Abstract
PURPOSE
Nivolumab 1 mg/kg plus ipilimumab 3 mg/kg (NIVO1+IPI3) is approved for first-line treatment of patients with advanced melanoma in several countries. We conducted a phase IIIb/IV study (CheckMate 511) to determine if nivolumab 3 mg/kg plus ipilimumab 1 mg/kg (NIVO3+IPI1) improves the safety profile of the combination.
PATIENTS AND METHODS
Patients (N = 360) age 18 years or older with previously untreated, unresectable stage III or IV melanoma were randomly assigned 1:1 to NIVO3+IPI1 or NIVO1+IPI3 once every 3 weeks for four doses. After 6 weeks, all patients received NIVO 480 mg once every 4 weeks until disease progression or unacceptable toxicity. The primary end point was a comparison of the incidence of treatment-related grade 3 to 5 adverse events (AEs) between groups. Secondary end points included descriptive analyses of objective response rate, progression-free survival, and overall survival. The study was not designed to formally demonstrate noninferiority of NIVO3+IPI1 to NIVO1+IPI3 for efficacy end points.
RESULTS
At a minimum follow-up of 12 months, incidence of treatment-related grade 3 to 5 AEs was 34% with NIVO3+IPI1 versus 48% with NIVO1+IPI3 (P = .006). In descriptive analyses, objective response rate was 45.6% in the NIVO3+IPI1 group and 50.6% in the NIVO1+IPI3 group, with complete responses in 15.0% and 13.5% of patients, respectively. Median progression-free survival was 9.9 months in the NIVO3+IPI1 group and 8.9 months in the NIVO1+IPI3 group. Median overall survival was not reached in either group.
CONCLUSION
The CheckMate 511 study met its primary end point, demonstrating a significantly lower incidence of treatment-related grade 3-5 AEs with NIVO3+IPI1 versus NIVO1+IPI3. Descriptive analyses showed that there were no meaningful differences between the groups for any efficacy end point, although longer follow up may help to better characterize efficacy outcomes.
INTRODUCTION
Combined inhibition of programmed death 1 (PD-1) and cytotoxic T lymphocyte antigen-4 (CTLA-4) with nivolumab and ipilimumab has demonstrated efficacy in several tumor types at different dosing schedules.1-5 In patients with advanced melanoma, nivolumab combined with ipilimumab was first evaluated in a phase I dose-escalation study.6,7 This study showed that nivolumab 1 mg/kg plus ipilimumab 3 mg/kg led to higher rates of objective response and greater aggregate clinical activity than did nivolumab 3 mg/kg plus ipilimumab 1 mg/kg, albeit with a higher incidence of treatment-related adverse events (AEs).6 On the basis of these findings, we selected nivolumab 1 mg/kg plus ipilimumab 3 mg/kg as the dosing regimen for additional clinical evaluation.
In patients with previously untreated, advanced melanoma, results of the phase II CheckMate 0698,9 and phase III CheckMate 0671,2 trials demonstrated a significant improvement in objective response rate (ORR) and progression-free survival (PFS) with nivolumab plus ipilimumab versus ipilimumab alone. Follow-up analyses from the CheckMate 067 trial showed a significant improvement in overall survival (OS) for nivolumab plus ipilimumab compared with ipilimumab alone.2 Recently, updated data from the CheckMate 067 trial showed 4-year OS rates of 53%, 46%, and 30% in the nivolumab plus ipilimumab, nivolumab alone, and ipilimumab alone groups, respectively.10
In both the CheckMate 069 and 067 studies, nivolumab was administered at 1 mg/kg and ipilimumab at 3 mg/kg, once every 3 weeks for four doses, followed by nivolumab monotherapy at 3 mg/kg once every 2 weeks. Results of these studies led to the approval of the combination as a first-line treatment in patients with advanced melanoma. In the initial report from CheckMate 067, at a minimum follow-up of 9 months, treatment-related grade 3 and 4 AEs were reported in 55% of patients who received combination therapy, 16% who received nivolumab alone, and 27% who received ipilimumab alone, which led to the discontinuation of treatment in 29%, 5%, and 13% of patients, respectively.1 The phase IIIb/IV CheckMate 511 study was conducted to determine whether nivolumab 3 mg/kg plus ipilimumab 1 mg/kg improves the safety profile of the approved dosing regimen in patients with advanced melanoma.
PATIENTS AND METHODS
Patients
Eligible patients were age 18 years or older with unresectable stage III or stage IV melanoma (per American Joint Committee on Cancer staging system 7th edition), an Eastern Cooperative Oncology Group performance status of 0 or 1, no prior systemic therapy for metastatic melanoma (prior adjuvant or neoadjuvant therapy was permitted if completed 6 weeks or more before random assignment), measurable disease per Response Evaluation Criteria In Solid Tumors (RECIST) v1.1, and tumor tissue available for biomarker analyses. Patients with active brain metastases, ocular melanoma, or autoimmune disease that required systemic treatment with corticosteroids or other immunosuppressive medications within 14 days of random assignment were excluded.
The trial was conducted in accordance with Good Clinical Practices as defined by the International Conference on Harmonization. The study was conducted in compliance with the protocol approved by the institutional review boards of each study center. All patients provided written, informed consent before enrollment.
Study Design and Treatment
This phase IIIb/IV, randomized, double-blind study was conducted at 57 sites in 13 countries. Patients were randomly assigned 1:1 and stratified by programmed death ligand 1 (PD-L1) status (5% or more v less than 5% tumor cell surface expression) and M stage (M0/M1a/M1b v M1c; Data Supplement). In part 1 of the study, patients received either nivolumab 3 mg/kg plus ipilimumab 1 mg/kg (NIVO3+IPI1) or nivolumab 1 mg/kg plus ipilimumab 3 mg/kg (NIVO1+IPI3) once every 3 weeks for four doses. NIVO was administered first as a 30-minute infusion, and after a 30-minute waiting period was followed by IPI as a 30-minute infusion. Patients who discontinued combination therapy as a result of toxicity did not enter the maintenance phase (part 2 of the study) in which open-label NIVO was administered as a 30-minute infusion at a flat dose of 480 mg once every 4 weeks until disease progression or unacceptable toxicity. The maintenance phase began 6 weeks after the last combination dose. Dose delays as a result of toxicity were permitted, but dose reductions for either drug were not allowed.
Assessments
The primary end point was to evaluate the rate of treatment-related grade 3 to 5 AEs in patients who received NIVO3+IPI1 and NIVO1+IPI3. Secondary end points included ORR, PFS, OS, and health-related quality of life (HRQoL) in both treatment groups. Exploratory end points included duration of response, time to response, and overall safety and tolerability.
AEs were graded according to National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.0), assessed from the first dose of study therapy to 30 days after the last dose. Safety was based on the frequency of deaths, AEs, serious AEs, and AEs leading to treatment discontinuation. In addition, analyses of select AEs—that is, those with a potential immunologic cause—included incidence, time to onset, and time to resolution with immune-modulating medications. All treated patients were evaluated for investigator-assessed response according to RECIST v1.1 at 12 weeks after random assignment, 8 weeks thereafter for the first 12 months, and then every 12 weeks until disease progression or treatment discontinuation. Tumor PD-L1 expression was assessed in pretreatment biopsy specimens at a central laboratory using a validated, automated immunohistochemical assay (PD-L1 IHC 28-8 pharmDx; Dako, Carpinteria, CA) as described previously.1
HRQoL was assessed with the use of the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-Core 30 (QLQ-C30) and the three-level version of the European Quality of Life-5 Dimensions (EQ-5D) questionnaire, as described previously.11 Analysis of HRQoL outcomes was performed on all treated patients who had a baseline assessment and at least one subsequent assessment on study. A clinically meaningful change from baseline score was defined as 10 for QLQ-C30 Global Health Status,12 0.08 for the EQ-5D utility index,13 and 7 for the EQ-5D Visual Analog Scale.13 For all assessments, a higher score represents a better quality of life.
Statistical Analysis
Statistical powering for the primary end point of treatment-related grade 3 to 5 AEs assumed that 340 total patients would be treated, 170 in each group. Given a two-sided α of .05, 340 patients would provide 80% power to show a statistically significant difference assuming a rate of 40% in the NIVO3+IPI1 group versus 55% in the NIVO1+IPI3 group. A difference of 15% was considered clinically meaningful and is consistent with results from the CheckMate 0671,2 and CheckMate 0698,9 studies. As the primary end point was a safety analysis that included all treated patients, secondary efficacy end points also included all treated patients. The study was not designed or powered to formally demonstrate noninferiority of NIVO3+IPI1 to NIVO1+IPI3 for the secondary end points; thus, efficacy data are for descriptive purposes only and were not adjusted for multiplicity. We calculated ORR and corresponding 95% CI using the Clopper-Pearson method and compared the two groups using a two-sided Cochran-Mantel-Haenszel test, stratified by PD-L1 and M stage. We estimated PFS and OS using the Kaplan-Meier method.
RESULTS
Patient Characteristics and Drug Exposure
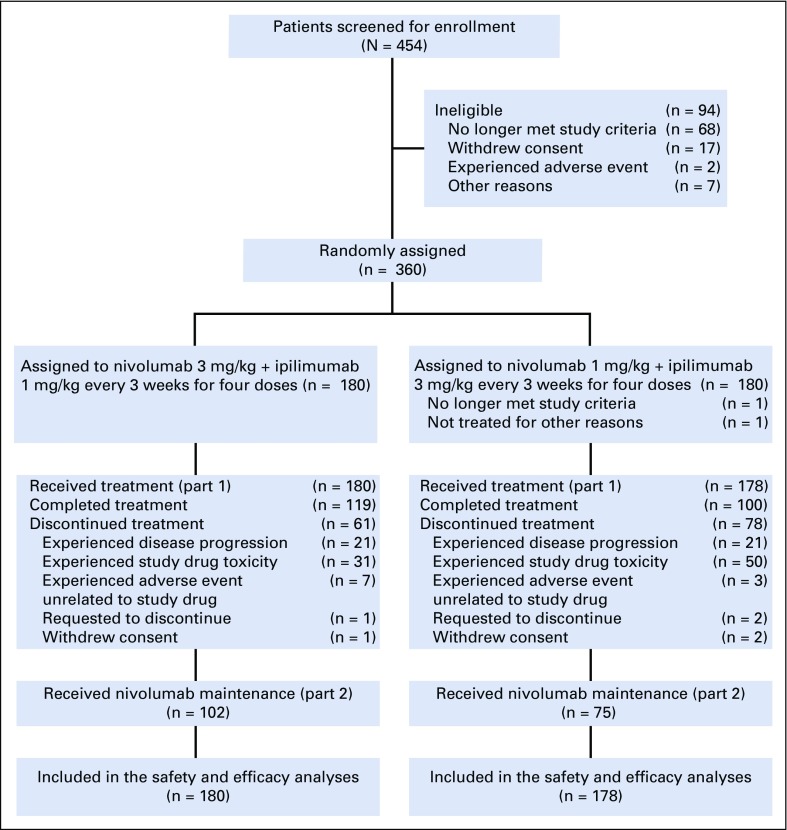
Patients were enrolled from April 4, 2016, to March 27, 2017. Results presented here are from a database lock on June 1, 2018, with a minimum patient follow-up of 12 months. Median follow-up was 18.8 months in the NIVO3+IPI1 group and 18.6 months in the NIVO1+IPI3 group. A total of 360 patients were randomly assigned, with 180 treated in the NIVO3+IPI1 group and 178 in the NIVO1+IPI3 group; 119 (66.1%) of 180 patients and 100 (56.2%) of 178 patients completed combination therapy in the NIVO3+IPI1 and NIVO1+IPI3 groups, respectively (Fig 1). The most common reason for discontinuation of combination therapy was study drug toxicity—17.2% in the NIVO3+IPI1 group and 28.1% in the NIVO1+IPI3 group.
FIG 1.
CONSORT diagram. Patient disposition as of June 1, 2018.
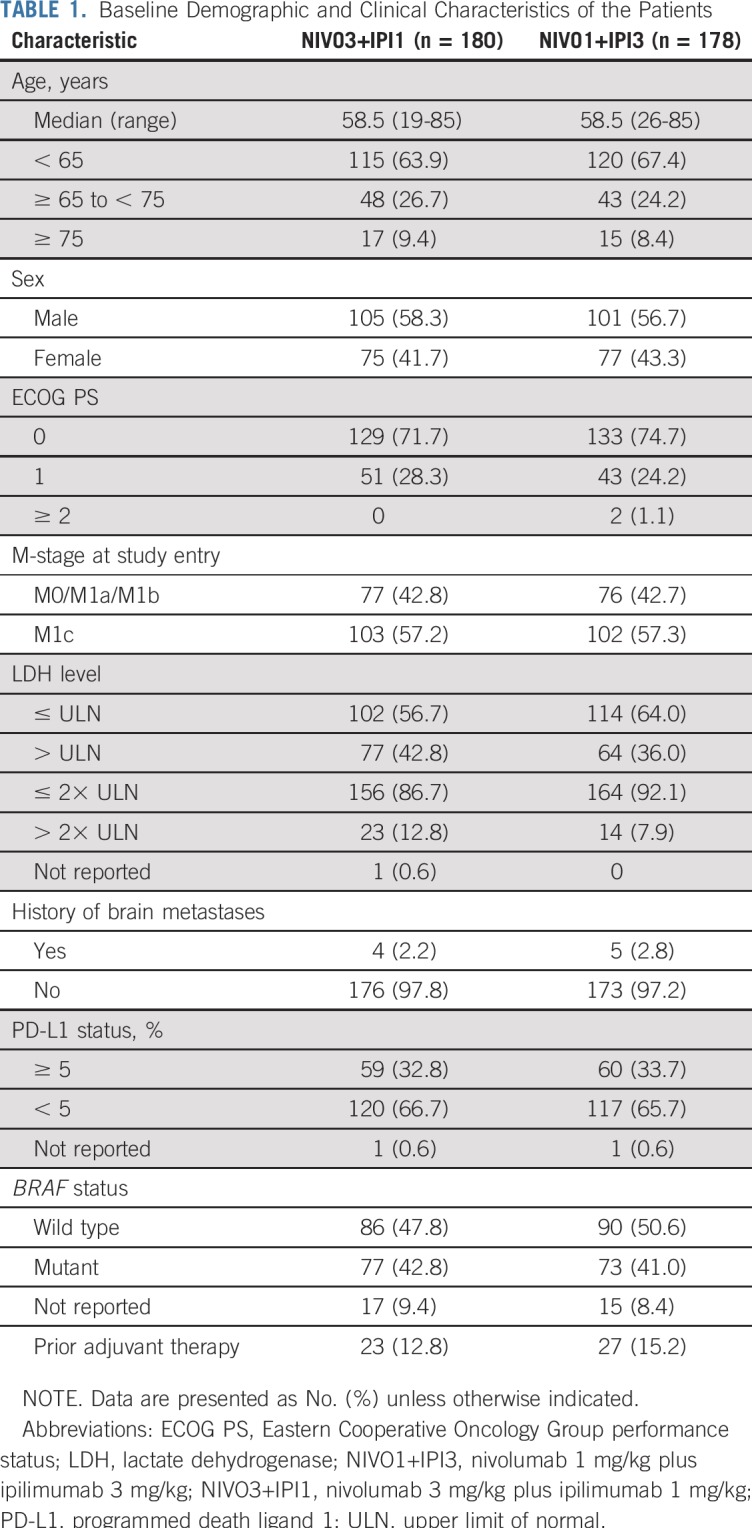
Baseline characteristics were generally well balanced between the two groups, with the exception of a higher percentage of patients with elevated lactate dehydrogenase (LDH) levels in the NIVO3+IPI1 group (Table 1). In the initial treatment period (part 1), patients in both groups received a median of four doses of NIVO (range, one to four doses) and four doses of IPI (range, one to four doses); 123 (68.3%) of 180 patients in the NIVO3+IPI1 group and 102 (57.3%) of 178 patients in the NIVO1+IPI3 group received all four doses of the combination. Median duration of therapy was 4.4 months and 2.3 months, respectively. During part 2 of the treatment period, 102 patients (56.7%) in the NIVO3+IPI1 group and 75 patients (42.1%) in the NIVO1+IPI3 group received NIVO maintenance therapy, with a median of 15.0 doses (range, one to 23 doses) and 16.0 doses (range, one to 23 doses), respectively. Median duration of therapy in part 2 of the study was 14.7 months in both groups. Subsequent systemic therapy was received by 43 patients (23.9%) in the NIVO3+IPI1 group and 41 patients (23.0%) in the NIVO1+IPI3 group.
TABLE 1.
Baseline Demographic and Clinical Characteristics of the Patients
Safety
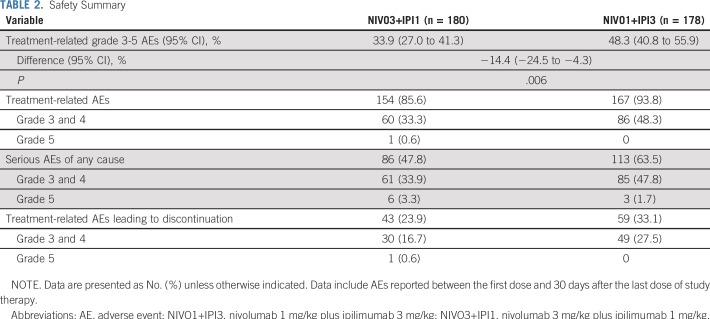
Incidence of treatment-related grade 3 to 5 AEs was significantly lower in the NIVO3+IPI1 group (61 [33.9%] of 180 patients; 95% CI, 27.0% to 41.3%) compared with the NIVO1+IPI3 group (86 [48.3%] of 178 patients; 95% CI, 40.8% to 55.9%; P = .006; Table 2). Grade 5 treatment-related AEs were reported in one patient in the NIVO3+IPI1 group (rhabdomyolysis and autoimmune myocarditis). One patient in the NIVO1+IPI3 group experienced treatment-related grade 2 hypophysitis and died 23 days after onset following an episode of sepsis. The investigator reported that hypophysitis and probable encephalitis contributed to the patient’s death. Grade 3 and 4 serious AEs were reported in 33.9% of patients in the NIVO3+IPI1 group and 47.8% in the NIVO1+IPI3 group, with treatment-related grade 3 and 4 AEs leading to discontinuation in 16.7% and 27.5% of patients, respectively (Table 2).
TABLE 2.
Safety Summary
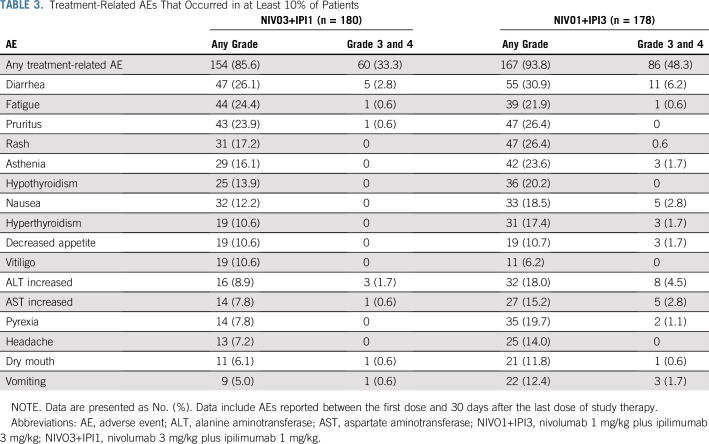
Rates of most treatment-related AEs were lower in the NIVO3+IPI1 group (Table 3); however, the overall lower incidence of treatment-related grade 3 and 4 AEs in the NIVO3+IPI1 group compared with the NIVO1+IPI3 group was primarily a result of lower rates of hepatic (7.2% v 16.3%), GI (6.1% v 10.7%), and endocrine (2.8% v 7.3%) AEs (Data Supplement). Median time to the onset of treatment-related select AEs of any grade ranged from 5.1 weeks to 15.7 weeks in the NIVO3+IPI1 group and from 2.4 weeks to 9.1 weeks in the NIVO1+IPI3 group (Data Supplement). Skin AEs occurred early relative to other AE categories, whereas renal and pulmonary AEs occurred later after treatment initiation. Most select AEs were manageable and resolved with immune-modulating medications (Data Supplement). For endocrine AEs of any grade, 30% and 40% resolved in the NIVO3+IPI1 and NIVO1+IPI3 groups, respectively, although unresolved AEs were manageable with sustained hormone-replacement therapy. Infusion-related reactions of any grade occurred in nine patients (5.0%) in the NIVO3+IPI1 group and in four patients (2.2%) in the NIVO1+IPI3 group.
TABLE 3.
Treatment-Related AEs That Occurred in at Least 10% of Patients
Among patients who received NIVO maintenance therapy, treatment-related AEs of any grade were reported in 78.4% in the NIVO3+IPI1 group and in 80.0% in the NIVO1+IPI3 group (Data Supplement). Treatment-related AEs of grade 3 and 4 were reported in 17.6% and 16.0% of patients in the NIVO3+IPI1 and NIVO1+IPI3 groups and led to discontinuation in 5.9% and 2.7%, respectively. There were no reported deaths as a result of study drug toxicity during maintenance therapy.
Efficacy
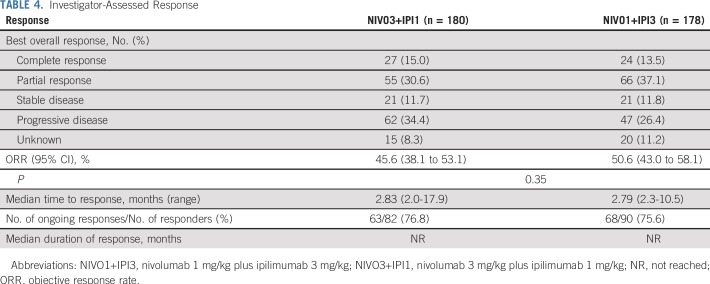
In descriptive analyses, investigator-assessed ORR was 45.6% (95% CI, 38.1% to 53.1%) in the NIVO3+IPI1 group and 50.6% (95% CI, 43.0% to 58.1%) in the NIVO1+IPI3 group, with complete responses in 15.0% and 13.5% of patients, respectively (Table 4). Median time to response was approximately 2.8 months in both groups. At the time of the current analysis, 63 (76.8%) of 82 responses in the NIVO3+IPI1 group and 68 (75.6%) of 90 responses in the NIVO1+IPI3 group were ongoing. Median duration of response was not reached in either group. Median reduction in tumor volume was −41.3% and −55.9% in the NIVO3+IPI1 and NIVO1+IPI3 groups, respectively (Data Supplement). ORR was similar between NIVO3+IPI1 and NIVO1+IPI3 across patient subgroups, including baseline LDH levels, BRAF mutation status, and PD-L1 status, although no definitive conclusions can be made from these analyses (Data Supplement).
TABLE 4.
Investigator-Assessed Response
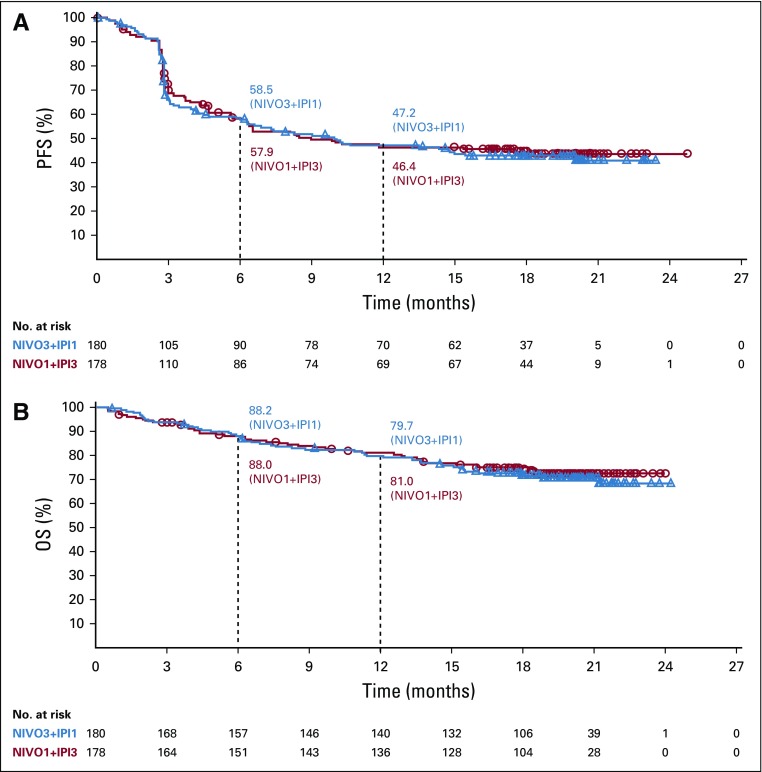
Survival outcomes seemed to be similar between the two treatment groups (Fig 2). Median PFS was 9.92 months in the NIVO3+IPI1 group and 8.94 months in the NIVO1+IPI3 group (HR, 1.06; 95% CI, 0.79 to 1.42). Twelve-month PFS rates were 47.2% and 46.4% in the NIVO3+IPI1 and NIVO1+IPI3 groups, respectively (Fig 2A). Median OS was not reached in either group (HR, 1.09; 95% CI, 0.73 to 1.62). Twelve-month OS rates were 79.7% and 81.0% in the NIVO3+IPI1 and NIVO1+IPI3 groups, respectively (Fig 2B).
FIG 2.
Kaplan-Meier plot of (A) progression-free survival (PFS) and (B) overall survival (OS) in patients who received NIVO3+IPI1 (nivolumab 3 mg/kg plus ipilimumab 1 mg/kg) or NIVO1+IPI3 (nivolumab 1 mg/kg plus ipilimumab 3 mg/kg). Symbols indicate censored observations. Median PFS was 9.92 months in the NIVO3+IPI1 group and 8.94 months in the NIVO1+IPI3 group (hazard ratio, 1.06; 95% CI, 0.79 to 1.42). Median OS was not reached in either group (hazard ratio, 1.09; 95% CI, 0.73 to 1.62).
HRQoL
Quality of life as measured by the European Organization for Research and Treatment of Cancer QLQ-C30 Global Health Status remained stable through week 40 in both groups, with no mean change in score from baseline reaching a clinically meaningful difference at any time point in either group (Data Supplement). Similarly, health status as measured by the EQ-5D utility index remained stable through week 40 in both groups, with no mean change in score from baseline reaching a minimally important difference at any time point in either group. For the EQ-5D Visual Analog Scale, we observed a clinically meaningful improvement in the NIVO3+IPI1 group from week 24 through week 40, whereas an improvement from baseline in the NIVO1+IPI3 group was only observed at week 28.
DISCUSSION
The CheckMate 511 study met its primary end point, demonstrating a significantly lower incidence of treatment-related grade 3 to 5 AEs with NIVO3+IPI1 compared with the approved regimen, NIVO1+IPI3, in patients with previously untreated, advanced melanoma. On the basis of descriptive analyses, there were no clinically meaningful differences between groups for ORR, PFS, or OS at the time of the current analysis. Analyses of HRQoL demonstrated little to no impact of NIVO3+IPI1 or NIVO1+IPI3 on quality of life in a clinically meaningful way.
Our results showed that duration of therapy during combination treatment was longer for NIVO3+IPI1 treatment than for NIVO1+IPI3, which is consistent with the higher rate of discontinuation as a result of treatment-related AEs in the NIVO1+IPI3 group. Despite discontinuation because of AEs, analyses from the CheckMate 069 and 067 studies have demonstrated that patients who discontinued NIVO1+IPI3 as a result of an AE can still derive benefit without additional treatment.10,14 The lower rate of discontinuation as a result of treatment-related AEs in the NIVO3+IPI1 group led to more patients entering the NIVO maintenance phase than in the NIVO1+IPI3 group. For those who entered the maintenance phase, duration of therapy and the number of NIVO doses received were similar in both groups.
The overall lower incidence of treatment-related AEs in the NIVO3+IPI1 group compared with the NIVO1+IPI3 group was a result of lower rates of several treatment-related grade 3 and 4 AEs, particularly diarrhea, colitis, increased ALT and AST, and endocrine AEs. For endocrine AEs, such as hypophysitis, a lower incidence may lead to fewer patients requiring long-term hormone-replacement therapy. Of interest, the incidence of treatment-related grade 3 and 4 AEs with NIVO1+IPI3 in CheckMate 511 was lower than that initially reported with NIVO1+IPI3 in CheckMate 067 (48% v 55%).1 It is hypothesized that this apparent difference is because of improved management of AEs with the combination as a result of earlier recognition and management of events and greater experience among investigators with established management guidelines.
In a recent meta-analysis of a WHO pharmacovigilance database and records from seven academic centers, toxicity-related deaths with anti–PD-1/anti–CTLA-4 combination therapies were most commonly a result of colitis and myocarditis.15 In CheckMate 511, one grade 5 treatment-related AE of myocarditis was reported in the NIVO3+IPI1 group, but no case of colitis had a fatal outcome. Of note, rates of grade 3 and 4 colitis at 2.2% in the NIVO3+IPI1 group and 4.5% in the NIVO1+IPI3 group were lower than that observed in other studies of the combination.2,7,9,10 During maintenance therapy with NIVO 480 mg once every 4 weeks, as recently approved in the United States and European Union, a manageable safety profile was observed in both groups with no new safety signals and no deaths as a result of study drug toxicity.
We reported an initial analysis of secondary efficacy end points which were descriptive according to the study design. Our results show that efficacy measures were largely consistent between groups. ORR was numerically lower in the NIVO3+IPI1 group compared with the NIVO1+IPI3 group, but the small 5% difference was not statistically significant. PFS and OS were similar between groups in CheckMate 511 and were consistent with results from CheckMate 067; however, as shown in CheckMate 067 with NIVO1+IPI3 and NIVO alone,2,10 longer follow-up may differentiate survival outcomes between groups in CheckMate 511. Recently, results of the phase II Optimal Neo-adjuvant Combination Scheme of Ipilimumab and Nivolumab study were reported that evaluated two dosing regimens of IPI plus NIVO in patients with resected stage III melanoma before complete lymph node dissection. A lower incidence of grade 3 and 4 immune-related AEs was reported in patients who received 2× IPI 1 mg/kg + NIVO 3 mg/kg than in patients who received 2× IPI 3 mg/kg + NIVO 1 mg/kg (20% v 40%), but with a similar pathologic response rate (77% v 80%).16 These results suggest that NIVO combined with a lower dose of IPI may be appropriate for neoadjuvant treatment of melanoma.
Dual inhibition of CTLA-4 and PD-1 continues to demonstrate durable, long-term immunologic memory and clinical benefit for patients with advanced melanoma. In a recent 4-year update of data from the CheckMate 067 study, 53% of patients who were treated with NIVO1+IPI3 were alive, and among these patients, 71% were off study therapy and had not received subsequent systemic therapy at the time of the analysis.10 A complete response was achieved by 21% of patients who were treated with NIVO1+IPI3 in this study.10 Whereas the results of the CheckMate 511 study provide evidence for the safety of NIVO in combination with a lower dose of IPI, long-term survival outcomes are supported by data with NIVO1+IPI3 in CheckMate 067.10 Moreover, NIVO1+IPI3 has demonstrated clinical benefit in patients with BRAF-mutated tumors,2,10 elevated LDH levels,2,10 brain metastases,17,18 and mucosal melanoma19 and may thus be considered as a first-line treatment of these patients.
The CheckMate 511 study was the first large, randomized trial, to our knowledge, to evaluate low-dose IPI in combination with the approved dose of an anti–PD-1 agent in advanced melanoma. Results of this study demonstrate that the safety profile of NIVO3+IPI1 is superior to that of NIVO1+IPI3, which is consistent with the results of other studies in earlier stages of melanoma. Whereas the analyses of efficacy end points are descriptive, this study provides important information for health care providers to consider regarding the benefit–risk profile of anti–PD-1 agents combined with IPI, particularly in certain populations—such as, elderly patients. Patients in CheckMate 511 will continue to be observed to assess long-term overall survival.
Data Sharing
Bristol-Myers Squibb’s policy on data sharing may be found online.20
ACKNOWLEDGMENT
The authors thank the patients who participated in the CheckMate 511 trial and the clinical study teams. The authors also thank Ana Moreno and Ans Valgaeren, who served as the study protocol managers; Ravi Dangeti, who served as the statistical programmer; and Andriy Moshyk for contributions to the health-related quality of life analyses. Professional medical writing and editorial assistance were provided by Ward A. Pedersen, PhD, and Cara Hunsberger at StemScientific, an Ashfield Company, funded by Bristol-Myers Squibb.
Footnotes
Presented in part at the European Society for Medical Oncology 2018 Congress, Munich, Germany, October 19-23, 2018.
Supported by Bristol-Myers Squibb (Princeton, NJ) and ONO Pharmaceutical Company (Osaka, Japan).
Processed as a Rapid Communication manuscript.
Clinical trial information: NCT02714218.
AUTHOR CONTRIBUTIONS
Conception and design: Celeste Lebbé, Caroline Robert, Dirk Schadendorf, Linda Rollin
Provision of study material or patients: Celeste Lebbé, Ivan Marquez-Rodas, Caroline Robert, Piotr Rutkowski, Alexander M. Menzies, Thomas Eigentler, Michael Smylie, Dirk Schadendorf, Mazhar Ajaz, Inge Marie Svane, Rene Gonzalez
Collection and assembly of data: Celeste Lebbé, Nicolas Meyer, Laurent Mortier, Ivan Marquez-Rodas, Caroline Robert, Piotr Rutkowski, Alexander M. Menzies, Thomas Eigentler, Paolo A. Ascierto, Dirk Schadendorf, Mazhar Ajaz, Rene Gonzalez, Linda Rollin, Abdel Saci, Jacopo Pigozzo
Data analysis and interpretation: Celeste Lebbé, Laurent Mortier, Ivan Marquez-Rodas, Caroline Robert, Piotr Rutkowski, Alexander M. Menzies, Paolo A. Ascierto, Michael Smylie, Dirk Schadendorf, Mazhar Ajaz, Inge Marie Svane, Rene Gonzalez, Linda Rollin, Jennifer Lord-Bessen, Abdel Saci, Elena Grigoryeva, Jacopo Pigozzo
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST AND DATA AVAILABILITY STATEMENT
Evaluation of Two Dosing Regimens for Nivolumab in Combination With Ipilimumab in Patients With Advanced Melanoma: Results From the Phase IIIb/IV CheckMate 511 Trial
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Celeste Lebbé
Honoraria: Roche, Bristol-Myers Squibb, Novartis, Amgen, MSD, Pierre Fabre, Pfizer, Incyte
Consulting or Advisory Role: Bristol-Myers Squibb, MSD, Novartis, Amgen, Roche
Speakers' Bureau: Roche, Bristol-Myers Squibb, Novartis, Amgen
Research Funding: Roche (Inst), Bristol-Myers Squibb (Inst)
Travel, Accommodations, Expenses: Bristol-Myers Squibb
Other Relationship: Avantis Medical Systems
Nicolas Meyer
Consulting or Advisory Role: Bristol-Myers Squibb, MSD Oncology, Genentech, Novartis, Pierre Fabre
Research Funding: Bristol-Myers Squibb (Inst), MSD Oncology (Inst)
Laurent Mortier
Travel, Accommodations, Expenses: Genentech, Novartis, Bristol-Myers Squibb
Ivan Marquez-Rodas
Consulting or Advisory Role: Bristol-Myers Squibb, MSD, Novartis, Roche, Amgen, Sanofi, AstraZeneca, Merck Serono, Incyte, Bioncotech, Pierre Fabre
Travel, Accommodations, Expenses: Bristol-Myers Squibb, MSD, Roche, Bioncotech
Caroline Robert
Consulting or Advisory Role: Bristol-Myers Squibb, Roche, Merck, Amgen, Novartis, GlaxoSmithKline, Pierre Fabre, Merck Serono
Piotr Rutkowski
Honoraria: Bristol-Myers Squibb, MSD, Novartis, Roche, Eli Lilly, Pfizer
Consulting or Advisory Role: Novartis, Blueprint Medicines, Bristol-Myers Squibb, Pierre Fabre, MSD, Amgen
Speakers' Bureau: Pfizer, Novartis
Research Funding: Novartis (Inst), Roche (Inst), Bristol-Myers Squibb (Inst)
Travel, Accommodations, Expenses: Orphan Europe, Pierre Fabre
Alexander M. Menzies
Consulting or Advisory Role: MSD Oncology, Novartis, Pierre Fabre, Bristol-Myers Squibb, Roche
Travel, Accommodations, Expenses: Bristol-Myers Squibb
Thomas Eigentler
Consulting or Advisory Role: Bristol-Myers Squibb, Roche
Speakers' Bureau: Roche, MSD, Bristol-Myers Squibb, Novartis
Paolo A. Ascierto
Consulting or Advisory Role: Bristol-Myers Squibb, Genentech, Merck Sharp & Dohme, Novartis, Amgen, Array BioPharma, Merck Serono, Pierre Fabre, Newlink Genetics, Genmab, Incyte, MedImmune, AstraZeneca, Syndax, Sun Pharma, Sanofi, Idera, Ultimovacs, Sandoz, Immunocore
Research Funding: Bristol-Myers Squibb (Inst), Genentech (Inst), Array BioPharma (Inst)
Travel, Accommodations, Expenses: Merck Sharp & Dohme
Michael Smylie
Honoraria: Bristol-Myers Squibb, Novartis, Merck, Roche
Consulting or Advisory Role: Merck, Bristol-Myers Squibb
Dirk Schadendorf
Honoraria: Genentech, Novartis, Amgen, Bristol-Myers Squibb, Merck Sharp & Dohme, Sysmex, Immunocore, Grünenthal Group, Merck Serono, Agenus, Array BioPharma, AstraZeneca, LEO Pharma, Incyte, Pfizer, Pierre Fabre, Philogen, Regeneron, 4SC, Mologen
Consulting or Advisory Role: Genentech, Novartis, Bristol-Myers Squibb, Merck Sharp & Dohme, Merck Serono, Amgen, Immunocore, Incyte, 4SC, Pierre Fabre, Mologen, Sanofi, Regeneron
Speakers' Bureau: Roche, Bristol-Myers Squibb, Merck Sharp & Dohme, Novartis, Amgen, Incyte, Pierre Fabre
Research Funding: Bristol-Myers Squibb (Inst), Novartis (Inst)
Travel, Accommodations, Expenses: Genentech, Bristol-Myers Squibb, Amgen, Merck, Merck Serono, Novartis
Mazhar Ajaz
Travel, Accommodations, Expenses: Bristol-Myers Squibb
Inge Marie Svane
Honoraria: MSD, Bristol-Myers Squibb, Novartis, Incyte, Sanofi, Pierre Fabre, AbbVie, Genentech
Consulting or Advisory Role: MSD, Novartis, Incyte, Pierre Fabre
Research Funding: Bristol-Myers Squibb (Inst)
Travel, Accommodations, Expenses: MSD, Novartis, Pfizer
Rene Gonzalez
Stock and Other Ownership Interests: AstraZeneca, GlaxoSmithKline, Eli Lilly, Johnson & Johnson, Merck, Novartis, Pfizer, Procter & Gamble, Sanofi
Consulting or Advisory Role: Array BioPharma, Genentech, Bristol-Myers Squibb, Novartis, Vavotar
Research Funding: Millennium Pharmaceuticals, Takeda, Boston Biomedical, Bristol-Myers Squibb, Checkmate Pharmaceuticals, Incyte, Syndax, Genentech, Tesaro
Travel, Accommodations, Expenses: Incyte, Novartis, Bristol-Myers Squibb, Array BioPharma, Genentech, Newlink Genetics, Pharmatech
Linda Rollin
Employment: Bristol-Myers Squibb
Stock and Other Ownership Interests: Bristol-Myers Squibb, Abbott Laboratories, AbbVie
Jennifer Lord-Bessen
Employment: Bristol-Myers Squibb
Abdel Saci
Employment: Bristol-Myers Squibb
Stock and Other Ownership Interests: Bristol-Myers Squibb
No other potential conflicts of interest were reported.
REFERENCES
- 1.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2017;377:1345–1356. doi: 10.1056/NEJMoa1709684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hellmann MD, Ciuleanu TE, Pluzanski A, et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med. 2018;378:2093–2104. doi: 10.1056/NEJMoa1801946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378:1277–1290. doi: 10.1056/NEJMoa1712126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Janjigian YY, Bendell J, Calvo E, et al. CheckMate-032 study: Efficacy and safety of nivolumab and nivolumab plus ipilimumab in patients with esophagogastric cancer. J Clin Oncol. 2018;36:2836–2844. doi: 10.1200/JCO.2017.76.6212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122–133. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Callahan MK, Kluger H, Postow MA, et al. Nivolumab plus ipilimumab in patients with advanced melanoma: Updated survival, response, and safety data in a phase I dose-escalation study. J Clin Oncol. 2018;36:391–398. doi: 10.1200/JCO.2017.72.2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Postow MA, Chesney J, Pavlick AC, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372:2006–2017. doi: 10.1056/NEJMoa1414428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hodi FS, Chesney J, Pavlick AC, et al. Combined nivolumab and ipilimumab versus ipilimumab alone in patients with advanced melanoma: 2-year overall survival outcomes in a multicentre, randomised, controlled, phase 2 trial. Lancet Oncol. 2016;17:1558–1568. doi: 10.1016/S1470-2045(16)30366-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hodi FS, Chiarion-Sileni V, Gonzalez R, et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol. 2018;19:1480–1492. doi: 10.1016/S1470-2045(18)30700-9. [DOI] [PubMed] [Google Scholar]
- 11.Schadendorf D, Larkin J, Wolchok J, et al. Health-related quality of life results from the phase III CheckMate 067 study. Eur J Cancer. 2017;82:80–91. doi: 10.1016/j.ejca.2017.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Osoba D, Rodrigues G, Myles J, et al. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol. 1998;16:139–144. doi: 10.1200/JCO.1998.16.1.139. [DOI] [PubMed] [Google Scholar]
- 13.Pickard AS, Neary MP, Cella D. Estimation of minimally important differences in EQ-5D utility and VAS scores in cancer. Health Qual Life Outcomes. 2007;5:70. doi: 10.1186/1477-7525-5-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schadendorf D, Wolchok JD, Hodi FS, et al. Efficacy and safety outcomes in patients with advanced melanoma who discontinued treatment with nivolumab and ipilimumab because of adverse events: A pooled analysis of randomized phase II and III trials. J Clin Oncol. 2017;35:3807–3814. doi: 10.1200/JCO.2017.73.2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang DY, Salem JE, Cohen JV, et al. Fatal toxic effects associated with immune checkpoint inhibitors: A systematic review and meta-analysis. JAMA Oncol. 2018;4:1721–1728. doi: 10.1001/jamaoncol.2018.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blank CU, Rozeman EA, Menzies AM, et al. OpACIN-neo: A multicenter phase II study to identify the optimal neo-adjuvant combination scheme of ipilimumab (IPI) and nivolumab (NIVO) Ann Oncol. 2018;29 suppl 8; abstr LBA42. [Google Scholar]
- 17.Long GV, Atkinson V, Lo S, et al. Combination nivolumab and ipilimumab or nivolumab alone in melanoma brain metastases: A multicentre randomised phase 2 study. Lancet Oncol. 2018;19:672–681. doi: 10.1016/S1470-2045(18)30139-6. [DOI] [PubMed] [Google Scholar]
- 18.Tawbi HA, Forsyth PA, Algazi A, et al. Combined nivolumab and ipilimumab in melanoma metastatic to the brain. N Engl J Med. 2018;379:722–730. doi: 10.1056/NEJMoa1805453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.D’Angelo SP, Larkin J, Sosman JA, et al. Efficacy and safety of nivolumab alone or in combination with ipilimumab in patients with mucosal melanoma: A pooled analysis. J Clin Oncol. 2017;35:226–235. doi: 10.1200/JCO.2016.67.9258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bristol-Myers Squibb Data sharing request process. https://www.bms.com/researchers-and-partners/independent-research/data-sharing-request-process.html