Healthy men in a multinational cohort were followed every 6 months for a median of 3.7 years to assess recurrence of genital human papillomavirus (HPV). We found that 20%–31% of genital HPV infections recurred. High-risk sexual behavior was associated with recurrent infections.
Keywords: human papillomavirus, recurrence, redetection, men, HIM Study
Abstract
Background
The purpose of this study was to assess genital recurrence of human papillomavirus (HPV) genotypes included in the 9-valent vaccine and to investigate factors associated with recurrence among men in the HPV Infection in Men (HIM) Study.
Methods
Men were followed every 6 months for a median of 3.7 years. HPV genotypes were detected using Roche linear array. Factors associated with type-specific HPV recurrence (infections occurring after a ≥12-month infection-free period) were assessed.
Results
In type-specific analyses, 31% of prior prevalent and 20% of prior incident infections recurred. Among prevalent infections, HPV types 52, 45, 16, 58, and 6 and among incident infections, HPV types 58, 52, 18, 16, and 11 had the highest rates of recurrence. New sexual partners (male or female) and frequency of sexual intercourse with female partners were associated with HPV-6, -16, -31, and -58 infection recurrence. In grouped analyses, lifetime and new male sexual partners were associated with recurrence of prior incident infection with any of the 9 HPV types.
Conclusions
Recurrence of genital HPV infections is relatively common among men and associated with high-risk sexual behavior. Further studies are needed to understand the role of HPV recurrence in the etiology of HPV-associated diseases.
Human papillomavirus (HPV) infection is one of the most common sexually transmitted infections in men and women [1]. In the prospective HPV Infection in Men (HIM) Study, average duration of a genital HPV infection was approximately 7.5 months for any HPV infection and 12.2 months for HPV-16 [1, 2]. After initial clearance (≥12 months without detection of HPV DNA), individuals may be susceptible for recurrence/redetection of HPV infection with the same HPV type. Recurrent HPV infection is an important component of the natural history of these infections and may help to elucidate the reasons for sustained genital HPV prevalence observed across the lifespan among men in numerous studies [1, 3–6].
No studies have evaluated HPV recurrence in men and only a few have examined recurrence of cervical HPV infection among women. In studies among women, 18.1%–19.5% of HPV infections were redetected [7–11]. High-risk sexual behaviors, such as having multiple sexual partners and new sexual partners, have been reported as some of the most important risk factors associated with redetection of cervical HPV infection [7–11]. However, sexual behavior does not explain all infection recurrences observed in women.
In the current study, we assessed recurrence of 9 HPV vaccine types (HPV-6, -11, -16, -18, -31, -33, -45, -52, and -58) in the HIM Study cohort (N = 4123). Our objective was to estimate type-specific recurrence of genital HPV infections and investigate factors associated with recurrence among men.
METHODS
Study Population
The HIM Study is an HPV natural history study among healthy men in the United States, Brazil, and Mexico. Details regarding participant recruitment and other methods have been published previously [2]. In brief, healthy men from each study site were followed every 6 months for a median follow-up time of 3.7 years. Demographic and sexual behavior information was assessed by computer-assisted self-interviewing. All eligible participants provided signed informed consent and approval was obtained from the human subjects committees of the University of South Florida (Tampa), Ludwig Institute for Cancer Research (São Paulo, Brazil), Centro de Referencia e Treinamento em Doencas Sexualmente Transmissíveis e AIDS (São Paulo, Brazil), and Instituto Nacional de Salud Publica de Mexico (Cuernavaca, Mexico).
HPV DNA Testing
Genital cell samples were collected with prewetted Dacron swabs, which were later combined to form a single sample [1, 2]. Specimens were stored at –80°C until analysis and HPV DNA testing was performed using polymerase chain reaction amplification of the L1 HPV gene fragment. Thirty-seven different types of genital HPV were detected using the Roche linear array assay [12]. Human β-globin was tested to assess specimen adequacy, with an overall β-globin positivity of 98%.
Statistical Analysis
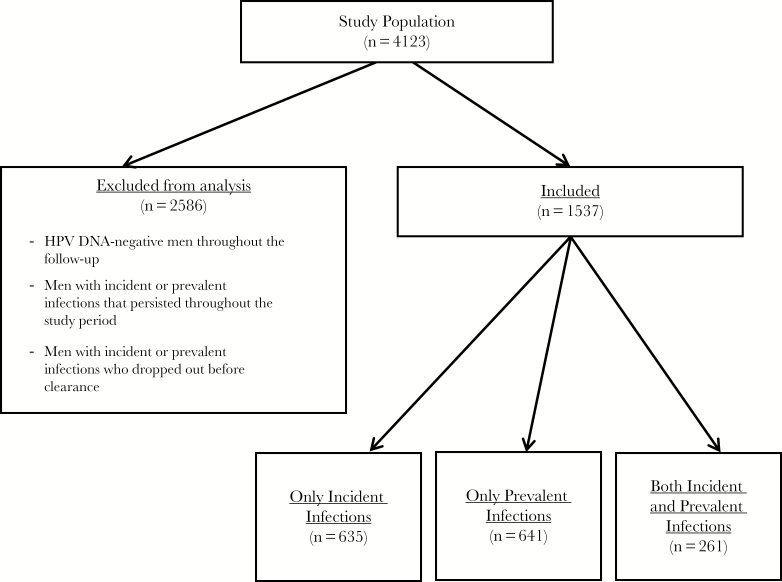
The 9 HPV types (HPV-6, -11, -16, -18, -31, -33, -45, -52, and -58) in the recently approved nonavalent HPV vaccine were included in this analysis [13]. Participants with prevalent HPV infections at baseline or an incident infection during the follow-up period were eligible for inclusion in the study. Participants who were HPV DNA negative throughout the study period, those with persistent infections throughout the study period, and those who dropped out of the study before infection clearance were excluded from the analysis (Figure 1). Two different analyses were carried out: (1) individual HPV genotype analysis for each of the 9 genotypes (infection-level analysis) and (2) to increase statistical power, grouped analyses (recurrent infection vs nonrecurrent infection with any of the 9 vaccine types). Recurrences assessed in both of these analyses were genotype concordant (type-specific).
Figure 1.
Study population included in analyses of recurrent genital human papillomavirus (HPV) infections in the HPV in Men (HIM) Study.
In individual HPV type (type-specific) analyses, infections were categorized into 2 groups: (1) infections that were first observed as incident and (2) infections observed as prevalent at the baseline visit. These groups were further categorized by infection duration as transient (observed at 1 visit only) or persistent (infection with duration of ≥6 months). Infection recurrence was defined as redetection of DNA of the same HPV genotype after 2 successive DNA-negative visits scheduled at 6-month intervals (≥12 months’ duration).
For each HPV genotype, the proportion of infections that recurred among individuals with a prior incident or prior prevalent infection was calculated. Visit-specific questionnaire data corresponding to 2 study visits following the last HPV DNA–positive visit (12-month at-risk period for recurrence) were utilized for nonrecurrent infections. For recurrent infections, questionnaire data from the visit at which recurrence was observed and data reported on the visit prior to the recurrence (total time period up to 12 months prior to the recurrence) were utilized in analyses. Variables of interest for these analyses included sexual behavior, smoking, alcohol use, and factors previously shown to be associated with genital incidence and prevalence in the HIM Study cohort [1, 2].
In grouped analysis, if individuals had recurrent infection of any of the 9 HPV types, they were grouped as “recurrent infections for any type.” Individuals in the “nonrecurrent infections” group did not have recurrences of any of the 9 HPV types. Subjects were further categorized into 3 groups: (1) only incident infection with any of the 9 types; (2) only prevalent infection with any of the 9 types; and (3) a combination of prevalent and incident infection with any of the 9 types (eg, accounting for multiple infections). Separate analyses were carried out for each of these 3 groups. Demographic characteristics for these groups were compared using χ2 tests with Monte Carlo estimation of exact P values. Men who had multiple HPV infections were at risk for recurrence of the individual HPV types at different susceptibility time periods during the follow-up. Therefore in grouped analysis, we characterized individual behavior based on participants’ overall pattern of responses (consistently reported behavior) throughout the study period. Logistic regression was utilized to evaluate associations between these variables and infection recurrence. Behavioral and demographic variables were evaluated in adjusted models. Selection of variables for inclusion in final models was based on the Akaike information criteria values.
RESULTS
Genital DNA samples were available for 4123 HIM study participants at baseline. A total of 635 men with incident infections, 641 men with prevalent infections, and 261 men with both an incident and prevalent infection with 1 or more of the 9 vaccine-type HPVs were included in analyses. The majority of men included in this analysis were from Brazil (45%), aged 18–30 years (49.1%), and were uncircumcised (65.5%); 4.7% were men who have sex with men, and 13.7% reported sex with both men and women. Median duration of follow-up was 3.7 years (range, 0.4–7.4 years; interquartile range, 3.4–4.0 years).
Individual HPV-Type Recurrence
Prevalent Infections
Overall, persistent infections had higher recurrence proportions compared to transient prevalent infections. Among men with prior transient prevalent infections, 3.9%–31.2% had recurrent infections, compared with 10.8%–36.8% of men with prior persistent prevalent infections. Among men with a prior transient infection, HPV-52 had the highest proportion of recurrence (31.2%), followed by HPV-45 (22.9%), HPV-58 (21.4%), HPV-6 (19.8%), and HPV-16 (19.6%) (Table 1). Among men with a prior persistent infection, HPV-45 had the highest recurrence proportion of recurrence (36.8%), followed by HPV-52 (32.4%), HPV-16 (23.5%), and HPV-6 (21.9%). The average time to clearance between the prevalent infection (either transient or persistent) and detection of the recurrent infection ranged between 17.2 and 26.2 months.
Table 1.
Recurrent Human Papillomavirus (HPV) Infection Proportions Among Men With a Prior Prevalent HPV-6, -11, -16, -18, -31, -33, -45, -52 or -58 Infection
| HPV Type | Total Prevalent Infections, No. | Transient Prevalenta Infections | Persistent Prevalentb Infections | ||
|---|---|---|---|---|---|
| Recurrentc/Total Transient Infections, No. | Proportion, % | Recurrentd/Total Persistent Infections, No. | Proportion, % | ||
| HPV-6 | 260 | 29/146 | 19.86 | 25/114 | 21.93 |
| HPV-11 | 58 | 1/26 | 3.85 | 6/32 | 18.75 |
| HPV-16 | 282 | 26/133 | 19.55 | 35/149 | 23.50 |
| HPV-18 | 103 | 10/57 | 17.54 | 9/46 | 19.57 |
| HPV-31 | 81 | 8/44 | 18.18 | 4/37 | 10.81 |
| HPV-33 | 25 | 1/13 | 7.69 | 2/12 | 16.67 |
| HPV-45 | 86 | 11/48 | 22.92 | 14/38 | 36.84 |
| HPV-52 | 161 | 29/93 | 31.18 | 22/68 | 32.35 |
| HPV-58 | 101 | 9/42 | 21.43 | 12/59 | 20.34 |
Abbreviation: HPV, human papillomavirus.
aTransient prevalent infection: a prevalent infection that was detected only at baseline.
bPersistent prevalent infection: persistence of prevalent infection for duration of ≥6 months that cleared at some point during follow-up.
cTransient prevalent recurrent: prevalent infections that cleared (2 successive negative DNA results) and then recurred during the follow-up period.
dPrevalent persistent recurrent: persistent prevalent infections that cleared (2 successive negative DNA results) and then recurred during the median follow-up time of 48 months.
Incident Infections
Similar to prevalent HPV infections, incident infections that were persistent had a higher proportion of recurrence compared to transient infections. Recurrence of incident infections ranged from 0.0% to 19.7% for transient incident infections and 0.0% to 26.3% for persistent incident infections. Among men whose incident infections were initially transient, the highest proportion of recurrence was for HPV-58 (19.7%), followed by HPV-52 (14.6%), HPV-18 (12.6%), and HPV-16 (11.8%) (Table 2). Among men with persistent infections, the highest recurrence proportion was observed for HPV-11 (26.3%), followed by HPV-52 (24.5%), HPV-18 (17.6%), and HPV-45 (13.6%). The average time between clearance of the incident infection (either transient or persistent) and the recurrent infection ranged between 16.8 and 21.3 months.
Table 2.
Recurrent Human Papillomavirus (HPV) Infection Proportions Among Men With a Prior Incident HPV-6, -11, -16, -18, -31, -33, -45, -52, and -58 Infection
| HPV Type | Total Incident Infections, No. | Transient Incidenta Infections | Incident Persistentb Infections | ||
|---|---|---|---|---|---|
| Recurrentc/Total Incident Infections, No. | Proportion, % | Recurrentd/Total Persistent Infections, No. | Proportion, % | ||
| HPV-6 | 211 | 15/164 | 9.15 | 6/47 | 12.77 |
| HPV-11 | 79 | 0/60 | 0.00 | 5/19 | 26.32 |
| HPV-16 | 249 | 22/195 | 11.28 | 4/54 | 7.41 |
| HPV-18 | 121 | 11/87 | 12.64 | 6/34 | 17.65 |
| HPV-31 | 90 | 6/66 | 9.09 | 3/24 | 12.50 |
| HPV-33 | 39 | 2/33 | 6.06 | 0/6 | 0.00 |
| HPV-45 | 121 | 10/99 | 10.10 | 3/22 | 13.64 |
| HPV-52 | 204 | 22/151 | 14.57 | 13/53 | 24.53 |
| HPV-58 | 104 | 14/71 | 19.72 | 3/33 | 9.09 |
Abbreviation: HPV, human papillomavirus.
aTransient incident: an incident infection that was detected at only 1 time point.
bIncident persistent infection: an incident infection that had an observed duration of ≥6 months.
cTransient incident recurrent: incident infections that cleared (2 successive negative results) and then recurred during the follow-up period.
dIncident persistent recurrent: persistent incident infections that cleared (2 successive negative results) and then recurred during the median follow-up time of 48 months.
Factors Associated With Genital HPV Infection Recurrence
Sexual behavior variables were consistently associated with recurrence of prevalent and incident infections. Among men with initial prevalent infections, HPV-6 infection recurrence was significantly associated with having ≥2 new female partners in the previous 6–12 months (P = .0007; Supplementary Table 1A). HPV 16 recurrence was associated with higher frequency of sexual intercourse with female partners (>30 times) in the past 6–12 months (P = .045). For HPV-31, men who reported having a new female sexual partner had higher rates of recurrence (38.5%) compared with men without a new sexual partner (P = .032; Supplementary Table 1B).
Among men with initially incident infections, having ≥1 new male sexual partners in the previous 6–12 months was significantly associated with higher recurrence of HPV-16 infections (P = .01; Supplementary Table 2A). Significantly higher rates of HPV-52 recurrence were observed among former smokers (27.3%) compared with current smokers (P = .048; Supplementary Table 2B).
HPV Infection Recurrence in Grouped Analysis
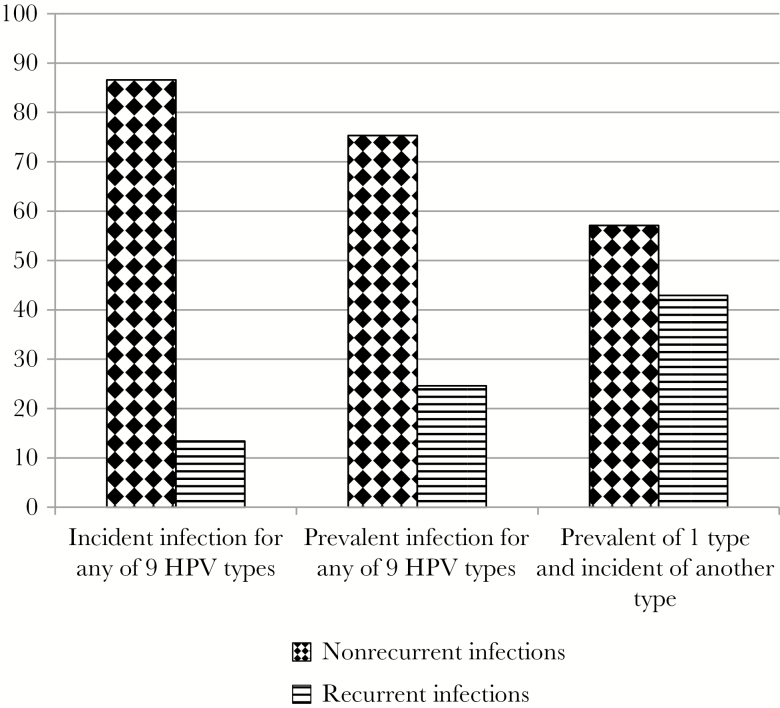
In grouped analyses, the number of men included in each of the 3 groups was 635 (only incident infection of any of the 9 types), 641 (only prevalent infection of any of the 9 types), and 261 (combination of prevalent and incident infections) (Figure 2). In this analysis, 13.4% of men with prior incident infections and 24.6% of men with prior prevalent infections had recurrence of any of the 9 HPV types. The highest recurrence was observed among men who had a combination of incident and prevalent infections of different HPV types (43.0%). White men had the highest proportion (17.6%) of incident infections that recurred (P = .036; Table 3). Recurrence of incident infections was also higher among men with greater numbers of lifetime male sexual partners (P = .012) and new male partners (P = .011). Among men with a combination of incident/prevalent infections, circumcised men had higher recurrences compared to uncircumcised men (P = .011). Among men with initially prevalent infections, none of the considered variables were significantly associated with infection recurrence.
Figure 2.
Proportion of incident and prevalent human papillomavirus (HPV) type 6, 11, 16, 18, 31, 33, 45, 52, and 58 infections that recurred according to infection status. Three groups compared here are incident infection of any of 9 HPV vaccine types, prevalent infections of any of 9 HPV vaccine types, prevalent infection of 1 HPV type, and incident infection of another HPV type (combination of prevalent and incident infections).
Table 3.
Demographic Characteristics of Men With Incident, Prevalent, or a Combination of Incident and Prevalent Human Papillomavirus (HPV) Infections by Recurrence Status for 1 or More of the 9 Types of HPV (HPV-6, -11, -16, -18, -31, -33, -45, -52, and -58)
| Demographic Variable | Only Incident Infections | Only Prevalent Infections | Combination of Incident and Prevalent Infections | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Nonrecurrent | Recurrent | P Valuea | Nonrecurrent | Recurrent | P Valuea | Nonrecurrent | Recurrent | P Valuea | |
| Country | |||||||||
| United States | 144 (84.2) | 27 (15.8) | .1383 | 139 (74.7) | 47 (25.3) | .7281 | 30 (51.7) | 28 (48.3) | .3084 |
| Brazil | 246 (85.4) | 42 (14.6) | 190 (74.2) | 66 (25.8) | 83 (56.1) | 65 (43.9) | |||
| Mexico | 160 (90.9) | 16 (9.1) | 154 (77.4) | 45 (22·6) | 36 (65.5) | 19 (34.5) | |||
| Age, y | |||||||||
| 18–30 | 255 (87.0) | 38 (13.0) | .7751 | 243 (78.9) | 65 (21.1) | .0892 | 90 (58.8) | 63 (41.2) | .8225 |
| 31–44 | 215 (87.0) | 32 (13.0) | 188 (73.2) | 69 (26.8) | 46 (54.8) | 38 (45.2) | |||
| 45–73 | 80 (84.2) | 15 (15.8) | 52 (68.4) | 24 (31.6) | 13 (54.2) | 11 (45.8) | |||
| Marital status | |||||||||
| Single | 240 (84.8) | 43 (15.2) | .4867 | 222 (77.6) | 64 (22.4) | .4352 | 84 (57.5) | 62 (42.5) | .7864 |
| Married/cohabiting | 307 (88.0) | 42 (12.0) | 209 (73.1) | 77 (26.9) | 50 (58.1) | 36 (41.9) | |||
| Divorced, separated, or widowed | 50 (89.3) | 6 (10.7) | 49 (75.4) | 16 (24.6) | 14 (50.0) | 14 (50.0) | |||
| Race | |||||||||
| White | 234 (82.4) | 50 (17.6) | .0359 | 227 (73.2) | 83 (26.8) | .4273 | 72 (56.7) | 55 (43.3) | .9721 |
| African American | 112 (88.9) | 14 (11.1) | 70 (77.8) | 20 (22.2) | 35 (56.5) | 27 (43.5) | |||
| Asian/Pacific Islander | 11 (91.7) | 1 (8.3) | 7 (63.6) | 4 (36.4) | 2 (66.7) | 1 (33.3) | |||
| Mixed race/other | 186 (91.2) | 18 (8.8) | 172 (78.2) | 48 (21.8) | 38 (59.4) | 26 (40.6) | |||
| Years of education | |||||||||
| ≤12 | 273 (86.4) | 43 (13.6) | .7707 | 230 (73.7) | 82 (26.3) | .1634 | 78 (58.6) | 55 (41.4) | .8409 |
| 13–15 | 141 (85.5) | 24 (14.5) | 112 (81.8) | 25 (18.2) | 30 (54.5) | 25 (45.5) | |||
| ≥16 | 135 (88.2) | 18 (11.8) | 140 (74.1) | 49 (25.9) | 40 (55.6) | 32 (44.4) | |||
| Alcohol usea | |||||||||
| <0.5 drinks/d | 174 (86.6) | 27 (13.4) | .9887 | 204 (79.7) | 52 (20.3) | .1014 | 38 (64.4) | 21 (35.6) | .2436 |
| 0.5 to <1.5 drinks/d | 157 (86.3) | 25 (13.7) | 114 (71.3) | 46 (28.8) | 38 (50.0) | 38 (50.0) | |||
| ≥1.5 drinks/d | 219 (86.9) | 33 (13.1) | 165 (73.3) | 60 (26.7) | 73 (57.9) | 53 (42.1) | |||
| Cigarette smokinga | |||||||||
| Never | 352 (85.4) | 60 (14.6) | .3288 | 319 (76.3) | 99 (23.7) | .6832 | 82 (54.3) | 69 (45.7) | .5879 |
| Former smoker | 30 (93.8) | 2 (6.3) | 34 (75.6) | 11 (24.4) | 8 (57.1) | 6 (42.9) | |||
| Current smoker | 168 (88.0) | 23 (12.0) | 130 (73.0) | 48 (27.0) | 59 (61.5) | 37 (38.5) | |||
| Circumcision | |||||||||
| No | 357 (87.7) | 50 (12.3) | .3357 | 320 (75.1) | 106 (24.9) | .9233 | 109 (62.6) | 65 (37.4) | .0111 |
| Yes | 193 (84.6) | 35 (15.4) | 163 (75.8) | 52 (24.2) | 40 (46.0) | 47 (54.0) | |||
| Sexual orientation | |||||||||
| MSW | 380 (86.4) | 60 (13.6) | .8233 | 340 (74.7) | 115 (25.3) | .7534 | 105 (57.7) | 77 (42.3) | .3327 |
| MSM | 29 (82.9) | 6 (17.1) | 20 (76.9) | 6 (23.1) | 9 (75.0) | 3 (25.0) | |||
| MSWM | 70 (85.4) | 12 (14.6) | 69 (78.4) | 19 (21.6) | 21 (51.2) | 20 (48.8) | |||
| Lifetime female sexual partnersa | |||||||||
| 0–1 partner | 66 (82.5) | 14 (17.5) | .4199 | 68 (81.0) | 16 (19.0) | .3773 | 13 (65.0) | 7 (35.0) | .1971 |
| 2–12 partners | 221 (88) | 30 (12) | 187 (76.0) | 59 (24.0) | 48 (64.0) | 27 (36.0) | |||
| ≥13 partners | 253 (87.2) | 37 (12.8) | 215 (73.6) | 77 (26.4) | 82 (52.6) | 74 (47.4) | |||
| No. of new female sexual partnersa | |||||||||
| 0 | 210 (84.0) | 40 (16.0) | .2127 | 211 (77.0) | 63 (23.0) | .4468 | 42 (61.8) | 26 (38.2) | .6439 |
| 1 | 205 (88.0) | 28 (12.0) | 150 (73.2) | 55 (26.8) | 54 (56.8) | 41 (43.2) | |||
| ≥2 | 131 (89.7) | 15 (10.3) | 94 (71.8) | 37 (28.2) | 50 (54.3) | 42 (45.7) | |||
| Frequency of sexual intercourse with female partners (occurrences per mo)a | |||||||||
| 0–5 | 93 (82.3) | 20 (17.7) | .2393 | 94 (77.7) | 27 (22.3) | .7540 | 26 (57.8) | 19 (42.2) | .9856 |
| 6–30 | 208 (88.9) | 26 (11.1) | 165 (74.0) | 58 (26.0) | 42 (57.5) | 31 (42.5) | |||
| ≥31 | 238 (86.5) | 37 (13.5) | 214 (75.6) | 69 (24.4) | 76 (56.3) | 59 (43.7) | |||
| Lifetime male sexual partnersa | |||||||||
| 0 | 466 (88.3) | 62 (11.7) | .0116 | 392 (74.0) | 138 (26.0) | .3708 | 119 (56.9) | 90 (43.1) | .9426 |
| 1–12 partners | 34 (87.2) | 5 (12.8) | 31 (79.5) | 8 (20.5) | 15 (57.7) | 11 (42.3) | |||
| ≥13 partners | 35 (72.9) | 13 (27.1) | 30 (83.3) | 6 (16.7) | 11 (52.4) | 10 (47.6) | |||
| No. of new male sexual partnersa | |||||||||
| 0 | 479 (88.4) | 63 (11.6) | .0111 | 403 (74.6) | 137 (25.4) | .8566 | 117 (56.8) | 89 (43.2) | .8745 |
| 1 | 22 (81.5) | 5 (18.5) | 30 (73.2) | 11 (26.8) | 28 (54.9) | 23 (45.1) | |||
| ≥2 | 36 (73.5) | 13 (26.5) | 22 (78.6) | 6 (21.4) | 0 (0.00) | 0 (0.00) | |||
Data are presented as No. (%) unless otherwise indicated. Subjects were categorized into 3 groups: (1) subjects with only incident infection with any of the 9 human papillomavirus (HPV) types; (2) subjects with only prevalent infection with any of the 9 types; and (3) a combination of prevalent and incident infection with any of the 9 types. In these 3 groups, subjects were further categorized based on recurrence of infection. Individuals with recurrent infection of any of the 9 HPV types were grouped as “recurrent infections for any type.” Individuals who did not have recurrences of any of the 9 HPV types were grouped as “nonrecurrent infections.” P values for categorical variables were calculated using the χ2 test with Monte Carlo estimation of P values, α = .05. P value represents global value for overall difference between incident and recurrent infections.
Abbreviations: MSM, men who have sex with men; MSW, men who have sex with women; MSWM, men who have sex with women and men.
aThese variables are based on the overall behavior pattern of an individual during the 3.7-year follow-up period. Subjects were categorized into a particular behavior category according to the most consistently reported behavior at 6-month interviews over the course of the study.
In logistic regression models (Table 4) utilizing overall behavior of the participants during the follow-up period, recurrence was significantly associated with report of >12 lifetime number of male sexual partners (adjusted odds ratio [aOR], 2.40; 95% confidence interval [CI], 1.19–4.84) and ≥2 new male sexual partners (aOR, 2.35; 95% CI, 1.16–4.74) among men with prior incident infections. Among men with a prior prevalent infection, moderate alcohol consumption (0.5 to <1.5 drinks per day) was significantly associated with recurrence (aOR, 1.59; 95% CI, 1.01–2.53) compared with mild alcohol consumption (<0.5 drinks/day). Among men with a combination of prior incident and prevalent infections, none of the considered variables yielded statistically significant results.
Table 4.
Factors Independently Associated With Recurrent Human Papillomavirus (HPV) Infections With Any 1 or More of the 9 HPV Types (HPV-6, -11, -16, -18, -31, -33, -45, -52, and -58) Among Men in the HPV Infection in Men (HIM) Study
| Only Incident Infections | Only Prevalent Infections | Combination of Prevalent and Incident Infections | ||||
|---|---|---|---|---|---|---|
| Variablea | Crude OR (95% CI) |
Adjusted ORb (95% CI) |
Crude OR (95% CI) |
Adjusted OR (95% CI) |
Crude OR (95% CI) |
Adjusted OR (95% CI) |
| Alcohol use | ||||||
| <0.5 drinks/d | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| 0.5 to <1.5 drinks/d | 1.03 (.57–1.84) | 1.01 (.55–1.91)b | 1.58 (1.00–2.50) | 1.59 (1.01–2.53)c | 1.81 (.90–3.63) | 1.97 (.96–4.06)d |
| ≥1.5 drinks/d | 0.97 (.56–1.68) | 0.90 (.50–1.60)b | 1.43 (.93–2.18) | 1.40 (.92–2.15)c | 1.31 (.69–2.49) | 1.22 (.63–2.36)d |
| Cigarette smoking | ||||||
| Never | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Former smoker | 0.39 (.09–1.68) | 0.43 (.10–1.87)b | 1.04 (.51–2.13) | 1.06 (.52–2.17)c | 0.88 (.30–2.69) | 0.89 (.29–2.76)d |
| Current smoker | 0.80 (.48–1.34) | 0.73 (.42–1.30)b | 1.19 (.80–1.78) | 1.21 (.81–1.81)c | 0.75 (.44–1.26) | 0.80 (.46–1.39)d |
| Lifetime female sexual partners | ||||||
| 0–1 partner | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| 2–12 partners | 0.64 (.32–1.28) | 0.73 (.32–1.66)b | 1.34 (.72–2.49) | 1.39 (.74–2.59)c | 1.05 (.37–2.93) | 1.13 (.39–3.25)d |
| ≥13 partners | 0.69 (.35–1.35) | 0.85 (.38–1.92)b | 1.52 (.83–2.78) | 1.50 (.82–2.75)c | 1.68 (.64–4.43) | 1.68 (.63–4.44)d |
| No. of new female sexual partners | ||||||
| 0 | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| 1 partner | 0.72 (.43–1.21) | 0.91 (.51–1.62)b | 1.22 (.80–1.86) | 1.23 (.81–1.87)c | 1.23 (.65–2.32) | 1.14 (.60–2.19)d |
| ≥2 partners | 0.60 (.32–1.13) | 0.69 (.35–1.35)b | 1.32 (.82–2.12) | 1.29 (.79–2.08)c | 1.36 (.72–2.57) | 1.19 (.61–2.33)d |
| Frequency of intercourse with female partners (occurrences per mo) | ||||||
| 0–5 | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| 6–30 | 0.58 (.31–1.09) | 0.93 (.39–2.23)b | 1.22 (.73–2.06) | 1.24 (.74–2.10)c | 1.01 (.48–2.14) | 1.03 (.47–2.21)d |
| ≥31 | 0.72 (.40–1.31) | 1.27 (.53–3.04)b | 1.12 (.67–1.86) | 1.12 (.68–1.87)c | 1.06 (.54–2.10) | 0.99 (.49–1.98)d |
| Lifetime male sexual partners | ||||||
| 0 | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| 1–12 partners | 1.11 (.42–2.93) | 1.02 (.38–2.73)e | 0.73 (.33–1.63) | 0.71 (.32–1.60)c | 0.97 (.43–2.21) | 1.12 (.48–2.62)d |
| ≥13 partners | 2.79 (1.40–5.56) | 2.40 (1.19–4.84)e | 0.57 (.23–1.39) | 0.53 (.21–1.32)c | 1.20 (.49–2.95) | 1.26 (.50–3.20)d |
| No. of new male sexual partners | ||||||
| 0 | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| 1 partner | 1.73 (.63–4.73) | 1.19 (.39–3.61)e | 1.08 (.53–2.21) | 1.04 (.50–2.15)c | 1.08 (.58–2.00) | 1.18 (.62–2.26)d |
| ≥2 partners | 2.75 (1.38–5.46) | 2.35 (1.16–4.74)e | 0.80 (.32–2.02) | 0.76 (.30–1.94)c | 0.00 (NE) | 0.00 (NE) |
Subjects were categorized into 3 groups: (1) subjects with only incident infection with any of the 9 human papillomavirus (HPV) types, (2) subjects with only prevalent infection with any of the 9 types; and (3) a combination of prevalent and incident infection with any of the 9 types. In these 3 groups, subjects were further categorized based on recurrence of infection. Individuals with recurrent infection of any of the 9 HPV types were grouped as “recurrent infections for any type.” Individuals who did not have recurrences of any of the 9 HPV types were grouped as “nonrecurrent infections.”
Abbreviations: CI, confidence interval; NE, not estimable; OR, odds ratio.
aThese variables are based on the overall behavior pattern of an individual during the 3.7-year follow-up period. Subjects were categorized into a particular behavior category according to the most consistently reported behavior at 6-month interviews over the course of the study.
bAdjusted for race and lifetime male sexual partners.
cAdjusted for country.
dAdjusted for country and circumcision status.
eAdjusted for race.
DISCUSSION
This is the first study to report HPV recurrence among men at the external genital skin. Our results indicate that 3.9%–31.2% of prior prevalent genital HPV infections recur with the same HPV type, with higher recurrence observed for oncogenic HPV types (HPV-16, 19.6%; HPV-45, 22.9%; HPV-52, 31.2%; and HPV-58, 21.4%).
In the current analysis, higher numbers of new male sexual partners were consistently associated with higher recurrence of incident infections in both type-specific (HPV-16) and grouped analyses. Other high-risk sexual behaviors (number of new female partners and frequency of sexual intercourse) were also associated with higher recurrence of prevalent HPV-6, -16, and -31 infections. Recurrence significantly differed by smoking and alcohol use only for HPV types 16, 52, and 58. As no prior study has described genital HPV recurrence among men, it is unclear if proportions reported here are unique to the cohort, or generalizable to a broader population.
The pattern of genital HPV recurrence observed in the current study of men is similar to that reported in the few studies conducted among women. In a study from Winer et al among young women, 19.4% of incident infections of multiple genotypes were redetected within a year [8]. In an international study of women aged 16–26 years, HPV recurrence ranged from 0 to 16%, with HPV-16 recurrence detected at 11% [9]. A study by Moscicki et al observed redetection of HPV-16 DNA in 18.1% of women [10]. This study also reported that having 1 or more sexual partners (aOR, 2.90; 95% CI, 1.22–6.90) and having a new sexual partner (aOR, 1.1; 95% CI, 1.02–1.19) were associated with increased risk of redetection [10]. Lower rates of HPV recurrence were observed (7.7% women with intervening negative results and 2.7% women with definite clearance) in an analysis of the Guanacaste cohort [11]. Finally, in a study by Trottier et al, a strong association between HPV recurrence and having new sexual partners was observed (relative risk, 3.7; 95% CI, 1.1–13.8) for all HPV types included in the analysis [7].
There are 3 possible explanations for the observed HPV infection recurrence: (1) sexual exposure from the same partner who has not cleared the infection; (2) exposure to a new partner who is infected with the same HPV type; and (3) reactivation of latent infections. Our results show that new male partners, new female partners, and higher numbers of lifetime sexual partners led to higher genital HPV infection recurrence. We do not, however, have information to differentiate whether the recurrence was acquired from a new partner or from the same partner. Partner studies as well as evaluation of HPV status with next-generation sequencing are needed to shed light on the origin of the recurrent infections. However, intratypic variant analysis may not identify the source of infection, as specific HPV variants are more prevalent in a particular geographic area [14–16]; thus, probability of infection from a new source is highest for a prevalent variant circulating in the community. As men appear not to develop immunity to HPV following natural infection [17], the probability of any given HPV type recurring will likely depend on the prevalence of the individual HPV types circulating in the community, the number of new sexual partners and their current infections, and perhaps the infectivity of the different HPV types. More research is needed to elucidate whether the rate of infectivity differs by HPV type at the genital epithelium in men. In addition, current methods cannot differentiate between latent and newly acquired infections [18]. Thus, analysis of recurrent infection presents a number of challenges and logistic issues.
Strengths of this study include large sample size, multinational nature of the cohort, long follow-up time, and sensitive HPV DNA genotyping methods [19, 20]. Furthermore, we carefully assessed behavior during the intervening cleared period (susceptibility period for infection reacquisition). We chose 9-valent vaccine type HPVs as these are the most commonly detected HPV types and contribute to significant disease burden among men and women. As these are the most common types, they also provide the statistical power for analyses and clinical relevance for current analyses.
The study also had several limitations that may have affected our results. We observed higher recurrence among the group of participants with prior prevalent infections. Baseline prevalent infections had a relatively longer observation period to allow for clearance and redetection compared with infections detected as incident at some point in the follow-up period. This greater opportunity to detect a recurrence may in part explain the larger number of recurrence events with prevalent infections. It is also likely that individuals with preexisting infections had high-risk sexual behavior and as a result were at higher risk of reacquiring HPV infections. However, analyses comparing differing recurrence proportions within groups of incident and prevalent infections are not affected by this limitation.
Our analysis was also restricted by small sample size of individual HPV-type recurrences during the follow-up period. To overcome this limitation, we conducted grouped analyses of factors associated with genital HPV recurrence. As recurrences assessed in this manner are not HPV-type specific, we could not conduct a person-year analysis. An additional limitation is the lack of information regarding HPV infection status prior to the study start; therefore, it is likely that participants may have acquired and cleared infections prior to entering the study. There is also the possibility that existing latent infection prior to study start or during the follow-up become reactivated and then detected as either an incident or recurrent infection [18, 21–24]. HPV latency is proposed based on the animal model whereby HPV is retained in a latent state in the basal epithelial stem cell pool [18]. Current HPV DNA detection methods may not be sensitive enough to detect low viral load of latent infections [22–24], and reactivation of latent infection is less likely in immunocompetent subjects (as in the HIM Study). Future studies are needed to further elucidate the role of latent HPV infections in men and women.
Misclassification of HPV infection status is also a possibility. Low viral load and long periods of time between the initial infection and assessment of HPV DNA may lead to misclassification of HPV infection status. However, such misclassification should play a minor role in our analyses, considering the robust methods used for HPV DNA detection [19, 20]. We did not have a sufficient number of recurrent infections to adequately power individual type-specific analyses, especially for less common HPV types (eg, HPV-11, -31, -33). We attempted to address this issue in our grouped analyses of all 9 HPV types. Furthermore, subjects excluded from the analysis did not differ significantly from study participants regarding sexual behavior and other important demographic variables.
In conclusion, men are susceptible to genital HPV infection recurrence, with up to 31% of prevalent and 20% of incident infections recurring over time. Recurrence of HPV infection among men is influenced by high-risk sexual behavior; including higher numbers of new sexual partners and lifetime sexual partners. Future studies are needed to further understand the role of HPV recurrence in the etiology of HPV-associated diseases, and to understand infection recurrence at other anatomic sites (eg, anal canal) where HPV causes cancer. To prevent these infections, gender-neutral HPV vaccine policies that target adolescent males and females are needed to protect against the initial HPV infection, recurrent infections, and importantly, the prevalence of disease-causing HPV types circulating in the overall community.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank the HIM Study teams and participants in the United States (Moffitt Cancer Center, Tampa, Florida), Brazil (Centro de Referência e Treinamento em DST/AIDS, Fundação Faculdade de Medicina Instituto do Câncer do Estado de São Paulo, Ludwig Institute for Cancer Research, São Paulo), and Mexico (Instituto Mexicano del Seguro Social, Instituto Nacional de Salud Pública, Cuernavaca).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Giuliano AR, Lee JH, Fulp W, et al. Incidence and clearance of genital human papillomavirus infection in men (HIM): a cohort study. Lancet 2011; 377:932–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Giuliano AR, Lazcano-Ponce E, Villa LL, et al. The human papillomavirus infection in men study: human papillomavirus prevalence and type distribution among men residing in Brazil, Mexico, and the United States. Cancer Epidemiol Biomarkers Prev 2008; 17:2036–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Giuliano AR, Nyitray AG, Kreimer AR, et al. EUROGIN 2014 roadmap: differences in human papillomavirus infection natural history, transmission and human papillomavirus-related cancer incidence by gender and anatomic site of infection. Int J Cancer 2015; 136:2752–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Partridge JM, Hughes JP, Feng Q, et al. Genital human papillomavirus infection in men: incidence and risk factors in a cohort of university students. J Infect Dis 2007; 196:1128–36. [DOI] [PubMed] [Google Scholar]
- 5. Franceschi S, Castellsagué X, Dal Maso L, et al. Prevalence and determinants of human papillomavirus genital infection in men. Br J Cancer 2002; 86:705–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Smith JS, Gilbert PA, Melendy A, Rana RK, Pimenta JM. Age-specific prevalence of human papillomavirus infection in males: a global review. J Adolesc Health 2011; 48:540–52. [DOI] [PubMed] [Google Scholar]
- 7. Trottier H, Ferreira S, Thomann P, et al. Human papillomavirus infection and reinfection in adult women: the role of sexual activity and natural immunity. Cancer Res 2010; 70:8569–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Winer RL, Hughes JP, Feng Q, et al. Early natural history of incident, type-specific human papillomavirus infections in newly sexually active young women. Cancer Epidemiol Biomarkers Prev 2011; 20:699–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Insinga RP, Perez G, Wheeler CM, et al. FUTURE I Investigators Incidence, duration, and reappearance of type-specific cervical human papillomavirus infections in young women. Cancer Epidemiol Biomarkers Prev 2010; 19:1585–94. [DOI] [PubMed] [Google Scholar]
- 10. Moscicki AB, Ma Y, Farhat S, et al. Redetection of cervical human papillomavirus type 16 (HPV16) in women with a history of HPV16. J Infect Dis 2013; 208:403–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rodríguez AC, Schiffman M, Herrero R, et al. Low risk of type-specific carcinogenic HPV re-appearance with subsequent cervical intraepithelial neoplasia grade 2/3. Int J Cancer 2012; 131:1874–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bouvard V, Baan R, Straif K, et al. WHO International Agency for Research on Cancer Monograph Working Group A review of human carcinogens—part B: biological agents. Lancet Oncol 2009; 10:321–2. [DOI] [PubMed] [Google Scholar]
- 13. Joura EA, Giuliano AR, Iversen OE, et al. Broad Spectrum HPV Vaccine Study A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N Engl J Med 2015; 372:711–23. [DOI] [PubMed] [Google Scholar]
- 14. Pérez-Gallego L, Moreno-Bueno G, Sarrió D, Suárez A, Gamallo C, Palacios J. Human papillomavirus-16 E6 variants in cervical squamous intraepithelial lesions from HIV-negative and HIV-positive women. Am J Clin Pathol 2001; 116:143–8. [DOI] [PubMed] [Google Scholar]
- 15. Barzon L, Militello V, Lavezzo E, et al. Human papillomavirus genotyping by 454 next generation sequencing technology. J Clin Virol 2011; 52:93–7. [DOI] [PubMed] [Google Scholar]
- 16. Arroyo LS, Smelov V, Bzhalava D, Eklund C, Hultin E, Dillner J. Next generation sequencing for human papillomavirus genotyping. J Clin Virol 2013; 58:437–42. [DOI] [PubMed] [Google Scholar]
- 17. Pamnani SJ, Sudenga SL, Viscidi R, et al. Impact of serum antibodies to HPV serotypes 6, 11, 16, and 18 to risks of subsequent genital HPV infections in men: the HIM Study. Cancer Res 2016; 76:6066–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gravitt PE. Evidence and impact of human papillomavirus latency. Open Virol J 2012; 6:198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stevens MP, Rudland E, Garland SM, Tabrizi SN. Assessment of MagNA pure LC extraction system for detection of human papillomavirus (HPV) DNA in PreservCyt samples by the Roche AMPLICOR and LINEAR ARRAY HPV tests. J Clin Microbiol 2006; 44:2428–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gravitt PE, Peyton CL, Apple RJ, Wheeler CM. Genotyping of 27 human papillomavirus types by using L1 consensus PCR products by a single-hybridization, reverse line blot detection method. J Clin Microbiol 1998; 36:3020–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lu B, Viscidi RP, Wu Y, et al. Prevalent serum antibody is not a marker of immune protection against acquisition of oncogenic HPV16 in men. Cancer Res 2012; 72:676–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rositch AF, Burke AE, Viscidi RP, Silver MI, Chang K, Gravitt PE. Contributions of recent and past sexual partnerships on incident human papillomavirus detection: acquisition and reactivation in older women. Cancer Res 2012; 72:6183–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Strickler HD, Burk RD, Fazzari M, et al. Natural history and possible reactivation of human papillomavirus in human immunodeficiency virus-positive women. J Natl Cancer Inst 2005; 97:577–86. [DOI] [PubMed] [Google Scholar]
- 24. Theiler RN, Farr SL, Karon JM, et al. High-risk human papillomavirus reactivation in human immunodeficiency virus-infected women: risk factors for cervical viral shedding. Obstet Gynecol 2010; 115:1150–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.