Abstract
Aims
Advances of cardiac computed tomography angiography (CTA) have been developed for dose reduction, but their efficacy in clinical practice is largely unknown. This study was designed to evaluate radiation dose exposure and utilization of dose-saving strategies for contrast-enhanced cardiac CTA in daily practice.
Methods and results
Sixty one hospitals from 32 countries prospectively enrolled 4502 patients undergoing cardiac CTA during one calendar month in 2017. Computed tomography angiography scan data and images were analysed in a central core lab and compared with a similar dose survey performed in 2007. Linear regression analysis was performed to identify independent predictors associated with dose. The most frequent indication for cardiac CTA was the evaluation of coronary artery disease in 89% of patients. The median dose-length product (DLP) of coronary CTA was 195 mGy*cm (interquartile range 110–338 mGy*cm). When compared with 2007, the DLP was reduced by 78% (P < 0.001) without an increase in non-diagnostic coronary CTAs (1.7% in 2007 vs. 1.9% in 2017 surveys, P = 0.55). A 37-fold variability in median DLP was observed between the hospitals with lowest and highest DLP (range of median DLP 57–2090 mGy*cm). Independent predictors for radiation dose of coronary CTA were: body weight, heart rate, sinus rhythm, tube voltage, iterative image reconstruction, and the selection of scan protocols.
Conclusion
This large international radiation dose survey demonstrates considerable reduction of radiation exposure in coronary CTA during the last decade. However, the large inter-site variability in radiation exposure underlines the need for further site-specific training and adaptation of contemporary cardiac scan protocols.
Keywords: Cardiac CT angiography, Radiation dose exposure, Dose-length product, Dose-saving strategies
Introduction
Cardiac computed tomography angiography (CTA) is an increasingly used non-invasive imaging method in cardiology.1,2 Due to the high diagnostic accuracy, coronary CTA clarifies the diagnosis of angina in patients with suspected coronary artery disease (CAD) in addition to standard clinical care3 and coronary CTA is able to rule out CAD with high negative predictive value.4 The improved diagnostic capabilities may alter downstream testing and clinical care in a significant proportion of patients, which may reduce cardiac events.3,5 Finally, coronary CTA carries significant prognostic information.6
Radiation exposure from cardiac CTA carries the potential risk of cancer induction in a dose-dependent manner.7 Accordingly, safety considerations of cardiac CTA are an ongoing concern and computed tomography (CT) imager should aim to reduce radiation dose exposure to be ‘as low as reasonably achievable’ (ALARA principle), while maintaining diagnostic image quality.8 One decade ago, the international dose survey PROTECTION I collected data from 1965 cardiac CT studies and evaluated the radiation exposure from cardiac CTA.9 Since then, advances in CT technology including new acquisition techniques and software algorithms, have been developed and societal guidelines as well as clinical studies have advocated for a consequent use of these techniques to lower radiation dose.10–15 However, the utilization and efficacy of modern techniques for radiation dose reduction in real-world clinical practice across the globe is currently unknown. Thus, the current dose survey was designed to investigate the radiation dose of cardiac CTA, the utilization and efficacy of established dose-saving strategies and the potential for further radiation dose reduction in a real-world setting in 2017.
Methods
Study protocol
The study design of the 2017 dose survey, entitled Prospective Multicenter Registry on RadiaTion Dose Estimates of Cardiac CT AngIOgraphy IN Daily Practice in 2017 (PROTECTION VI), has been described previously.16 The dose survey is an international, industry-independent, multi-vendor, prospective, observational study. With the objective to garner a representative worldwide sampling, a total of 435 clinicians from 62 different countries were invited as identified by literature research and by membership of the Society of Cardiovascular Computed Tomography (SCCT). The study collaborators enrolled all consecutive patients undergoing cardiac CTAs during one month between March and December 2017. Computed tomography studies before transfemoral aortic valve replacements were excluded, due to necessity to image the entire aorta and the heterogeneity in acquisition protocols. Cardiac CTAs were carried out according to local standard of clinical care. Data was analysed in a central core laboratory. Each study site consulted the responsible local ethics committee to evaluate the study protocol, which had to be approved prior to patient enrolment. All patients gave written informed consent as required at the individual study sites and data was obtained prospectively. An Executive Steering Committee composed of a group of physicians with expertise in cardiac CTA, clinical research and statistics supervised the study. The study has been registered with clinicaltrials.gov (NCT02996903).
Strategies for reduction of radiation dose
The selection of strategies for radiation dose reduction was at the discretion of the local study investigators. The conventional retrospectively electrocardiogram (ECG)-gated helical scan protocol is a robust scan technique, which is least radiation efficient. Prospectively ECG-triggered scan techniques, including the axial and high-pitch scan modes, deliver the radiation efficiently only during a short fraction of the R–R interval and is recommended for patients with sinus rhythm and heart rates ≤65 b.p.m.11 Reducing the tube potential from the conventionally used 120 kVp to 100 kVp lowers radiation exposure without compromising diagnostic image quality and has been recommended for patients with a body mass index (BMI) ≤30 kg/m2.12 The combination of iterative image reconstruction (IR) with reduced radiation tube currents has also been shown to reduce radiation exposure during coronary CTA without compromising diagnostic image quality.10
Estimation of radiation dose
The parameters relevant to radiation dose were obtained from the dose report generated by the CT system after each cardiac CTA study. The total dose-length product (DLP), which includes the radiation exposure of the entire CT investigation including among others the localizer and timing bolus, was the main outcome measure. The DLPCTA represents the radiation exposure, which was delivered for the acquisition of the CT angiography only. The DLP equals the CT dose index (CTDIvol) multiplied by the respective scan length.
Image quality
Diagnostic image quality of coronary CTAs was assessed by the local investigators. Non-diagnostic image quality was defined by severe vessel blurring or vessel discontinuity secondary to reconstruction artefacts, which did not allow the exclusion of obstructive coronary lesions. Coronary CTAs were considered as non-diagnostic when at least one coronary artery was of non-diagnostic image quality.
Statistical analysis
Variables are expressed as counts with percentages or medians with interquartile ranges (IQRs). Comparison of groups was performed with Wilcoxon–Mann–Whitney U-test or χ2 test as appropriate. Multivariable linear regression analysis with backward variable elimination was performed to identify predictors significantly associated with radiation dose in coronary CTA. A logistic regression analysis with the endpoint of performance to a diagnostic reference level was additionally performed. A generalized estimation equation model was used to account for the clustering effect of this multicentre trial. A P-value <0.05 was considered to be statistical significant. Statistical analysis was performed using R version 3.4.1.
Results
Patient and study site characteristics
Clinicians of 61 international sites (42 university hospitals, 19 community hospitals) from 32 different countries participated in the study (see Supplementary material online, Table S1 for regional enrolment). These sites contributed 4502 patients undergoing diagnostic cardiac CTA (median of 51 patients per site during the month of enrolment; IQR 27–91 patients). The median site experience for the performance of cardiac CTA studies was 10.5 years (IQR 7–13 years). Patient and study site characteristics of the 2017 dose survey are listed in Table 1; the characteristics of the 2007 dose survey are listed for comparison. In 2017, median patient’s age was 60 years (IQR 51–69 years) and their median BMI was 26.8 kg/m2 (IQR 24.1–30.1 kg/m2). Ninety percent of patients (4055 patients) were examined in sinus rhythm and beta blockers were administered in 66% (2973 patients) resulting in a median heart rate of 60 b.p.m. (IQR 55–67 b.p.m.).
Table 1.
Patient and study site characteristics
| 2007 dose survey (1965 patients) | 2017 dose survey (4502 patients) | |
|---|---|---|
| Patient characteristics | ||
| Age (years) | NA | 60 (51–69) |
| Gender male, % (n) | NA | 58 (2623) |
| Patient height (m) | 1.70 (1.63–1.77) | 1.70 (1.60–1.80) |
| Patient weight (kg) | 77 (66–87) | 78 (67–90) |
| BMI (kg/m2) | 26.2 (23.8–28.8) | 26.8 (24.1–30.1) |
| Indication for cardiac CTA, % (n) | ||
| Coronary artery evaluation | 82 (1611) | 89 (4006) |
| EP planning study | 2 (38) | 4 (177) |
| CABG | 12 (225) | 3 (122) |
| Othera | 4 (91) | 4 (197) |
| ß-Blocker medication, % (n) | ||
| None | 42 (828) | 33 (1501) |
| Taking daily | 12 (233) | 13 (603) |
| Administration for CTA | 46 (904) | 53 (2370) |
| Unknown | 0 | 0.6 (28) |
| Sinus rhythm, % (n) | 95 (1874) | 90 (4055) |
| Heart rate (b.p.m.) | 61 (55–75) | 60 (55–67) |
| Study site characteristics | ||
| Site experience (years) | 3 (1.5–5.5) | 10.5 (7.0–13.0) |
| Number of cardiac CTAs/month | 26 (10–46) | 51 (27–93) |
| CT system, % (n) | ||
| 16-slice CT | 4% (72) | 0% |
| 64-slice CT | 96% (1893) | 9% (387) |
| ≥128-slice CT | NA | 91% (4115) |
| CT manufacturer, % (n) | ||
| GE | 24 (466) | 26 (1168) |
| Philips | 8 (159) | 13 (574) |
| Siemens | 59 (1155) | 48 (2160) |
| Toshiba | 9 (185) | 13 (600) |
Values are median (interquartile range) or % (number of patients).
CABG, coronary artery bypass grafting; NA, not available.
Other indications for cardiac CTA included among others triple-rule-out CTs, visualization of the cardiac anatomy and coronary anomalies.
The main indication for cardiac CTA was the evaluation of the coronary arteries (coronary CTA) in 89% of patients. Planning of electrophysiological procedures (4%) and visualization of bypass grafts (3%) were less frequent indications. All four major CT manufacturers were represented with examination of at least 13% of the enrolled patients. At the time of data collection modern CT scanners were used in both surveys with 96% and 91% of scans performed 64-slice and ≥128-slice CT scanners in the 2007 and 2017 dose surveys, respectively.
Radiation dose
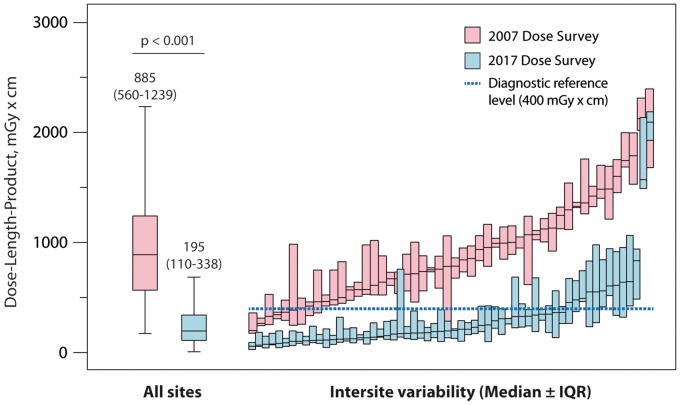
The median total DLP of all 4502 patients included in the 2017 dose survey was 252 mGy*cm (IQR 154–412 mGy*cm). The median total DLP for coronary CTAs was 246 mGy*cm (IQR 153–402 mGy*cm) with 79% of the radiation exposure resulting from the coronary CTA (DLPCTA 195 mGy*cm, IQR 110–338 mGy*cm, Table 2). The observed DLP of 195 mGy*cm corresponds to effective doses of 2.7 or 5.1 mSv, estimated using the thoracic or the recently published cardiac DLP to effective dose conversion factor of 0.014 or 0.026 mSv/mGy*cm, respectively.17,18 Compared with the 2007 survey, a significant 78% reduction in DLPCTA was observed in 2017 (P < 0.001). The regional development of radiation exposure from 2007 to 2017 is presented in Table 2. Take home figure summarizes the reduction in radiation dose and the variability of DLPCTA between study sites in the 2007 and 2017 dose surveys. While a seven-fold difference was observed in the 2007 dose survey between the study sites with lowest and highest median DLPCTA (lowest and highest DLPCTA in 2007: 331 and 2146 mGy*cm, respectively), this dose variability increased to a 37-fold difference in the 2017 dose survey (lowest and highest median DLPCTA in 2017: 57 and 2090 mGy*cm, respectively). DLPCTA data stratified for gender and CT manufacturer are displayed in Figure 1 (further stratification according to CT model is given in Supplementary material online, Figure S1).
Table 2.
Scan characteristics for coronary CT angiographies
| 2007 dose survey (1611 patients) | 2017 dose survey (4006 patients) | P-value | |
|---|---|---|---|
| Scan length (mm) | 131 (118–144) | 137 (125–157) | <0.001 |
| Total DLP (mGy*cm) | NA | 246 (153–402) | NA |
| CTDIvolCTA (mGy) | 54 (38–74) | 14 (8–24) | <0.001 |
| DLPCTA (mGy*cm) | 885 (560–1239) | 195 (110–338) | <0.001 |
| Regional DLP for coronary CTA only | |||
| Europe (mGy*cm) | 814 (537–1151) | 176 (93–312) | <0.001 |
| North America (mGy*cm) | 993 (292–1343) | 199 (124–340) | <0.001 |
| Latin and South America (mGy*cm) | 1556 (711–1932) | 295 (189–624) | <0.001 |
| Middle East (mGy*cm) | 1799 (1482–2138) | 244 (132–400) | <0.001 |
| East Asia and Australia (mGy*cm) | 940 (599–1130) | 169 (96–276) | <0.001 |
| Dose saving strategies | |||
| Tube potential ≤100kV, % (n) | 5 (82) | 56 (2226) | <0.001 |
| Tube potential <100 kV, % (n) | 0 (0) | 14 (564) | NA |
| Retrospectively ECG-gated helical scan protocol, % (n) | 94 (1512) | 11 (447) | <0.001 |
| Subgroup with ECG-correlated modulation of tube current, % (n) | 95 (1440) | 73 (325) | <0.001 |
| Prospectively ECG-triggered axial scan protocol, % (n) | 6 (99) | 78 (3094) | <0.001 |
| Prospectively ECG-triggered high-pitch helical scan protocol, % (n) | 0 (0) | 11 (449) | NA |
| Iterative image reconstruction, % (n) | 0 (0) | 83 (3306) | NA |
Values are median (interquartile range) or % (number of patients).
NA, not available.
Figure 3.
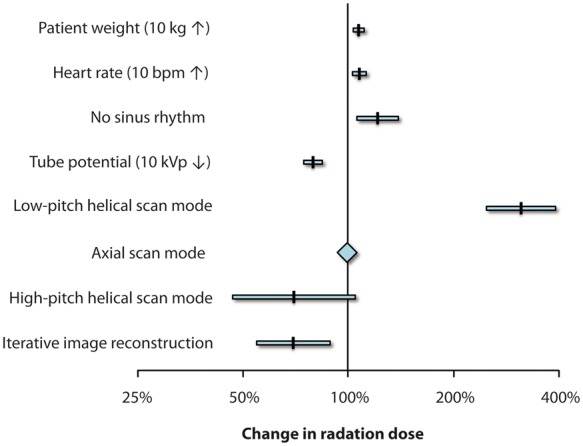
Independent predictors of radiation dose from coronary computed tomography angiographies. Change of radiation dose in coronary computed tomography angiography by independent patient and scan-associated factors as identified by a multivariable linear regression analysis. Retrospective electrocardiogram-gated low-pitch helical and prospective electrocardiogram-triggered high-pitch helical scan modes were compared with the axial scan technique.
Take home figure.
Reduction of dose-length product from coronary computed tomography angiographies and variation between study sites. Left: box plots illustrate dose-length product of all coronary computed tomography angiographies. Right: variability of median dose-length product (± interquartile range) between the study sites in the 2007 and 2017 dose surveys, respectively.
Compared with the 2007 dose survey, the reduction in radiation dose did not increase the rate of non-diagnostic CTA studies in 2017. The rates of non-diagnostic coronary CTAs were 1.7% and 1.9% in 2007 and 2017, respectively (P = 0.55).
Use of dose saving strategies
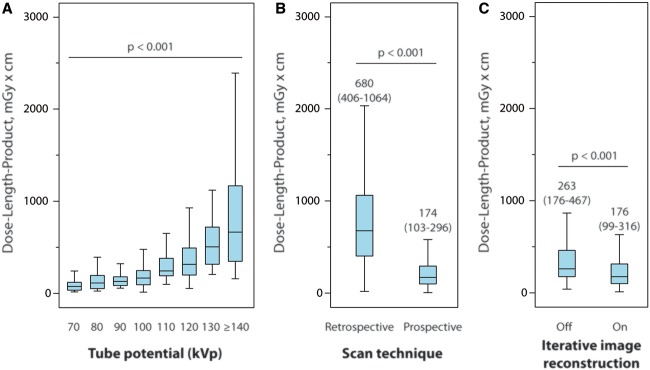
The relationship between radiation tube potential and DLPCTA is displayed in Figure 2A. Considering the cohorts of both surveys, the use of a tube potential of ≤100 kVp increased significantly from 5% to 56% in the 2007 and 2017 surveys. When a BMI threshold of ≤30 kg/m2 is considered for the eligibility of a ≤100 kVp tube potential, 6% and 70% of eligible patients were studied with a ≤100 kVp scan protocol in the 2007 and 2017 dose surveys, respectively. A tube potential of less than 100 kVp, which has not been used in 2007, was applied in 14% of patients in 2017.
Figure 1.
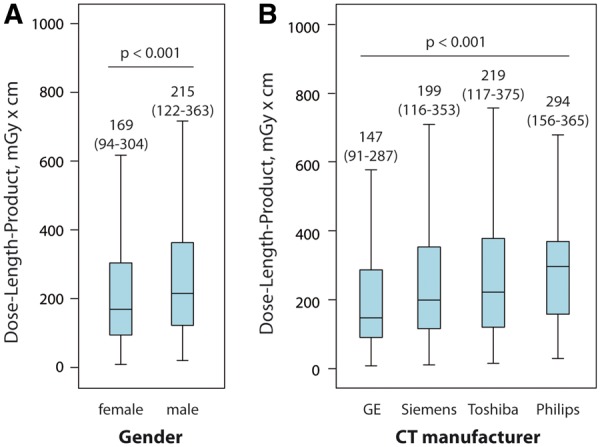
Differences in dose-length product of coronary computed tomography angiography by gender (A) and computed tomography manufacturer (B).
In the 2007 dose survey, 94% of patients were scanned using retrospectively ECG-gated helical imaging and only 6% of patients were examined by prospectively ECG-triggered axial imaging. In the 2017 dose survey, retrospective helical imaging was decreasingly utilized in only 11% of patients and prospectively ECG-triggered axial scanning was favoured in 78% of patients. ECG-triggered high-pitch helical imaging, which is available on dual-source CT systems, was applied in 11%. Compared with retrospectively ECG-gated helical scanning, the prospectively ECG-triggered scan modes (axial or high-pitch) resulted in a 74% reduction in radiation dose in the 2017 dose survey (P < 0.001; Figure 2B). Image reconstruction methods, which were not available in 2007, were used in 83% of patients in the 2017. Application of IR techniques resulted in a 33% reduction of radiation dose, when compared with standard filtered back projection (P < 0.001, Figure 2C).
Predictors for radiation dose
In the multivariable linear regression model, three patient-related and three scan-related variables of a total of 11 included parameters (see Supplementary material online, Table S2) were identified as independent predictors associated with radiation dose of coronary CTA. An increase in body weight of 10 kg, an increase in heart rate of 10 b.p.m., and the absence of sinus rhythm were associated with an increase of radiation dose of 7%, 8%, and 21%, respectively (all P < 0.01; Figure 3). A decrease in the tube potential of 10 kVp and the use of IR were associated with a dose reduction of 21% and 30%, respectively (both P < 0.01). Finally, the use of the ECG-gated low-pitch helical scan technique resulted in an increase of radiation dose by 313% (P < 0.001), while the use of the ECG-triggered high-pitch scan technique was associated with 30% dose reduction (P = 0.08) when compared with the axial scan technique. The results were confirmed in a second logistic regression model, which addressed the performance to the proposed diagnostic reference level (see also Discussion section and Supplementary material online, Table S3).
Figure 2.
Effect of dose saving strategies with tube potential reduction (A), scan mode (B), and application of iterative image reconstruction (C) on dose-length product in coronary computed tomography angiography.
Discussion
Cardiovascular CT has become an established and increasingly used technique mainly for the diagnostic assessment of CAD burden in patients with chest pain. Although several studies have evaluated strategies to reduce the radiation exposure of cardiac CT imaging, there is still concern about the delivered dose in daily practice. The current international dose survey demonstrates that the radiation exposure associated with cardiovascular CT has been tremendously reduced by 78% over the last decade. This progress contributed to the establishment of cardiac CT as frequently used non-invasive imaging method supported by national guidelines.19 The determined international median DLP of coronary CTA corresponds to a recent national dose survey performed in the UK.20 The achieved dose reduction can be attributed to several important factors: (i) the increasing awareness about radiation safety and the growing experience and knowledge of CT imagers in cardiovascular CT, (ii) the publication and adherence to best practice guidelines for cardiac CT imaging,13 and (iii) the availability of scan protocols in modern CT scanners, which are radiation dose efficient.
Scan protocols with a reduced tube potential and prospectively ECG-triggered scan protocols were, among others, the main contributor to the reduced radiation exposure in the 2017 dose survey. The use of 100 kVp instead of the conventional 120 kVp scan protocols in non-obese patients has been shown to reduce radiation exposure by 29% in a randomized comparison without compromising diagnostic image quality.12 Over the last decade, the frequency of use of ≤100 kVp scan protocols has raised by over 10-fold from 5% in 2007 to 56% in 2017. Similarly, the use of prospectively ECG-triggered scan modes has shown to reduce radiation exposure by at least 69% without compromising diagnostic image quality.11,21 These prospectively triggered scan modes were applied in 89% of patients undergoing coronary CTA in 2017, while the frequency was only 6% in 2007.
The observed low median DLPCTA of 195 mGy*cm for a coronary CTA corresponds to an effective dose estimate of 2.7 or 5.1 mSv depending on the applied conversion factor (k = 0.014 or 0.026 mSv/mGy*cm).17,18 The effective dose estimates of coronary CTA are considerably lower than the recently published median effective dose estimates of 10.0 mSv for myocardial perfusion imaging, obtained from a comparable worldwide dose survey.22 This difference may result in an improvement in population safety, if coronary CTA would be preferably used over myocardial perfusion imaging in patients with suspected CAD. The current dose survey allow for the determination of a new diagnostic reference level for coronary CTA. The diagnostic reference level, which is typically set at the 75th percentile dose level for a typical-sized patient and for a certain radiological procedure, is not the recommended or preferred dose, but rather an action level at which additional investigation into the dose used should be performed. Based on the current results, a new diagnostic reference level of 400 mGy*cm should be considered for coronary CTA. In 2017, median DLPs for coronary CTA were above this proposed diagnostic reference level in 13 of 61 participating centres (see Supplementary material online, Figure S2). This observation but even more the 37-fold difference in median DLPs between the study sites with the lowest and highest median DLPs underlines the need and potential for further education around dose reduction strategies and standardization of coronary CTA scan protocols. Considering the 89% use of prospectively ECG-triggered scan protocols (axial or high-pitch) in the current survey, a further increase in use will be difficult to achieve, when the presence of a stable sinus rhythm and a heart rate of ≤65 b.p.m. are respected as selection criteria. In contrast, a scan protocol with ≤100 kVp tube potential was selected in ‘only’ 70% of eligible patients, if a conservative BMI threshold of ≤30 kg/m2 was considered as eligibility criterion. Although this rate increased considerably from 2007 to 2017, this also indicates, that another 30% of patients with a BMI of ≤30 kg/m2 would have qualified for this dose saving scan protocol. A tube potential of 100 kVp has been successfully applied in some studies in patients up to a body weight of even 100 kg without compromising image quality.23 If this body weight threshold would have been exploited as eligibility criterion, then even 36% of patients from the 2017 dose survey would have qualified for a low-dose 100 kVp scan protocol. Finally, scan protocols with tube potentials less than 100 kVp have demonstrated a large potential for further dose reductions,24 but they have been used only in 14% of patients in the current dose survey.
The results of the 2017 dose survey for cardiovascular CT have relevant implications on different levels of our health systems: On a patient level, the radiation doses should not discourage patients from undergoing coronary CTAs, when clinically indicated. In fact, an effective dose of 5 mSv in a 60-year-old patient (median age in the current survey) adds only a small, negligible additional risk to life-time cancer risk, but the diagnostic information and the clinical consequences resulting from a coronary CTA may outweigh this very small theoretical additional cancer risk. This becomes even more evident when the 0.05% estimated risk of fatal malignancy from a 10 mSv CT scan is compared with the 50% reduction in fatal and non-fatal myocardial reduction observed already 3 years after a coronary CTA.5,25 However, the large variability in radiation doses between participating study sites indicates that patients may select certified non-invasive imaging centres for their CT studies, because the likelihood of obtaining low-dose CT studies might be higher in certified than non-certified imaging centres. On a physician and institutional level, a continuous monitoring of patient’s radiation exposure as well as the participation in dose surveys20,26 will allow for benchmarking with other physicians and institutions. The participation in such programmes has been shown to improve ‘best practice’ performance.26 On a societal level, the 2017 radiation survey reveals the importance of education. The publication of guidelines as well as the organization of educational sessions on radiation exposure will improve the adherence to best practice recommendations.27 Finally, on an industry level, the current study results claim for the set-up of default scan protocols, which are dose-efficient, as well as the development of applications supporting the automated selection of optimal low-dose scan parameters, which will secure low-dose cardiac imaging for the individual patient.
Limitations
The lack of financial support for study conduction allowed the gathering of data without bias, but limited also the participation of additional sites.
Conclusion
In conclusion, the 2017 radiation dose survey demonstrates that the radiation exposure from cardiac CTA has been considerably reduced over the last 10 years. This was accomplished by an increased use of (i) low tube potential scan protocols, (ii) prospectively ECG-triggered axial and high-pitch scan protocols, and (iii) iterative image reconstruction. However, a large 37-fold inter-site variability in median radiation dose was observed, which underlines the need for further site-specific training and adaptation of contemporary cardiac scan protocols.
Supplementary Material
Acknowledgments
Conflict of interest: T.J. Stocker, S. Deseive, M. Hadamitzky, R. Rubinshtein, M. Heckner, X. Fang, E. Grove, J. Lesser, P. Maurovich-Horvat, J. Otton, S. Shin, G. Pontone, H. Marques, C. Nomura, R. Tabbalat, A. Schmermund, J. Kang, C. Naoum, M. Atkins, E. Martuscelli and S. Massberg report no relevant disclosures. J.J. Bax reports grants from Biotronik, Medtronic, Boston Scientific, GE Healthcare and Edwards Lifesciences, outside the submitted work. J. Hausleiter reports research support and speaker honoraria from Abbott Vascular and Edwards LifeSciences, J. Leipsic reports personal fees from Heartflow and Circl CVI outside the submitted work, M. Chen reports non-financial support from Canon Medical systems outside the submitted work, B. Chow reports non-financial support from TeraRecon, grants from Ausculsciences and grants from CV Diagnostix during the conduct of the study.
Appendix
PROTECTION VI Investigators (sorted by country)
Patricia Carrascosa and Alejandro Deviggiano, Diagnóstico Maipú, Buenos Aires, Argentina
Christopher Naoum and John Magnussen, Macquarie University Hospital, Sydney, Australia
James Otton and Anthony Kaplan, Spectrum Radiology Liverpool, Sydney, Australia
Gudrun Feuchtner and Fabian Plank, Medizinische Universität, Innsbruck, Austria
Kristof De Smet and Nico Buls, Universitair Ziekenhuis, Brussel, Belgium
Roberto Caldeira Cury and Marcio Sommer Bittencourt, Delboni/DASA, Sao Paulo, Brazil
Cesar Higa Nomura and Roberto Nery Dantas Junior, Heart Institute—InCor, Sao Paulo, Brazil
Jonathon Leipsic and Philipp Blanke, University of British Columbia, Vancouver, Canada
Carl Chartrand-Lefebvre and Anne Chin, University of Montreal, Montreal, Canada
Gary Small and Benjamin Chow, University of Ottawa Heart Institute, Ottawa, Canada
Claudio Silva F, Clinica Alemana de Santiago, Santiago, Chile
Marcelo Godoy Z. and Claudio Silva F., Clinica Alemana de Temuco, Temuco, Chile
Xiang-Ming Fang and Wang Jie, Wuxi People's Hospital, Wuxi, China
Alberto Cadena, Clínica de la Costa, Barranquilla, Colombia
Theodor Adla and Vojtech Suchanek, Motol University Hospital, Prague, Czech Republic
Erik Lerkevang Grove and Kamilla Bech Pedersen, Aarhus University Hospital, Aarhus, Denmark
Jess Lambrechtsen and Mirza Husic, OUH-Svendborg, Svendborg, Denmark
Juhani Knuuti and Teemu Maaniitty, Turku University Hospital, Turku, Finnland
Bernhard Bischoff and Elisabeth Arnoldi, Klinikum der Universität München, München, Germany
Axel Schmermund and Joachim Eckert, CCB, Frankfurt, Germany
Martin Hadamitzky and Tom Finck, Deutsches Herzzentrum München, München, Germany
Michaela Hell and Mohamed Marwan, Universitätsklinikum Erlangen-Nürnberg, Erlangen, Germany
Fabian Bamberg and Stefanie Mangold, Universitätsklinikum Tübingen, Tübingen, Germany
Thomas Schlosser and Johannes Ludwig, Universitätsklinikum Essen, Essen, Germany
Maria Mylona and Spyros Skiadopoulos, Olympion Hospital, Patras, Greece
Pál Maurovich-Horvat and Bálint Szilveszter, MTA-SE Cardiovascular Imaging Research Group, Heart and Vascular Center, Budapest, Hungary
Uday Jadav and Brian V. Pinto, MGM New Bombay Hospital, Vashi New Mumbai, India
Ronen Rubinshtein and Essam Hussein, Lady Davis Carmel Medical Center, Haifa, Israel
Daniele Andreini and Gianluca Pontone, Centro Cardiologico Monzino, Milan, Italy
Eugenio Martuscelli and Massimiliano Sperandio, Policlinico di Tor Vergata, Rome, Italy
Kakuya Kitagawa and Naoki Nagasawa, Mie University Hospital, Tsu, Japan
Ramzi Tabbalat and Rami A. Farhan, Khalidi Hospital, Amman, Jordan
Lilia M. Sierra Galán and Leovigildo A. Delgado, American British Cowdray Medical Center Observatory Campus, Mexico City, Mexico
Lilia M. Sierra Galán and Marco A. Reza Orozco, American British Cowdray Medical Center Santa Fe Campus, Mexico City, Mexico
Francisco C. Castellón and Mariana D. Zamudio, Instituto Ignacio Chavez, Mexico City, Mexico
Andres Preciado-Anaya and Rafael P. Gómez, Hospital Siena, Leon, Mexico
Signe Helene Forsdahl and Grete Anita Hansen, University Hospital North Norway, Tromsø, Norway
Anne Günther and Joanna F. Kristiansen, Oslo University Hospital Rikshospitalet, Oslo, Norway
Edith Chavez Huapalla and Percy Teran Chavez, Complejo Hospitalario San-Pablo, Surco, Peru
Hugo Marques and Pedro de A. Goncalves, UNICA (cardiovascular CT and MRI Unit), Hospital da Luz, Lisbon, Portugal
Subramaniyan Ramanathan, Al Wakra Hospital, Doha, Qatar
Valentin Sinitsyn and Maria Glazkova, Federal Center of Medicine and Rehabilitation, Moscow, Russia
Rami Abazid and Osama A. Smettei, Prince Sultan Cardiac Center, Burydah, Saudi Arabia
Ahmed Dawood and Salama Hussain Omar, Dr Erfan and Bagedo Hospital, Jeddah, Saudi Arabia
Joon-Won Kang and Dong Hyun Yang, Asan Medical Center, Seoul, South Korea
Tae Hoon Kim and Chul Hwan Park, Gangnam Severance Hospital, Seoul, South Korea
Sanghoon Shin and Seok Jong Ryu, Ilsan Hospital, Goyang-si, South Korea
Jose R. Palomares and Hug Cuellar, Hospital Val d'Hebron, Barcelona, Spain
Jeroen J. Bax and Alexander van Rosendael, Leiden University Medical Center, Leiden, Netherlands
Michelle C. Williams, David Newby and Edwin J. R. van Beek, University of Edinburgh, Edinburgh, UK
Russell Bull and Kavin Jayawardhana, Royal Bournemouth Hospital, Bournemouth, UK
Patricia Dickson and Jennifer Espey, Capital Cardiology Associates, Albany, USA
John Lesser and B. Kelly Han, Minneapolis Heart Institute, Minneapolis, USA
Renée Bullock-Palmer, Deborah Heart and Lung Center, Browns Mills, USA
Mark Rabbat and Nancy Schoenecker, Loyola University Medical Center, Maywood, USA
Dustin M. Thomas and Rosco S. Gore, San Antonio Military Medical, San Antonio, USA
Melany Atkins, Fairfax Radiological Consultants, Fairfax, USA
Marcus Y. Chen and Sujata M. Shanbhag, National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, USA
John Mahmarian, Houston Methodist Hospital, Houston, USA
Jeannie Yu, Long Beach VA Healthcare System, Long Beach, USA
Todd C. Villines and Binh Nguyen, Walter Reed National Military Medical Center, Bethesda, USA
Footnotes
See page 3724 for the editorial comment on this article (doi: 10.1093/eurheartj/ehy552)
Contributor Information
PROTECTION VI investigators:
Patricia Carrascosa, Alejandro Deviggiano, Christopher Naoum, John Magnussen, James Otton, Anthony Kaplan, Gudrun Feuchtner, Fabian Plank, Kristof De Smet, Nico Buls, Roberto Caldeira Cury, Marcio Sommer Bittencourt, Cesar Higa Nomura, Roberto Nery Dantas, Jonathon Leipsic, Philipp Blanke, Carl Chartrand-Lefebvre, Anne Chin, Gary Small, Benjamin Chow, Claudio Silva F, Marcelo Godoy Z, Claudio Silva F, Xiang-Ming Fang, Wang Jie, Alberto Cadena, Theodor Adla, Vojtech Suchanek, Erik Lerkevang Grove, Kamilla Bech Pedersen, Jess Lambrechtsen, Mirza Husic, Juhani Knuuti, Teemu Maaniitty, Bernhard Bischoff, Elisabeth Arnoldi, Axel Schmermund, Joachim Eckert, Martin Hadamitzky, Tom Finck, Michaela Hell, Mohamed Marwan, Fabian Bamberg, Stefanie Mangold, Thomas Schlosser, Johannes Ludwig, Maria Mylona, Spyros Skiadopoulos, Pál Maurovich-Horvat, Bálint Szilveszter, Uday Jadav, Brian V Pinto, Ronen Rubinshtein, Essam Hussein, Daniele Andreini, Gianluca Pontone, Eugenio Martuscelli, Massimiliano Sperandio, Kakuya Kitagawa, Naoki Nagasawa, Ramzi Tabbalat, Rami A Farhan, Lilia M Sierra Galán, Leovigildo A Delgado, Lilia M Sierra Galán, Marco A Reza Orozco, Francisco C Castellón, Mariana D Zamudio, Andres Preciado-Anaya, Rafael P Gómez, Signe Helene Forsdahl, Grete Anita Hansen, Anne Günther, Joanna F Kristiansen, Edith Chavez Huapalla, Percy Teran Chavez, Hugo Marques, Pedro de A Goncalves, Subramaniyan Ramanathan, Valentin Sinitsyn, Maria Glazkova, Rami Abazid, Osama A Smettei, Ahmed Dawood, Salama Hussain Omar, Joon-Won Kang, Dong Hyun Yang, Tae Hoon Kim, Chul Hwan Park, Sanghoon Shin, Seok Jong Ryu, Jose R Palomares, Hug Cuellar, Jeroen J Bax, Alexander van Rosendael, Michelle C Williams, David Newby, Edwin J R van Beek, Russell Bull, Kavin Jayawardhana, Patricia Dickson, Jennifer Espey, John Lesser, B Kelly Han, Renée Bullock-Palmer, Mark Rabbat, Nancy Schoenecker, Dustin M Thomas, Rosco S Gore, Melany Atkins, Marcus Y Chen, Sujata M Shanbhag, John Mahmarian, Jeannie Yu, Todd C Villines, and Binh Nguyen
References
- 1. Hamilton-Craig CR, Friedman D, Achenbach S.. Cardiac computed tomography—evidence, limitations and clinical application. Heart Lung Circ 2012;21:70–81. [DOI] [PubMed] [Google Scholar]
- 2. Montalescot G, Sechtem U, Achenbach S, Andreotti F, Arden C, Budaj A, Bugiardini R, Crea F, Cuisset T, Di MC, Ferreira JR, Gersh BJ, Gitt AK, Hulot JS, Marx N, Opie LH, Pfisterer M, Prescott E, Ruschitzka F, Sabate M, Senior R, Taggart DP, van der Wall EE, Vrints CJ, Zamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol C, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Tendera M, Torbicki A, Wijns W, Windecker S, Knuuti J, Valgimigli M, Bueno H, Claeys MJ, Donner BN, Erol C, Frank H, Funck BC, Gaemperli O, Gonzalez-Juanatey JR, Hamilos M, Hasdai D, Husted S, James SK, Kervinen K, Kolh P, Kristensen SD, Lancellotti P, Maggioni AP, Piepoli MF, Pries AR, Romeo F, Ryden L, Simoons ML, Sirnes PA, Steg PG, Timmis A, Wijns W, Windecker S, Yildirir A, Zamorano JL.. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J 2013;34:2949–3003. [DOI] [PubMed] [Google Scholar]
- 3. SCOT-HEART investigators. CT coronary angiography in patients with suspected angina due to coronary heart disease (SCOT-HEART): an open-label, parallel-group, multicentre trial. Lancet 2015;385:2383–2391. [DOI] [PubMed] [Google Scholar]
- 4. Budoff MJ, Dowe D, Jollis JG, Gitter M, Sutherland J, Halamert E, Scherer M, Bellinger R, Martin A, Benton R, Delago A, Min JK.. Diagnostic performance of 64-multidetector row coronary computed tomographic angiography for evaluation of coronary artery stenosis in individuals without known coronary artery disease: results from the prospective multicenter ACCURACY (Assessment by Coronary Computed Tomographic Angiography of Individuals Undergoing Invasive Coronary Angiography) trial. J Am Coll Cardiol 2008;52:1724–1732. [DOI] [PubMed] [Google Scholar]
- 5. Williams MC, Hunter A, Shah ASV, Assi V, Lewis S, Smith J, Berry C, Boon NA, Clark E, Flather M, Forbes J, McLean S, Roditi G, van Beek EJR, Timmis AD, Newby DE.. Use of coronary computed tomographic angiography to guide management of patients with coronary disease. J Am Coll Cardiol 2016;67:1759–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cho I, Al’Aref SJ, Berger A, Ó Hartaigh B, Gransar H, Valenti V, Lin FY, Achenbach S, Berman DS, Budoff MJ, Callister TQ, Al-Mallah MH, Cademartiri F, Chinnaiyan K, Chow BJW, DeLago A, Villines TC, Hadamitzky M, Hausleiter J, Leipsic J, Shaw LJ, Kaufmann PA, Feuchtner G, Kim Y-J, Maffei E, Raff G, Pontone G, Andreini D, Marques H, Rubinshtein R, Chang H-J, Min JK.. Prognostic value of coronary computed tomographic angiography findings in asymptomatic individuals: a 6-year follow-up from the prospective multicentre international CONFIRM study. Eur Heart J 2018;39:934–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Einstein AJ, Moser KW, Thompson RC, Cerqueira MD, Henzlova MJ.. Radiation dose to patients from cardiac diagnostic imaging. Circulation 2007;116:1290–1305. [DOI] [PubMed] [Google Scholar]
- 8. Prasad KN, Cole WC, Haase GM.. Radiation protection in humans: extending the concept of as low as reasonably achievable (ALARA) from dose to biological damage. Br J Radiol 2004;77:97–99. [DOI] [PubMed] [Google Scholar]
- 9. Hausleiter J, Meyer T, Hermann F, Hadamitzky M, Krebs M, Gerber TC, McCollough C, Martinoff S, Kastrati A, Schomig A, Achenbach S.. Estimated radiation dose associated with cardiac CT angiography. JAMA 2009;301:500–507. [DOI] [PubMed] [Google Scholar]
- 10. Deseive S, Chen MY, Korosoglou G, Leipsic J, Martuscelli E, Carrascosa P, Mirsadraee S, White C, Hadamitzky M, Martinoff S, Menges AL, Bischoff B, Massberg S, Hausleiter J.. Prospective randomized trial on radiation dose estimates of CT angiography applying iterative image reconstruction: the PROTECTION V study. JACC Cardiovasc Imaging 2015;8:888–896. [DOI] [PubMed] [Google Scholar]
- 11. Hausleiter J, Meyer TS, Martuscelli E, Spagnolo P, Yamamoto H, Carrascosa P, Anger T, Lehmkuhl L, Alkadhi H, Martinoff S, Hadamitzky M, Hein F, Bischoff B, Kuse M, Schomig A, Achenbach S.. Image quality and radiation exposure with prospectively ECG-triggered axial scanning for coronary CT angiography: the multicenter, multivendor, randomized PROTECTION-III study. JACC Cardiovasc Imaging 2012;5:484–493. [DOI] [PubMed] [Google Scholar]
- 12. Hausleiter J, Martinoff S, Hadamitzky M, Martuscelli E, Pschierer I, Feuchtner GM, Catalán-Sanz P, Czermak B, Meyer TS, Hein F, Bischoff B, Kuse M, Schömig A, Achenbach S.. Image quality and radiation exposure with a low tube voltage protocol for coronary CT angiography results of the PROTECTION II Trial. JACC Cardiovasc Imaging 2010;3:1113–1123. [DOI] [PubMed] [Google Scholar]
- 13. Halliburton SS, Abbara S, Chen MY, Gentry R, Mahesh M, Raff GL, Shaw LJ, Hausleiter J.. SCCT guidelines on radiation dose and dose-optimization strategies in cardiovascular CT. J Cardiovasc Comput Tomogr 2011;5:198–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hirshfeld JW Jr, Ferrari VA, Bengel FM, Bergersen L, Chambers CE, Einstein AJ, Eisenberg MJ, Fogel MA, Gerber TC, Haines DE, Laskey WK, Limacher MC, Nichols KJ, Pryma DA, Raff GL, Rubin GD, Smith D, Stillman AE, Thomas SA, Tsai TT, Wagner LK, Wann LS.. 2018 ACC/HRS/NASCI/SCAI/SCCT expert consensus document on optimal use of ionizing radiation in cardiovascular imaging: best practices for safety and effectiveness: a report of the American College of Cardiology Task Force on Expert Consensus Decision Pathways. J Am Coll Cardiol 2018;71:e283–e351. [DOI] [PubMed] [Google Scholar]
- 15. Raff GL, Chinnaiyan KM, Share DA, Goraya TY, Kazerooni EA, Moscucci M, Gentry RE, Abidov A.. Radiation dose from cardiac computed tomography before and after implementation of radiation dose-reduction techniques. JAMA 2009;301:2340–2348. [DOI] [PubMed] [Google Scholar]
- 16. Stocker TJ, Deseive S, Chen M, Leipsic J, Hadamitzky M, Rubinshtein R, Grove EL, Fang XM, Lesser J, Maurovich-Horvat P, Marques H, Andreini D, Tabbalat R, Kang JW, Eckert J, Dickson P, Forsdahl SH, Lambrechtsen J, Cury RC, Hausleiter J.. Reduction in radiation exposure in cardiovascular computed tomography imaging: results from the PROspective multicenter registry on radiaTion dose Estimates of cardiac CT angIOgraphy iN daily practice in 2017 (PROTECTION VI). J Cardiovasc Comput Tomogr 2018;12:81–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shrimpton PC, Hillier MC, Lewis MA, Dunn M.. National survey of doses from CT in the UK: 2003. Br J Radiol 2006;79:968–980. [DOI] [PubMed] [Google Scholar]
- 18. Trattner S, Halliburton S, Thompson CM, Xu Y, Chelliah A, Jambawalikar SR, Peng B, Peters MR, Jacobs JE, Ghesani M, Jang JJ, Al-Khalidi H, Einstein AJ.. Cardiac-specific conversion factors to estimate radiation effective dose from dose-length product in computed tomography. JACC Cardiovasc Imaging 2018;11:64–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Moss AJ, Williams MC, Newby DE, Nicol ED.. The updated NICE Guidelines: cardiac CT as the first-line test for coronary artery disease. Curr Cardiovasc Imaging Rep 2017;10:15.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Castellano IA, Nicol ED, Bull RK, Roobottom CA, Williams MC, Harden SP.. A prospective national survey of coronary CT angiography radiation doses in the United Kingdom. J Cardiovasc Comput Tomogr 2017;11:268–273. [DOI] [PubMed] [Google Scholar]
- 21. Deseive S, Pugliese F, Meave A, Alexanderson E, Martinoff S, Hadamitzky M, Massberg S, Hausleiter J.. Image quality and radiation dose of a prospectively electrocardiography-triggered high-pitch data acquisition strategy for coronary CT angiography: the multicenter, randomized PROTECTION IV study. J Cardiovasc Comput Tomogr 2015;9:278–285. [DOI] [PubMed] [Google Scholar]
- 22. Einstein AJ, Pascual TN, Mercuri M, Karthikeyan G, Vitola JV, Mahmarian JJ, Better N, Bouyoucef SE, Hee-Seung Bom H, Lele V, Magboo VP, Alexanderson E, Allam AH, Al-Mallah MH, Flotats A, Jerome S, Kaufmann PA, Luxenburg O, Shaw LJ, Underwood SR, Rehani MM, Kashyap R, Paez D, Dondi M.. Current worldwide nuclear cardiology practices and radiation exposure: results from the 65 country IAEA Nuclear Cardiology Protocols Cross-Sectional Study (INCAPS). Eur Heart J 2015;36:1689–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Achenbach S, Marwan M, Ropers D, Schepis T, Pflederer T, Anders K, Kuettner A, Daniel WG, Uder M, Lell MM.. Coronary computed tomography angiography with a consistent dose below 1 mSv using prospectively electrocardiogram-triggered high-pitch spiral acquisition. Eur Heart J 2010;31:340–346. [DOI] [PubMed] [Google Scholar]
- 24. Hell MM, Bittner D, Schuhbaeck A, Muschiol G, Brand M, Lell M, Uder M, Achenbach S, Marwan M.. Prospectively ECG-triggered high-pitch coronary angiography with third-generation dual-source CT at 70 kVp tube voltage: feasibility, image quality, radiation dose, and effect of iterative reconstruction. J Cardiovasc Comput Tomogr 2014;8:418–425. [DOI] [PubMed] [Google Scholar]
- 25. Gerber TC, Carr JJ, Arai AE, Dixon RL, Ferrari VA, Gomes AS, Heller GV, McCollough CH, McNitt-Gray MF, Mettler FA, Mieres JH, Morin RL, Yester MV.. Ionizing radiation in cardiac imaging: a science advisory from the American Heart Association Committee on Cardiac Imaging of the Council on Clinical Cardiology and Committee on Cardiovascular Imaging and Intervention of the Council on Cardiovascular Radiology and Intervention. Circulation 2009;119:1056–1065. [DOI] [PubMed] [Google Scholar]
- 26. Chinnaiyan KM, Peyser P, Goraya T, Ananthasubramaniam K, Gallagher M, Depetris A, Boura JA, Kazerooni E, Poopat C, Al-Mallah M, Saba S, Patel S, Girard S, Song T, Share D, Raff G.. Impact of a continuous quality improvement initiative on appropriate use of coronary computed tomography angiography. Results from a multicenter, statewide registry, the Advanced Cardiovascular Imaging Consortium. J Am Coll Cardiol 2012;60:1185–1191. [DOI] [PubMed] [Google Scholar]
- 27. Abbara S, Blanke P, Maroules CD, Cheezum M, Choi AD, Han BK, Marwan M, Naoum C, Norgaard BL, Rubinshtein R, Schoenhagen P, Villines T, Leipsic J.. SCCT guidelines for the performance and acquisition of coronary computed tomographic angiography: a report of the Society of Cardiovascular Computed Tomography Guidelines Committee: endorsed by the North American Society for Cardiovascular Imaging (NASCI). J Cardiovasc Comput Tomogr 2016;10:435–449. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.