Does adding low-dose primaquine to mass drug administration of artemisinin-based combination therapy improve malaria control? On a highly endemic African island, >99% effectiveness of falciparum malaria reduction followed 3 monthly rounds of artemisinin and piperaquine, with or without low-dose primaquine.
Keywords: malaria control, Plasmodium falciparum, artemisinin-based combination therapy, PfK13 Kelch-propeller gene, Comoros Islands
Abstract
Background
Mass drug administration (MDA), with or without low-dose primaquine (PMQLD), is being considered for malaria elimination programs. The potential of PMQLD to block malaria transmission by mosquitoes must be balanced against liabilities of its use.
Methods
Artemisinin–piperaquine (AP), with or without PMQLD, was administered in 3 monthly rounds across Anjouan Island, Union of Comoros. Plasmodium falciparum malaria rates, mortality, parasitemias, adverse events, and PfK13 Kelch-propeller gene polymorphisms were evaluated.
Results
Coverage of 85 to 93% of the Anjouan population was achieved with AP plus PMQLD (AP+PMQLD) in 2 districts (population 97164) and with AP alone in 5 districts (224471). Between the months of April–September in both 2012 and 2013, average monthly malaria hospital rates per 100000 people fell from 310.8 to 2.06 in the AP+PMQLD population (ratio 2.06/310.8 = 0.66%; 95% CI: 0.02%, 3.62%; P = .00007) and from 412.1 to 2.60 in the AP population (ratio 0.63%; 95% CI: 0.11%, 1.93%; P < .00001). Effectiveness of AP+PMQLD was 0.9908 (95% CI: 0.9053, 0.9991), while effectiveness of AP alone was 0.9913 (95% CI: 0.9657, 0.9978). Both regimens were well tolerated, without severe adverse events. Analysis of 52 malaria samples after MDA showed no evidence for selection of PfK13 Kelch-propeller mutations.
Conclusions
Steep reductions of malaria cases were achieved by 3 monthly rounds of either AP+PMQLD or AP alone, suggesting potential for highly successful MDA without PMQLD in epidemiological settings such as those on Anjouan. A major challenge is to sustain and expand the public health benefits of malaria reductions by MDA.
Malaria infects ~210 million people and kills nearly 0.5 million people annually, with ~90% of malaria deaths in Africa [1]. In the Union of Comoros, Plasmodium falciparum infections have historically accounted for 15–30% of hospitalizations and 15–20% of registered deaths in the pediatric services [2]. To eliminate this burden, the Comoros National Health Development Plan for 2010–2014 aimed to reduce parasite carriage rates to <5% [3]. Objectives included access for all Comorians to long-lasting insecticide–treated nets, indoor residual spraying, intermittent presumptive treatment for pregnant women, medical care for malaria, and implementations of mass drug administration (MDA).
An important goal of MDA is the comprehensive treatment of asymptomatic parasite carriers who silently sustain transmission in endemic populations [4, 5]. MDA regimens have therefore included primaquine (PMQ) to kill the P. falciparum gametocytes that infect mosquitoes. A single dose of PMQ (0.25 mg base/ kg) may clear P. falciparum gametocytes without serious complications from G6PD deficiency [6]. In a modeling study, low-dose PMQ (PMQLD; 9 mg adult dose) was predicted to enhance the transmission blocking effect of MDA with artemisinin-based combination therapy (ACT) [7]. Indeed, MDA of artemisinin–piperaquine (AP; Artequick) plus PMQLD (AP + PMQLD) rapidly and dramatically reduced the parasite carriage rates in 3653 individuals in 17 villages of Kampong Speu Province, Cambodia [8].
MDA regimens of ACT, with or without PMQLD, are under evaluation for use in malaria elimination programs [9]. The potential of these regimens spurs interest in questions that include: (1) where and when is MDA application most effective and sustainable, (2) in what settings can inclusion of PMQLD as a gametocidal drug improve MDA outcomes, (3) how do the benefits of the individual drugs balance against the liabilities of their use and attendant possibilities of adverse events, including the risks and contraindications of primaquine in conditions of G6PD-deficiency and early pregnancy, and (4) what are the possibilities of inducing drug resistance? Here we report on MDA of AP with or without PMQLD in 2 cohorts totaling 85–93% of the ~322000 inhabitants of Anjouan Island, Union of Comoros. We compare MDA outcomes with malariometrics in a population of ~420000 on Grande Comore Island, where control programs of bednet distributions, diagnosis, and treatment continued without MDA. Finally, we survey the occurrences of mutations in the PfK13 Kelch-propeller sequence, which has been associated with a P. falciparum ring-stage artemisinin survival phenotype in Southeast Asia [10].
METHODS
Study Areas
Anjouan Island (Ndzuwani) includes an area of 424 km2 with 321635 inhabitants residing in 97 villages of 7 districts (Supplementary Figure 1); nearby Grande Comore Island (Ngazidja) has an area of 1147 km2 with ~420000 inhabitants (2012 estimates). Malaria on Anjouan is transmitted principally by Anopheles gambiae and Anopheles funestus, with strong regional and seasonal variations, whereas transmission on Grande Comore is by An. gambiae and largely hyperendemic [11, 12]. Nearly all reported malaria cases are from P. falciparum infections [13].
Community Permission, Informed Consent, Engagement and Information Distribution
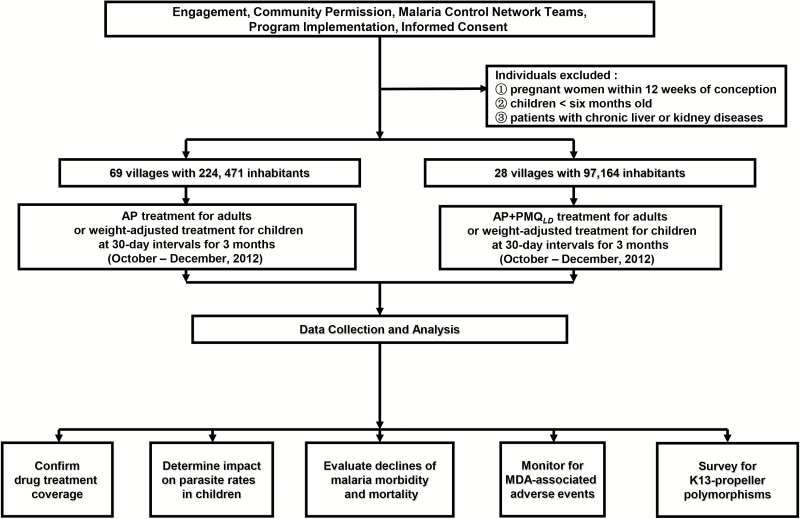
The protocol and informed consent forms for this study were approved by the Ethics Committees of Comoros Ministry of Health (No. 12521/MSSPG/CAB) and Guangzhou University of Chinese Medicine (No. 2012L0816). MDA programs were established with the approval of village leaders and religious elders under community permission [14], in collaboration with representatives of the National Malaria Control Programme of Comoros, the Guangzhou University of Chinese Medicine, and the Comoros Ministry of Health (Figure 1). Informed consent was provided by individuals, parents, or guardians, as appropriate. Consent forms were kept for 3 years after completion of the MDA.
Figure 1.
Diagram of study organization, recruitment, data collection, and analyses.
A campaign to inform and engage Anjouan inhabitants was launched in concert with the Comoros Ministry of Health and National Malaria Control Programme. Information and discussions were promoted through announcements about the MDA programs on radio stations (300 times), on television stations (20 times), in newspapers (90 times), on posters (3500 items), in brochures (10100 pieces), and on banners (340 items); provision of MDA explanations to women and students by international and local non-profit organizations (Women’s Human Rights Organization, Women’s Family, Women’s Legal Aid Center, the Red Cross, middle schools, and university venues); and MDA discussions with village leaders and religious elders after presentations by the government officials of Comoros and the malaria control team of Guangzhou University of Chinese Medicine (155 events).
Malaria Control Network and MDA Administration
Malaria control teams consisting of 4100 village malaria volunteers, 236 village malaria workers, and 14 district malaria workers was organized and assigned the responsibility to visit every household, keep villagers informed of MDA activities, administer treatments, and monitor for MDA-associated adverse events. A “village-district-county-island” malaria surveillance-response system was concurrently established, with 7 diagnostic laboratories plus 7 district- and 97 county-level malaria control centers.
After a comprehensive census of Anjouan households, MDA of either AP alone or AP+PMQLD was assigned in 3 monthly rounds from October–December 2012 (medicines supplied by Artepharm, People’s Republic of China). The decision of which districts were to receive PMQ was based on logistics and population numbers, with attention to clear instructions to staff and organized data collection. Risk of possible adverse events from PMQ was a further consideration. After discussions with regional and national health authorities, the decision was made to include PMQ in MDA for a smaller population of 97164 residents (~1/3 of the total) in 28 villages of the Pomoni and Domoni districts, while AP alone was offered to the 224471 residents of 69 villages in the Mutsamudu, Ouani, Sima, Tsembehou, and Miremani districts. Medicine doses (Supplementary Table 1) were given under direct observation. Pregnant women within 3 months of conception, children <6 months old, and patients with liver or kidney diseases were excluded from MDA and received care as recommended by the Comoros Ministry of Health.
Microscopic Diagnosis of Parasitemias
Informed consent was obtained from adults or the legal guardians of children, and finger-prick blood samples were collected for peripheral thick and thin blood smears. After preparation and staining with 10% Giemsa solution, the smears were sent to the malaria laboratories in local district hospitals for examination by certified malaria microscopists. A negative smear was recorded if no parasite was observed in at least 200 fields with an oil immersion lens (100×). Of the negative smears, 10% were randomly selected and re-examined by a second microscopist to confirm the quality of the determinations. Any discrepancies were reviewed in conference and resolved, if necessary, by the readings of a third microscopist.
Monitoring and Treatment of Travelers
We monitored travelers at the airport (1) and wharfs (98) of the Island. Each traveler was asked to complete an information form and provided with an AP dose to take either immediately (recommended) or later if fever developed. Individual travelers were also asked to visit a malaria diagnostic laboratory to have a free microscopic check for Plasmodium infection.
Surveillance for MDA-associated Adverse Events
On days 1 and 4 after the start of each MDA round, subjects were interviewed for adverse events, including any appearance of dark urine, and these individuals were clinically evaluated if indicated. In addition to a standard symptom questionnaire, subjects were asked to answer open-ended questions such as “Do you feel different in any way since starting the drug treatment course?” Subjects experiencing adverse events were attended on site by a doctor or referred to a local district hospital for care.
PCR Amplification and Analysis of PfK13 Propeller Sequences
Blood samples before or after MDA were obtained from subsets of patients who presented with symptoms and P. falciparum parasitemia. Whole blood (1 ml) was collected in an EDTA-containing vacutainer tube (Sangon Bio Inc., Shanghai, China) and stored at −20°C. DNA was isolated from 100 μl of each blood sample using the Takara DNA Blood Mini Kit (Takara, Japan). After polymerase chain reaction (PCR) amplification (Supplementary Table 2), nucleotide sequencing was performed with an ABI PRISM3730 DNA sequencer (Sangon Bio Inc., Shanghai, China).
Statistical Analyses
Data from April–September 2012 and from April–September 2013 (Supplementary Table 3) were analyzed by a quasi-Poisson model as described in Supplementary Appendix 1.
RESULTS
MDA Coverage
Table 1 summarizes the groups given 3 monthly rounds of either AP or AP+PMQLD on Anjouan Island in October–December 2012. Average coverage rates of 85.7–93.2% and 84.7–92.9% of the whole district populations were achieved with AP and AP+PMQLD, respectively, with improved participation in each round. The rounds of MDA excluded 2.3–2.6% of the population as women pregnant < 3 months, 3.1–3.6% as infants <6 months old, and 0.03–0.04% as patients with liver or kidney diseases (Supplementary Table 4).
Table 1.
Mass Drug Administration Coverage in 7 Districts of Anjouan Island, the Union of Comoros
| MDA Treatmenta | District | Registered Populationb | Number Treated (%) | ||
|---|---|---|---|---|---|
| Round 1 | Round 2 | Round 3 | |||
| AP | Ouani | 44788 | 37982 (84.8) | 39959 (89.2) | 41528 (92.7) |
| Miremani | 67162 | 56088 (83.5) | 60448 (90.0) | 63337 (94.3) | |
| Mutsamudu | 59388 | 51969 (87.5) | 54121 (91.1) | 53689 (90.4) | |
| Tsembehou | 25338 | 22509 (88.8) | 23618 (93.2) | 24050 (94.9) | |
| Sima | 27795 | 23714 (85.3) | 25188 (90.6) | 26550 (95.5) | |
| Total | 224471 | 192262 (85.7) | 203334 (90.6) | 209154 (93.2) | |
| AP+PMQLD | Domoni | 59535 | 51022 (85.7) | 54059 (90.8) | 54955 (92.3) |
| Pomoni | 37629 | 31310 (83.2) | 33720 (89.6) | 35298 (93.8) | |
| Total | 97164 | 82332 (84.7) | 87779 (90.3) | 90253 (92.9) | |
Abbreviations: AP, artemisinin and piperaquine; MDA, mass drug administration; PMQLD, low-dose primaquine.
aTreatment schedules are listed in Supplementary Table 1.
bNumbers of inhabitants are from 2012 census data compiled by the malaria control teams in the Union of Comoros.
A total of 81212 travelers were recorded at the airport and wharfs for the 27-month period of October 2012–December 2014. Of these travelers, 82.3% (66837) took a therapeutic dose of AP immediately; 10.6% (8608) opted to carry the dose and treat themselves if they developed fever during their journey; and 7.1% either avoided an interview or declined to participate.
Fast, Dramatic Reductions of Malaria Cases and Deaths With MDA of AP+PMQLD or AP
Among the 97164 individuals in the districts covered by AP+PMQLD MDA, hospital malaria cases dropped from a rate of 310.8 per 100000 (1812 total cases) in April–September 2012, to a rate of 2.06 per 100000 (12 total cases) in April–September 2013. Likewise, case rates among the 224471 Anjouan individuals in the districts covered by AP MDA dropped from 412.1 per 100000 (5550 total cases) in April–September 2012, to 2.60 per 100000 in April–September 2013 (35 total cases; Supplementary Table 3). District-by-district analyses were performed by quasi-Poisson modeling and compared to malaria rates on Grand Comore Island, where control programs of bednet distributions, diagnosis, and treatment were continued without MDA in a setting of higher Plasmodium indices and anthropogenic reservoirs of transmission [11, 12]. Results of these analyses (Supplementary Appendix 1) indicated an estimated AP+PMQLD effectiveness of 0.9908 compared to the no-MDA control population on Grande Comore Island (95% CI: 0.9053, 0.9991). Similarly, the effectiveness of MDA with AP alone was 0.9913 compared to the no-MDA controls (95% CI: 0.9657, 0.9978). In terms of post-treatment malaria case rates as a fraction of baseline rates, we obtained ratios of 0.00662 and 0.00631 after AP+PMQLD and AP, respectively. While these 2 ratios are not significantly different (0.00662/0.00631 = 1.0501; 95% CI: 0.07027, 15.69363; P = .9717), we note that proof of the same outcome after AP+PMQLD and AP (within a confidence interval of 0.80–1.20) would require repetition of the full MDA study 241 times, each time with a similar estimate of the ratio and its variance (Supplementary Appendix 1).
No malaria-associated deaths were reported on Anjouan Island in the 18 months after completion of the MDA programs, compared to 6 deaths in April–September 2012, prior to MDA (Supplementary Table 3). In contrast, mortality on Grande Comore Island remained virtually unchanged: 10 malaria-associated deaths were reported in April–September 2012, and 9 such deaths in April–September 2013. Quasi-Poisson cluster analysis of these deaths could not be performed as for the non-fatal malaria cases: the models do not converge because there were no deaths among the AP+PMQLD and AP only groups in the post-treatment months.
Reduction of Parasitemia Rates in Children
Before MDA, parasitemia rates among children 0.5–16 years old ranged from 9.8% (52/531, Domoni) to 15.8% (69/436, Pomoni), and averaged 13.5% (383/2841) across the 7 districts (Supplementary Table 5). In the 2 districts treated with AP+PMQLD, average parasitemia rates in children decreased to 1.2% (12/994), 0.7% (7/985), and 0.6% (6/991) at 6, 12, and 18 months post-MDA, respectively. Similarly, in the 5 districts treated with AP, average parasitemia rates in children decreased to 1.1% (22/1925) at 6 months post-MDA, 0.7% (13/1879) at 12 months post-MDA, and 0.5% (9/1792) at 18 months post-MDA. Among the samples examined 6 months post-MDA, gametocytes were detected by microscopy in 7 of the 994 (0.7%) children treated with AP+PMQLD and in 9 of the 1925 (0.5%) children treated with AP (Fisher’s exact test, 2-sided P = .434).
MDA-associated Adverse Events
A total of 865114 treatments of AP+PMQLD or AP were monitored for MDA-associated adverse events (260364 for AP+PMQLD and 604750 for AP). There were 504 mild adverse events recorded within 4 days of the MDA rounds (153 after AP+PMQLD and 351 after AP), amounting to an overall incidence of 0.58/1000 persons (Supplementary Table 6). Headache was most common (0.12/1000 persons), followed by loss of appetite (0.11/1000 persons), dizziness (0.11/1000 persons), and nausea (0.09/1000 persons). No MDA-associated deaths or other serious adverse events were reported.
Prevalence of PfK13 Propeller Polymorphisms in P. falciparum Isolates
Table 2 lists the PfK13 polymorphisms found in 248 successful PCR products from 271 P. falciparum–infected blood samples collected before or after MDA. None of these polymorphisms showed a statistically significant difference in prevalence between the 2 periods (Supplementary Appendix 1).
Table 2.
P. falciparum K13 Sequence Polymorphisms on Anjouan Island
| Mutations | Amino Acid Change and Positiona |
Genetic Changea | Number of Changes Detected (%)b | |
|---|---|---|---|---|
| Before MDA (n = 196)c | After MDA (n = 52)d | |||
| Synonymous | Y500Y | TAT→TAC | 2 (1.0) | 2 (3.8) |
| N531N | AAT→AAC | 3 (1.5) | 0 (0) | |
| Non-synonymous | D464H | GAT→CAT | 5 (2.6) | 2 (3.8) |
| S477Y | TCT→TAT | 0 (0) | 1 (1.9) | |
| N490H | AAT→CAT | 6 (3.1) | 1 (1.9) | |
| V520A | GTT→GCT | 0 (0) | 1 (1.9) | |
| N554H | AAT→CAT | 3 (1.5) | 1 (1.9) | |
| A578S | GCT→TCT | 2 (1.0) | 0 (0) | |
Abbreviations: MDA, mass drug administration; PCR, polymerase chain reaction.
aAmino acid and nucleotide polymorphisms are indicated in bold type.
bNo changes are statistically different between the 2 periods (Fisher Exact Test).
cThe 196 sequences are from amplified PCR products of samples from Pomoni (41), Miremani (60), and Domoni (95), collected during August–September 2012.
dThe 52 sequences are from amplified PCR products of samples from Pomoni (10), Miremani (7), Domoni (8), Mutsamudu (7), Ouani (9), Sima (8), and Tsembehou (3), collected during January 2013–March 2015.
DISCUSSION
In endemic settings, PMQLD is effective against P. falciparum gametocytes and is recommended by the World Health Organization for transmission control [15–18]. Recent studies found that posttreatment gametocytemias and gametocyte carriage times were reduced by addition of low PMQ doses to different ACTs (artemether–lumefantrine or dihydroartemisinin–piperaquine) [19, 20]; however, a dry season study in Eastern Sudan found that the addition of PMQ to MDA of artesunate–sulfadoxine–pyrimethamine did not contribute to the reduction of gametocyte carriage and parasitemia rates [21]. Recent modeling has predicted limited advantages from the addition of PMQLD to MDA in highly malarious regions [22]. In agreement with this modeling, our comparison on Anjouan Island of AP+PMQLD vs. AP alone found effectiveness of >99% for both programs, with no substantive contribution of PMQLD on MDA outcomes.
Together, the MDA programs of AP+PMQLD and AP alone were associated with a reduction from 7362 total cases among 321635 Anjouan residents in April–September 2012, before the start of MDA, to 47 total cases 1 year later, in April–September 2013 (99.4% decrease). Among the ~420000 individuals on Grand Comore Island, where standard malaria control programs were maintained but no MDA programs were yet implemented, hospital malaria cases in these same periods decreased by only 27.8%, from 36449 to 26324 cases (Table 2). Success of MDA in the Anjouan setting involved training and programmatic operations similar to those for programs of Fast Elimination of Malaria by Source Eradication (FEMSE) in Cambodia [8, 23] and on Moheli Island [24], as well as for earlier programs in China [25–27]. Operations included: (1) institution of an integrated malaria control network, including diagnostic laboratories, district- and county-level malaria control centers, and a “village-district-county-island” surveillance response system; (2) presentations of the MDA programs and discussions of malaria transmission by mass media in concert with international/local non-profit organizations, religious elders, and local representatives, including village leaders; and (3) recruitment and activation of malaria control teams, including village- and district-level workers responsible for door-to-door visits and MDA distributions, with attention to local customs and sensitivities conducive to trust and participation. All MDA operations on Anjouan Island were instituted in harmony with ongoing programs of long-lasting insecticidal net distributions, indoor residual spraying, active case detection, and focal outbreak investigations.
Malaria parasite rates among randomly selected Anjouan children decreased from an average of 13.5% prior to MDA to 1.2%, 0.7% and 0.5% at 6, 12 and 18 months after MDA, respectively. These reductions in parasite rate (92–96%) were somewhat less than the 99.4% and 100% reductions achieved for morbidity and mortality. Likely explanations for the greater reductions of morbidity and mortality, as well as the trend to lower parasitemia rates 6, 12, and 18 months after MDA, include: (1) the success of continued follow-up case detections and outbreak management by the malaria control teams; (2) heightened malaria awareness and treatment-seeking, encouraged by the malaria educational programs; and (3) integration of malaria control activities on Anjouan Island within the overall Comoros National Health Development Plan [3, 28]. Residual parasite rates after MDA in this study were comparable to those found after 2 rounds of AP+PMQLD on Moheli Island in 2007 (1.4% and 0.33% at 2 and 4 months after MDA, respectively, from an average rate of 23% before MDA) [24].
The MDA doses of AP+PMQLD and AP in this study were well tolerated without serious untoward drug reactions or adverse events. Safety and a similar absence of severe adverse events after treatments of various artemisinin derivatives plus piperaquine, with or without PMQLD, have been reported previously [8, 24, 29, 30].
Treatment of P. falciparum malaria in the Comoros Archipelago has not yet encountered the drug resistance to ACTs that is now common in regions of Southeast Asia [31–33]. Whether MDA regimens may promote or reduce the emergence and spread of anti-malarial drug resistance remains an open question [34–36]. Polymorphisms C580Y, R539T, Y493H, and I543T of the PfK13 propeller sequence are considered to be markers of a P. falciparum ring-stage artemisinin survival phenotype that may be associated with parasite clearance rates [37]; however, none of these polymorphisms were detected in the present study. Results instead identified 6 non-synonymous polymorphisms (D464H, S477Y, N490H, V520A, N554H, and A578S) already reported from other islands of the Comorian Archipelago [38, 39]. None of these polymorphisms showed significant differences in their frequencies before and after MDA.
Continued attention to robust control measures will be required to improve on these gains from MDA, and to eliminate the residual parasites that threaten renewed outbreaks of transmission. Inter-island migration acts as a continual source of malaria infections in the Comorian Archipelago, including the French Island of Mayotte where antimalarial programs have entered the elimination phase [40]. The steep decline of malaria and eventual entry of Anjouan into the elimination phase is thus an important contribution, integrated with other malaria control activities in the Comoros National Health Development Plan [3, 24, 28, 41]. In other malarious regions with similar epidemiological conditions, rapid control may likewise be achievable by appropriately-implemented MDA regimens of ACT without PMQLD. Questions remain: for example, will PMQLD show important benefit in programs where the number of rounds or drug combinations of MDA are different, or in instances where malaria endemicities and transmission conditions are distinct from those on Anjouan? Will PMQLD prove useful against low-incidence outbreaks in post-MDA and low-transmission settings? The challenges now are to address these questions, build on the potential of MDA, and proceed to elimination.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. C. D. and B. H. contributed equally to this report. J. S. designed, organized and directed the programs. C. D., B. H., Q. W., S. Z., H. Z., D. L., Q. X., Z. W., L. L., J. Z., H. Y., F. L., X. W., X.-b. L., S. H., and X.-h. L. collected data. C. D., B. H., Q. W., W. W., S. Z., H. Z., D. L., D. F., G. L., L. X., T. Y., F. T., F. M., A. B., K. S. A., R. A. K., A. M. M., M. M., and F. O. carried out the field work. B. H., C. D., X.-z. S., T. E. W., M. P. F., and J. S. analyzed data. B. H., X.-z. S., M. P. F., and T. E. W. wrote and revised the manuscript. All authors read and approved the final manuscript.
Acknowledgements. We thank the patients, their relatives, participating doctors and nurses at the field sites, and the malaria control teams from the Union of Comoros and Guangzhou University of Chinese Medicine for their contributions to the project. We are deeply grateful to Professor Guoqiao Li of Guangzhou University of Chinese Medicine, the President of Comoros, the Ministry of Health in Comoros, the Embassy of the People’s Republic of China in the Union of Comoros, the Ministry of Commerce of the People’s Republic of China, and the State Administration of Traditional Chinese of the People’s Republic of China for discussions, encouragement, and assistance.
Disclaimer. The funding agencies had no role in study design, data collection and analyses, decision to publish, or preparation of the manuscript.
Financial support. This work was supported by grants from Guangdong Provincial Science and Technology Program [grant number 2015B020234003] and China-UK Global Health Support Programme, funded by UK Government Department for International Development [DFID, grant number 202708], to J. S.; the National Science Foundation of China [grant number 81702020], Guangdong Provincial Science Foundation [grant number 2015A030310107], and Guangdong Provincial Medicine Science Foundation [grant number A2016315] to B. H.; the YangFan Innovative and Entrepreneurial Research Team Project [grant number 2014YT02S008] to C. D.; the Guangdong Provincial Science and Technology Program [grant number 2013B090800024] to Q. W.; and the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health (X.-z. S., M. P. F., T. E. W.).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization Global Malaria Programme. World malaria report 2015. Geneva (Switzerland): WHO; 2015. Report No.: 978 92 4 156483 0. [Google Scholar]
- 2. Ouledi A. Epidemiology and control of malaria in the Federal Islamic Republic of Comoros. Sante 1995; 5:368–71. [PubMed] [Google Scholar]
- 3. Union of Comoros Ministry of Health Solidarity and Promotion of Gender. National health development plan 2010–2014. Available at: http://www.nationalplanningcycles.org/sites/default/files/country_docs/Comoros/pnds_05_mai_2010_documentvf_en.pdf. Accessed 14 July 2016. [Google Scholar]
- 4. Ouédraogo AL, Bousema T, Schneider P, et al. Substantial contribution of submicroscopical Plasmodium falciparum gametocyte carriage to the infectious reservoir in an area of seasonal transmission. PLoS One 2009; 4:e8410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Okell LC, Ghani AC, Lyons E, Drakeley CJ. Submicroscopic infection in Plasmodium falciparum-endemic populations: a systematic review and meta-analysis. J Infect Dis 2009; 200:1509–17. [DOI] [PubMed] [Google Scholar]
- 6. White NJ. Primaquine to prevent transmission of falciparum malaria. Lancet Infect Dis 2013; 13:175–81. [DOI] [PubMed] [Google Scholar]
- 7. Maude RJ, Socheat D, Nguon C, et al. Optimising strategies for Plasmodium falciparum malaria elimination in Cambodia: primaquine, mass drug administration and artemisinin resistance. PLoS One 2012; 7:e37166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Song J, Socheat D, Tan B, et al. Rapid and effective malaria control in Cambodia through mass administration of artemisinin-piperaquine. Malar J 2010; 9:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gosling RD, Okell L, Mosha J, Chandramohan D. The role of antimalarial treatment in the elimination of malaria. Clin Microbiol Infect 2011; 17:1617–23. [DOI] [PubMed] [Google Scholar]
- 10. Ariey F, Witkowski B, Amaratunga C, et al. A molecular marker of artemisinin-resistant Plasmodium falciparum malaria. Nature 2014; 505:50–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Blanchy S, Julvez J, Mouchet J. Epidemiological stratification of malaria in the Comoro archipelago. Bull Soc Pathol Exot 1999; 92:177–84. [PubMed] [Google Scholar]
- 12. Chakir I, Said AI, Affane B, Jambou R. Control of malaria in the Comoro Islands over the past century. Malar J 2017; 16:387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Papa Mze N, Ahouidi AD, Diedhiou CK, et al. Distribution of Plasmodium species on the island of Grande Comore on the basis of DNA extracted from rapid diagnostic tests. Parasite 2016; 23:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Diallo DA, Doumbo OK, Plowe CV, Wellems TE, Emanuel EJ, Hurst SA. Community permission for medical research in developing countries. Clin Infect Dis 2005; 41:255–9. [DOI] [PubMed] [Google Scholar]
- 15. Dicko A, Brown JM, Diawara H, et al. Primaquine to reduce transmission of Plasmodium falciparum malaria in Mali: a single-blind, dose-ranging, adaptive randomised phase 2 trial. Lancet Infect Dis 2016; 16:674–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Greenwood B, Tine R. Primaquine to stop transmission of falciparum malaria. Lancet Infect Dis 2016; 16:623–4. [DOI] [PubMed] [Google Scholar]
- 17. World Health Organization. Policy brief on single dose primaquine as a gametocytocide in Plasmodium falciparum malaria. Geneva (Switzerland): WHO; 2015. Available at: http://www.who.int/malaria/publications/atoz/policy-brief-single-dose-primaquine-pf/en/. Accessed 29 August 2016. [Google Scholar]
- 18. Gonçalves BP, Tiono AB, Ouédraogo A, et al. Single low dose primaquine to reduce gametocyte carriage and Plasmodium falciparum transmission after artemether-lumefantrine in children with asymptomatic infection: a randomised, double-blind, placebo-controlled trial. BMC Med 2016; 14:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Okebe J, Bousema T, Affara M, et al. The gametocytocidal efficacy of different single doses of primaquine with dihydroartemisinin-piperaquine in asymptomatic parasite carriers in The Gambia: a randomized controlled trial. EBioMedicine 2016; 13:348–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Eziefula AC, Bousema T, Yeung S, et al. Single dose primaquine for clearance of Plasmodium falciparum gametocytes in children with uncomplicated malaria in Uganda: a randomised, controlled, double-blind, dose-ranging trial. Lancet Infect Dis 2014; 14:130–9. [DOI] [PubMed] [Google Scholar]
- 21. El-Sayed B, El-Zaki SE, Babiker H, et al. A randomized open-label trial of artesunate- sulfadoxine-pyrimethamine with or without primaquine for elimination of sub-microscopic P. falciparum parasitaemia and gametocyte carriage in eastern Sudan. PLoS One 2007; 2:e1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gerardin J, Eckhoff P, Wenger EA. Mass campaigns with antimalarial drugs: a modelling comparison of artemether-lumefantrine and DHA-piperaquine with and without primaquine as tools for malaria control and elimination. BMC Infect Dis 2015; 15:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Song J, Duong S, Tan B, et al. A pilot project on fast eliminating malaria by eradicating source in a highly malaria endemic area. J Guangzhou Univ Tradit Chin Med 2006; 23:89–94. [Google Scholar]
- 24. Li G, Song J, Deng C, et al. One-year report on the Fast Elimination of Malaria by Source Eradication (FEMSE) project in Moheli Island of Comoros. J Guangzhou Univ Tradit Chin Med 2010; 27:90–8. [Google Scholar]
- 25. Tang L. Progress in malaria control in China. Chin Med J (Engl) 2000; 113:89–92. [PubMed] [Google Scholar]
- 26. Tang LH, Qian HL, Xu SH. Malaria and its control in the People’s Republic of China. Southeast Asian J Trop Med Public Health 1991; 22:467–76. [PubMed] [Google Scholar]
- 27. Yip K. Antimalarial work in China: a historical perspective. Parassitologia 1998; 40:29–38. [PubMed] [Google Scholar]
- 28. Kassim SA, James PB, Alolga RN, et al. Major decline in malaria morbidity and mortality in the Union of Comoros between 2010 and 2014: the effect of a combination of prevention and control measures. S Afr Med J 2016; 106:709–14. [DOI] [PubMed] [Google Scholar]
- 29. Baiden R, Oduro A, Halidou T, et al. Prospective observational study to evaluate the clinical safety of the fixed-dose artemisinin-based combination Eurartesim® (dihydroartemisinin/piperaquine), in public health facilities in Burkina Faso, Mozambique, Ghana, and Tanzania. Malar J 2015; 14:160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Smithuis F, Kyaw MK, Phe O, et al. Effectiveness of five artemisinin combination regimens with or without primaquine in uncomplicated falciparum malaria: an open-label randomised trial. Lancet Infect Dis 2010; 10:673–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Saunders DL, Vanachayangkul P, Lon C; U.S. Army Military Malaria Research Program; National Center for Parasitology, Entomology, and Malaria Control (CNM); Royal Cambodian Armed Forces Dihydroartemisinin-piperaquine failure in Cambodia. N Engl J Med 2014; 371:484–5. [DOI] [PubMed] [Google Scholar]
- 32. Spring MD, Lin JT, Manning JE, et al. Dihydroartemisinin-piperaquine failure associated with a triple mutant including kelch13 C580Y in Cambodia: an observational cohort study. Lancet Infect Dis 2015; 15:683–91. [DOI] [PubMed] [Google Scholar]
- 33. Amaratunga C, Lim P, Suon S, et al. Dihydroartemisinin-piperaquine resistance in Plasmodium falciparum malaria in Cambodia: a multisite prospective cohort study. Lancet Infect Dis 2016; 16:357–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. von Seidlein L, Greenwood BM. Mass administrations of antimalarial drugs. Trends Parasitol 2003; 19:452–60. [DOI] [PubMed] [Google Scholar]
- 35. World Health Organization Evidence Review Group. Mass drug administration, mass screening and treatment and focal screening and treatment for malaria. Geneva (Switzerland): WHO; 2015. Available at: http://www.who.int/malaria/mpac/mpac-sept2015-erg-mda-report.pdf. Accessed 13 July 2016. [Google Scholar]
- 36. White NJ. Does antimalarial mass drug administration increase or decrease the risk of resistance?Lancet Infect Dis 2017; 17:e15–20. [DOI] [PubMed] [Google Scholar]
- 37. World Health Organization. Status report on artemisinin and ACT resistance. Geneva (Switzerland): WHO; 2015. Available at: http://www.who.int/malaria/publications/atoz/status-rep-artemisinin-resistance-sept2015.pdf. Accessed 25 August 2016. [Google Scholar]
- 38. Huang B, Deng C, Yang T, et al. Polymorphisms of the artemisinin resistant marker (K13) in Plasmodium falciparum parasite populations of Grande Comore Island 10 years after artemisinin combination therapy. Parasit Vectors 2015; 8:634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Torrentino-Madamet M, Collet L, Lepere JF, et al. K13-propeller polymorphisms in Plasmodium falciparum isolates from patients in Mayotte in 2013 and 2014. Antimicrob Agents Chemother 2015; 59:7878–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Maillard O, Lernout T, Olivier S, et al. Major decrease in malaria transmission on Mayotte Island. Malar J 2015; 14:323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Toyb M, Ouledi A, Gauzere BA, Aubry P. Malaria in the Comoros Archipelago in 2015: status after 15 years of fight. Bull Soc Pathol Exot 2016; 109:107–13. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.