] [PubMed] [Google Scholar]
- [41].Lefaucheur JP, Ayache SS, Sorel M, Farhat WH, Zouari HG, Ciampi de Andrade D, Ahdab R, Ménard-Lefaucheur I, Brugières P, Goujon C. Analgesic effects of repetitive transcranial magnetic stimulation of the motor cortex in neuropathic pain: influence of theta burst stimulation priming. Eur J Pain 2012;16:1403–13. [DOI] [PubMed] [Google Scholar]
- [42].Lewis GN, Rice DA, McNair PJ. Conditioned pain modulation in populations with chronic pain: a systematic review and meta-analysis. J Pain 2012;13:936–44. [DOI] [PubMed] [Google Scholar]
- [43].Maarrawi J, Peyron R, Mertens P, Costes N, Magnin M, Sindou M, Laurent B, Garcia-Larrea L. Brain opioid receptor density predicts motor cortex stimulation efficacy for chronic pain. PAIN 2013;154:2563–8. [DOI] [PubMed] [Google Scholar]
- [44].Mercier C, Gagné M, Reilly KT, Bouyer LJ. Effect of experimental cutaneous hand pain on corticospinal excitability and short afferent inhibition. Brain Sci 2016;6:E45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Moana-Filho EJ, Herrero Babiloni A, Theis-Mahon NR. Endogenous pain modulation in chronic orofacial pain: a systematic review and meta-analysis. PAIN 2018;159:1441–55. [DOI] [PubMed] [Google Scholar]
- [46].Moisset X, de Andrade DC, Bouhassira D. From pulses to pain relief: an update on the mechanisms of rTMS-induced analgesic effects. Eur J Pain 2016;20:689–700. [DOI] [PubMed] [Google Scholar]
- [47].Molina-Luna K, Pekanovic A, Röhrich S, Hertler B, Schubring-Giese M, Rioult-Pedotti MS, Luft AR. Dopamine in motor cortex is necessary for skill learning and synaptic plasticity. PLoS One 2009;4:e7082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Moloney TM, Witney AG. Transcranial direct current stimulation (tDCS) priming of 1Hz repetitive transcranial magnetic stimulation (rTMS) modulates experimental pain thresholds. Neurosci Lett 2013;534:289–94. [DOI] [PubMed] [Google Scholar]
- [49].Moloney TM, Witney AG. Pressure pain thresholds increase after preconditioning 1 Hz repetitive transcranial magnetic stimulation with transcranial direct current stimulation. PLoS One 2014;9:e92540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Nahman-Averbuch H, Martucci KT, Granovsky Y, Weissman-Fogel I, Yarnitsky D, Coghill RC. Distinct brain mechanisms support spatial vs temporal filtering of nociceptive information. PAIN 2014;155:2491–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Niesters M, Proto PL, Aarts L, Sarton EY, Drewes AM, Dahan A. Tapentadol potentiates descending pain inhibition in chronic pain patients with diabetic polyneuropathy. Br J Anaesth 2014;113:148–56. [DOI] [PubMed] [Google Scholar]
- [52].O'Brien AT, Deitos A, Triñanes Pego Y, Fregni F, Carrillo-de-la-Peña MT. Defective endogenous pain modulation in fibromyalgia: a meta-analysis of temporal summation and conditioned pain modulation paradigms. J Pain 2018;19:819–36. [DOI] [PubMed] [Google Scholar]
- [53].Pagano RL, Fonoff ET, Dale CS, Ballester G, Teixeira MJ, Britto LR. Motor cortex stimulation inhibits thalamic sensory neurons and enhances activity of PAG neurons: possible pathways for antinociception. PAIN 2012;153:2359–69. [DOI] [PubMed] [Google Scholar]
- [54].Parker RS, Lewis GN, Rice DA, McNair PJ. Is motor cortical excitability altered in people with chronic pain? A systematic review and meta-analysis. Brain Stimul 2016;9:488–500. [DOI] [PubMed] [Google Scholar]
- [55].Piché M, Arsenault M, Rainville P. Cerebral and cerebrospinal processes underlying counterirritation analgesia. J Neurosci 2009;29:14236–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Rossini PM, Zarola F, Stalberg E, Caramia M. Pre-movement facilitation of motor-evoked potentials in man during transcranial stimulation of the central motor pathways. Brain Res 1988;458:20–30. [DOI] [PubMed] [Google Scholar]
- [57].Rossini PM, Caramia MD, Iani C, Desiato MT, Sciarretta G, Bernardi G. Magnetic transcranial stimulation in healthy humans: influence on the behavior of upper limb motor units. Brain Res 1995;676:314–24. [DOI] [PubMed] [Google Scholar]
- [58].Rossini PM, Burke D, Chen R, Cohen LG, Daskalakis Z, Di Iorio R, Di Lazzaro V, Ferreri F, Fitzgerald PB, George MS, Hallett M, Lefaucheur JP, Langguth B, Matsumoto H, Miniussi C, Nitsche MA, Pascual-Leone A, Paulus W, Rossi S, Rothwell JC, Siebner HR, Ugawa Y, Walsh V, Ziemann U. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee. Clin Neurophysiol 2015;126:1071–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Schabrun SM, Christensen SW, Mrachacz-Kersting N, Graven-Nielsen T. Motor cortex reorganization and impaired function in the transition to sustained muscle pain. Cereb Cortex 2016;26:1878–90. [DOI] [PubMed] [Google Scholar]
- [60].Schwenkreis P, Witscher K, Janssen F, Dertwinkel R, Zenz M, Malin JP, Tegenthoff M. Changes of cortical excitability in patients with upper limb amputation. Neurosci Lett 2000;293:143–6. [DOI] [PubMed] [Google Scholar]
- [61].Schwenkreis P, Janssen F, Rommel O, Pleger B, Völker B, Hosbach I, Dertwinkel R, Maier C, Tegenthoff M. Bilateral motor cortex disinhibition in complex regional pain syndrome (CRPS) type I of the hand. Neurology 2003;61:515–9. [DOI] [PubMed] [Google Scholar]
- [62].Schwenkreis P, Scherens A, Rönnau AK, Höffken O, Tegenthoff M, Maier C. Cortical disinhibition occurs in chronic neuropathic, but not in chronic nociceptive pain. BMC Neurosci 2010;11:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Sprenger C, Bingel U, Büchel C. Treating pain with pain: supraspinal mechanisms of endogenous analgesia elicited by heterotopic noxious conditioning stimulation. PAIN 2011;152:428–39. [DOI] [PubMed] [Google Scholar]
- [64].Starr A, Caramia M, Zarola F, Rossini PM. Enhancement of motor cortical excitability in humans by non-invasivenelectrical stimulation appears prior to voluntary movement. Electroencephalogr Clin Neurophysiol 1988;70:26–32. [DOI] [PubMed] [Google Scholar]
- [65].Staud R. Abnormal endogenous pain modulation is a shared characteristic of many chronic pain conditions. Expert Rev Neurother 2012;12:577–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Turgut N, Altun BU. Cortical disinhibition in diabetic patients with neuropathic pain. Acta Neurol Scand 2009;120:383–8. [DOI] [PubMed] [Google Scholar]
- [67].Vaegter HB, Handberg G, Emmeluth C, Graven-Nielsen T. Preoperative hypoalgesia after cold pressor test and aerobic exercise is associated with pain relief 6 Months after total knee replacement. Clin J Pain 2017;33:475–84. [DOI] [PubMed] [Google Scholar]
- [68].van den Bos MA, Geevasinga N, Menon P, Burke D, Kiernan MC, Vucic S. Physiological processes influencing motor-evoked potential duration with voluntary contraction. J Neurophysiol 2017;117:1156–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Van Oosterwijck J, Nijs J, Meeus M, Paul L. Evidence for central sensitization in chronic whiplash: a systematic literature review. Eur J Pain 2013;17:299–312. [DOI] [PubMed] [Google Scholar]
- [70].Vidor LP, Torres IL, Medeiros LF, Dussán-Sarria JA, Dall'agnol L, Deitos A, Brietzke A, Laste G, Rozisky JR, Fregni F, Caumo W. Association of anxiety with intracortical inhibition and descending pain modulation in chronic myofascial pain syndrome. BMC Neurosci 2014. ;15:42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Weissman-Fogel I, Granovsky Y, Crispel Y, Ben-Nun A, Best LA, Yarnitsky D, Granot M. Enhanced presurgical pain temporal summation response predicts post-thoracotomy pain intensity during the acute postoperative phase. J Pain 2009;10:628–36. [DOI] [PubMed] [Google Scholar]
- [72].Werhahn KJ, Kunesch E, Noachtar S, Benecke R, Classen J. Differential effects on motorcortical inhibition induced by blockade of GABA uptake in humans. J Physiol 1999;517:591–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Woolf CJ, Thompson SW. The induction and maintenance of central sensitization is dependent on N-methyl-D-aspartic acid receptor activation; implications for the treatment of post-injury pain hypersensitivity states. PAIN 1991;44:293–9. [DOI] [PubMed] [Google Scholar]
- [74].Yarnitsky D. Quantitative sensory testing. Muscle Nerve 1997;20:198–204. Review. [DOI] [PubMed] [Google Scholar]
- [75].Yarnitsky D, Crispel Y, Eisenberg E, Granovsky Y, Ben-Nun A, Sprecher E, Best LA, Granot M. Prediction of chronic post-operative pain: pre-operative DNIC testing identifies patients at risk. PAIN 2008;138:22–8. [DOI] [PubMed] [Google Scholar]
- [76].Yarnitsky D, Arendt-Nielsen L, Bouhassira D, Edwards RR, Fillingim RB, Granot M, Hansson P, Lautenbacher S, Marchand S, Wilder-Smith O. Recommendations on terminology and practice of psychophysical DNIC testing. Eur J Pain 2010;14:339. [DOI] [PubMed] [Google Scholar]
- [77].Yarnitsky D, Granot M, Nahman-Averbuch H, Khamaisi M, Granovsky Y. Conditioned pain modulation predicts duloxetine efficacy in painful diabetic neuropathy. PAIN 2012;153:1193–8. [DOI] [PubMed] [Google Scholar]
- [78].Yarnitsky D, Granot M, Granovsky Y. Pain modulation profile and pain therapy: between pro- and antinociception. PAIN 2014;155:663–5. [DOI] [PubMed] [Google Scholar]
- [79].Yarnitsky D, Bouhassira D, Drewes AM, Fillingim RB, Granot M, Hansson P, Landau R, Marchand S, Matre D, Nilsen KB, Stubhaug A, Treede RD, Wilder-Smith OH. Recommendations on practice of conditioned pain modulation (CPM) testing. Eur J Pain 2015;19:805–6. [DOI] [PubMed] [Google Scholar]
- [80].Yilmaz P, Diers M, Diener S, Rance M, Wessa M, Flor H. Brain correlates of stress-induced analgesia. PAIN 2010;151:522–9. [DOI] [PubMed] [Google Scholar]