Supplemental Digital Content is Available in the Text.
Keywords: Vulvodynia, Dyspareunia, Pain measurement, Neuropathic and nociceptive pain
Abstract
Objectives:
To evaluate self-reported sensory pain scores of women with generalized vulvodynia (GV) and provoked vestibulodynia (PVD), characterize pain phenotypes, and assess feasibility of using the Internet for recruitment and data collection among women with vulvodynia.
Methods:
Descriptive online survey. Data collected using an online survey accessed via a link on the National Vulvodynia Association web site. Convenience sample, 60 women aged 18 to 45 years (mean = 32.7 ± 5.5); 50 white, 2 black/African American, 4 Hispanic/Latino, and 4 Native American/Alaskan Native, diagnosed with vulvodynia, not in menopause. Pain assessment and medication modules from PAINReportlt.
Results:
Women with GV (n = 35) compared to PVD (n = 25). Estimated mean pain sites (2.5 ± 1.4 vs 2.2 ± 1.0, P = 0.31), mean current pain (8.7 ± 1.4 vs 5.5 ± 4.0, P = 0.0008), worst pain (8.1 ± 1.8 vs 6.1 ± 3.6, P = 0.02), and least pain in the past 24 hours (4.4 ± 1.8 vs 2.0 ± 2.0, P < 0.0001). Average pain intensity (7.1 ± 1.2 vs 4.6 ± 2.9, P = 0.0003) on a scale of 0 to 10, mean number of neuropathic words (8.3 ± 3.6 vs 7.7 ± 5.0), and mean number of nociceptive words (6.9 ± 4 vs 7.5 ± 4.4). Nineteen (54%) women with GV compared to 9 (38%) with PVD were not satisfied with pain levels.
Conclusion:
Women with GV reported severe pain, whereas those with PVD reported moderate to severe pain. Pain quality descriptors may aid a clinician's decisions about whether to prescribe adjuvant drugs vs opioids to women with vulvodynia.
1. Introduction
Vulvodynia is “vulvar pain of at least 3-month duration without clear identifiable cause, which may have potentially associated factors.”2 There are 2 major subtypes of vulvodynia: generalized vulvodynia (GV) and provoked vestibulodynia (PVD). The pain of GV may affect the whole vulva as well as the inner thighs and perineum, and it may be spontaneous and/or provoked. Pain with PVD is confined to the vulvar vestibule and vaginal introitus.2 It may be provoked by sexual intercourse, tampon insertion, tight clothing, or sitting. Up to 7 million American women have this debilitating chronic pain syndrome. It is accompanied by dyspareunia (pain with vaginal penetration that renders sexual intercourse nearly impossible).16 Also, women with vulvodynia experience relationship difficulties due to their inability to have sexual intercourse.1,4 No treatment is consistently effective,15,37 and only 25% of women diagnosed with vulvodynia attain remission.30 Evidence suggests vulvodynia is a complex pain phenomenon with both neuropathic and nociceptive characteristics.14,22,33 Few studies describe vulvodynia pain, and most are limited by lack of valid, reliable, and comprehensive pain measures. The development of effective pain treatment strategies has been impeded by lack of characterization of women's perceptions of their vulvodynia pain. Also, there is insufficient knowledge about the nature of the 2 subtypes. In this pilot study, we begin to address these gaps by presenting self-reported sensory pain scores and characterization of the pain phenotypes of 60 women diagnosed with either GV or PVD. The first aim of this study was to describe the sensory elements (location, intensity, quality, and pattern) and nociceptive and neuropathic components of GV and PVD pain. In other patient populations, the McGill Pain Questionnaire (MPQ) pain quality descriptors have been used to diagnose neuropathic pain.42,45 Use of the pain quality descriptors in the characterization of GV and PVD may help guide development of appropriate and new treatments.
To inform a future large-scale national online study, our second aim was to determine the feasibility of using the Internet for recruitment and collection of data from women who have vulvodynia. We expected to recruit 50 women who would complete more than 90% of all questionnaire items.
2. Method
2.1. Design
An online survey pilot study was conducted over a 3-month period from November 2016 to January 2017. This study was approved by the University of Illinois at Chicago institutional review board.
2.2. Sample
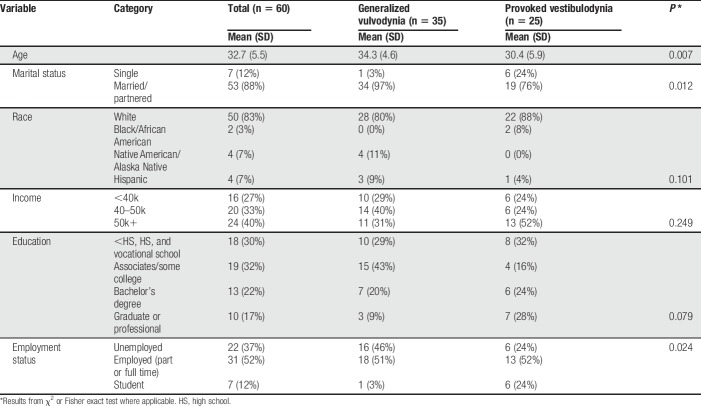
A convenience sample of women with self-identified diagnosis of GV or PVD meeting the following criteria were eligible to participate: (1) between 18 and 45 years of age; (2) not pregnant; (3) not in menopause; and (4) able to read English. To deter women without vulvodynia from participating, interested participants were asked the following bogus screening question21: “What happens when your vulvodynia flares up?”; possible responses included: I get a rash on my arms and legs, I get short of breath, I get bad diarrhea, all of the above, or none of the above. Eligible, potential participants were screened for exclusionary conditions: endometriosis, untreated vaginal/cervical infections, pain from pelvic inflammatory disease, vulvar skin diseases/vulvar conditions causing pain, vulvar cancer/precancer, neurological problems causing pelvic pain such as pudendal nerve entrapment or herpetic neuralgia, ovarian cysts, fibroids, painful scar tissue, pain from a previous genital injury, or pain from a cut/tear to the genitals that occurred during childbirth/an operation. Of the 121 women screened, 87 were eligible (64%). Sixty of these (50%) completed the study; 18 did not. The average age of the 60 participants was 32.7 ± 5.5 years, and they self-identified as white (n = 50), black/African American (n = 2), Hispanic/Latino (n = 4), or Native American/Alaska Native (n = 4). Demographic characteristics are in Table 1.
Table 1.
Patient characteristics by vulvodynia subtype.
2.3. Procedures
Potential participants found the survey link on the National Vulvodynia Association web site at NVA.org. The study purpose and procedures were explained in writing, and eligibility questions were completed. After screening for eligibility, online written informed consent was obtained. Participants who completed the study received a code to access a $30 gift card from an online retailer.
2.4. Instruments
Instruments used to measure the sensory pain experience of study participants were from the pain assessment and medication modules from PAINReportlt (Nursing Consultant LLC, Seattle, WA).18,41,43 PAINReportlt is a computerized version of the 1970 MPQ,26 which is a multidimensional measure of pain intensity, location, quality, and pattern. The equivalence of the paper MPQ and PAINReportlt has been demonstrated.18 It has been validated in many pain patient groups including those with vulvodynia.8,25,44,45,48 The survey took approximately 20 minutes to complete.
In PAINReportlt, participants mark the locations of their pain on a body outline; the number of pain sites is considered a location measure and is used to calculate the multidimensionality of a patient's pain. We modified the coding of pain locations for the vulvodynia population. Two expert women's health practitioners (urogynecologist and certified nurse midwife) determined the specific pain sites relevant to vulvodynia pain using the body outline image, establishing content validity for the location index.26 A maximum of 14 pain sites were identified. The interrater agreement rate across all body sites between separate coders ranged from 67% to 100%, and 12 of the body site kappa statistics showed moderate (0.41) or better agreement (for all 14 sites, kappa ranged from 0.12 to 1.0).20 Interrater reliability for the count of the number of pain sites was excellent (intraclass correlation coefficient = 0.956, P < 0.0001). We derived the total number of vulvodynia pain sites per person based on consensus coding between the 2 raters, when disagreements were evident.
Pain intensity was assessed by asking participants to assign a number to their current pain, and their worst and least pain in the past 24 hours, using a scale of 0 (no pain) to 10 (pain as bad as it could be) as well as compared with their worst headache, toothache, and stomachache.47 The mean of these items constituted the pain intensity scale, which has established acceptable concurrent (r = 0.80–0.89)28,39 and construct10,19 validity, and reliability and sensitivity.6,11,19,39
Participants reported their vulvodynia pain quality by selecting from among 78 pain quality descriptors, divided into 20 pain quality categories, containing words that represent a range of intensity. Endorsed descriptors were coded using 3 methods summarizing different constructs. Melzack's original method of summing the number of endorsed words was used to create the following scales: (1) sensory (PRI-S, 42 words); (2) affective (PRI-A, 14 words); (3) evaluative (PRI-E, 5 words); (4) miscellaneous (PRI-M, 17 words); and (5) a total score (PRI-T, 78 words).26 Test–retest reliability for all 4 subscales (0.31–0.82)44 and construct validity (r = 0.53–0.89)25 have been established with high internal consistency (α = 0.92) in a PVD sample.7
The number of words chosen (NWC) shows how many of the 20 groups of pain quality categories are represented, 1 word is chosen from each group (1–20), which enables understanding of the quality of the vulvodynia pain without intensity being a factor. For the pain quality descriptors, test–retest reliability (0.62–0.7),26,44 construct validity (r = 0.89),25,26 and stability (70.3%)26 have been demonstrated. There is substantial documentation that neuropathic and nociceptive pain may be differentiated using the pain quality descriptors from the MPQ.3,5,9,23,24,26,27,31,32,38,42,45 Others have demonstrated 81% sensitivity to neuropathic and 59% sensitivity to nociceptive pain with the MPQ.44 Measures were scored by counting the number of neuropathic (0–28) and nociceptive words (0–26) chosen by each participant.12,34
The temporal pain pattern represents how pain changes over time and helps determine the optimal timing for the administration of pain medications and therapies. Temporal pain pattern scores were calculated by having participants select from 3 pain patterns each comprised of 3 descriptors: continuous (continuous, steady, or constant); intermittent (rhythmic, periodic, or intermittent); and transient (brief, momentary, or transient).45,46 Each pain pattern was assigned a numeric value: 3 for continuous, 2 for intermittent, and 1 for transient. We calculated a total pain pattern score by summing the values of the 3 pain patterns. A total pain pattern score ranging from 0 (no pain pattern descriptors selected) to 6 (at least 1 descriptor selected for each pain pattern) was derived. Reliability and validity of the pain patterns have been established.29
The Composite Pain Index (CPI) was developed to denote the multidimensionality of participants' pain using a single score, ranging from 0 to 100. It is calculated by converting the number of pain sites, pain intensity (current, least, and worst), PRI-T, and pain pattern scores to proportional scores and then averaging them.46 The CPI has been shown to have adequate internal consistency for the 4 pain measures (Cronbach's alpha = 0.71 for baseline data and 0.69 at posttest). Test–retest reliability over 3 to 4 weeks was 0.52 in a sample of outpatients with cancer.46
Within the PAINReportlt medication module, participants chose from lists of analgesic medications used to reduce pain. Analgesic categories were: nonopioid (eg, nonsteroidal anti-inflammatories, acetaminophen, and aspirin); adjuvants (eg, antidepressants and membrane stabilizers); and opioids. The number of medications used in each category was summed. Participants provided demographic information including age, race, and education.
2.5. Statistical analysis
Data were exported from the PAINReportlt structured query language database into Microsoft Excel and imported into SAS 9.4 for statistical analyses. Descriptive statistics (means, variability measures, frequencies, and percentages) and inferential tests (the independent t test, χ2 test, Fisher exact test, and Pearson correlation coefficients) were used to examine the relationships between variables and the vulvodynia subtypes (GV or PVD). We did not undertake multivariable analyses controlling for confounding because this was a feasibility pilot study with a small sample size. Statistical significance was set at an α level P < 0.05.
3. Results
3.1. Feasibility of recruitment
Of the 121 women screened, 34 did not meet eligibility criteria: 11 skipped all screening questions, 18 had 1 or more exclusionary criteria, and 5 failed the bogus screening question. Of the 87 women deemed eligible (64%), 60 completed the study (77%), and 27 did not start the survey (23%), 9 of whom did not consent. Thus, we exceeded our goal of 50 completed surveys.
Although we attempted to prevent women without vulvodynia from participating by having them respond to the bogus screening question, we acknowledge that it was still possible to guess correctly and be admitted to the study. Another limitation is that the same woman may have entered the study more than once using different email addresses.
3.2. Completion of study measures and missing data
All questionnaires were completed by the 60 participants. Four women (7%) did not mark their pain locations on the body outline, and thus the CPI could not be calculated on those 4 participants. One woman did not report current, least, and worst pain in the past 24 hours, or average pain intensity (API). All other study measures were completed in their entirety. Overall, there was less than 1% missing data.
3.3. Univariate results
3.3.1. Vulvodynia pain location
Women reported pain sites on a full body outline without specific genitalia locations. This lack of specificity prevented us from differentiating vulvodynia pain location by the subtype on the body outline. The areas we coded and the proportion who endorsed them were: women (n = 50, 89%) who drew a mark where they approximated the vulva to be, then upper thighs (n = 35, 63%), coccyx (n = 35, 63%), pelvis (n = 30, 54%), sacral iliac joint (n = 30, 54%), and sacrum (n = 27, 48%). Only 4 women marked nonvulvodynia sites (2%) that included the head and wrist (n = 1), neck, shoulders, upper back (n = 1), and mid back (n = 2).
3.3.2. Intensity
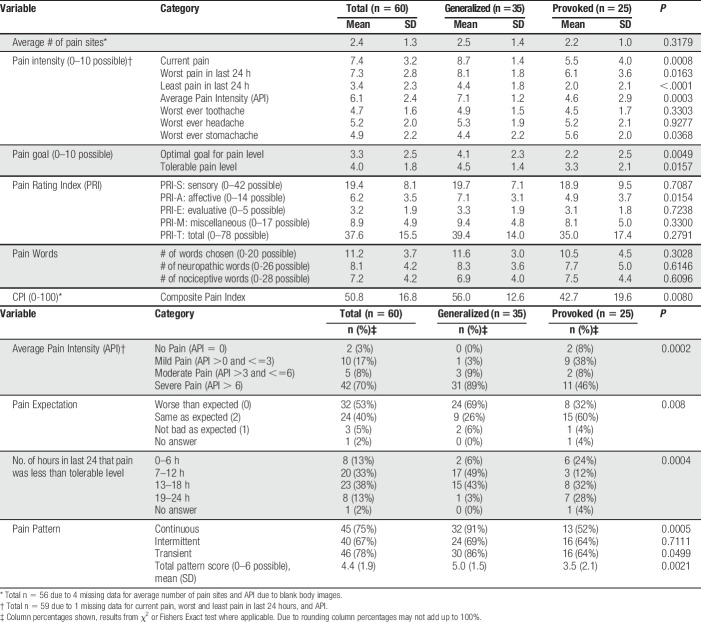
Pain scores for the total sample and the 2 vulvodynia subtypes are in Table 2. Among women with GV, none had an API score of 0. Mild pain (API >0 and ≤3/10) was reported by 1 woman (3%), and moderate pain (API >3 and ≤6/10) was reported by 3 women (9%). Severe pain (API >6 and ≤10/10) was reported by 31 women (89%). The MPQ includes a single item aimed at measuring a person's satisfaction in living with their level of chronic pain.26 The majority (54%) of women were not satisfied with their level of pain, 11 women (31%) were unsure whether they were satisfied or not satisfied, and 5 women (14%) were satisfied with their level of pain.
Table 2.
Descriptive statistics for the pain measures.
Among those women with PVD, 2 had an API of 0 (8%). Nine women (38%) reported mild pain (API >0 and ≤3/10). Two women (8%) reported moderate pain (API >3 and ≤6/10). Eleven women (46%) reported severe pain (API >6 and ≤10/10). Nine women (38%) were not satisfied with their level of pain, thirteen women (54%) were unsure whether they were satisfied or not satisfied, and 2 women (8%) were satisfied with their level of pain.
3.3.3. Quality
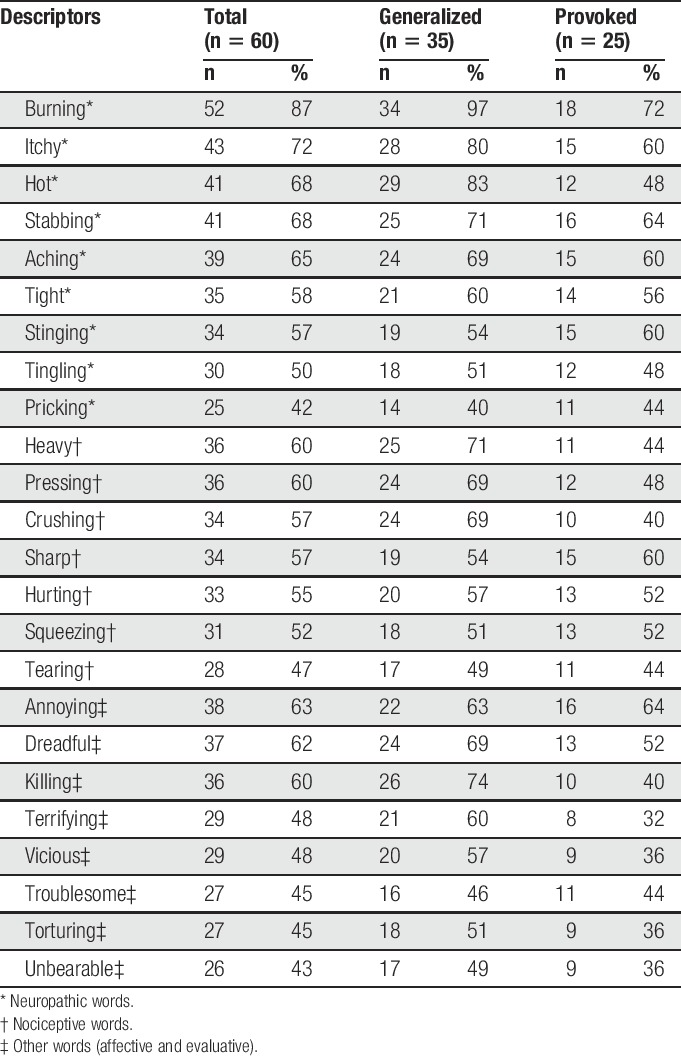
Table 2 also shows descriptive pain quality scores for the total sample and the 2 vulvodynia subtypes. Women with GV had PRI-T scores ranging from 7 to 64 (mean 39.4 ± 14.0). The mean NWC was 11.6 ± 3.0 with 6.9 ± 4.0 of these being neuropathic pain quality descriptors. Women with PVD had PRI-T scores that ranged from 0 to 58 (mean 35.0 ± 17.4). The mean NWC was 11.2 ± 3.7; they selected 7.2 ± 4.2 neuropathic pain quality descriptors (Table 2). The frequency of sensory (nociceptive or neuropathic), affective, and evaluative pain quality descriptors chosen by at least 40% of women by the vulvodynia subtype for the total sample is in Table 3; Supplemental Digital Content 1, Table (available at http://links.lww.com/PR9/A39) shows all pain quality descriptors chosen by the total sample (n = 60).
Table 3.
Frequency of selected McGill pain quality descriptors chosen by ≥40% of women by the vulvodynia subtype.
3.3.4. Temporal pain patterns
Pain pattern scores for the total sample and the 2 vulvodynia subtypes are in Table 2. A large majority of 35 women with GV (91%) reported their pain pattern as continuous and selected the pain pattern descriptors continuous, steady, or constant. Thirty women (86%) reported their pain pattern as rhythmic and selected the pain pattern descriptors rhythmic, periodic, or intermittent. Twenty-four women (69%) reported their pain pattern as transient and selected the pain pattern descriptors brief, momentary, or transient. Their total mean pain pattern score was 5.0 ± 1.5. Thirteen women (52%) with PVD reported their pain pattern as continuous and selected the pain pattern descriptors continuous, steady, or constant. Sixteen women (64%) reported their pain pattern as rhythmic and selected the pain pattern descriptors rhythmic, periodic, or intermittent. Sixteen women (64%) reported their pain pattern as transient and selected the pain pattern descriptors brief, momentary, or transient. Their total mean pain pattern score was 3.5 ± 2.1 (Table 2).
3.3.5. Composite Pain Index
Mean CPI scores for the total sample and 2 vulvodynia subtypes are in Table 2. Scores for women with GV and PVD were significantly different. Women with GV had a CPI score range between 14.0 and 76.9 with a mean of 56.0 ± 12.6. Women with PVD had a CPI score range between 5.5 and 66.0 with a mean CPI score of 42.7 ± 19.6.
3.3.6. Analgesics
For the total sample, 2 women (3.3%) did not report their medication use. For nonopioid analgesics in the total sample, women reported using a range of 0 to 8 medications (mean = 2.5 ± 1.9). Thirty-three women with GV (97%) reported using a range of 0 to 8 (mean 2.8 ± 1.9) nonopioid analgesics, and 17 women with PVD (71%) reported using a range between 0 and 6 (mean 2.0 ± 2.0) nonopioid medications.
For adjuvant analgesics in the total sample, women reported using a range of 0 to 5 (mean 1.8 ± 1.2) medications. Twenty-eight women with GV (82%) reported using a range of 0 to 5 (mean 1.9 ± 1.4) adjuvant analgesics, and 21 women with PVD (88%) reported using a range of 0 to 4 (mean 2 ± 1.1) adjuvant analgesics.
For opioid analgesics in the total sample, women reported using a range of 0 to 4 (mean 1.1 ± 1.1) medications. Twenty-seven women with GV (80%) reported using a range of 0 to 3 (mean 1.4 ± 1.0) opioid analgesics, and 10 women with PVD (42%) reported using a range of 0 to 4 (mean 0.8 ± 1.2) opioid analgesics.
3.4. Bivariate results
Women with GV compared to women with PVD were more likely to have pelvic pain sites (71% vs 36%; x2 (1, 56) = 6.39, P = 0.0115) and upper thigh pain sites (82% vs 45%; x2 (1, 56) = 8.3, P = 0.0039). The correlation between API and the number of pain sites was moderate and highly significant (Pearson's r = 0.41, P < 0.0016); therefore, as the number of pain sites increased, the API increased.
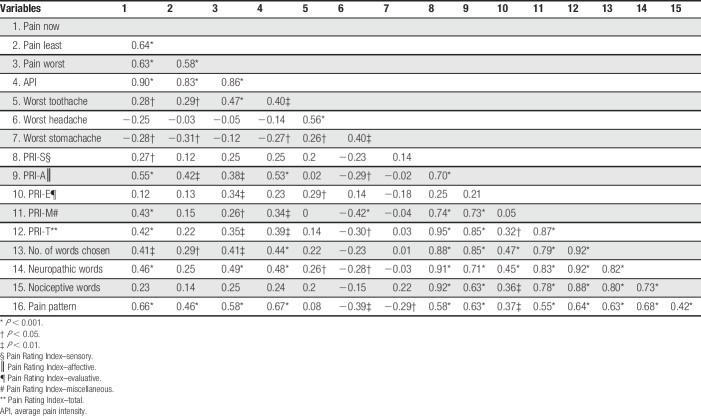
Correlations between the sensory pain variables are in Table 4. There were multiple strong positive and significant correlations among the sensory pain variables. The PRI-S and PRI-A positively correlate (P < 0.001). The PRI-S, PRI-A, and PRI-T also positively correlate with the NWC (P < 0.001) and for the number of words, associated with neuropathic and nociceptive pain (P < 0.001).
Table 4.
Correlations among sensory pain variables in women with vulvodynia.
The mean current pain score was significantly higher for women with GV than for women with PVD (t27 = 3.8, P = 0.0008). The mean least pain score was significantly higher for women with GV than for women with PVD (t57 = 4.6, P < 0.0001). The mean worst pain score was significantly higher for women with GV than for women with PVD (t31 = 2.5, P = 0.0163). Although women with both subtypes of vulvodynia reported significantly different vulvodynia pain intensity, reported pain intensities for worst toothache (t57 = 0.98, P = 0.33) and worst headache (t57 = 0.09, P = 0.92) did not differ. However, their pain intensities did differ significantly for worst stomachache (t57 = −2.1, P = 0.0368). The mean pain pattern temporal score was significantly higher for women with GV than for women with PVD (t58 = 3.2, P = 0.002). Women with GV had significantly higher constant, continuous, or steady pain (x2 (1, 60) = 12.09, P = 0.0005) as well as momentary, transient, or brief pain (x2 (1, 60) = 3.84, P = 0.05) than for women with PVD, but did not differ significantly in their reports of intermittent, periodic, or rhythmic pain (x2 (1, 60) = 0.13, P = 0.71).
4. Discussion
This pilot project was designed to obtain recruitment feasibility, participants completing all measures, missing data, and parameter estimates for variables of interest. It provides initial subtype comparisons of pain intensity, location, quality, and pattern. The women with GV and PVD were experiencing moderate to severe current and worst pain intensities. It is notable that the mean pain scores did not appear to be a function of response bias because mean common pain intensities for worst toothache and worst headache did not differ by the vulvodynia subtype; pain intensity for worst stomachache did differ, but this could be a spurious finding and needs to be assessed in a larger sample.
An important limitation of our study is that data were not collected on the date of the participant's last menstrual period nor did we inquire whether their vulvodynia pain was cyclic relative to the menstrual cycle. These variables may have affected reported pain parameters and should be included in future studies on vulvodynia. Also, although a diagnosis of endometriosis was an exclusionary criterion, there is an interval of 7 to 8 years between symptom onset and diagnosis.13 Therefore, some study participants may have had undiagnosed endometriosis, which may have potentiated their pain in addition to vulvodynia.
We identified an importation limitation of the body outline pain location tool (ie, the lack of genitalia to more specifically identify the location of genital pain). As a result, we are developing an additional module for PAINReportlt that will feature a drawing of the external female genitalia. This more detailed anatomical drawing will allow women with vulvodynia to select all areas of genital pain, allowing for further differentiation between the symptoms of GV and PVD. Regardless of the subtype, women reported severe pain in the vulva in addition to several other body sites. The higher the API, the more pain sites women with both types of vulvodynia reported. Although women with GV reported more sites than women with PVD, the difference was not significant. In the future, a difference may be found if women are able to draw all pain sites on a drawing of the external genitalia. Upper thigh and pelvic pain was reported by more than 50% of women with GV vs PVD.
The MPQ pain quality descriptors have been used to determine the presence of neuropathic and nociceptive pain in diabetic peripheral neuropathy, lung cancer, sickle cell disease, and other chronic pain conditions.3,24,26,27,35,38,40,42,45 Dargie et al.7 recently performed a preliminary investigation of 4 measures used to assess neuropathic pain in women with PVD, the Leeds Assessment of Neuropathic Pain and Symptoms (S-LANSS), the Neuropathic Pain Symptoms Inventory, and the Pain Quality Assessment Scale. They concluded that these 4 instruments produced inconsistent findings and that more exploration into pain mechanisms of PVD were needed.7 However, Dargie et al. did not explore the MPQ's 78 pain quality descriptors for their ability to discern between neuropathic and nociceptive pain. Our study is not only the first to examine the use of the MPQ pain quality descriptors for determining the presence of neuropathic and nociceptive pain in women with vulvodynia but also in GV as well as PVD.
In this small sample of women with GV and PVD, we successfully used the MPQ pain quality descriptors to assess for the presence of neuropathic and nociceptive pain and summed the number of nociceptive and neuropathic words chosen to create subscale scores.3,12,24,40,42,45 We found that both women with GV and those with PVD selected neuropathic and nociceptive pain quality descriptors, which may suggest the presence of both types of pain.
Study limitations are that our sample size is small and, although appropriate for a pilot study, did not allow us to control for potential confounders when comparing pain results between the 2 vulvodynia subtypes. Also, as a convenience sample, women were self-selected for enrollment and so it is difficult to generalize their experiences to the entire vulvodynia population. Similarly, a larger number of women with GV than with PVD participated, which may be due to the fact that they had severe and constant pain and were more motivated to participate in a survey. In other words, our sample does not match the proportions expected for the diagnostic subtypes (GV, 20% and PVD, 80%).2 Also, the sample included few Hispanic, African-American, or other minority women, which limits the transferability of our findings.
We successfully recruited 60 women who self-reported a diagnosis of vulvodynia and completed the study, which is greater than our goal of 50. However, it is possible that women who deceivingly self-reported a diagnosis of vulvodynia answered the bogus screening question correctly and entered the study, or women with or without vulvodynia may have participated multiple times to receive more than 1 online gift card, as we did not check computer IP addresses.
5. Conclusion
Our findings of the sensory pain characteristics of women with vulvodynia are novel. They add to the growing body of evidence, suggesting that women with vulvodynia are also experiencing neuropathic pain. We also found that it was feasible to perform an online survey; however, we cannot assess if the range of pain experiences was represented or if only those with severe pain were represented. This pilot study needs to be replicated with a larger nationwide sample of women with vulvodynia in an attempt to ensure a representative sample. Further exploration of the use of the MPQ pain quality descriptors in women with GV and PVD is needed to provide a more in-depth interpretation of the subscales and how these might relate to determining optimal drug treatment regimens for women with vulvodynia. Also, future randomized controlled trials to determine efficacy of adjuvant drugs and nonaddictive therapies such as acupuncture36 and physical therapy17 should be performed because despite women with GV and PVD reporting using multiple pain medications including opioids, their pain is not controlled.
Disclosures
P.D. Thornton, D. Hartmann, M.L. Suarez, and H.A. Pauls have nothing to disclose. J.M. Schlaeger reports grants from NICHD and NINR. C.L. Patil reports a grant through NINR. A.D. Steffen reports grants through NICHD, NHLBI, NIDDK, NINR, NIA, and HRSA. W.H. Kobak reports a grant through NICHD. Y. Yao reports grants through NHLBI, NCI, and PCORI. K.L. Roach reports grants through NHLBI and NIA. T.L. Hughes reports a grant through NIAAA. D.J. Wilkie reports grants through NICHD, NHLBI, NCI, NIA, NINR, PCORI, and AHRQ; and is a Founder and the Chairman of eNursingllc, a company that does not own tools used in the research.
Supplementary Material
Acknowledgement
This research was supported by a grant from the University of Illinois at Chicago, Office of the Vice Chancellor for Research, Campus Research Board Award. This publication was made possible by Grant Number R01HD091210 from the National Institutes of Health, National Institute of Child Health and Human Development (NICHD). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NICHD. The final peer-reviewed manuscript is subject to the National Institutes of Health Public Access Policy.
Appendix A. Supplemental digital content
Supplemental digital content associated with this article can be found online at http://links.lww.com/PR9/A39.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.painrpts.com).
References
- [1].Basson R. The recurrent pain and sexual sequelae of provoked vestibulodynia: a perpetuating cycle. J Sex Med 2012;9:2077–92. [DOI] [PubMed] [Google Scholar]
- [2].Bornstein J, Goldstein AT, Stockdale CK, Bergeron S, Pukall C, Zolnoun D, Coady D, Goldstein A, Bachmann GA, Bissonnette I. 2015 ISSVD, ISSWSH, and IPPS consensus terminology and classification of persistent vulvar pain and vulvodynia. J Sex Med 2016;13:607–12. [DOI] [PubMed] [Google Scholar]
- [3].Boureau F, Doubrère JF, Luu M. Study of verbal description in neuropathic pain. PAIN 1990;42:145–52. [DOI] [PubMed] [Google Scholar]
- [4].Brotto LA, Yong P, Smith KB, Sadownik LA. Impact of a multidisciplinary vulvodynia program on sexual functioning and dyspareunia. J Sex Med 2015;12:238–47. [DOI] [PubMed] [Google Scholar]
- [5].Cherny NI, Foley KM. Current approaches to the management of cancer pain: a review. Ann Acad Med Singap 1994;23:139–59. [PubMed] [Google Scholar]
- [6].Coward DD, Wilkie DJ. Metastatic bone pain: meanings associated with self-report and self-management decision making. Cancer Nurs 2000;23:101–8. [DOI] [PubMed] [Google Scholar]
- [7].Dargie E, Gilron I, Pukall CF. Self-reported neuropathic pain characteristics of women with provoked vulvar pain: a preliminary investigation. J Sex Med 2017;14:577–91. [DOI] [PubMed] [Google Scholar]
- [8].Dargie E, Holden RR, Pukall CF. The Vulvar Pain Assessment Questionnaire Inventory. PAIN 2016;157:2672–86. [DOI] [PubMed] [Google Scholar]
- [9].Dobratz MC. Patterns of advanced cancer pain in home hospice patients. Cancer Nurs 2001;24:294–9. [DOI] [PubMed] [Google Scholar]
- [10].Downie WW, Leatham PA, Rhind VM, Wright V, Branco JA, Anderson JA. Studies with pain rating scales. Ann Rheum Dis 1978;37:378–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Du Pen SL, Du Pen AR, Polissar N, Hansberry J, Kraybill BM, Stillman M, Panke J, Everly R, Syrjala K. Implementing guidelines for cancer pain management: results of a randomized controlled clinical trial. J Clin Oncol 1999;17:361. [DOI] [PubMed] [Google Scholar]
- [12].Epstein JB, Wilkie DJ, Fischer DJ, Kim YO, Villines D. Neuropathic and nociceptive pain in head and neck cancer patients receiving radiation therapy. Head Neck Oncol 2009;1:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Falcone T, Flyckt R. Clinical management of endometriosis. Obstet Gynecol 2018;131:557–71. [DOI] [PubMed] [Google Scholar]
- [14].Giesecke J, Reed BD, Haefner HK, Giesecke T, Clauw DJ, Gracely RH. Quantitative sensory testing in vulvodynia patients and increased peripheral pressure pain sensitivity. Obstet Gynecol 2004;104:126–33. [DOI] [PubMed] [Google Scholar]
- [15].Haefner HK, Collins ME, Davis GD, Edwards L, Foster DC, Hartmann ED, Kaufman RH, Lynch PJ, Margesson LJ, Moyal-Barracco M, Piper CK, Reed BD, Stewart EG, Wilkinson EJ. The vulvodynia guideline. J Low Genit Tract Dis 2005;9:40–51. [DOI] [PubMed] [Google Scholar]
- [16].Harlow BL, Kunitz CG, Nguyen RH, Rydell SA, Turner RM, MacLehose RF. Prevalence of symptoms consistent with a diagnosis of vulvodynia: population-based estimates from 2 geographic regions. Am J Obstet Gynecol 2014;210:40.e41–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hoerle T, Dominguez S, Fitzgerald S, Ault K. Physical therapy is an effective treatment for vulvodynia [30q]. Obstetrics Gynecol 2018;131:192S. [Google Scholar]
- [18].Huang HY, Wilkie DJ, Zong SPS, Berry D, Hairabedian D, Judge MK, Farber S, Chabal C. Developing a computerized data collection and decision support system for cancer pain management. Comput Inform Nurs 2003;21:206–17. [DOI] [PubMed] [Google Scholar]
- [19].Jensen MP, Karoly P, Braver S. The measurement of clinical pain intensity: a comparison of six methods. PAIN 1986;27:117–26. [DOI] [PubMed] [Google Scholar]
- [20].Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977;33:159–74. [PubMed] [Google Scholar]
- [21].Lavrakas PJ. Encyclopedia of survey research methods. Los Angeles, CA: Sage Publications, 2008. [Google Scholar]
- [22].Lowenstein L, Vardi Y, Deutsch M, Friedman M, Gruenwald I, Granot M, Sprecher E, Yarnitsky D. Vulvar vestibulitis severity—assessment by sensory and pain testing modalities. PAIN 2004;107:47–53. [DOI] [PubMed] [Google Scholar]
- [23].Martin LA, Hagen NA. Neuropathic pain in cancer patients: mechanisms, syndromes, and clinical controversies. J Pain Symptom Manage 1997;14:99–117. [DOI] [PubMed] [Google Scholar]
- [24].Masson EA, Hunt L, Gem JM, Boulton AJ. A novel approach to the diagnosis and assessment of symptomatic diabetic neuropathy. PAIN 1989;38:25–8. [DOI] [PubMed] [Google Scholar]
- [25].McGuire DB. Assessment of pain in cancer inpatients using the McGill Pain Questionnaire. Oncol Nurs Forum 1984;11:32–7. [PubMed] [Google Scholar]
- [26].Melzack R. The McGill Pain Questionnaire: major properties and scoring methods. PAIN 1975;1:277–99. [DOI] [PubMed] [Google Scholar]
- [27].Melzack R, Terrence C, Fromm G, Amsel R. Trigeminal neuralgia and atypical facial pain: use of the McGill Pain Questionnaire for discrimination and diagnosis. PAIN 1986;27:297–302. [DOI] [PubMed] [Google Scholar]
- [28].Murphy DF, McDonald A, Power C, Unwin A, MacSullivan R. Measurement of pain: a comparison of the visual analogue with a nonvisual analogue scale. Clin J Pain 1987;3:197–200. [Google Scholar]
- [29].Ngamkham S, Holden JE, Wilkie DJ. Differences in pain location, intensity, and quality by pain pattern in outpatients with cancer. Cancer Nurs 2011;34:228–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Nguyen RH, Mathur C, Wynings EM, Williams DA, Harlow BL. Remission of vulvar pain among women with primary vulvodynia. J Low Genit Tract Dis 2015;19:62–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Payne R. Cancer pain. Anatomy, physiology, and pharmacology. Cancer 1989;63(11 suppl):2266–74. [DOI] [PubMed] [Google Scholar]
- [32].Portenoy RK. The physical examination in cancer pain assessment. Semin Oncol Nurs 1997;13:25–9. [DOI] [PubMed] [Google Scholar]
- [33].Pukall CF, Binik YM, Khalifé S, Amsel R, Abbott FV. Vestibular tactile and pain thresholds in women with vulvar vestibulitis syndrome. PAIN 2002;96:163–75. [DOI] [PubMed] [Google Scholar]
- [34].Robley L. TNEEL: toolkit for nurturing excellence at end-of-life transition, Version 1.0. J Assoc Nurses AIDS Care 2003;14:69–70. [Google Scholar]
- [35].Savedra MC, Tesler MD, Holzemer WL, Wilkie DJ, Ward JA. Pain location: validity and reliability of body outline markings by hospitalized children and adolescents. Res Nurs Health 1989;12:307–14. [DOI] [PubMed] [Google Scholar]
- [36].Schlaeger JM, Xu N, Mejta CL, Park CG, Wilkie DJ. Acupuncture for the treatment of vulvodynia: a randomized wait-list controlled pilot study. J Sex Med 2015;12:1019–27. [DOI] [PubMed] [Google Scholar]
- [37].Stockdale CK, Lawson HW. 2013 Vulvodynia Guideline update. J Low Genit Tract Dis 2014;18:93–100. [DOI] [PubMed] [Google Scholar]
- [38].Twycross R. Cancer pain classification. Acta Anaesthesiol Scand 1997;41(1 pt 2):141–5. [DOI] [PubMed] [Google Scholar]
- [39].Wilkie D, Lovejoy N, Dodd M, Tesler M. Cancer pain intensity measurement: concurrent validity of three tools—finger dynamometer, pain intensity number scale, visual analogue scale. Hosp J 1990;6:1–13. [DOI] [PubMed] [Google Scholar]
- [40].Wilkie DJ, Corless I, Farber S, Forrest J, Holstein M. Toolkit for nurturing excellence at the end-of-life transition for educators (TNEEL) (Version1.1), Available at: https://tneel.ahc.ufl.edu/TNEEL/index. Accessed September 30, 2018.
- [41].Wilkie DJ, Huang HY, Berry DL, Schwartz A, Lin YC, Ko NY, Chen A, Gralow J, Lindsley SK, Fitzgibbon D. Cancer symptom control: feasibility of a tailored, interactive computerized program for patients. Fam Community Health 2001;24:48–62. [PubMed] [Google Scholar]
- [42].Wilkie DJ, Huang HY, Reilly N, Cain KC. Nociceptive and neuropathic pain in patients with lung cancer: a comparison of pain quality descriptors. J Pain Symptom Manage 2001;22:899–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Wilkie DJ, Judge MKM, Berry DL, Dell J, Zong S, Gilespie R. Usability of a computerized PAINReportlt in the general public with pain and people with cancer pain. J Pain Symptom Manage 2003;25:213–24. [DOI] [PubMed] [Google Scholar]
- [44].Wilkie DJ, Keefe FJ. Coping strategies of patients with lung cancer-related pain. Clin J Pain 1991;7:292–9. [DOI] [PubMed] [Google Scholar]
- [45].Wilkie DJ, Molokie R, Boyd-Seal D, Suarez ML, Kim YO, Zong S, Wittert H, Zhao Z, Saunthararajah Y, Wang ZJ. Patient-reported outcomes: descriptors of nociceptive and neuropathic pain and barriers to effective pain management in adult outpatients with sickle cell disease. J Natl Med Assoc 2010;102:18–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Wilkie DJ, Molokie RE, Suarez ML, Ezenwa MO, Wang ZJ. Composite Pain Index: reliability, validity, and sensitivity of a patient-reported outcome for research. Pain Med 2015;16:1341–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Wilkie DJ, Savedra MC, Holzemer WL, Tesler MD, Paul SM. Use of the McGill Pain Questionnaire to measure pain: a meta-analysis. Nurs Res 1990;39:36–41. [PubMed] [Google Scholar]
- [48].Wilkie DJ, Williams AR, Grevstad P, Mekwa J. Coaching persons with lung cancer to report sensory pain. Literature review and pilot study findings. Cancer Nurs 1995;18:7–15. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.