Abstract
Surgical therapy is vital for thoracolumbar burst fracture in restoring vertebral height, correcting kyphosis, decompressing nervous, and maintaining stability. Patients have unexpectedly lower hemoglobin levels postoperatively, which is remarkably inconsistent with the measured blood loss. However, hidden blood loss (HBL) is often neglected.
To investigate HBL during perioperative period and determine its influential factors after surgery.
A total of 68 patients who underwent surgery in our institution between January 2015 and January 2017 were included in the study. The demographic information, including the patients’ age, gender, weight, height, duration of symptoms, surgery approach, time of operation, volume of drainage, classification of fracture, percentage of vertebral height loss and restoration, was collected. HBL was calculated according to the Gross formula. Influential factors were further analyzed using multivariate linear regression analysis.
The mean HBL was 303.5 (range 18.4–803.5) mL, accounting for 67.5% of total blood loss. It indicated that the amount of HBL was much higher than we expected. Multiple and stepwise regression analysis revealed that blood loss, preoperative activated partial prothrombin time (APPT), percentage of anterior and medium vertebral height restoration were positively correlated with HBL. The association between HBL and the influential factors was analyzed based on the regression model equation: HBL = [1 + e [216.737 + 0.627∗blood loss + 10.817∗APTT + 207.549∗anterior height restoration + 20.002∗medium height restoration]]−1.
HBL during perioperative period accounted for a substantial portion of the total blood loss and was much larger than what we thought. The blood loss, preoperative APPT, percentage of anterior and medium vertebral height restoration were positively correlated with HBL. Therefore, more attention needs to be paid to HBL to ensure patients’ safety.
Keywords: hidden blood loss, influential factors, multiple linear stepwise regression, surgery, thoracolumbar burst fracture
1. Introduction
Thoracolumbar burst fractures, accounting for 25% to 50% of all spinal fractures,[1–4] are frequently associated with kyphotic deformity and can have a great impact on the patients’ daily physical activities. Surgical treatment allows early rehabilitation, facilitates nursing care, and reduces the occurrence of complications associated with prolonged bed rest. Surgical therapy can also restore vertebral height, correct kyphosis, decompress nerves, and maintain stability.[5–6]
Hidden blood loss (HBL) refers to the obvious decrease of hemoglobin (HB) during the perioperative period after the fracture, which is inconsistent with the dominant blood loss. The concept of HBL was first put forward by Sehat in 2000.[7] The HBL during perioperative period is often neglected in clinical practice, but it is important for the prognosis of patients, especially the elderly patients.[8–9]
Patients with thoracolumbar burst fractures tend to have a lower postoperative HB level than anticipated after surgery despite the apparently satisfactory perioperative management of blood loss. Since intraoperative dominant blood loss could be excluded as the cause of the HB decrease, we concluded that these patients might have HBL. Having a correct understanding and paying more attention to HBL can ensure an accurate estimate of total blood loss (TBL) and ensure the patients’ safety during perioperative period. Due to the absence of published literature regarding HBL in patients with thoracolumbar burst fracture after surgery, we conducted a study using multiple linear stepwise regression to investigate the amount of HBL during surgery and explore the influential factors causing HBL.
2. Materials and methods
2.1. Patient selection
This retrospective study was conducted in accordance with the principles of the Declaration of Helsinki.[10] Approval to perform the study was obtained from the ethics committee in our institution. All the participants signed the informed consents prior to the study. The study was registered in December 2017. Records of patients who received surgery in our institution for symptomatic thoracolumbar burst fractures between January 2015 and January 2017 were collected and analyzed.
The inclusion criteria for the study were:
-
(1)
age of >18 years old;
-
(2)
burst fracture (AO classification);
-
(3)
fracture level: T11 to L3;
-
(4)
surgical method: posterior instrumentation;
-
(5)
informed consent.
The exclusion criteria were:
-
(1)
pathological fracture caused by infection or tumor;
-
(2)
history of surgery for spinal disorder.
All eligible patients were required to have complete clinical data, demographic data, clinical characteristics, and radiographic parameters. The patients with data missing were excluded. All the patients’ surgeries were performed by the same spine surgeon. The demographic and clinical characteristics included the patient's age, gender, weight, height, duration of symptoms, surgery approach, time of operation, blood loss, use of anticoagulant drug, use of bone cement, fracture levels, preoperative, and postoperative hematocrit (HCT), HB, red blood cell (RBC), prothrombin time (PT), activated partial thromboplastin time (APTT), platelet count (PLT), volume of drainage, percentage of vertebral height loss (HL), percentage of vertebral height restoration (HR), and classification of fracture. The volume of transfused and reinfused blood was also recorded, respectively.
2.2. Intraoperative treatment
All patients were under general anesthesia and placed in the prone position. A standard posterior midline or Wiltse approach was used to explore the spine. Pedicle screws were inserted in the fractured vertebra and the vertebra above with the positions confirmed by the C-arm. A distraction force was applied using spreader forceps to restore lordosis and body height. A trocar in a cannula was inserted into the defect of the fractured body through the pedicle. On the fourteenth postoperative day, the patients were encouraged to sit and rehabilitation began in preparation for ambulation. Patients over 60 years old received BMD exam after the operation. All patients were protected by a brace for 3 months.
2.3. Calculation of the HBL
We calculated the HBL by deducting the measured blood loss from the calculated TBL. To calculate the TBL, we estimated preoperative blood volume (PBV) in milliliters. It was estimated according to the formula of Nadler.[11] The patient's gender, weight, and height were taken into account. The formula used was as follows:
PBV = k1∗height (m)3 + k2∗weight (kg) + k3; where k1 = 0.3669, k2 = 0.03219, and k3 = 0.6041 for men, and k1 = 0.3561, k2 = 0.03308, and k3 = 0.1833 for women.
The TBL in the perioperative period was reflected by the reduction of HCT. It was calculated according to the method of Gross,[12] using preoperative HCT, postoperative HCT and PBV. The formula used was as follows:
TBL = PBV∗(HCTpre− HCTpost)/HCTave; where HCTpre referred to the preoperative HCT, HCTpost referred to the HCT on postoperative day 2 or 3, and HCTave referred to the average of HCTpre and HCTpost.
We calculated the HBL according to the method of Sehat.[1] If allogenic transfusion or reinfusion was performed, the true TBL calculated based on the change in HCT was smaller than what was expected, as transfusion rendered the patient with a higher HCT than they otherwise would have had. Thus, the TBL was equal to the loss calculated from the change in Hct plus the volume transfused or reinfused. The formula used was as follows: HBL= TBL-volume of blood infused-measured blood loss. When transfusion was performed, a unit of red cell concentrate containing 200 mL standard red blood corpuscles was used.
2.4. Evaluation of the clinical outcome
We used the VAS score to measure the scale of pain after the surgery. VAS, a reliable and valid measurement of pain, had a 100-mm-long horizontal line, with “no pain” recorded on the left end (score: 0) and “pain as bad as it could be” on the right end (score: 10). Patients were asked to place a hatch mark that corresponded to their current level of pain (both at rest and at the most painful movement) on the line. The VAS score was then determined by measuring the distance between the left endpoint and the marked point. Health-related quality of life was measured using the Oswestry disability index (ODI) questionnaire, which was widely used to assess the quality of life.
2.5. Evaluation of the percentage of vertebral HL and restoration
We calculated the percentage of vertebral HL and HR using the method of Lee.[13] We recorded the anterior, medium, and posterior preoperative height of the fractured vertebrae as a0, m0, and p0, respectively. The anterior, medium, and posterior height of 2 adjacent vertebrae were recorded as a1 and a2, m1 and m2, p1 and p2, respectively. The original height of the vertebral fracture was predicted as the follow formula: A = (a1 +a2)/2, M = (m1 + m2)/2 and P = (p1 + p2)/2, respectively. The percentage of vertebral HL was calculated using the follow formula: HLA = (A − a0)/A × 100%, HLM = (M − m0)/M × 100% and HLP = (P − p0)/P × 100%. We recorded the anterior, medium and posterior postoperative height of the fractured vertebrae as a3, m3, and p3, respectively. The percentage of vertebral HR was calculated using the following formula: HRA = (a3 − a0)/a0 × 100%, HRM = (m3 − m0)/m0 × 100%, and HRP = (p3 − p0)/p0 × 100%, respectively.
2.6. Perioperative treatment
Patients were confined to bed postoperatively. If the drainage volume was less than 50 mL/24 h without cerebrospinal fluid leakage, extubation was performed. All patients on antibiotics had a complete blood count regularly. The follow-up time was 2 months.
2.7. Statistical analysis
All data analyses were performed with the SPSS 20.0 statistical software package (SPSS Inc, Chicago, IL). Mean, standard deviation, median, and quartiles for continuous variables, and frequency for categorical variables was calculated based on the patient's demographic, clinical characteristics, and radiographic parameter. Mann–Whitney U test was performed for continuous variable as appropriate. Categorical variables were evaluated using Fisher exact test or Chi-square test as appropriate. Multiple and stepwise linear regression analysis was used to analyze the significant independent variables and model the effect of influential factors on HBL, including qualitative variables (age, height, weight, duration of symptoms, time of operation, blood loss, volume of drainage, preoperative HCT, HB, RBC, PT, APTT, PLT, percentage of vertebral HL, and HR) and quantitative variables (gender, Wiltse approach or not, use of anticoagulant drug, use of bone cement, classification of fracture). All independent variables were incorporated into the model using the method of “Enter.” The level of statistical significance was set as P < .05.
3. Results
3.1. Demographic and clinical characteristics
A total of 68 patients who received surgery for thoracolumbar burst fractures between January 2015 and January 2017 were included in the study. Among these patients were 25 males and 43 females, with a mean age of 57.8 (range 25–84) years. The mean preoperative duration before surgery was 11.9 (range 2–45) days. The Wiltse approach was used in 27 patients to explore the spine. A total of 11 patients had osteoporosis and were treated with antiosteoporosis therapy. Bone cement was used in 12 patients in the treatment of fracture level. The mean blood loss was 151.2 (range 50–600) mL. The mean VAS score was 6.7 ± 0.3. The mean ODI score was 27.33 ± 7.88. Table 1 summarizes the demographic and clinical characteristics. Table 2 shows the height of fracture vertebra, percentage of vertebral HL, and HR, respectively. The mean TBL was 438.4 (range 78.1–1138.1) mL. The mean HBL was 303.5 (range 18.4–803.5) mL, accounting for a mean of 67.5% for TBL), indicating a considerable amount of HBL, which was much higher than we had expected.
Table 1.
Patient's demographic information (n = 68).
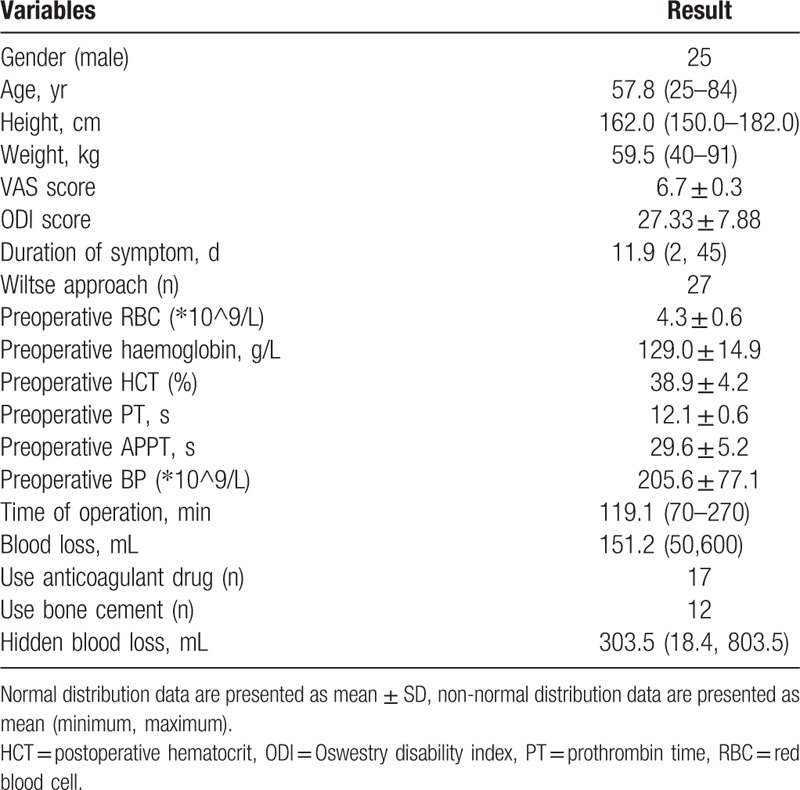
Table 2.
Preoperative and postoperative height of the fractured vertebral body.

3.2. Multiple stepwise linear regression model analysis
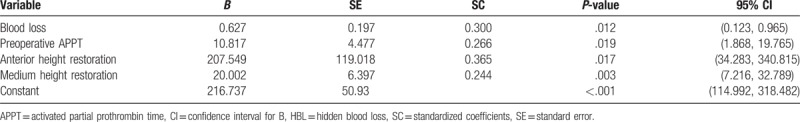
We performed multiple and stepwise linear regression analysis to explore the association between HBL and the influential factors mentioned earlier. Among all the characteristics, 4 variables—the blood loss (P = .012), preoperative APPT (P = .019), percentage of anterior vertebral HR (P = .017), and percentage of medium vertebral HR (P = .003)—showed statistical significance, indicating their positive correlation with HBL. The result showed that the patients with more blood loss had more HBL than those with less blood loss, the patients with higher preoperative APPT had more HBL than those with lower preoperative APPT, and the patients with a higher percentage of anterior and medium vertebral HR had more HBL than those with a lower percentage. Furthermore, it was indicated that age, gender, height, weight, duration of symptoms, time of operation, volume of drainage, preoperative HCT, HB, RBC, PT, PLT, Wiltse approach or not, use of anticoagulant drug, use of bone cement, and classification of fracture were not significantly correlated with HBL. For the B value of the 4 variables and constant, this was calculated to be 0.627, 10.817, 207.549, 20.002, and 216.737, respectively. The standardized coefficients of the 4 variables were 0.300, 0.266, 0.365, and 0.244, respectively. The association between HBL and the influential factors was analyzed based on the regression model equation as follows: HBL = [1 + e [216.737 + 0.627∗blood loss + 10.817∗ APTT + 207.549∗anterior HR + 20.002∗medium HR]]−1. Table 3 demonstrates the coefficient, P-value and 95% confidence interval of the regression analysis.
Table 3.
Multiple linear regression analysis on influential factors of HBL.
4. Discussion
This study showed that a substantial amount of hidden or unmeasured blood loss frequently occurred after the thoracolumbar burst fracture surgery, and the amount was much larger than expected. However, the factors correlated to the amount of HBL were not clarified. In our study, we investigated and analyzed the influential factors of HBL after surgery using multiple and stepwise regression. Our results indicated that the blood loss, preoperative APPT, percentage of anterior vertebral HR, and percentage of medium vertebral HR were positively correlated with HBL.
Some scholars showed that HBL for patients who received total hip arthroplasty was positively associated with BMI, blood transfusion volume, length of incision, change of HCT between preoperation and postoperation but negatively with age.[9] To the best of our knowledge, few studies have dealt with HBL in spine surgery. Primary decompression and posterior fusion patients had a mean hidden loss of 600 mL, accounting for 42% of the total loss. After revision posterior spinal fusion surgery, the mean hidden loss was 631 mL, about 39% of the total loss.[18] Patients with severe vertebral HL, better vertebral HR, fresh fractures, bone cement leakage, and multi segmental fractures had a higher HBL after PKP. Some influential factors are consistent to the result of Wu.[19]
A large amount of HBL during perioperative period may lead to serious and adverse consequences, such as excitement of sympathetic adrenal medulla system, strengthening the cardiac contractile force, increasing the workload of heart in response to postoperative stress reaction. Because the amount of HBL is much higher than expected and clinicians often neglect HBL, heart failure might be induced in patients with basic heart diseases. In addition, due to inadequate renal blood perfusion, the renal ischemia can easily lead to the dysfunction.[14] HBL in large amount also can lead to postoperative infection, delay of incision and fracture healing and a prolonged rehabilitation process
The mechanisms of HBL are not fully clear. Previous studies have provided some possible explanations for the mechanisms of hidden loss. Pattison believed that the postoperative HBL might be, at least in part, caused by hemolysis.[15] Faris demonstrated that the use of unwashed, filtered and reinfused blood increased hemolysis.[16] A mean volume of 1.3 L blood was reinfused and produced a plasma HB level of 50 g/L in the patient. This level of hemolysis weakened the effects of the re-infused blood. Furthermore, Erskine showed that the infiltration of the blood into the tissue compartment during the operation was attributable to the unexplained hidden loss.[17]
It is easy to find that HBL is directly related to a large amount of blood loss in intraoperative period and a higher level of APTT can also increase HBL, which is probably due to hemolysis. In addition, when postoperative reinfusion with autologous blood is used, a further deficit may be caused by hemolysis in the reinfused blood. Consistently, our results showed that the percentage of vertebral HR was related to HBL, which could presumably be ascribed to blood infiltration into the tissue compartments. Namely, the higher the percentage of vertebral HR was, the more penetrable tissue compartments could be formed.
We speculate that another explanation for HBL is the “empty shell theory” for the fractured vertebral body.[20] It means that the more the vertebral height is restored, the bigger the “cavity” will be. Meanwhile, with increased HR, there will be a larger fracture gap around the vertebral walls, which may lead to more infiltration of blood into the tissue compartments. Our statistical analysis showed that the standardized coefficients of the 4 variables were 0.300, 0.266, 0.365, and 0.244, respectively. We showed that vertebral HR was the most influential factor of the variables.
Several notable strengths should be considered in our study. First, this is the first, to the best of our knowledge, to explore the intraoperative dominant blood loss. With this objective in mind, we conducted a study to investigate the amount of HBL during surgical procedure and its influential factors. Second, the statistical method we used is accurate and reasonable. Some limitations should also be considered in our present results. First, this study is a review of clinical data with a small sample size, which might impact the accuracy of some parameters. The results will be more reliable in prospective trials with larger sample size. Second, some elderly patients in this study are long-term users of aspirin, warfarin or other drugs, which may have resulted in an increase in HBL and disturbed the data of study. Another limitation is that 5 patients who had epidural sac injury and cerebrospinal fluid leakage during the surgical procedure were not excluded. So, the recorded volume of perioperative drainage included a portion of the cerebrospinal fluid, and the actual amount of drainage was less than the recorded amount, which might have increased the bias of the results.
5. Conclusions
In conclusion, this study has shown that HBL for patients who have thoracolumbar burst fracture during perioperative period is much more than expected, and it accounts for a substantial portion of the TBL. The blood loss, preoperative APPT, percentage of anterior vertebral HR, percentage of medium vertebral HR are positively correlated to HBL. A predictive model formed by these parameters has the potential to improve decision-making about the postoperative complications and promote the overall safety.
Author contributions
Conceptualization: Jinhai Xu, Quan Huang.
Data curation: Mengchen Yin, Jian Yang.
Formal analysis: Guanghui Chen.
Resources: Jian Yang.
Software: Zhengyi Tong.
Writing – original draft: Quan Huang.
Writing – review and editing: Juming Ma, Wen Mo.
Footnotes
Abbreviations: APTT = activated partial thromboplastin time, HB = hemoglobin, HBL = hidden blood loss, HCT = postoperative hematocrit, PBV = preoperative blood volume, PLT = platelet count, PT = prothrombin time, RBC = red blood cell, TBL = total blood loss.
MY, GC, and JY contributed equally to this work.
MY designed the study; GC and JY collected the data; JX and ZT did the data analysis; MY wrote the manuscript; QH and WM revised the manuscript; JM and WM decided to submit the manuscript for publication.
This work was sponsored by research grants from National Nature Science Foundation of China (81603635 and 81403419); Research project of Shanghai Health and Family Planning Commission (20164Y0081, ZYKC201701003 and 201840010); Research project of Shanghai Science and Technology Commission (16401930600 and 17401934400); Research project of Shanghai Shenkang Hospital Development Center (16CR3074B and 16CR4011A); Research project of National TCM clinical research base of Longhua Hospital (LYTD-60).
The authors have no conflicts of interest to disclose.
References
- [1].Holdsworth F. Fractures, dislocations, and fracture-dislocations of the spine. J Bone Joint Surg Am 1970;52:1534–51. [PubMed] [Google Scholar]
- [2].Denis F. The three column spine and its significance in the classification of acute thoracolumbar spinal injuries. Spine (Phila Pa 1976) 1983;8:817–31. [DOI] [PubMed] [Google Scholar]
- [3].Thomas KC, Bailey CS, Dvorak MF, et al. Comparison of operative and nonoperative treatment for thoracolumbar burst fractures in patients without neurological deficit: a systematic review. J Neurosurg Spine 2006;4:351–8. [DOI] [PubMed] [Google Scholar]
- [4].Tian NF, Wu YS, Zhang XL, et al. Fusion versus nonfusion for surgically treated thoracolumbar burst fractures: a meta-analysis. PLoS One 2013;8:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Gnanenthiran SR, Adie S, Harris IA. Nonoperative versus operative treatment for thoracolumbar burst fractures without neurologic deficit: a meta-analysis. Clin Orthop Relat Res 2012;470:567–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Yi L, Jingping B, Gele J, et al. Operative versus non-operative treatment for thoracolumbar burst fractures without neurological deficit. Cochrane Database Syst Rev 2006;18:1–8. [DOI] [PubMed] [Google Scholar]
- [7].Sehat KR, Evans R, Newman JH. How much blood is really lost in total knee arthroplasty? Correct blood loss management should take hidden loss into account. Knee 2000;7:151–5. [DOI] [PubMed] [Google Scholar]
- [8].Sehat KR, Evans RL, Newman JH. Hidden blood loss following hip and knee arthroplasty. Correct management of blood loss should take hidden loss into account. J Bone Joint Surg Br 2004;86:561–5. [PubMed] [Google Scholar]
- [9].Miao K, Ni S, Zhou X, et al. Hidden blood loss and its influential factors after total hip arthroplasty. J Orthop Surg Res 2015;18:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Fuson RL, Sherman M, Van Vleet J, et al. The conduct of orthopaedic clinical trials. J Bone Joint Surg Am 1997;79:1089–98. [DOI] [PubMed] [Google Scholar]
- [11].Nadler SB, Hidalgo JH, Bloch T. Prediction of blood volume in normal human adults. Surgery 1962;51:224–32. [PubMed] [Google Scholar]
- [12].Gross JB. Estimating allowable blood loss: corrected for dilution. Anesthesiology 1983;58:277–80. [DOI] [PubMed] [Google Scholar]
- [13].Lee ST, Chen JF. Closed reduction vertebroplasty for the treatment of osteoporotic vertebral compression fractures. Technical note. J Neurosurg 2004;1004 Suppl Spine:392–6. [DOI] [PubMed] [Google Scholar]
- [14].Wang J, Wei J, Wang M. The risk factors of perioperative hemoglobin and hematocrit drop after intramedullary nailing treatment for intertrochanteric fracture patients. J Orthop Sci 2005;20:163–7. [DOI] [PubMed] [Google Scholar]
- [15].Pattison E, Protheroe K, Pringle RM, et al. Reduction in haemoglobin after knee joint surgery. Ann Rheum Dis 1973;32:582–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Faris PM, Ritter MA, Keating EM, et al. Unwashed filtered shed blood collected after knee and hip arthroplasties. A source of autologous red blood cells. J Bone Joint Surg Am 1991;73:1169–78. [PubMed] [Google Scholar]
- [17].Erskine JG, Fraser C, Simpson R, et al. Blood loss with knee joint replacement. J R Coll Surg Edinb 1981;26:295–7. [PubMed] [Google Scholar]
- [18].Smorgick Y, Baker KC, Bachison CC, et al. Hidden blood loss during posterior spine fusion surgery. Spine J 2013;13:877–81. [DOI] [PubMed] [Google Scholar]
- [19].Wu YS, Zhang H, Zheng WH, et al. Hidden blood loss and the influential factors after percutaneous kyphoplasty surgery. Eur Spine J 2017;26:1878–83. [DOI] [PubMed] [Google Scholar]
- [20].Guglielmino A, Sorbello M, Barbagallo G, et al. Osteoporotic vertebral compression fracture pain (back pain): our experience with balloon kyphoplasty. Minerva Anestesiol 2007;73:77–100. [PubMed] [Google Scholar]


