Abstract
Background
In learning and memory tasks, requiring visual spatial memory (VSM), males exhibit superior performance to females (a difference attributed to the hormonal influence of estrogen). This study examined the influence of phytoestrogens (estrogen-like plant compounds) on VSM, utilizing radial arm-maze methods to examine varying aspects of memory. Additionally, brain phytoestrogen, calbindin (CALB), and cyclooxygenase-2 (COX-2) levels were determined.
Results
Female rats receiving lifelong exposure to a high-phytoestrogen containing diet (Phyto-600) acquired the maze faster than females fed a phytoestrogen-free diet (Phyto-free); in males the opposite diet effect was identified. In a separate experiment, at 80 days-of-age, animals fed the Phyto-600 diet lifelong either remained on the Phyto-600 or were changed to the Phyto-free diet until 120 days-of-age. Following the diet change Phyto-600 females outperformed females switched to the Phyto-free diet, while in males the opposite diet effect was identified.
Furthermore, males fed the Phyto-600 diet had significantly higher phytoestrogen concentrations in a number of brain regions (frontal cortex, amygdala & cerebellum); in frontal cortex, expression of CALB (a neuroprotective calcium-binding protein) decreased while COX-2 (an inducible inflammatory factor prevalent in Alzheimer's disease) increased.
Conclusions
Results suggest that dietary phytoestrogens significantly sex-reversed the normal sexually dimorphic expression of VSM. Specifically, in tasks requiring the use of reference, but not working, memory, VSM was enhanced in females fed the Phyto-600 diet, whereas, in males VSM was inhibited by the same diet. These findings suggest that dietary soy derived phytoestrogens can influence learning and memory and alter the expression of proteins involved in neural protection and inflammation in rats.
Background
Estrogen as a neuroprotective and neurotrophic factor: 1) influences memory and cognition [1,2], 2) attenuates the extent of cell death resulting from brain injuries (i.e., from cerebrovascular stroke and neurotrauma) [1-5] and, 3) decreases the risk and delays the onset of neurological disorders such as, Alzheimer's disease [4,5]. Numerous studies indicate that estrogen is essential for optimal brain function [1-5], as estrogen has been shown to increase cerebral blood flow [1-5], act as an anti-inflammatory agent [1-5], and enhance activity at neuronal synapses [1-5]. Through a variety of mechanisms, estrogen strongly influences memory and cognition; a type of cognition, which is especially dependant on gonadal hormones (i.e. estradiol), is visual spatial memory (VSM) [6-12].
In learning and memory tasks, which require the use of visual spatial cues, males acquire and exhibit superior performance to females [13-20]. This sex difference in rats has been attributed to the hormonal influence of estrogen (presumably by way of its in situ conversion from testosterone by the aromatase enzyme in brain) [19-22], and is observable morphologically in visually spatial critical brain areas (i.e. frontal cortex & hippocampus [15-17]
In this regard, endocrine disrupters are environmental chemicals that mimic or modulate the physiological effects of steroid hormones, especially that of estrogens [23,24]. Of all of the considered estrogenic endocrine disrupters examined thus far, phytoestrogens have been extensively studied [23,24] (however, little research has been conducted examining VSM in relationship to phytoestrogens). Phytoestrogens are plant compounds that are structurally and functionally similar to estradiol and have the ability to selectively bind estrogen receptors (estrogen receptor β greater than estrogen receptor α; [25]). Phytoestrogens have received increased investigative attention due to their potential protective effects against age-related diseases (e.g. cardiovascular disease and osteoporosis) and hormone-dependent cancers (i.e., breast and prostate cancer; [26-37]). These estrogen-like molecules (with a diphenolic nonsteroidal structure) are found in many plants but are especially abundant in soy products [26-37] which are used as the major protein source in all natural-ingredient, commercially available, rodent diets (ranging from 200 to 600 μg phytoestrogens/gram diet [36,38]). Therefore, animals ingesting these diets are continually exposed to endocrine-active compounds [36,39-47].
The available research regarding cognitive function and phytoestrogens suggests that large amounts of phytoestrogens, consumed as tofu, have an adverse influence on cognitive ability in men, where decreased brain weight, increased ventricular size and dementia have been reported [44]. In ovariectomized female rats, on the other hand, phytoestrogen treatments resulted in a dose-dependent improvement of VSM [45]. This improvement in cognitive ability in phytoestrogen treated females may be due in part to the increased presence of choline acetyltransferase messenger RNA in the frontal cortex, which has been shown to be associated with protection and enhancement of cognitive function [45]. Furthermore, we have shown that phytoestrogens significantly affect the brain calcium-binding protein calbindin (CALB), which acts as a buffer by binding intracellular calcium and plays an important role in mediating cell proliferation, programmed cell death (apoptosis), and neurotoxicity [48-52]. This neuroprotective mechanism via CALB appears to be important in neurological disorders such as Parkinson's disease and Huntington's disease [48-52]. Additionally, Cyclooxygenase-2 (COX-2) seems to play an important role in mediating functional neuronal maturation and responses to certain stimuli in the brain [53-55]. The expression of COX-2 is associated with key pathophysiologic events in Alzheimer's disease: deposition of beta-amyloid protein in neuritic plaques within the hippocampus and cortex [53-55]. Of particular importance to this study is the fact that COX-2 has been shown to be influenced by steroidal hormones [53-55]. Therefore, the expression of COX-2 in the frontal cortex may also be hormonally regulated and relevant to cognitive decline.
The design of this study was to examine the influence of phytoestrogens (present via soy in rodent diets) on VSM in three separate experiments utilizing radial arm maze methods to examine varying aspects of memory. Additionally, brain regions critical for diverse parameters of VSM (working vs. reference memory) were assayed to determine phytoestrogen, CALB and COX-2 levels. Our laboratory has previously reported the phytoestrogen levels in the diets used in this study and circulating plasma and brain phytoestrogen levels obtained in rats exposed to these diets [39-43]. Notably, we have shown that rats fed the Phyto-600 diet have phytoestrogen plasma levels similar to that of a human Asian population consuming high amounts of soy protein per day and the low levels of plasma phytoestrogens from the Phyto-free diet fed animals are reflective of a Western population where soy foods are rarely consumed [22,40,42]. Therefore, we have established an animal model that approaches the human population in circulating plasma phytoestrogen levels based upon consumption of these phytoestrogen (soy-rich vs. soy-free) diets.
Results
Acquisition of the Radial Arm Maze (shaping to criterion)
Experiment 1
Males fed the Phyto-600 diet (lifelong) required significantly more trials (1 trial/day) to acquire the maze than males fed the Phyto-free diet (lifelong) (t(30) = 2.118, p < 0.05; see figure 1a).
Figure 1.
Dietary soy phytoestrogens influence on acquisition (shaping to criterion) of the radial arm maze. Male (a; experiment 1) and female (b; experiment 2) Long-Evans rats were fed either a phytoestrogen rich (Phyto-600) diet or a phytoestrogen free (Phyto-free) diet lifelong (from conception to adulthood). a. Number of trials (Mean + SEM; one trial per day) required for males (experiment 1) to reach a set criterion level of performance in the eight-arm radial maze. * Phyto-600 fed females reached criterion significantly earlier than Phyto-free fed females (p < 0.05) b. Number of trials (Mean + SEM; one trial per day) required for females (experiment 2) to reach a set criterion level of performance in the eight-arm radial maze. * Phyto-free fed males reached criterion significantly earlier than Phyto-600 fed males (p < 0.05)
Experiment 2
In a separate study, in complete opposition to experiment 1 (in experiment 2), phytoestrogens, within diet, significantly altered acquisition of the maze in females. Females fed the Phyto-600 diet (lifelong) acquired the maze in significantly fewer trials than did females fed the Phyto-free diet (lifelong) (t(30) = 2.173, p < 0.05). These results are presented in figure 1b.
Experiment 3
In this study, male and female rats were examined together. Examining sexually dimorphic VSM, in animals fed the Phyto-600 diet, males acquired the maze in significantly fewer trials than females (F(1,22) = 16.58, p < 0.05), (before the diet switch). As can be seen in figure 2, males acquired the maze, on average, 4 days earlier than females.
Figure 2.
Dietary soy phytoestrogens influence on acquisition (shaping to criterion) of the radial arm maze. Male and female (experiment 3) Long-Evans rats received life-long exposure to a high-phytoestrogen containing diet (Phyto-600 diet, from conception to adulthood). Number of trials (Mean + SEM; one trial per day) required for males and females to reach a set criterion level of performance in the eight-arm radial maze by Long-Evans rats. * males reached criterion significantly earlier than females (p < 0.05)
Eight-Arm Task
Experiments 1 & 2
Dietary phytoestrogens did not alter working memory as assessed by the 8-arm task in either females or males (p > 0.05 respectively; data not shown) when these animals by diet were tested separately (by sex). Therefore, an across sex by diet comparison was not appropriate for these separate data sets.
Experiment 3
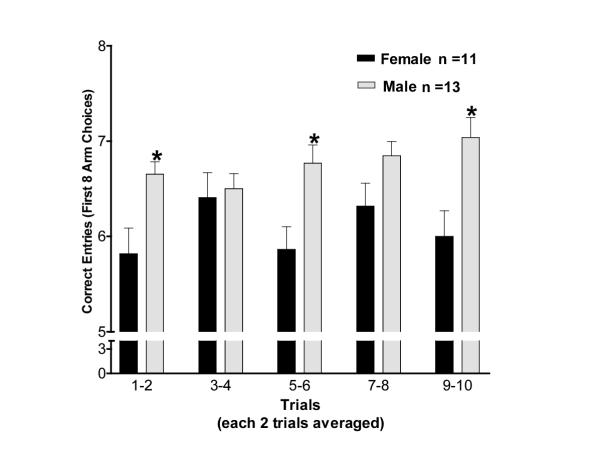
When males and females were tested within the same experiment (and exposed to the same diet, Phyto-600, again before the diet switch), consistent with previous reports, on sexual dimorphism in the radial arm maze, males performed with more accuracy than females in the 8-arm task. A significant sex difference was found on maze performance in the 8-arm task (p < 0.05). Males entered significantly more correct arms than females during trials 1–2, 5–6 and trials 9–10. These results are presented in figure 3.
Figure 3.
Dietary soy phytoestrogens influence on an 8-arm (working memory) task in the radial arm maze. Long-Evans rats (males and females) received life-long exposure to a high-phytoestrogen containing diet (Phyto-600, from conception to adulthood). Number of correct arm choices, in the first eight arm entries (Mean + SEM; average of 2 trials), made by male and female rats (experiment 3) in the eight-arm radial maze. A correct arm choice was defined as an entry into an arm not yet visited in the trial. * males made significantly more correct choices than females (p < 0.05)
Experiment 3 Four-Arm Task (baited/unbaited)
In contrast, on the 4-arm task, male VSM dominance appeared to be dependant upon diet (or after the diet switch). Multivariate analysis of variance resulted in a significant interaction of sex and diet (p < 0.05), see figure 4. The Phyto-600 diet improved performance in females while hampering performance in males. Further analysis via post hoc comparisons revealed that Phyto-600 females made significantly more correct arm choices than females switched to the Phyto-free diet during trial 10–12 (p < 0.05) and Phyto-600 males (on trials 10–12 and 13–15; p < 0.05). Males switched to the Phyto-free diet displayed significantly more correct arm choices than females switched to the Phyto-free diet (trials 7–9 and 13–15; p < 0.05) and lifelong fed Phyto-600 males (trials 10–12 and 13–15; p < 0.05). These results are shown in figure 4. Further multivariate statistical analysis and post hoc comparisons were used to identify differences in errors committed in the radial-arm maze. These data revealed a significant interaction of sex and diet on the incidence of reference errors during trials 10–12 and 13–15 (p < 0.05) but no significant differences in working or working/reference errors (p > 0.05). Pairwise comparisons revealed that lifelong fed Phyto-600 males committed significantly more reference memory errors than males switched to the Phyto-free diet and lifelong fed Phyto-600 females (p < 0.05) data presented in figure 5a. Additionally, in analyzing the amount of time required for subjects to enter the first 4 arms in a given trial it was found that a significant diet effect (independent of sex) was apparent where animals fed the Phyto-600 diet traveled through the maze significantly faster than animals fed the Phyto-free diet (trials 10–12; p < 0.05) figure 5b.
Figure 4.
Dietary soy phytoestrogen's influence on a 4-arm (working and reference memory) task (baited/unbaited) in the radial arm maze. Long-Evans rats (males and females) received a life-long exposure to a high-phytoestrogen containing diet (Phyto-600, from conception to adulthood). In adulthood, one-half of the total number of male or female (random cycling) rats were either: 1) kept on the original high phytoestrogen diet (Phyto-600) or 2) changed to a phytoestrogen-free (Phyto-free) diet. Number of correct arm choices, made in the first four arm entries (Mean ± SEM; average of 3 trials). A correct choice was defined as an entry into a baited arm not yet visited in the trial. a. Phytoestrogen-600 females made significantly more correct choices than Phytoestrogen-free females a' and Phytoestrogen-600 males a" (p < 0.05) on trials 1–12. b. Phytoestrogen-600 females made significantly more correct choices than Phytoestrogen-600 males b' (p < 0.05) on trials 10–12. c. Phytoestrogen-free males made significantly more correct choices than Phytoestrogen-free females c' (p < 0.05) on trials 7–9. d. Phytoestrogen-free males made significantly more correct choices than Phytoestrogen-600 males d' (p < 0.05) on trials 10–12. e. Phytoestrogen-free males made significantly more correct choices than Phytoestrogen-600 males e" and Phytoestrogen-free females e' (p < 0.05) on trials 13–15.
Figure 5.
a. Number of reference errors (Mean ± SEM), in the first four arm entries (of the baited/unbaited four-arm task), made by males fed either the Phyto-600 diet (lifelong) or the Phyto-600 diet from conception and then changed to the Phyto-free diet, in adulthood, for 40 days. * Males fed the Phyto-free diet scored significantly fewer reference errors than males fed the Phyto-600 diet (p < 0.05). A Reference Error was scored when an animal entered a non-baited arm b. The amount of time, in seconds (Mean ± SEM), male and female animals fed either the Phyto-600 diet (lifelong) or the Phyto-600 diet from conception and then changed to the Phyto-free diet in adulthood, required to traverse to the ends of their first 4-arm entries in the radial maze. * Phyto-600 animals traveled to the ends of their first 4-arm entries significantly faster than Phyto-free fed animals (p < 0.05).
Brain Content-Phytoestrogen Levels
Brain phytoestrogen levels are displayed in Table 1. In general, in all brain regions examined (except the hippocampus) Phyto-600 fed males displayed significantly higher phytoestrogen levels compared to Phyto-free fed males. In the amygdala, phytoestrogen levels were 3-fold higher in Phyto-600 males compared to Phyto-free values. Whereas, in the hippocampus there was not a significant difference between the Phyto-600 and Phyto-free fed males. However, when the frontal cortex and cerebellum were examined, where an abundance of ER-β receptors are present, Phyto-600 males displayed a 47-fold and 9-fold higher phytoestrogens levels, respectively, compared to Phyto-free male values. In each brain site examined (except the cerebellum), the major phytoestrogen metabolite was equol and there were relatively low levels of daidzein and genistein.
Table 1.
Brain Phytoestrogen Concentrations (Mean ± SEM) determined in tissues isolated from the frontal cortex and hippocampus (brain structures critical for VSM) along with the amygdala and cerebellum brain regions, by time-resolved fluoroimmunoassay of male rats (pooled by treatment, n = 4 per group).
| Phyto-600 | daidzein ng/g | genistein ng/g | equol ng/g | total ng/g |
| Frontal cortex | 271.0 (± 29.8)* | 295.5 (± 35.4)* | 705.3 (± 63.4)* | 1,271.8 (± 128.6)* |
| Hippocampus | 3.4 (± 0.4) | 3.1 (± 0.3) | 28.5 (± 3.4) | 35.0 (± 4.1) |
| Amygdala | 5.5 (± 0.6)* | 9.9 (± 1.1)* | 57.5 (± 5.2)* | 72.9 (± 6.9)* |
| Cerebellum | 58.8 (± 29.8)* | 126.8 (± 29.8)* | 33.4 (± 29.8)* | 219.0 (± 29.8)* |
| Phyto-free | daidzein ng/g | genistein ng/g | equol ng/g | total ng/g |
| Frontal cortex | 2.5 (± 0.3) | 1.2 (± 0.1) | 23.4 (± 2.8) | 27.1 (± 3.2) |
| Hippocampus | 1.4 (± 0.1) | BDL | 25.5 (± 3.1) | 26.9 (± 3.2) |
| Amygdala | 1.9 (± 0.2) | 0.6 (± 0.1) | 22.7 (± 2.5) | 25.2 (± 2.8) |
| Cerebellum | 2.2 (± 0.2) | 0.8 (± 0.1) | 20.7 (± 2.1) | 23.7 (± 2.4) |
Phyto-600 refers to animals exposed to the Phyto-rich diet from 50 days of age until time of sacrifice at 120 days of age. Phyto-free refers to animals exposed to the Phyto-free diet from 50 days of age until sacrifice at 120 days of age. The phytoestrogen content that the animals were exposed to from conception until 50 days of age was approximately 300 ug/g of diet. BDL = below detectable limits (0.5 ng/g) *Phyto-600 fed males displayed significantly higher phytoestrogen levels compared to Phyto-free fed males.
Frontal Cortex and Hippocampal Calbindin
The frontal cortical CALB results are displayed in figure 6. The abundance of CALB from male Phyto-600 rats was significantly lower than males on the Phyto-free diet (F(1,10) = 30.7, p < 0.05). This suggests that high levels of dietary phytoestrogens significantly decreased frontal cortical CALB levels in male rats, however, in females, there was no significant diet effect on CALB levels and the band intensities were similar to males fed the Phyto-600 diet.
Figure 6.
Western analysis of frontal cortex CALB abundance in adult male rats exposed to either the Phyto-600 or Phyto-free diets. A. Densiometric analysis of CALB frontal cortex Western autoradiograms. B. Abundance of cytochromeoxidase subunit 4 served as controls (for loading). C. Histogram of CALB abundance by treatment. For each immunoblot, the cytochromeoxidase band was divided into the CALB band within each lane, then the lowest intensity band was assigned an arbitrary number (1) and all other bands were expressed as a fraction of this value. The immunoblots analyzed represent the Mean ± SEM, averaging the band values from 4 to 6 independent blots). * Calbindin levels are significantly decreased in Phyto-600 males compared to Phyto-free male values.
In examining hippocampal CALB levels between Phyto-600 vs. Phyto-free fed males, there were no significant alterations in CALB levels in the CA 1, CA 3 or dentate gyms regions by the diet treatments (data not shown).
Frontal Cortex COX-2
Western analysis of the same frontal cortex samples (as performed for the CALB Western analysis) revealed significantly lower COX-2 levels in Phyto-free males compared to Phyto-600 males (F(1,10) = 30.7, p < 0.05). These results are presented in figure 7. This suggests that high levels of dietary phytoestrogens significantly increased the abundance of COX-2 in the male rat frontal cortex. In females, similar to the CALB results above, there was no significant diet effect on COX-2 levels and the band intensities were almost identical to that of male Phyto-free values.
Figure 7.
Western analysis of frontal cortex COX-2 abundance in adult male rats exposed to either the Phyto-600 or Phyto-free diets. A. Densiometric analysis of COX-2 frontal cortex Western autoradiograms. B. Abundance of cytochromeoxidase subunit 4 served as controls (for loading). C. Histogram of COX-2 abundance by treatment. For each immunoblot, the cytochromeoxidase band was divided into the COX-2 band within each lane, then the lowest intensity band was assigned an arbitrary number (1) and all other bands were expressed as a fraction of this value. The immunoblots analyzed represent the Mean ± SEM, averaging the band values from 4 to 6 independent blots). * COX-2 levels are significantly decreased in Phyto-free males compared to Phyto-600 male values. Note: Due to the relatively low abundance of phytoestrogens in the hippocampus, and the ability to detect COX-2 via Western analysis this was not preformed in this brain tissue site.
Note: Due to the low abundance of phytoestrogens in the hippocampus, in both diet treatments, analysis of COX-2 was not preformed. Furthermore, in preliminary experiments hippocampal COX-2 signals, via Western analysis, were too weak to quantify.
Discussion
Phytoestrogen research has grown rapidly in recent years due to its potential health benefits in preventing age-related and hormone-dependent cancers [26-37]. In contrast very little research has been conducted to identify phytoestrogen effects on cognitive behavior. Because phytoestrogens are present in virtually all natural-ingredient rodent diets that use soy as a source of protein [36,38], and since these compounds are endocrine-active [27,41-49], it is important to determine whether the amounts present in rodent diets are sufficient to affect sexually dimorphic spatial ability.
The present study addressed these issues, in part, by investigating the influence of dietary phytoestrogens (present in a normal rodent diet), on visual spatial ability. In tasks requiring the use of spatial skills, a sexually dimorphic difference has been consistently demonstrated in which males reliably outperform females [6-12]. This sex difference is most likely due to the presence of testosterone or more likely its metabolite estradiol, in brain [13-20]. Because phytoestrogens have the ability to bind estrogen receptors and alter many of the biological responses that are evoked by physiological estrogens [25,39-46], we determined phytoestrogens affects on VSM in adult rats. Measurements of accuracy in acquisition (determined as trials required for shaping to a predetermined criterion) of the radial maze were diet dependent in that males (in experiment 1) fed the phytoestrogen-free diet and females (in experiment 2) fed the phytoestrogen-rich diet acquired the maze in fewer days than females or males fed the alternate diet. It should be noted that the dietary effects on body weight are the same for both male and female rats and therefore, do not account for the sexually dimorphic maze performance [39]. There was, however, no significant dietary effects observed in the 8-arm task performance in either males (experiment 1) or females (experiment 2). Measures of accuracy on the baited/unbaited four-arm task (experiment 3) demonstrated that a diet change in young adult animals (a change from Phyto-600 to Phyto-free) had a positive influence on the accuracy in males, but a negative influence on the accuracy in females. In a companion paper, the impact of hormonal manipulation and dietary phytoestrogens' influence on VSM is examined and validates several aspects of the findings from the present study.
The discrepancies in maze performance were expressed in two ways: (1) Phyto-600 males (exposed lifelong to the diet) committed more reference errors than males switched to the Phyto-free diet and (2) lifelong Phyto-600 fed males and females had increased mobility within the maze which seemed to be advantageous to females but disruptive to males. It is intriguing to speculate that, the increase in reference errors, but not working or working/reference errors, suggests that disruption of maze performance is occurring within the frontal cortex (but not the hippocampus) of lifelong Phyto-600 fed males. The drop in performance is directly correlated to phytoestrogen levels in brain and the effects of phytoestrogens on brain CALB and COX-2 levels, in that phytoestrogens were identified as having a negative relationship with CALB (-0.942) and a positive relationship with COX-2 (0.974). In all other studies conducted previously the determination of phytoestrogens in the diet, circulating in plasma and especially in brain have not been performed together. The exception to this line of investigation has been our laboratory, where, in general, we obtained similar values previously to those quantified in the present study [39,42], when overlapping diet exposure and brain regions were examined in Long-Evans and Sprague-Dawley rats [40,51].
As reported, phytoestrogens were 47 times higher in the frontal cortex of Phyto-600 vs. Phyto-free fed males where there is an abundance of ER-β receptors [2], while phytoestrogen levels were similar in these animals in the hippocampal region. Furthermore males fed the Phyto-600 diet displayed a significant decrease in frontal cortical CALB levels in comparison to males receiving the Phyto-free diet. As CALB plays an important role in regulating intraneuronal cellular calcium, which protects against neurodegenerative disease and defends against apoptosis [48-52], a decrease in frontal cortex CALB may result in cognitive impairment due to cell death or abnormal calcium homeostasis [48-52]. Furthermore, frontal cortical COX-2 was shown to significantly increase in males fed the Phyto-600 diet. Because the expression of COX-2 is associated with key pathophysiologic event(s) in Alzheimer's disease [53-55], the significant increase in COX-2 expression in the frontal cortex may be relevant to the cognitive decline seen in the Phyto-600 males. In this regard, it is known that the hormonal action of estrogens may be mediated by the differential expression of estrogen receptors alpha vs betta in brain structures that activate or inhibit cell death mechanisms [56]. Although, this parameter was not directly investigated in this study, the differing influence that dietary phytoestrogens had on CALB (significant decrease) and COX-2 (significant increase) in the male frontal cortex, suggests that phytoestrogens may activate programmed cell death. In support of this notion, since the Phyto-600 fed males displayed a 47-fold greater phytoestrogen levels in the frontal cortex (vs. Phyto-free males) and the greater affinity of phytoestrogens is for ER-β > ER-α, it has been shown that estrogen-related neuronal apoptosis is determined by ER-β and the Fas/Fas ligand system [57]. In fact isoflavones have been shown to cause apoptosis in rat primary cortical neurons in vitro via a calcium dependant mechanism [58]. Furthermore, the action of phytoestrogens may be tissue site specific. As our results show phytoestrogens are more prevalent and have greater affinity for certain brain structures and areas, therefore, phytoestrogens may act both as agonist and antagonist in a site-specific manner or in other words as natural SERM-type molecules [35,38-42].
Taken together these findings suggest that soy dietary phytoestrogens present in the animal diets or the lack thereof, for a relatively short interval even in young adult animals, can significantly influence sexually dimorphic cognitive behavior. Dietary phytoestrogens sex reversed VSM, as expressed in the radial-arm maze by enhancing spatial memory in females but inhibited this ability in males, this finding corresponds to research regarding increased dementia observed in aged men consuming high tofu levels [44]. However, while it is important to establish the influence of dietary phytoestrogens on brain function and behavior in animal models, the true significance in humans remains to be determined as it relates to the present findings.
Finally, as established by the above findings, of particular importance which deserves emphasis is that the Phyto-600 diet, used in this study, is a typical rat chow formulation that is similar to other rodent diet products that are used in many laboratories, whereas, phytoestrogen-free (Phyto-free) diets are rarely used. As previously reported, Thigpen et al. [38] and Brown and Setchell [36] determined the source and concentration of phytoestrogens in rodent diets. The results of this study showed that phytoestrogen concentrations vary widely among diets (between 200 μg/g and 600 μg/g of phytoestrogens). Additionally, one of our laboratories has quantified phytoestrogen plasma levels in a variety of physiologic conditions validating the importance of soy-derived phytoestrogens via rodent chow diets [36]. Therefore not all diets have the same phytoestrogen levels, however, all diets, which have soy as their main protein source, contain phytoestrogens. It is very unusual to consider the influence of diet in hormone sensitive research investigations. This may be a critical error due to the complicating and/or confounding role phytoestrogens play. For example, we have previously shown that soy dietary phytoestrogens significantly alter body weight, food and water intake levels, puberty onset in females and prostate weights [39]. Also, we have preliminary (unpublished) data demonstrating the effects of soy-derived phytoestrogens on several endocrine and metabolic parameters. However, brain aromatase and circulating plasma estradiol levels in male rats does not appear to be influenced by dietary phytoestrogens [22,41,51]. As the results from this research imply, phytoestrogens have considerable effects on hormonally sensitive parameters and their influence may be, in part, responsible for many reported sexual dimorphisms such as, the establishment and plasticity of sexually dimorphic brain structures like the sexually dimorphic nucleus of the preoptic area (SDN-POA) and the anteroventral periventricular nucleus (AVPV) [40,41].
Therefore, dietary soy derived phytoestrogens can influence brain, cognition and memory in a manner that was previously unknown, where soy phytoestrogens enhance memory in intact females but inhibit memory in intact males. Further research is warranted in order to examine this important and growing research field of endocrine disruptors to determine the true significance of phytoestrogens influence that appears to be hormonal and gender dependent.
Conclusions
Male rats acquire and exhibit significantly better performance compared to females in learning and memory task requiring the use of visual spatial cues. This study, examined the influence of phytoestrogens, present in rodent diets, on VSM and identified phytoestrogens, CALB, and COX-2 expression in the rat brain. Within the radial arm maze dietary phytoestrogens significantly sex-reversed the normal sexually dimorphic expression of VSM. Specifically, in tasks requiring the use of reference, but not working, memory VSM was enhanced in females fed the Phyto-600 diet, whereas, in males VSM was inhibited by the same diet. It was determined (via TR-FIA) that males fed the Phyto-600 diet had significantly higher levels of phytoestrogens in a number of brain regions (frontal cortex, hypothalamus & cerebellum, but not hippocampus) compared to males fed the Phyto-free diet. Since reference memory is thought to be mediated in the frontal cortex and because males fed the Phyto-600 diet had decreased expression of CALB (a neuroprotectant factor) and increased COX-2 (an inflammatory factor prevalent in Alzheimer's disease) levels in this brain region compared to males fed the Phyto-free diet, these findings suggest that phytoestrogens, present in soy dietary sources, may alter VSM via there presence and action in the frontal cortex.
Materials and Methods
Experiment 1 examined males, while Experiment 2 examined females
Experiment 1 and 2 were conducted independent of each other, however, they were conducted at similar time intervals and each study used the same diet treatment and maze procedure (see below). Animals Ten 50 day-old Long-Evans females were purchased from Charles River Laboratories (Wilmington, MA, USA) for breeding. These animals were caged individually and housed in the Brigham Young University Bio-Ag vivarium and maintained on a 10-hour dark 14-hour light schedule (lights on 1400–0400). The animals and methods for these studies were approved by the Animal Care and Use Committee (IACUC) at Brigham Young University. Upon arrival all animals were allowed ad libitum access to either a commercially available diet with high phytoestrogen levels (Harlan Teklad Rodent Diet 8604, Madison, WI, USA) containing 600 micrograms of phytoestrogens/gram of diet; referred to hereafter as the Phyto-600 diet, or a custom phytoestrogen-free diet; referred to hereafter as the Phyto-free diet, obtained from Ziegler Bros. (Gardner, PA, USA) and water. For the Phyto-free diet, the phytoestrogen concentrations were below the detectable limits of HPLC analysis [40]. The content and nutrient composition of these diets are described in detail elsewhere [40]. At 80–85 days of age, the animals were time mated within their respective diet treatments so that the offspring of these pairings would be exposed solely to either the Phyto-600 or Phyto-free diet. Thirty days following birth, the animals were weaned and separated by sex into colony cages (4 animals per cage, males were tested in experiment 1, whereas, females were tested separately in experiment 2). The animals were fed the same diet as their mother (Phyto-600 or Phyto-free). At 40 days of age, animals were singly caged and remained on the assigned diet treatments.
Experiment 3: Animals
Male and female rats utilized in experiment 3 received lifelong exposure to only the Phyto-600 diet (using a diet treatment design similar to that described above for experiments 1 and 2). However, the diet treatments were switched after acquisition and eight-arm maze procedures (see below) in order to examine the plasticity of visual spatial memory performance in adult animals.
Apparatus
The maze utilized in this research study was an 8-arm radial maze obtained from Columbus Instruments (Columbus, OH, USA). The stainless steel maze consists of eight arms (length 45 cm, width 13 cm, wall height 13 cm) extending outward, at equal angles around a center platform. Five cm from the end of each arm a small plastic receptacle was placed to hold the food out of view from the center of the maze. Above the maze a video camera was suspended (at approximately 5 feet) to record each trial for analysis.
Maze procedure (acquisition and eight-arm task; working memory) examining animals in Experiments 1, 2 and 3
At 50-days of age rats (males tested in experiment 1 or females tested in experiment 2) were put on a limited feeding schedule and maintained at approximately 90% of normal body weight; however, animals were allowed to gain an additional 5 g per week to account for normal growth. One week later, the rats were introduced to the radial arm maze. On 3 consecutive days each rat was placed into the maze for 5 minutes to explore. Following this introduction, rats were placed, alone, in the center of the maze. Three Froot Loops (FL) were placed at the start of each arm. If the rat retrieved FL from at least 5 of the piles within 3 minutes, then on the following day a single FL was placed at the start of each arm. If 5 of the 8 FL were retrieved within 3 minutes, then the following day a single FL was placed 5 cm further out on the arms. In this manner, the FLs were gradually placed farther and farther out on the arms in a systematic manner. Training was completed for a given rat when it retrieved FLs from the end of at least 5 of the 8 arms within 3 minutes on 3 consecutive trials. The number of trials required to reach this criterion was recorded for each rat. Once this criterion level was reached, for a given rat, it was tested on this task for 10 additional trials, one/day. Each trial was considered complete when all 8 arms were visited, or 3 minutes had passed. An arm choice was scored if the rat traveled three-fourths of the way down the length of an arm. A working memory error was recorded if an animal reentered a previously visited arm. Following the eight-arm task the animals in experiments 1 and 2 were sacrificed and blood was taken to determine circulating phytoestrogen levels and brain samples were collected for CALB and COX-2 determination.
In experiment 3, following the 8-arm task trials described above these animals were returned to their home cages and a dietary change was initiated. One-half the total number of male or female (random cycling) rats was kept on the original Phyto-600 diet (long-term) and the other half was assigned to the Phyto-free diet (short-term). This ad lib feeding continued for 15 days. Following this ad lib period of feeding, rats were again reduced to 90% of normal body weight for 10 days.
Maze procedure (four-arm task; reference and working memory) examining animals in experiment 3
Following the reduction to 90% of normal body weight, each rat was tested for an additional 15 days, one trial/day, during which only 4 arms were baited (four-arm task). To begin these trial, each animal was placed in a bottomless, opaque box in the center of the maze. The box was removed opening all arms simultaneously to the rat. These trials were considered complete when the 4 baited arms (arms 2,3,6 and 8) had been visited or 3 minutes had passed. During all trials the amount of time required to visit the initial 4 arms and the order that each rat entered the arms were recorded. Again an arm entry was noted if a rat traversed at least three-fourths the length of the arm. A working memory error was recorded if a rat reentered a baited arm, a reference memory error was recorded if an animal entered an arm, which was not baited, and a working/reference memory error was recorded if an animal reentered an arm, which was not baited. All testing and analysis was accomplished without knowledge of the diet treatments.
Circulating phytoestrogen levels
The concentration and types of phytoestrogens in collected plasma samples were analyzed by gas-chromatography/mass spectrometry (GC/MS), as previously performed in our laboratories [28,40,51]. This was accomplished by liquid-solid extraction and liquid-gel chromatographic techniques to isolate the phytoestrogen fractions using standard assay methods with internal controls [28].
Brain calbindin, COX-2 and phytoestrogen levels
In Long-Evans rats, brains tissues were collected for phytoestrogen, CALB and COX-2 analysis. Brain phytoestrogen concentrations (i.e., daidzein, genistein and equol) were determined in tissues isolated from the frontal cortex and hippocampus (brain structures critical for VSM) along with the amygdala and cerebellum brain regions, by time-resolved fluoroimmunoassay (TR-FIA) using standard methods based on previously published and validated methods for plasma and urine [59,60]. The brain tissue method (in brief) follows: The brain samples were lyophilized (pooled by treatment, n = 4 per group) from male rats used as breeders that were on the diet treatments from 50 to 120 days of age. Following addition of 300 μl of water and mixing, the samples were left at room temperature for 10 min, and thereafter carefully mixed and sonicated for 10 min. Tritiated estradiol glucuronide was added as an internal standard and 700 μl of methanol were mixed with samples. Fatty material was precipitated overnight at -20°C. The samples were then centrifuged (3500 rpm) for 10 min at -10°C and the supernatant was decanted into another tube. The procedure was repeated by adding an additional 1 ml, ice-cold 70% methanol, mixing, centrifugating in the cold, and decanting the supernatant into the tube containing the extract. Methanol was evaporated under a stream of nitrogen until only water remained. The samples were extracted once with 2.5 ml of n-hexane, which removed the remaining fat (the remaining n-hexane was removed with nitrogen). Helix Pomatia enzyme was purified with activated charcoal and used for enzymatic hydrolysis of the phytoestrogen conjugates. The hydrolysis (2 h at 60°C) was carried out using ascorbic acid in 0.15 M acetate buffer. The samples were then extracted twice with 3 ml of diethylether, freezing separated the phases and the ether fractions were combined and evaporated. After adding 300 μl of TRIS buffer pH 7.76 (TR-FIA buffer) a sample was taken for measurement of recovery in a β-counter. The final assay was carried out as described elsewhere [60,61]. Equol was determined in the same way genistein and daidzein, using an antiserum to 4'-O-carboxymethyl-equol-bovine serum albumin and the Europium label was synthesized using the same derivative of equol [62]. The cross-reactivities of the genistein and daidzein antisera with other isoflavone metabolites have been described previously [60]. The equol antiserum showed a cross reactivity of 0% for genistein, 12% for dihydrogenistein, 4% for dihydrodaidzein and less than 1% for daidzein and its other metabolites. The intra-assay CV determined, from a pool of rat brains, varied from 9.7 to 19.5%. The relatively high CV was due to the fact that the samples were never completely homogeneous and the differences in phytoestrogen concentrations are great in different brain regions.
In male and female rats from experiments 1 and 2, CALB and COX-2 levels in the frontal cortex and hippocampus (CALB only) were determined using Western blot analysis, as reported elsewhere [49,55]. In brief, homogenized samples (30 μg per lane for frontal cortex and hippocampus- by treatment) were resolved on 14% Tri-Glycine gels and transferred onto Millipore Immobilon-P membranes (Millipore, Bedford, MA, USA) by electroblotting. The membranes were probed with rat calbindin antibody (1:50,000 obtained from Dr. Anthony Iacopino, Marquette University, USA) or human COX-2 antibody (1:3,000) (obtained from Dr. Daniel Simmons, BYU, USA) and detected by the enhanced chemiluminescence (ECL) Western blotting system (Amersham, Arlington Height, IL, USA). The immunoreactive bands were quantified by optical density measurements using an imaging system (Fotodyne, Hartland, WI, USA) and analyzed on an IBM Pentium II 350 computer using the public domain NIH Image program Scion Image Beta 4.02 (at http://www.scioncorp.com:8080/Downloads/fr_login.htm). The obtained results represent data derived from 3 to 4 independent immunoblots per brain site.
Maze statistical analysis experiments 1, 2 and 3 (acquisition and 8-arm tasks)
Acquisition
A one-way analysis of variance (ANOVA) was used to evaluate acquisition of the radial-arm maze based on the number of trials needed for each rat to meet the specified level of performance.
Accuracy (8 arm-task)
A multivariate analysis of variance (MANOVA; sex over trials 2 × 5) was used to determine accuracy of the initial 10 trials (each 2 trials were averaged). Accuracy was defined as the number of correct arm choices (an arm not yet chosen in that trial) in the first eight arm entries.
Maze statistical analysis experiment 3(4-arm task)
Accuracy (4 arm-task)
Accuracy on the four-arm task was determined by the number of correct arm choices (baited arms not yet chosen in the trial) made in the first 4 arm entries. A MANOVA (sex by diet change over trials 2 × 2 × 5) was used to interpret these findings (each 3 trials were averaged). Also a sex by diet over trials MANOVA was completed to determine the number and types of errors committed in the initial 4 arm entries.
All analyses were performed using SPSS statistical software package and all significant main effects were followed by Bonferroni post hoc comparisons. The alpha level was set at 0.05.
Acknowledgments
Acknowledgements
This work was supported, in part, by grants from The National Science Foundation, IBN-9507972 and DBI-9912126 (to EDL), BYU Research Office 19-223566 (to EDL) and The BYU Neuroscience Dean's Graduate Fellowship (to TDL). Development of the tissue phytoestrogen method was supported by the EU contract QLRT-2000-00266 (PHYTOPREVENT), and by the Sigrid Juselius Foundation, Helsinki, Finland, (H.A).
Contributor Information
Trent D Lund, Email: Neuroscience@byu.edu.
Timothy W West, Email: Neuroscience@byu.edu.
Lilyan Y Tian, Email: Neuroscience@byu.edu.
Lihong H Bu, Email: tlund@colostate.edu.
Daniel L Simmons, Email: dan_simmons@byu.edu.
Kenneth DR Setchell, Email: KSetchell@aol.com.
Herman Adlercreutz, Email: herman.adlercreutz@helsinki.fi.
Edwin D Lephart, Email: Edwin_Lephart@byu.edu.
References
- Wise PM, Dubal DB, Wilson ME, Rau SW, Böttner M. Minireview: neuroprotective effects of estrogen-new insights into mechanisms and action. Endocrinology. 2001;142:969–973. doi: 10.1210/en.142.3.969. [DOI] [PubMed] [Google Scholar]
- Sughrue PJ, Merchenthaler I. Estrogen is more than just a "sex hormone": novel sites for estrogen action in the hippocampus and cerebral cortex. Front Neuroendocrinol. 2000;21:95–101. doi: 10.1006/frne.1999.0190. [DOI] [PubMed] [Google Scholar]
- Wise PM, Dubal DB. Estradiol protects against ischemic brain injury in middle-aged rats. Biol Reprod. 2000;63:982–985. doi: 10.1095/biolreprod63.4.982. [DOI] [PubMed] [Google Scholar]
- Toran-Allerand CD, Singh M, Setalo G. Novel mechanisms of estrogen action in the brain: new players in an old story. Front Neuroendocrinol. 1999;20:97–121. doi: 10.1006/frne.1999.0177. [DOI] [PubMed] [Google Scholar]
- Roof RL, Hall ED. Gender differences in acute CNS trauma and stroke: neuroprotective effects of estrogen and progesterone. J Neurotrauma. 2000;17:367–388. doi: 10.1089/neu.2000.17.367. [DOI] [PubMed] [Google Scholar]
- Halpern DF. Sex Differences in Cognitive Abilities 3rd Edition, San Bernadino CA, LEA press; 2000.
- Harris LJ. Sex differences in spatial ability: possible environmental, genetic, and neurological factors. In M Kinsbourne, Asymmetrical Function of the Brain, Cambridge University Press, NY; 1981.
- Luine VN, Richards ST, Wu VY, Beck KD. Estradiol enhances learning and memory in a spatial memory task and effects levels of monoaminergic neurotransmitters. Horm and Behav. 1998;34:149–162. doi: 10.1006/hbeh.1998.1473. [DOI] [PubMed] [Google Scholar]
- Williams CL, Meck WH. The organizational effects of gonadal steroids on sexually dimorphic spatial ability. Psychneuroendo. 1991;16:155–176. doi: 10.1016/0306-4530(91)90076-6. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Fader AJ, Spencer AL, Dohanich GP. Estrogen enhances performance of female rats during acquisition of a radial arm maze. Horm Behav. 1997;32:217–225. doi: 10.1006/hbeh.1997.1433. [DOI] [PubMed] [Google Scholar]
- Frick KM, Burlingame LA, Arters JA, Berger-Sweeney J. Reference memory, anxiety and estrous cyclicity in C57BL/6NIA mice are affected by age and sex. Neuroscience. 2000;95:293–307. doi: 10.1016/S0306-4522(99)00418-2. [DOI] [PubMed] [Google Scholar]
- Warren SG, Juraska JM. Spatial and nonspatial learning across the rat estrous cycle. Behav Neurosc. 1997;111:259–266. doi: 10.1037//0735-7044.111.2.259. [DOI] [PubMed] [Google Scholar]
- Frye CA, Sturgis JD. Neurosteroids affect spatial/reference, working, and long-term memory of female rats. Neurobio Learn Mem. 1995;64:83–96. doi: 10.1006/nlme.1995.1046. [DOI] [PubMed] [Google Scholar]
- Rissman EF, Wersinger SR, Fugger HN, Foster TC. Sex with knockout models: behavioral studies of estrogen receptor alpha. Brain Res. 1999;835:80–90. doi: 10.1016/S0006-8993(99)01452-3. [DOI] [PubMed] [Google Scholar]
- Juraska JM. Sex differences in "cognitive" regions of the rat brain. Psychoneuroendocrinology. 1991;16:105–119. doi: 10.1016/0306-4530(91)90073-3. [DOI] [PubMed] [Google Scholar]
- Kesner RP, Hunt ME, Williams JM, Long JM. Prefrontal cortex and working memory for spatial response, spatial location, and visual object information in the rat. Cerebral Cortex. 1996;6:311–318. doi: 10.1093/cercor/6.2.311. [DOI] [PubMed] [Google Scholar]
- Olton DS. Frontal cortex, timing and memory: Neuropsychologia. 1989;27:121–130. doi: 10.1016/0028-3932(89)90094-8. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Burgess N, O'Keefe JO. Human spatial navigation: cognitive maps, sexual dimorphism, and neural substrates. Curt Opin Neurobiol. 1999;9:171–177. doi: 10.1016/S0959-4388(99)80023-3. [DOI] [PubMed] [Google Scholar]
- Roof RL. Neonatal exogenous testosterone modifies sex difference in radial arm and Morris water maze performance in prepubescent and adult rats, Beh Brain Res. 1993;53:1–10. doi: 10.1016/s0166-4328(05)80261-x. [DOI] [PubMed] [Google Scholar]
- Tan U, Tan M. The curvelinear correlations between the total testosterone levels and fluid intelligence in men and women. Int J Neurosci. 1998;94:55–61. doi: 10.3109/00207459808986438. [DOI] [PubMed] [Google Scholar]
- Lephart ED. A review of brain aromatase cytochrome P450. Brain Res Rev. 1996;22:1–26. [PubMed] [Google Scholar]
- Weber KS, Setchell KDR, Lephart ED. Maternal and perinatal brain aromatase: effects of dietary soy phytoestrogens. Dev Brain Res. 2001;126:217–221. doi: 10.1016/S0165-3806(00)00138-3. [DOI] [PubMed] [Google Scholar]
- Crews D, Willingham E, Skipper JK. Endocrine disruptors: present issues, future directions. The Q Rev Biol. 2000;75:243–260. doi: 10.1086/393498. [DOI] [PubMed] [Google Scholar]
- Laessig SA, McCarthy MM, Silbergeld EK. Neurotoxic effects of endocrine disruptors. Curr Opin Neurol. 1999;12:745–751. doi: 10.1097/00019052-199912000-00015. [DOI] [PubMed] [Google Scholar]
- Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, van der Burg B, Gustafsson JA. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998;139:4252–4263. doi: 10.1210/en.139.10.4252. [DOI] [PubMed] [Google Scholar]
- Adlercreutz H. Evolution, nutrition, intestinal microflora, and prevention of cancer: a hypothesis. Proc Soc Exp Bio Med. 1997;217:241–246. doi: 10.3181/00379727-217-44228. [DOI] [PubMed] [Google Scholar]
- Adlercreutz H, Mazur W. Phyto-estrogens and western diets. Ann Med. 1997;29:95–120. doi: 10.3109/07853899709113696. [DOI] [PubMed] [Google Scholar]
- Coward L, Barnes NC, Setchell KDR, Barnes S. Genistein, daidzein, and their glycoside conjugates: antitumor isoflavones in soybean foods from American and Asian diets. J Agr Food Chem. 1993;41:1961–1967. [Google Scholar]
- Hol T, Cox MB, Bryant HU, Draper MW. Selective estrogen receptor modulators and postmenopausal women's health. J Womens Health. 1997;6:523–531. doi: 10.1089/jwh.1997.6.523. [DOI] [PubMed] [Google Scholar]
- Knight DC, Eden JA. A review of the clinical effects of phytoestrogens. Obstet Gyneco. 1996;187:897–904. [PubMed] [Google Scholar]
- Murkies AL, Wilcox G, Davis SR. Clinical review-phytoestrogens. J Clin Endo Metab. 1998;83:297–303. doi: 10.1210/jc.83.2.297. [DOI] [PubMed] [Google Scholar]
- Setchell KDR, Zimmer-Nechemias L, Cai J, Heubi JE. Exposure of infants to phyto-estrogens from soy-based infant formula. Lancet. 1997;350:23–27. doi: 10.1016/S0140-6736(96)09480-9. [DOI] [PubMed] [Google Scholar]
- Setchell KDR. Phytoestrogens: biochemistry, physiology and implications for human hearth of soy isoflavones. Am J Clin Nutr. 1998;129:1333S–1346S. doi: 10.1093/ajcn/68.6.1333S. [DOI] [PubMed] [Google Scholar]
- Setchell KDR, Cassidy A. Dietary isoflavones: biological effects and relevance to human health. J Nutr. 1999;129:758–767. doi: 10.1093/jn/129.3.758S. [DOI] [PubMed] [Google Scholar]
- Brzezinski A, Debi A. Phytoestrogens: the 'natural' selective estrogen receptor modulators? Euro J Obstet Gynec Reprod Biol. 1999;85:47–51. doi: 10.1016/S0301-2115(98)00281-4. [DOI] [PubMed] [Google Scholar]
- Brown NM, Setchell KDR. Animal models impacted by phytoestrogens in commercial chow: implications for pathways influenced by hormones. Lab Invest. 2001;81:735–747. doi: 10.1038/labinvest.3780282. [DOI] [PubMed] [Google Scholar]
- Halbreich U, Kahn LS. Selective oestrogen receptor modulators-current and future brain and behavior applications. Expert Opin Pharmacother. 2000;1:1385–1398. doi: 10.1517/14656566.1.7.1385. [DOI] [PubMed] [Google Scholar]
- Thigpen JE, Setchell KDR, Ahlmark KB, Locklear J, Spahr T, Caviness GF, Goelz MF, Haseman JK, Newbold RR, Forsythe DB. Phytoestrogen content of purified, open- and closed-formula laboratory diets. Lab Anim Sci. 1999;49:530–536. [PubMed] [Google Scholar]
- Lund TD, Lephart ED. Dietary soy phytoestrogens produce anxiolytic effects in the elevated plus-maze. Brain Res. 2001;913:180–184. doi: 10.1016/S0006-8993(01)02793-7. [DOI] [PubMed] [Google Scholar]
- Lund TD, Rhees RW, Setchell KD, Lephart ED. Altered sexually dimorphic nucleus of the preoptic area (SDN-POA) volume in adult Long-Evans rats by dietary soy phytoestrogens. Brain Res. 2001;914:92–99. doi: 10.1016/S0006-8993(01)02779-2. [DOI] [PubMed] [Google Scholar]
- Lephart ED, Adlercreutz H, Lund TD. Dietary soy phytoestrogen effects on brain structure and aromatase in Long-Evans rats. NeuroReport. 2001;12:3451–3455. doi: 10.1097/00001756-200111160-00015. [DOI] [PubMed] [Google Scholar]
- Lephart ED, West TW, Weber KS, Rhees RW, Setchell KDR, Adlercreutz H, Lund TD. Neurobehavioral effects of dietary soy phytoestrogens. Neurotox Terat. 2002. [DOI] [PubMed]
- Lephart ED, Lund TD, Horvath TL. Brain androgen and progesterone metabolizing enzymes: biosynthesis, distribution and function. Brain Res Rev. 2001;37:25–37. doi: 10.1016/S0165-0173(01)00111-4. [DOI] [PubMed] [Google Scholar]
- White LR, Petrovitch H, Ross GW, Masaki K, Hardman J, Nelson J, Davis D, Markesbery W. Brain aging and midlife tofu consumption. J Am Coll Nutr. 2000;19:242–55. doi: 10.1080/07315724.2000.10718923. [DOI] [PubMed] [Google Scholar]
- Pan Y, Anthony M, Clarkson TB. Effects of estradiol and soy phytoestrogens on choline acetyltransferase and nerve growth factor mRNAs in the frontal cortex and hippocampus of female rats. Proc Soc Exp Biol Med. 1999;221:118–125. doi: 10.1046/j.1525-1373.1999.d01-64.x. [DOI] [PubMed] [Google Scholar]
- Patisaul HB, Dindo M, Whitten PL. Soy isoflavone supplements antagonize reproductive behavior and estrogen receptor α- and β-dependent gene expression in the brain. Endocrinology. 2001;142:2946–2952. doi: 10.1210/en.142.7.2946. [DOI] [PubMed] [Google Scholar]
- Boettger-Tong H, Murthy L, Chiappetta C, Kirkland JL, Goodwin B, Adlercreutz H, Stancel GM, Makela S. A case of a laboratory animal feed with high estrogenic activity and its impact in in vivo responses to exogenously administered estrogens – commentary. Environ Health Perspect. 1998;106:369–373. doi: 10.1289/ehp.98106369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor H, Quintero EM, Iacopino AM, Lephart ED. Phytoestrogens alter hypothalamic calbindin-D28K levels during prenatal development. Dev Brain Res. 1999;114:277–281. doi: 10.1016/S0165-3806(99)00038-3. [DOI] [PubMed] [Google Scholar]
- Watson MA, Taylor H, Lephart ED. Androgen-dependent modulation of calbindin-D28K in hypothalamic tissue during prenatal development. Neurosci Res. 1998;32:97–101. doi: 10.1016/S0168-0102(98)00068-6. [DOI] [PubMed] [Google Scholar]
- Stuart E, Lephart ED. Dimorphic expression of MBH-POA calbindin mRNA levels during perinatal development and adult brain tissue distribution of calbindin mRNA, Mole Brain Res, 1999;73:60–67. doi: 10.1016/S0169-328X(99)00236-3. [DOI] [PubMed] [Google Scholar]
- Lephart ED, Thompson JM, Setchell KDR, Adlercreutz H, Weber KS. Phytoestrogens decrease brain calcium-binding protiens but do not alter hypothalamic androgen metabolizing enzymes in adult male rats. Brain Res. 2000;859:123–131. doi: 10.1016/S0006-8993(00)01968-5. [DOI] [PubMed] [Google Scholar]
- Iacopino AM, Quintero EM, Miller EK. Calbindin-D28K a potential neuroprotective protein. Neurodegen. 1994;3:1–20. [Google Scholar]
- O'Banion MK. COX-2 and Alzheimer's disease: potential roles in inflammation and neurodegeneration. Expert Opin Investig Drugs. 1999;8:1521–1536. doi: 10.1517/13543784.8.10.1521. [DOI] [PubMed] [Google Scholar]
- Pasinetti GM, Aisen PS. Cyclooxygenase-2 expression is increased in frontal cortex of Alzheimer's disease brain. Neurosci. 1998;87:319–324. doi: 10.1016/S0306-4522(98)00218-8. [DOI] [PubMed] [Google Scholar]
- Zhang M, Harris RC, McKanna JA. Regulation of cyclooxygenase (COX-2) in rat renal cortex by adrenal glucocorticoid and mineralocorticoids. Pro Natl Acad Sci USA. 1999;96:15280–15285. doi: 10.1073/pnas.96.26.15280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodson JC, Gorski RA. Structural sex differences in the mammalian brain: Reconsidering the male/female dichotomy, In Sexual Differentiation of the Brain, A Matsumoto (ed), CRC Press, Boca Raton, FL, 2000. pp. 230–247.
- Nilsen J, Mor G, Naftolin F. Estrogen-regulated developmental neuronal apoptosis is determined by estrogen receptor subtype and the Fas/Fas ligand system. J Neurobiol. 2000;43:64–78. doi: 10.1002/(SICI)1097-4695(200004)43:1<64::AID-NEU6>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Linford NJ, Yang Y, Cook DG, Dorsa DM. Neuronal apoptosis resulting from high doses of the isoflavone genistein: role for calcium and p42/44 mitogen-activated protein kinase. J Pharmacol Exp Ther. 2001;299:67–75. [PubMed] [Google Scholar]
- Wang GJ, Lapcik O, Hampl R, Uehara M, Al-Maharik N, Stumpf K, Mikola H, Wahala K, Adlercreutz H. Time-resolved fluoroimmunoassay of plasma daidzein and genistein. Steroids. 2000;65:339–48. doi: 10.1016/S0039-128X(00)00089-1. [DOI] [PubMed] [Google Scholar]
- Wang GL, Lapcik O, Hampl R, Uehara M, AlMaharik N, Strumpf K, Mikola H, Wahala K, Adlercreutz H. Time-resolved fluoroimmunoassay of plasma daidzein and genistein. Steroids. 2000;65:339–348. doi: 10.1016/S0039-128X(00)00089-1. [DOI] [PubMed] [Google Scholar]
- Uehara M, Lapcik O, Hampl R, AlMaharik N, Makela T, Wahala K, Mikola H, Adlercreutz H. Rapid analysis of phytoestrogens in human urine by time-resolved fluoroimmunoassay. J Steroid Biochem Mol Biol. 2000;72:273–282. doi: 10.1016/S0960-0760(00)00045-5. [DOI] [PubMed] [Google Scholar]
- Brouwers E, L'homme R, Lapcik O, Hampi R, Adlercreutz H. Time-resolved fluroimmunoassay of equol in plasma and urine. submitted. [DOI] [PubMed]