Abstract
Scope:
The fetal programming paradigm posits that the origins of obesity can be traced, in part, to the intrauterine period of life. However, the mechanisms underlying fetal programming are not well understood, and few studies have measured offspring adiposity in the neonatal period. The aim of this study was to identify maternal metabolites, and their determinants, that are associated with neonatal adiposity.
Methods and Results:
We applied a targeted metabolomics approach to analyze plasma samples collected across gestation from a well-characterized cohort of 253 pregnant women participating in a prospective study at the University of California, Irvine. Whole-body Dual X-Ray Absorptiometry (DXA) imaging of body composition was obtained in N=121 newborns. Statistical models were adjusted for potential confounders and multiple testing.
We identified six alkyl-linked phosphatidylcholines (PCae), containing fatty acid 20:4, that were significantly and negatively associated with neonatal body fat percentage. Factors indicating higher socioeconomic status, non-Hispanic ethnicity and higher non-esterified fatty acid percentages were positively associated with these PCae.
Conclusions:
The polyunsaturated fatty acid 20:4 contained in PCae may exert a beneficial effect with respect to future propensity for obesity development. Pre- and early-pregnancy factors are determinants of these PCae, highlighting the importance of addressing pre-conceptional conditions for fetal programming of newborn adiposity.
Keywords: Metabolomics, pregnancy, obesity, adiposity, fetal programming
Introduction
Women who enter pregnancy with a raised BMI are significantly more likely to have obese offspring, giving rise to an inter-generational cycle of obesity risk (1–3). The conventional paradigm posits that individual susceptibility for, and inter-generational transmission of, obesity is largely attributable to genetic makeup. However, currently-identified genetic variants account for only a modest proportion of variation in BMI (4). Meanwhile, a rapidly growing and convergent body of evidence in humans and animals suggests that the origins of obesity can be traced, in part, to the intrauterine period of life (5), at which time the developing fetus responds to ‘suboptimal’ conditions by producing structural and functional changes in cells, tissues and organ systems (i.e., the concept of fetal programming) (6–10). While the mechanisms for early programming of human metabolism and obesity risk have yet to be fully elucidated, recent advances in the “omics” technologies facilitate a deeper insight to this process. Metabolomics provides potential to analyze the biological effects of genetic and environmental factors, since metabolites are the downstream product of biochemical pathways, and thus, closely related to the phenotype of interest. Previous studies have utilized metabolomics to investigate various pre- and postnatal programming mechanisms, but are lacking in standardization or statistical power (11, 12). Consistently, maternal omega-3 (n-3) fatty acid status, but not dietary n-3 fatty acid intake (13–15), has been found to be protective against offspring obesity risk (16–19). However, most of these studies used infant or childhood BMI as an outcome and, thus, the influence of the intra-uterine environment cannot be separated from obesity-related postnatal factors. To date, no human study has prospectively investigated the association between maternal metabolomic profiles during pregnancy with newborn adiposity.
The primary aim of the present study was to examine – using an unbiased approach - the prospective association between key plasma metabolites representative of maternal lipid and energy metabolism in early, mid and late gestation with newborn adiposity in a longitudinal, prospective birth cohort at the University of California, Irvine (UCI) (20, 21). Dual-energy X-ray absorptiometry (DXA) whole body imaging was used to directly quantify newborn body composition (percent fat mass) rather than relying on commonly-used weight- and length-based proxy measures of infant adiposity (e.g., weight-for-length ratio). The second aim was to identify maternal determinants of variation in the specific metabolites that predict newborn adiposity in this cohort.
Materials and Methods
Study Participants
The UCI Development, Health and Disease Research Program conducted a longitudinal, prospective study of 253 pregnant women and their babies beginning in early gestation. The overarching aim of this observational study was to determine the interplay of psychosocial and physiological stress during pregnancy and the implications for fetal programming of infant metabolic and neurodevelopmental health outcomes. Women were eligible for inclusion if they were >18 years of age with a singleton, intrauterine pregnancy. Maternal exclusionary criteria were uterine anomalies and pre-existing major medical co-morbidities (hypertension, infection or diabetes). Newborn exclusionary criteria were congenital malformations, chromosomal abnormalities, major perinatal complications, and preterm birth < 34 completed weeks. All aspects of this study were approved by the UCI Institutional Review Board (HS#2002–2316 and HS#2010–7530). Written, informed consent was obtained from all study participants.
Participants attended up to three visits during pregnancy at a mean ± SD gestational age of 12.5±1.7 weeks (N=206), 20.5±1.4 weeks (N=219) and 30.4±1.4 weeks (N=217). A 10ml EDTA tube was drawn by standard venipuncture technique by a phlebotomist and then centrifuged for 15 min at 1200 g. Separated plasma aliquots of 0.5ml were transferred to 2ml screw top plastic vials, labeled and stored at −80°C until analysis.
The study participants completed structured interviews and questionnaires related to sociodemographic characteristics, medical/obstetric history, and maternal behaviors. At each visit, participants were asked to self-report their pre-pregnancy weight. Height was measured in the upright supine position to the nearest 0.1 cm using a calibrated stadiometer. The average values for pre-pregnancy weight and measured height across pregnancy were calculated, and pre-pregnancy BMI was estimated using the formula weight (kg) / height (m)2. Although pre-pregnancy weight was not measured in the laboratory, the average self-reported pregnancy weight highly correlated with measured weight on the first visit (r=0.985, p<0.001) and therefore considered a reliable measure. The average value for self-reported pre-pregnancy weight was subtracted from measured weight at each visit to calculate total weight gain at each trimester.
Information on clinical conditions present during pregnancy, including diabetes (n=8), hypertensive disorders (n=7), anemia (n=8) and severe infections (n=9), was obtained from a combination of maternal self-report and medical records. The sociodemographic, pregnancy and newborn birth outcome characteristics of the study population are presented in Table 1.
Table 1.
Population demographics, anthropometric measures and newborn body composition for N=121 mother-offspring pairs with available DXA and metabolomics data.
| Maternal characteristics | Median [IQR] or n (%) |
|---|---|
| Age [years] | 29 [8] |
| Marital status (Married) | 72 (59.5%) |
| Employment (yes) | 66 (54.55%) |
| Yearly Income ($) | |
| <30,000 | 34 (29.05%) |
| 30000–50,000 | 27 (23.08%) |
| >50,000 | 56 (47.86%) |
| Education level achieved | |
| Primary, Elementary or Middle School | 5 (4.13%) |
| High school or General Education Development | 18 (14.88%) |
| Technical or Vocational School | 13 (10.74%) |
| Some College but no Degree | 45 (37.19%) |
| Associates Degree | 5 (4.13%) |
| Bachelor Degree | 19 (15.7%) |
| Graduate Degree | 13 (10.74%) |
| Hispanic Ethnicity | 54 (45%) |
| Smoking in Pregnancy (yes) | 12 (10.08%) |
| Parity | |
| 0 | 43 (35.83%) |
| 1 | 34 (28.33%) |
| 2+ | 43 (35.84%) |
| Pre-pregnancy BMI [kg/cm2] | 25.83 [8.37] |
| Total gestational weight gain [kg] | 13.61 [9.08] |
| Newborn characteristics | |
| Gestational age at birth [weeks] | 39.43 [1.71] |
| Sex (male) | 65 (53.72%) |
| Birth weight [g] | 3430 [601] |
| Age at DXA scan [weeks] | 3.0 [3.0] |
| Newborn percentage body fat | 12.88 [7.97] |
Dietary data
Dietary intake was assessed in each trimester via three 24-hour dietary recalls, conducted by a trained researcher over the telephone. Dietary data was entered to the Nutrition Data System for Research (NDSR) software version 2011 (Nutrition Coordinating Center (NCC), University of Minnesota, Minneapolis, MN), which includes over 18,000 foods with options to specify brand name, ingredient choice and preparation method. The NDSR program generates nutrient intake values based on the NCC Food and Nutrient Database.
Output files for nutrient and food group intakes were generated for each subject’s dietary recall from the NDSR program and imported to IBM SPSS for Windows, version 22, for data handling. A daily recall with total energy intake <1000 or >4000kcal was considered an under-reporter or over-reporter, respectively, and excluded from the analyses. Data was then aggregated to calculate mean daily intakes within each trimester for each subject. Diet quality was evaluated by calculating the Alternate Healthy Eating Index adapted for pregnancy (AHEI-P), as previously described (22), with minor modifications as follows: The ‘Nut & Soy’ category was expanded to include intake of any food group contributing a major source of vegetable protein, i.e. nuts and seeds, nut and seed butters, legumes, and meat alternatives; the ‘Fruit’ category excluded fruit juices.
Insulin and glucose measures
Serum glucose was quantitatively determined enzymatically using reagents from Vital Diagnostics (Lincoln, RI). Samples were incubated with reagent at 37°C and the absorbance read at 340/380 nm. Insulin was measured using an RIA procedure developed by EMD Millipore (St Charles, MO). The homeostasis model of insulin resistance (HOMA-IR) was computed using the formula: (glucose in mg/dl x insulin in μU/ml) / 405 (23).
Metabolomics analysis
Metabolomics analysis was performed at LMU Munich. Amino acids (AA), non-esterified fatty acids (NEFA), carboxylic acids (CA), acylcarnitines (acyl-Carn) and phospholipids (PL) were measured in the plasma samples taken in the first (n=206), second (n=219) and third trimester (n=217).
The measurement of metabolites available in the different trimesters was performed in two steps. The first set of 472 samples was shipped to Munich in May 2014 and analyzed in August 2014. The second set of 170 samples was shipped to Munich in December 2015 and analyzed in July 2016. We report all metabolite concentrations in μmol/L. As a point of note, the analytical technique used here is not capable of determining the position of the double bonds and the distribution of carbon atoms between fatty acid side chains. The acyl-Carn, PL and NEFA are mentioned as X:Y. In this nomenclature, X is the length of the carbon chain, Y is the number of double bonds. A detailed description on the metabolomics analysis can be found in the supplemental materials and methods.
Newborn Dual-energy X-ray absorptiometry (DXA) imaging
To quantify infant body fat, a whole body DXA scan was obtained using a Hologic Discovery Scanner (A, QDR 4500 series, Hologic Inc., Bedford, MA, USA) in infant scan mode. Calibration using Hologic’s anthropomorphic Spine QC Phantom was performed before each scan. During the scan, infants lay supine while sleeping, wearing only a disposable diaper and swaddled in a light cotton blanket. If the infant moved during the scan, a single repeat was performed. Children were, on average, 25.7 days old (mean (± 11.6 (SD) days) at the time of the scans. This age was selected to allow for stabilization of fluid shifts in the newborn that occur in the first 2–3 weeks following birth (24).
Statistical analyses
Statistical analyses were performed using R statistical software, version 3.0.1. Initially, individual outlying values for a given metabolite were detected if >3 times the SD of the second highest value. Further possible outliers were detected through inspection of boxplots for each metabolite at each time point of measurement and individual outlying values were removed from the dataset as appropriate.
Each metabolite was standardized by converting it to a z-score, using the mean and SD values within each batch of measurements, according to the formula: (metabolite value – within-batch mean value for given metabolite) / within-batch SD for given metabolite. Thus, the mean of each metabolite per batch was “0” and the SD was “1”.
In addition to single metabolite levels, PCA of all metabolites was performed to combine co-correlated metabolites into one component. The first ten trimester-specific principle components received by PCA were handled like single metabolites in the following analysis and are also included when mentioning “metabolites”.
For our first aim, to identify metabolomic marker related to newborn percent body fat (%BF), two different approaches were used: (I) Bivariate linear regression models with %BF as depended and the metabolite as independent variable, and (II) multivariate linear regression models, including adjustment for potential confounders.
To identify potential confounders of newborn body composition, bivariate linear models were applied with %BF as the dependent variable and the potential confounder (Supplemental methods) or metabolites as independent variables. Besides maternal determinates, newborn variables were also considered in the model because controlling for them may decrease the standard errors.
Forward stepwise model selection by the akaike information criterion (AIC) was performed with %BF as the dependent variable and one metabolite, sex, and ethnicity as fixed independent variables and the previous identified potential confounders (p<0.2 in bivariate models) as independent variables. This procedure was repeated 20 times for the 20 metabolites most significantly associated to %BF in the bivariate analysis. If a confounder was identified for more than 4 metabolite associations to %BF, this confound was used in the final multivariate model. The final multivariate model was adjusted for ethnicity of the mother, sex of the children, parity, gestational age at birth, marital status and age of the newborn at the time the DXA scan was performed.
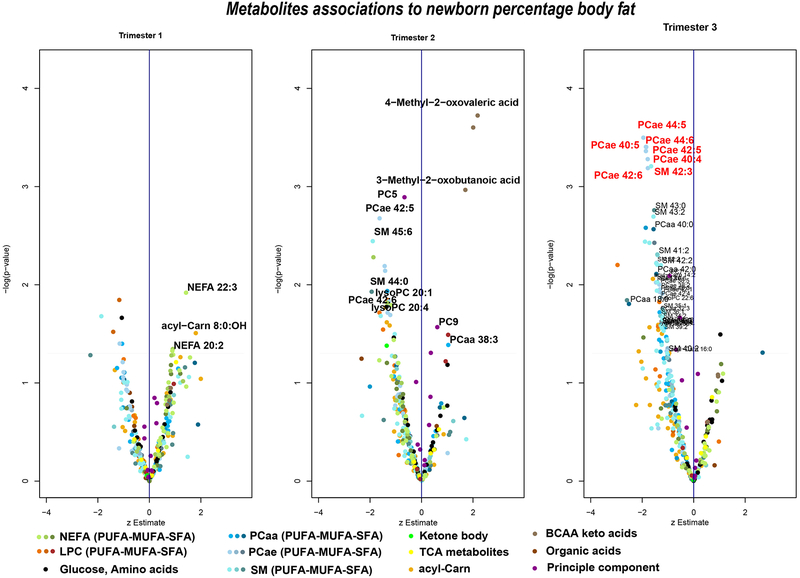
Associations were visualized by “volcano plots”, whereby the estimate is plotted on the x-axis and the negative log10(P) values on the y-axis. To correct for multiple testing, a false discovery rate (FDR) of less than 5% was used as the significance level (25), which was applied to each analysis performed in each trimester with a new outcome variable. These associations are highlighted with red text in the respective volcano plots (Figure 1). Associations, which did pass the FDR corrected significance level, but exhibit an uncorrected p-value below 0.05, are mentioned as tendencies.
Figure 1.
Associations of metabolites in each trimester to newborn percentage body fat. Negative log transformed p-values are plotted for each metabolite on the y-axis, while estimate of the z-score metabolite are plotted on the x-axis. Higher values represented in upper part present a higher association between metabolite and newborn body fat percentages. P-values were calculated by linear regression models with newborn body fat percentages obtained by DXA scans as dependent variable and the each metabolite as independent variables. The linear models were adjusted for infant sex, maternal ethnicity, gestational age at birth, parity, and marital status of the mother. Metabolite names highlighted in red depict significant associations after FDR correction for multiple testing. All newborn body fat percentages ~metabolite associations which were significant (without correction for multiple testing) after the sensitivity analysis are plotted with point and name. AA, amino acids; NEFA, nonesterified fatty acids; acyl-Carn, acylcarnitines; lysoPC, lysophosphatidylcholines; MUFA, monounsaturated fatty acid; PCaa, diacyl-phosphatidylcholines; PCae, alkyl-linked phosphatidylcholines; PUFA, polyunsaturated fatty acid; SFA, saturated fatty acid; SM, sphingomyelins; TCA, tricarboxylic acid.
Furthermore, significant associations identified were checked for potential influence by outliers (which persisted after initial outlier detection and removal) by two methods: 1) visual exploration of scatter plots, and 2) by removing the three highest and lowest metabolite values and checking if the significance remained stable. Metabolite associations which did not pass these sensitivity checks are not highlighted in the respective volcano plots.
For our second aim, to determine maternal determinates influencing metabolomic markers identified for aim 1, bivariate linear regression models were performed with the single identified metabolite as the dependent variable and maternal characteristics as independent variables (Supplemental Table 4.1–4.3). To test associations with categorical maternal characteristics, (ethnicity (Hispanic/Non-Hispanic), employment (yes/no) and education), logistic regression was used. The eight original education categories were summarized to three new categories to facilitate an appropriate number per group: Lower education – “Primary, Elementary or Middle School” and “High school or General Education Development”; Middle education – “Some College but no Degree” and “Technical or Vocational School”; Higher education – “Associates Degree”, “Bachelors Degree”, and “Graduate degree”.
Results
Metabolite data from cases identified as being obtained from non-fasting samples (9 cases at trimester 1, 5 cases at trimester 2 and 7 cases at trimester 3) were excluded from analysis. Of the remaining 227 participants with viable metabolomics data from at least one time point, there were data for 197 subjects at trimester 1, 214 subjects at trimester 2 and 210 subjects at trimester 3, and 175 women had data available at all three time points. Maternal glucose and insulin data were available for 194 (trimester 1), 210 (trimester 2) and 207 (trimester 3) women at each time point. DXA body composition data was available for a total of N=121 infants. Corresponding to the maternal participants with metabolomic data per trimester, newborn body composition data were available for infants born to 109, 115 and 114 women, in trimesters 1, 2 and 3 respectively. Maternal or infant characteristics did not differ between subsets of women with metabolomics data available at different prenatal assessment time points. Our cohort included women with a diagnosis of diabetes (n=8), hypertensive disorders (n=7), anemia (n=8) and severe infections (n=9), but these conditions had no effect on the results presented.
The sociodemographic, anthropometric and newborn characteristics for the cohort of N=121 mother-infant dyads with available data for analysis, are presented in Table 1. 53.7% of the pregnant women were classified as overweight or obese (pre-pregnancy BMI ≥25 kg/m2) and 53% gained gestational weight in excess of the Institute of Medicine criteria for their given pre-pregnancy BMI category.
395 metabolites were measured with inter-batch coefficient of variance of no more than 20% in the first and the second shipment, respectively, including 22 NEFA, 47 acyl-Carn, 258 PL, 14 CA, 23 AA and the sum of hexoses. Since only metabolites with at least 20 observations were used in the data analysis, the final linear regression models were performed with 308 metabolites and 10 principle components per trimester.
Metabolites associated with newborn body fat
In the bivariate models, no metabolite was significantly associated with newborn %BF after FDR correction for multiple testing (Supplemental Table 1, Supplemental Figure 1). Without correction for multiple testing, several PL in the second and last trimester tended to a negative association to %BF.
In the multivariate model (Figure 1, Supplemental Table 1), seven plasma PL in the last trimester were negatively associated with newborn %BF after FDR correction, namely alkyl-linked phosphatidylcholines (PCae) 40:4, PCae 40:5, PCae 42:5, PCae 42:6, PCae 44:5, PCae 44:6, and sphingomyelin (SM) 42:3 (padj=0.038, respectively). The standardized estimates for the associations ranged from −1.78 to −1.96, indicating that a reduction of 1 SD of these PL results in almost 2% greater body fat in the newborn. Newborns whose mother were in the lowest quartile of PCae 44:6 concentrations, exhibited a mean ±SD %BF of 15.8±5.8%, while newborns of mothers in the highest quartile had a %BF of 10.9±5.2%.
Additionally, the plasma keto-acids 3-Methyl-2-oxobutanoic acid, 3-Methyl-2-oxovalveric acid, and 4-Methyl-2-oxovaleric acid in the second trimester were positively associated with newborn %BF, but in the sensitivity analysis 3-Methyl-2-oxovaleric acid was no longer significant and the scatter-plots and associations of the other two keto-acids were also affected by outliers. A common characteristic of these outlying subjects could not be detected. Among the metabolite principle components, principle component 5 in the second trimester (padj=0.065, representing non-essential AA and long-chained acyl-Carn (Supplemental Table 2)) tended to be negatively related to newborn %BF.
Molecular identification of fatty acids in phospholipids
With FIA-MS/MS, we are unable to distinguish between ether- and odd-chain acyl bonds and could not identify which single fatty acid species are bound to the PL backbone. To identify which exact fatty acid are bound by which binding, we ran identification analyses for PL of interest by a modified LC-MS/MS method (26). With the LC-MS/MS method using 184 Da (choline group) as product ion, only one peak for the molecular mass of PCae 44.6 (875.7 Da) was detected, excluding the possibility of co-eluents. In negative ionization mode, the acetic acid masks the positive charge of the choline head group and fragmentation enables isolation of fatty acids as fragments, and thus, a unique identification was possible. In the multiple reaction monitoring scan of acetate adducts, we found only the mass 303.6 Da (fatty acid 20:4) as fragment of 934.7 Da (875.7 + 59 Da), implying that the second fatty acid is bound by an ether bond. Thus, the molecular mass 875.7 Da is most likely PCae 20:4/24:2 (Table 2). Fatty acid 20:4 was also identified in the mass transitions of acetate adducts of PCae 40:4 (882.7 Da), 40:5 (880.7 Da), 42:5 (908.7 Da),42:6 (906.7 Da) and 44:5 (936.7 Da) (Table 2).
Table 2.
Associations between maternal factors and pregnancy parameters and ether-linked phosphatidylcholine (PCae) speciesa
| Maternal sociodemographic factors | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PL in Trimester 3 | LC-MS/MS | Ethnicity | Employment | Education | ||||||||||||||
| FA a/e | p | p | p | |||||||||||||||
| PCae C40:4 | 20:4/20:0 | <0.001 | 0.407 | 0.040 | ||||||||||||||
| PCae C40:5 | 20:4/20:1 | <0.001 | 0.269 | 0.002 | ||||||||||||||
| PCae C42:5 | 20:4/22:0 | <0.001 | 0.124 | 0.002 | ||||||||||||||
| PCae C42:6 | 20:4/22:2 | <0.001 | 0.025 | 0.000 | ||||||||||||||
| PCae C44:5 | 20:4/24:1 | <0.001 | 0.018 | 0.007 | ||||||||||||||
| PCae C44:6 | 20:4/24:2 | <0.001 | 0.041 | 0.040 | ||||||||||||||
| Prenatal glycemic profiles | ||||||||||||||||||
| PL in Trimester 3 | Insulin (μU/ml) | HOMA-IR | Glucose (mg/dl) | |||||||||||||||
| Trimester 1 | Trimester 2 | Trimester 3 | Trimester 1 | Trimester 2 | Trimester 3 | Trimester 1 | Trimester 2 | Trimester 3 | ||||||||||
| B | p | B | p | B | p | B | p | B | p | B | p | B | p | B | p | B | p | |
| PCae C40:4 | −0.02 | 0.011 | −0.03 | 0.021 | −0.02 | 0.024 | −0.12 | 0.019 | −0.12 | 0.036 | −0.08 | 0.050 | −0.01 | 0.512 | 0.00 | 0.791 | −0.01 | 0.186 |
| PCae C40:5 | −0.03 | 0.002 | −0.03 | 0.011 | −0.03 | 0.003 | −0.15 | 0.002 | −0.13 | 0.019 | −0.12 | 0.006 | −0.01 | 0.376 | 0.00 | 0.730 | −0.02 | 0.087 |
| PCae C42:5 | −0.03 | 0.006 | −0.03 | 0.036 | −0.02 | 0.023 | −0.14 | 0.006 | −0.12 | 0.038 | −0.10 | 0.020 | −0.01 | 0.373 | −0.01 | 0.307 | −0.03 | 0.010 |
| PCae C42:6 | −0.03 | 0.003 | −0.02 | 0.051 | −0.02 | 0.052 | −0.15 | 0.004 | −0.11 | 0.064 | −0.09 | 0.042 | −0.02 | 0.272 | −0.01 | 0.371 | −0.03 | 0.005 |
| PCae C44:5 | −0.02 | 0.024 | −0.01 | 0.243 | −0.01 | 0.126 | −0.11 | 0.027 | −0.06 | 0.246 | −0.07 | 0.093 | 0.00 | 0.880 | −0.01 | 0.544 | −0.03 | 0.008 |
| PCae C44:6 | −0.03 | 0.008 | −0.02 | 0.177 | −0.01 | 0.171 | −0.13 | 0.014 | −0.07 | 0.219 | −0.06 | 0.156 | 0.00 | 0.855 | −0.01 | 0.571 | −0.03 | 0.009 |
| Dietary intake factors | ||||||||||||||||||
| PL in Trimester 3 | Omega-3 fatty acids (g/day) | Omega-6 fatty acids (g/day) | Glycemic Load | |||||||||||||||
| Trimester 1 | Trimester 2 | Trimester 3 | Trimester 1 | Trimester 2 | Trimester 3 | Trimester 1 | Trimester 2 | Trimester 3 | ||||||||||
| B | p | B | p | B | p | B | p | B | p | B | p | B | p | B | p | B | p | |
| PCae C40:4 | 0.11 | 0.518 | 0.26 | 0.074 | 0.07 | 0.648 | 0.03 | 0.150 | 0.02 | 0.167 | 0.02 | 0.393 | 0.01 | 0.042 | 0.00 | 0.528 | 0.01 | 0.064 |
| PCae C40:5 | −0.03 | 0.854 | 0.28 | 0.059 | 0.01 | 0.943 | 0.01 | 0.455 | 0.03 | 0.118 | 0.02 | 0.372 | 0.00 | 0.153 | 0.00 | 0.359 | 0.00 | 0.105 |
| PCae C42:5 | 0.09 | 0.587 | 0.39 | 0.007 | 0.11 | 0.496 | 0.03 | 0.142 | 0.04 | 0.014 | 0.04 | 0.081 | 0.01 | 0.022 | 0.01 | 0.078 | 0.01 | 0.062 |
| PCae C42:6 | 0.19 | 0.248 | 0.37 | 0.011 | 0.17 | 0.279 | 0.04 | 0.035 | 0.04 | 0.013 | 0.03 | 0.157 | 0.01 | 0.038 | 0.00 | 0.095 | 0.00 | 0.305 |
| PCae C44:5 | 0.19 | 0.231 | 0.38 | 0.006 | 0.17 | 0.269 | 0.04 | 0.038 | 0.04 | 0.006 | 0.04 | 0.043 | 0.01 | 0.016 | 0.01 | 0.049 | 0.00 | 0.157 |
| PCae C44:6 | 0.22 | 0.191 | 0.44 | 0.002 | 0.22 | 0.154 | 0.04 | 0.029 | 0.05 | 0.003 | 0.04 | 0.058 | 0.01 | 0.029 | 0.01 | 0.053 | 0.00 | 0.411 |
a only those PCae species significantly associated to newborn body fat percentages after FDR correction were included. Bivariate linear regression models were performed with PCae species as the dependent variable and maternal factors as independent variables, where B = parameter estimate and P = p-value. Light Grey background: p-value < 0.05. Dark grey background: p-value < 0.001. FA, fatty acid; HOMA-IR, homeostasis model assessment of insulin resistance.
Associations between NEFA composition and phospholipids in the third trimester
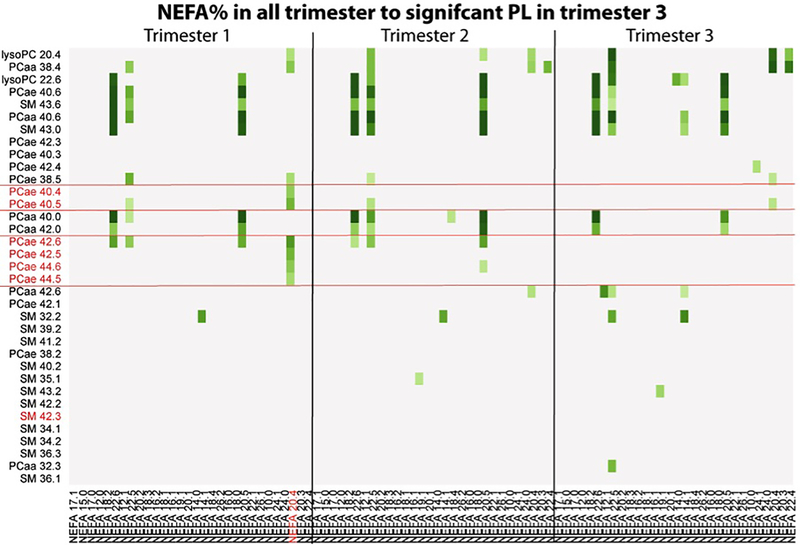
Figure 2 presents the associations between relative NEFA values (mol%) per trimester and the PL found to exhibit an uncorrected p-value <0.05 in the association to newborn %BF (Supplemental Table 3). The six PCae of the third trimester, which were significantly associated after FDR correction with newborn %BF, showed the strongest association with NEFA 20:4 percentages in the first trimester (p=3.3×10−5 - 1.1×10−6).
Figure 2.
Heatmap of associations between nonesterified fatty acid (NEFA) percentages (mol%) and phospholipid species PL found to exhibit a p-value <0.05 in association to newborn body fat percentages after sensitivity analysis. Displayed are the negative log transformed p-values (-logp) which were calculated with a bivariate linear regression models with single PL species as depended variable and relative NEFA as independent variables. Metabolite names highlighted in red depict significant associations to new-born %BF after FDR correction for multiple testing. Darkest green means a -logp greater 5 (p< 0.00001). Steps for lighter greens were 0.5. –logp < 3.9 (multiple testing level) are not colored.
Maternal determinates of metabolomics profiles associated with newborn %BF
Among all tested maternal sociodemographic (Supplemental Table 4.1), pregnancy obstetric risk (Supplemental Table 4.2) and dietary factors (Supplemental Table 4.3), only maternal ethnicity showed significant associations after Bonferroni correction with the six identified PCae species (Table 2). The six PCae were higher in the Non-Hispanic population (mean=1.36–6.43 μM) compared to Hispanics (mean=1.01–5.07 μM). However, no interaction effect between ethnicity and the PCae on %BF was observed (p>0.3) and ethnicity-stratified analysis showed no differences in metabolite associations to %BF between the groups (BHispanic=−2.14 − (−1.42), BNon-Hispanic=−2.79 − (−1.78)), implying that only the levels of the PCae, but not the relation between PCae and %BF was affected by ethnicity. Non-Hispanics had a higher intake of calories from total fat (33.9% vs 30.5%, p=0.002) and monounsaturated fat (11.8% vs 10.3%, p=0.003) only in the first trimester, but no differences in polyunsaturated fat intake compared to Hispanics in any of the three trimesters.
Table 2 summarizes other maternal factors (fasting insulin in the first trimester, n-3 intake in the second trimester, n-6 intake in the first and second trimester), which tended to be associated to most or all six PCae, when correction for multiple testing was not considered.
Employed participants had higher levels of the PCae 42.6 (2.50 μM vs 2.17 μM), PCae 44.5 (1.28 μM vs 1.11 μM), and PCae 44.6 (2.89 μM vs. 2.62 μM), without adjustment for multiple comparisons. Similar to the case of ethnicity, employment showed no effect on the associations between the six PCae and %BF (BEmployed=−2.19 − (−0.79), BNon-Employed=−2.28 − (−1.92)), and no interaction effect could be observed. Employed women had a higher dietary intake of n-3 FA (p=0.003), n-6 FA (p=0.005), and PUFA (p=0.031) in the first trimester, but not in the second and third trimester.
Higher education was associated with higher levels of the six PCae (all p values <0.05; Supplemental Table 4.1 and Supplemental Figure 2). Education exhibited no interaction effect with the six PCae on %BF and in the stratified analysis, the association between the PCae and %BF was weaker in the low education group (B=−1.04 – 0.83) compared to middle (B=−2.50 – (−1.61)) or higher educated women (B=−2.83 – (−1.59)). Women with higher education showed a tendency toward higher total energy (p=0.014) and higher n-3 fatty acid intake (p=0.031) in the first trimester.
Fasting insulin and HOMA-IR levels, particularly in the first trimester, as well as fasting glucose levels in the last trimester, were negatively associated with the six PCae, while dietary glycemic load in the first trimester and n-3 and n-6 fatty acid intake, particularly in the second trimester, were positively related to the six PCae.
Discussion
This study provides, to the best of our knowledge, the first findings regarding the relationship of different maternal metabolites measured across gestation, which are involved in lipid- and energy metabolism, with the development of offspring fat tissue accrual during the intrauterine period of life. Only one study could be identified to relate maternal metabolites to newborn adiposity focusing their research on maternal obesity related metabolites in mid-pregnancy in a European study (27). Thus, no studies exist in the fetal programming of obesity context that consider trimester-specific analysis, a wide range of metabolites, or the influence of other maternal determinants, except adiposity. Our major finding is that maternal phospholipid status was related to newborn adiposity and more specifically, phosphatidylcholines containing an ether-bond and the fatty acid 20:4n-6 appear to be protective against higher newborn body fat.
Most studies target the fatty acid composition of PL but lack information about the molecular structure of the PL, which exhibit a large degree of heterogeneity. Interestingly, in our study the sum of 22:6 or 20:4 species of diacyl-phosphatidylcholines (PCaa), the dominant species in PL, showed no associations with newborn %BF. While this seems to be in contrast to previous studies, we targeted newborn body composition, as opposed to birthweight, body composition or body weight in later infancy or childhood, as reported in other studies (16, 17, 19, 28–30), which allow only limited conclusions on influences of intra-uterine development. For instance, a recent study reported no associations between maternal metabolites and infant birth weight after correction for multiple testing, but did identify three fetal (cord blood) LPC metabolites (14:0, 16:1, 18:0) to be positively correlated (28). However, this study did not include any direct or proxy measures of infant adiposity.
The strong association between third-trimester PCae with %BF is not surprising since fetal fat deposition is amplified during this period and, thus, metabolites are expected to exert the strongest effect at this time of intrauterine development. However, maternal factors affecting the PCae concentrations in the last trimester were apparent in the first trimester (NEFA%, insulin, HOMA) and also from non-pregnancy related sociodemographic factors (education and employment), highlighting the importance of the pre-conceptional state and early gestational period not only for the PL levels, but also for fetal programming mechanisms.
Several publications present data to support beneficial effects of maternal n-3 fatty acid status on offspring body composition and adverse associations with n-6 fatty acid, but our findings may significantly advance our understanding of broader lipid class associations and possible underlying mechanisms. Key results from our study suggest that both 20:4n-6 and 20:5n-3/22:6n-3 may have a beneficial role for fetal growth and are protective against elevated neonatal %BF. However, controversial results have been published regarding PUFA and offspring growth. Firstly, several studies have reported enhanced infant adiposity outcomes associated with maternal n-6 fatty acid status. For instance, linoleic acid (18:2n-6) intake measured across 4 generations of rats increased AT mass over generations (31). Dihomo-γ-linolenic acid (DGLA, 20:3n-6), the precursor of 20:4n-6, is related to maternal pre-pregnancy BMI (21) and to offspring adiposity at 7 years (16). In contrast, maternal 20:5n-3 and 22:6n-3 levels in the second trimester were not related to childhood %BF determined by skin fold measurement at 3 years, while maternal dietary intake of n-3 PUFA showed negative associations (17). Another study showed that late pregnancy maternal n-6 PUFA were positively associated to offspring fat mass at 4 and 6 years, but no associations of n-3 PUFA were found (18), adjusting for a parameter depicting quality of child’s diet at 3 years weakened this effect.
Both n-6 and n-3 fatty acids are essential fatty acids, requiring alpha-linolenic acids (18:3n-3) and linoleic acid (18:2n-6) as precursors for their synthesis in human metabolism. Although dietary intake of n-3 and n-6 fatty acids in the second trimester were positively related to the PCae levels in the third trimester, much stronger determinates of these PCae were NEFA percentages, in particular 20:4% in the first trimester. Given that adipose tissue fatty acid composition and, thus, NEFA composition in fasting state (32) are markers for long-term dietary fat intake, we suggest that pre-pregnancy diet is a stronger determinant of prenatal PUFA status compared to dietary intake during pregnancy. This is also in line with our previous publication reporting that pre-pregnancy BMI is a strong determinant of NEFA throughout gestation, in particular n-6 NEFA, although not related to n-3 NEFA (21).
Our results further highlight a special role of ether-linked phosphatidylcholines in relation to offspring growth, which has not been previously reported. PCae are characterized by an ether or ether-vinyl bound on the sn-1 position of the glycerol backbone. Together with ether-linked phosphatidylethanolamines, PCae form the group of plasmalogens (33). They are synthesized in peroxisomes and usually contain 20:4 or 22:6 at the sn-2 position (34). In contrast to PCaa, the fatty acids in PCae are parallel decreasing fluidity and promoting the formation of non-bilayer phases. Furthermore, the ether bond also reduces hydrophilicity, is prone to oxidation and may work as an anti-oxidant for the sn-2 PUFA or other PL in membranes. Their enrichment in 20:4 and 22:6 and their unique properties due to their molecular structure may suggest that plasmalogens play a crucial role in lipid transport across the placenta. Maternal fasting insulin and HOMA in the first trimester tended to be associated with the third trimester PCae levels. Thus, we assume a general mechanistic association between PCae and insulin homeostasis which needs further clarification. The lack of associations of fasting insulin in the second and third trimester might be due to general increase of insulin resistance in pregnancy, attenuating the effect of any individual variation in insulin resistance from the pre-conception or early gestational period.
Despite lower levels of the six protective PCae in Hispanic women, the effect size of associations between the PCae and %BF in this subgroup was similar, implying that Hispanic ethnicity does not influence fetal programming of newborn body composition. Higher educational attainment and employment status were associated with higher concentrations of these six PCae, potentially due to better diet quality among women with higher socioeconomic status, as highlighted by the higher n-3 and n-6 dietary fatty acid intake of employed women in early pregnancy. In women with lower education, however, the concentration of the protective six PCae was too small to detect associations with %BF. This additionally emphasizes the importance of pre-conceptional factors in this fetal programming pathway for infant adiposity.
Our study has several strengths, including direct measurement of newborn adiposity by DXA scans, availability of detailed metabolic data at multiple gestational time points, and determination of molecular composition of specific lipid species. Most studies which focus on maternal metabolism and metabolites and its relation to offspring growth or body composition use birth weight (28, 35–37) or weight and length based proxy measures of body composition later in life as an outcome (16, 17, 19, 28, 29). Association studies with child body composition or growth parameters obtained after the perinatal period (i.e. after 1 month postnatal) can only draw limited conclusions about influences of the intra-uterine development, since postnatal conditions like infant feeding practices, occurrence of illnesses, medical treatments etc. may influence the offspring adipose tissue development. Furthermore, the already existing studies in this field commonly focus on fatty acid composition of TAG or PL. The presented results depict, for the first time, how different maternal metabolites involved in lipid- and energy metabolism influence the development of lean and fat tissue during intra-uterine life. However, there are also some limitations which should be considered in future studies. Firstly, due to our analytical approach, we may have missed potentially important metabolites such as ether-linked phosphatidylethanolamines or triacylglyceride species, and the applied techniques were also not able to distinguish between n-3 and n-6 fatty acids of the same chain-length and degree of saturation. Secondly, we do not have available relevant pre-pregnancy maternal data to complement our analysis. To help unravel the more nuanced mechanisms behind our results, it would be advisable that future studies obtain data relating to the pre-pregnancy period, including maternal diet and weight change. However, we found no associations between pre-pregnancy BMI and %BF in our confounder analysis. Although previous studies showed a positive association between these factors (38–40), a systematic review of diabetic mothers found no evidence of an association between pre-pregnancy BMI and infant adiposity (41). A potential reason is that extreme obesity was not included in the present study. McCarthy et al showed that a pre-pregnancy BMI >35 Kg/m2 resulted in 1 unit increase in %BF, while offspring of overweight and normal women had only minor difference in %BF (39).
In conclusion, this study demonstrates that maternal phospholipid status is related to newborn adiposity, which may have implications for childhood obesity risk. Phosphatidylcholines containing an ether-bond and 20:4n-6 appear to be protective against higher newborn %BF. In turn, these metabolites are especially affected by maternal sociodemographic, dietary and glycemic exposures in pre- or early pregnancy, highlighting the importance of pre-conceptional conditions and the potential for interventions to influence fetal development and neonatal body composition. PCae containing 22:6n-3 also exhibit a protective effect towards newborn adiposity. The role of ether-linked PL in fetal programming mechanisms may be underestimated and requires further clarification concerning its role in maternal, placental and fetal metabolism for developmental programming of offspring adiposity.
Supplementary Material
Acknowledgements
The work was support from the Commission of the European Communities (FP7–289346, H2020–633595, H2020-SC1–2016-RTD and ERC-2012-AdG–No.322605), the German Ministry of Education and Research (01 GI 0825), and the US National Institutes of Health (R01 HD-060628, R01 HD-065825, R01 MH-091351 and R21 RDK-098765).
Abbreviations:
- %BF
percent body fat
- AA
amino acids
- BMI
body mass index
- CA
carboxylic acid
- DXA
dual x-ray absorptiometry
- FDR
false discovery rate
- HOMA-IR
homeostasis model assessment of insulin resistance
- n-3
omega-3
- n-6
omega-6
- NEFA
non-esterified fatty acids
- PCaa
diacyl-phosphatidylcholine
- PCae
alkyl-linked phosphatidylcholine
- PCA
principle component analysis
- PL
phospholipid
- PUFA
polyunsaturated fatty acid
- SM
sphingomyelin
- UCI
University of California, Irvine
Footnotes
Conflict of interest
The authors have declared no conflict of interest.
References
- 1.Fuemmeler BF, Wang L, Iversen ES, Maguire R, Murphy SK, Hoyo C. Association between Prepregnancy Body Mass Index and Gestational Weight Gain with Size, Tempo, and Velocity of Infant Growth: Analysis of the Newborn Epigenetic Study Cohort. Childhood Obesity. 2016, 12, 210–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Samuelsson AM, Matthews PA, Argenton M, Christie MR, McConnell JM, Jansen EH, Piersma AH, Ozanne SE, Twinn DF, Remacle C, Rowlerson A, Poston L, Taylor PD. Diet-induced obesity in female mice leads to offspring hyperphagia, adiposity, hypertension, and insulin resistance: a novel murine model of developmental programming. Hypertension. 2008, 51, 383–392. [DOI] [PubMed] [Google Scholar]
- 3.Yu Z, Han S, Zhu J, Sun X, Ji C, Guo X. Pre-pregnancy body mass index in relation to infant birth weight and offspring overweight/obesity: a systematic review and meta-analysis. PLoS One. 2013, 8, e61627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Locke AE, Kahali B, Berndt SI, Justice AE, Pers TH, Day FR, Powell C, Vedantam S, Buchkovich ML, Yang J, Croteau-Chonka DC, Esko T, Fall T, Ferreira T, Gustafsson S, Kutalik Z, Luan J, Mägi R, Randall JC, Winkler TW, Wood AR, Workalemahu T, Faul JD, Smith JA, Zhao JH, Zhao W, Chen J, Fehrmann R, Hedman ÅK, Karjalainen J, Schmidt EM, Absher D, Amin N, Anderson D, Beekman M, Bolton JL, Bragg-Gresham JL, Buyske S, Demirkan A, Deng G, Ehret GB, Feenstra B, Feitosa MF, Fischer K, Goel A, Gong J, Jackson AU, Kanoni S, Kleber ME, Kristiansson K, Lim U, Lotay V, Mangino M, Leach IM, Medina-Gomez C, Medland SE, Nalls MA, Palmer CD, Pasko D, Pechlivanis S, Peters MJ, Prokopenko I, Shungin D, Stančáková A, Strawbridge RJ, Sung YJ, Tanaka T, Teumer A, Trompet S, van der Laan SW, van Setten J, Van Vliet-Ostaptchouk JV, Wang Z, Yengo L, Zhang W, Isaacs A, Albrecht E, Ärnlöv J, Arscott GM, Attwood AP, Bandinelli S, Barrett A, Bas IN, Bellis C, Bennett AJ, Berne C, Blagieva R, Blüher M, Böhringer S, Bonnycastle LL, Böttcher Y, Boyd HA, Bruinenberg M, Caspersen IH, Chen YI, Clarke R, Daw EW, de Craen AJM, Delgado G, Dimitriou M, Doney ASF, Eklund N, Estrada K, Eury E, Folkersen L, Fraser RM, Garcia ME, Geller F, Giedraitis V, Gigante B, Go AS, Golay A, Goodall AH, Gordon SD, Gorski M, Grabe HJ, Grallert H, Grammer TB, Gräßler J, Grönberg H, Groves CJ, Gusto G, Haessler J, Hall P, Haller T, Hallmans G, Hartman CA, Hassinen M, Hayward C, Heard-Costa NL, Helmer Q, Hengstenberg C, Holmen O, Hottenga JJ, James AL, Jeff JM, Johansson Å, Jolley J, Juliusdottir T, Kinnunen L, Koenig W, Koskenvuo M, Kratzer W, Laitinen J, Lamina C, Leander K, Lee NR, Lichtner P, Lind L, Lindström J, Lo KS, Lobbens S, Lorbeer R, Lu Y, Mach F, Magnusson PKE, Mahajan A, McArdle WL, McLachlan S, Menni C, Merger S, Mihailov E, Milani L, Moayyeri A, Monda KL, Morken MA, Mulas A, Müller G, Müller-Nurasyid M, Musk AW, Nagaraja R, Nöthen MM, Nolte IM, Pilz S, Rayner NW, Renstrom F, Rettig R, Ried JS, Ripke S, Robertson NR, Rose LM, Sanna S, Scharnagl H, Scholtens S, Schumacher FR, Scott WR, Seufferlein T, Shi J, Smith AV, Smolonska J, Stanton AV, Steinthorsdottir V, Stirrups K, Stringham HM, Sundström J, Swertz MA, Swift AJ, Syvänen AC, Tan ST, Tayo BO, Thorand B, Thorleifsson G, Tyrer JP, Uh HW, Vandenput L, Verhulst FC, Vermeulen SH, Verweij N, Vonk JM, Waite LL, Warren HR, Waterworth D, Weedon MN, Wilkens LR, Willenborg C, Wilsgaard T, Wojczynski MK, Wong A, Wright AF, Zhang Q; LifeLines Cohort Study, Brennan EP, Choi M, Dastani Z, Drong AW, Eriksson P, Franco-Cereceda A, Gådin JR, Gharavi AG, Goddard ME, Handsaker RE, Huang J, Karpe F, Kathiresan S, Keildson S, Kiryluk K, Kubo M, Lee JY, Liang L, Lifton RP, Ma B, McCarroll SA, McKnight AJ, Min JL, Moffatt MF, Montgomery GW, Murabito JM, Nicholson G, Nyholt DR, Okada Y, Perry JRB, Dorajoo R, Reinmaa E, Salem RM, Sandholm N, Scott RA, Stolk L, Takahashi A, Tanaka T, van ‘t Hooft FM, Vinkhuyzen AAE, Westra HJ, Zheng W, Zondervan KT; ADIPOGen Consortium; AGEN-BMI Working Group; CARDIOGRAMplusC4D Consortium; CKDGen Consortium; GLGC; ICBP; MAGIC Investigators; MuTHER Consortium; MIGen Consortium; PAGE Consortium; ReproGen Consortium; GENIE Consortium; International Endogene Consortium, Heath AC, Arveiler D, Bakker SJL, Beilby J, Bergman RN, Blangero J, Bovet P, Campbell H, Caulfield MJ, Cesana G, Chakravarti A, Chasman DI, Chines PS, Collins FS, Crawford DC, Cupples LA, Cusi D, Danesh J, de Faire U, den Ruijter HM, Dominiczak AF, Erbel R, Erdmann J, Eriksson JG, Farrall M, Felix SB, Ferrannini E, Ferrières J, Ford I, Forouhi NG, Forrester T, Franco OH, Gansevoort RT, Gejman PV, Gieger C, Gottesman O, Gudnason V, Gyllensten U, Hall AS, Harris TB, Hattersley AT, Hicks AA, Hindorff LA, Hingorani AD, Hofman A, Homuth G, Hovingh GK, Humphries SE, Hunt SC, Hyppönen E, Illig T, Jacobs KB, Jarvelin MR, Jöckel KH, Johansen B, Jousilahti P, Jukema JW, Jula AM, Kaprio J, Kastelein JJP, Keinanen-Kiukaanniemi SM, Kiemeney LA, Knekt P, Kooner JS, Kooperberg C, Kovacs P, Kraja AT, Kumari M, Kuusisto J, Lakka TA, Langenberg C, Marchand LL, Lehtimäki T, Lyssenko V, Männistö S, Marette A, Matise TC, McKenzie CA, McKnight B, Moll FL, Morris AD, Morris AP, Murray JC, Nelis M, Ohlsson C, Oldehinkel AJ, Ong KK, Madden PAF, Pasterkamp G, Peden JF, Peters A, Postma DS, Pramstaller PP, Price JF, Qi L, Raitakari OT, Rankinen T, Rao DC, Rice TK, Ridker PM, Rioux JD, Ritchie MD, Rudan I, Salomaa V, Samani NJ, Saramies J, Sarzynski MA, Schunkert H, Schwarz PEH, Sever P, Shuldiner AR, Sinisalo J, Stolk RP, Strauch K, Tönjes A, Trégouët DA, Tremblay A, Tremoli E, Virtamo J, Vohl MC, Völker U, Waeber G, Willemsen G, Witteman JC, Zillikens MC, Adair LS, Amouyel P, Asselbergs FW, Assimes TL, Bochud M, Boehm BO, Boerwinkle E, Bornstein SR, Bottinger EP, Bouchard C, Cauchi S, Chambers JC, Chanock SJ, Cooper RS, de Bakker PIW, Dedoussis G, Ferrucci L, Franks PW, Froguel P, Groop LC, Haiman CA, Hamsten A, Hui J, Hunter DJ, Hveem K, Kaplan RC, Kivimaki M, Kuh D, Laakso M, Liu Y, Martin NG, März W, Melbye M, Metspalu A, Moebus S, Munroe PB, Njølstad I, Oostra BA, Palmer CNA, Pedersen NL, Perola M, Pérusse L, Peters U, Power C, Quertermous T, Rauramaa R, Rivadeneira F, Saaristo TE, Saleheen D, Sattar N, Schadt EE, Schlessinger D, Slagboom PE, Snieder H, Spector TD, Thorsteinsdottir U, Stumvoll M, Tuomilehto J, Uitterlinden AG, Uusitupa M, van der Harst P, Walker M, Wallaschofski H, Wareham NJ, Watkins H, Weir DR, Wichmann HE, Wilson JF, Zanen P, Borecki IB, Deloukas P, Fox CS, Heid IM, O’Connell JR, Strachan DP, Stefansson K, van Duijn CM, Abecasis GR, Franke L, Frayling TM, McCarthy MI, Visscher PM, Scherag A, Willer CJ, Boehnke M, Mohlke KL, Lindgren CM, Beckmann JS, Barroso I, North KE, Ingelsson E, Hirschhorn JN, Loos RJF, Speliotes EK. Genetic studies of body mass index yield new insights for obesity biology. Nature. 2015, 518, 197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Entringer S, Buss C, Swanson JM, Cooper DM, Wing DA, Waffarn F, Wadhwa PD. Fetal programming of body composition, obesity, and metabolic function: the role of intrauterine stress and stress biology. J Nutr Metab. 2012, 2012, 632548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barker DJ. Fetal programming of coronary heart disease. Trends Endocrinol Metab. 2002, 13, 364–8. [DOI] [PubMed] [Google Scholar]
- 7.Godfrey KM, Barker DJ. Fetal programming and adult health. Pub Health Nutr. 2001, 4, 611–624. [DOI] [PubMed] [Google Scholar]
- 8.Oken E, Gillman MW. Fetal origins of obesity. Obes Res. 2003, 11, 496–506. [DOI] [PubMed] [Google Scholar]
- 9.Gillman MW, Ludwig DS. How early should obesity prevention start? N Engl J Med. 2013, 369, 2173–5. [DOI] [PubMed] [Google Scholar]
- 10.Silventoinen K, Jelenkovic A, Sund R, Hur YM, Yokoyama Y, Honda C, Hjelmborg J, Möller S, Ooki S, Aaltonen S, Ji F, Ning F, Pang Z, Rebato E, Busjahn A, Kandler C, Saudino KJ, Jang KL, Cozen W, Hwang AE, Mack TM, Gao W, Yu C, Li L, Corley RP, Huibregtse BM, Christensen K, Skytthe A, Kyvik KO, Derom CA, Vlietinck RF, Loos RJ, Heikkilä K, Wardle J, Llewellyn CH, Fisher A, McAdams TA, Eley TC, Gregory AM, He M, Ding X, Bjerregaard-Andersen M, Beck-Nielsen H, Sodemann M, Tarnoki AD, Tarnoki DL, Stazi MA, Fagnani C, D’Ippolito C, Knafo-Noam A, Mankuta D, Abramson L, Burt SA, Klump KL, Silberg JL, Eaves LJ, Maes HH, Krueger RF, McGue M, Pahlen S, Gatz M, Butler DA, Bartels M, van Beijsterveldt TC, Craig JM, Saffery R, Freitas DL, Maia JA, Dubois L, Boivin M, Brendgen M, Dionne G, Vitaro F, Martin NG, Medland SE, Montgomery GW, Chong Y, Swan GE, Krasnow R, Magnusson PK, Pedersen NL, Tynelius P, Lichtenstein P, Haworth CM, Plomin R, Bayasgalan G, Narandalai D, Harden KP, Tucker-Drob EM, Öncel SY, Aliev F, Spector T, Mangino M, Lachance G, Baker LA, Tuvblad C, Duncan GE, Buchwald D, Willemsen G, Rasmussen F, Goldberg JH, Sørensen TI, Boomsma DI, Kaprio J. Genetic and environmental effects on body mass index from infancy to the onset of adulthood: an individual-based pooled analysis of 45 twin cohorts participating in the COllaborative project of Development of Anthropometrical measures in Twins (CODATwins) study. Am J Clin Nutr. 2016, 104, 371–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rauschert S, Kirchberg FF, Marchioro L, Koletzko B, Hellmuth C, Uhl O. Early Programming of Obesity Throughout the Life Course: A Metabolomics Perspective. Ann Nutr Metab. 2017, 70, 201–209. [DOI] [PubMed] [Google Scholar]
- 12.Hivert MF, Perng W, Watkins SM, Newgard CS, Kenny LC, Kristal BS, Patti ME, Isganaitis E, DeMeo DL, Oken E, Gillman MW. Metabolomics in the developmental origins of obesity and its cardiometabolic consequences. J Dev Origins Health Dis. 2015, 6, 65–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brei C, Stecher L, Much D, Karla MT, Amann-Gassner U, Shen J, Ganter C, Karampinos DC, Brunner S, Hauner H. Reduction of the n-6:n-3 long-chain PUFA ratio during pregnancy and lactation on offspring body composition: follow-up results from a randomized controlled trial up to 5 y of age. Am J Clin Nutr. 2016, 103, 1472–1481. [DOI] [PubMed] [Google Scholar]
- 14.Muhlhausler BS, Yelland LN, McDermott R, Tapsell L, McPhee A, Gibson RA, Makrides M. DHA supplementation during pregnancy does not reduce BMI or body fat mass in children: follow-up of the DHA to Optimize Mother Infant Outcome randomized controlled trial. Am J Clin Nutr. 2016, 103, 1489–1496. [DOI] [PubMed] [Google Scholar]
- 15.Tanentsapf I, Heitmann BL, Adegboye AR. Systematic review of clinical trials on dietary interventions to prevent excessive weight gain during pregnancy among normal weight, overweight and obese women. BMC Pregnancy Childbirth. 2011, 11, 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Vries PS, Gielen M, Rizopoulos D, Rump P, Godschalk R, Hornstra G, Zeegers MP. Association between polyunsaturated fatty acid concentrations in maternal plasma phospholipids during pregnancy and offspring adiposity at age 7: The MEFAB cohort. Prostaglandins, Leukot Essent Fatty Acids.. 2014, 91, 81–85. [DOI] [PubMed] [Google Scholar]
- 17.Donahue SM, Rifas-Shiman SL, Gold DR, Jouni ZE, Gillman MW, Oken E. Prenatal fatty acid status and child adiposity at age 3 y: results from a US pregnancy cohort. Am J Clin Nutr. 2011, 93, 780–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moon RJ, Harvey NC, Robinson SM, Ntani G, Davies JH, Inskip HM, Godfrey KM, Dennison EM, Calder PC, Cooper C; SWS Study Group. Maternal plasma polyunsaturated fatty acid status in late pregnancy is associated with offspring body composition in childhood. J Clin Endocrinol Metab. 2013, 98, 299–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vidakovic AJ, Gishti O, Voortman T, Felix JF, Williams MA, Hofman A, Demmelmair H, Koletzko B, Tiemeier H, Jaddoe VW, Gaillard R. Maternal plasma PUFA concentrations during pregnancy and childhood adiposity: the Generation R Study. Am J Clin Nutr. 2016, 103, 1017–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lindsay KL, Hellmuth C, Uhl O, Buss C, Wadhwa PD, Koletzko B, Entringer S. Longitudinal Metabolomic Profiling of Amino Acids and Lipids across Healthy Pregnancy. PLoS One. 2015, 10, e0145794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hellmuth C, Lindsay KL, Uhl O, Buss C, Wadhwa PD, Koletzko B, Entringer S. Association of maternal prepregnancy BMI with metabolomic profile across gestation. Int J Obes (Lond). 2017, 41, 159–169. [DOI] [PubMed] [Google Scholar]
- 22.Rodriguez-Bernal CL, Rebagliato M, Iniguez C, Vioque J, Navarrete-Munoz EM, Murcia M, Bolumar F, Marco A, Ballester F. Diet quality in early pregnancy and its effects on fetal growth outcomes: the Infancia y Medio Ambiente (Childhood and Environment) Mother and Child Cohort Study in Spain. Am J Clin Nutr. 2010, 91, 1659–1666. [DOI] [PubMed] [Google Scholar]
- 23.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985, 28, 412–419. [DOI] [PubMed] [Google Scholar]
- 24.Toro-Ramos T, Paley C, Pi-Sunyer FX, Gallagher D. Body composition during fetal development and infancy through the age of 5 years. Eur J Clin Nutr. 2015, 69, 1279–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bejamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J Royal Stat Soc. 1995, 57, 289–300. [Google Scholar]
- 26.Uhl O, Glaser C, Demmelmair H, Koletzko B. Reversed phase LC/MS/MS method for targeted quantification of glycerophospholipid molecular species in plasma. J Chromatogr B Analyt Technol Biomed Life Sci. 2011, 879, 3556–3564. [DOI] [PubMed] [Google Scholar]
- 27.Sandler V, Reisetter AC, Bain JR, Muehlbauer MJ, Nodzenski M, Stevens RD, Ilkayeva O, Lowe LP, Metzger BE, Newgard CB, Scholtens DM, Lowe WL Jr; HAPO Study Cooperative Research Group. Associations of maternal BMI and insulin resistance with the maternal metabolome and newborn outcomes. Diabetologia. 2017, 60, 518–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu Y-P, Reichetzeder C, Prehn C, Yin L-H, Yun C, Zeng S, Chu C, Adamski J, Hocher B. Cord blood lysophosphatidylcholine 16:1 is positively associated with birth weight. Cell Physiol Biochem. 2018, 45, 614–624. [DOI] [PubMed] [Google Scholar]
- 29.Moco S, Collino S, Rezzi S, Martin FP. Metabolomics perspectives in pediatric research. Pediatr Res. 2013, 73, 570–576. [DOI] [PubMed] [Google Scholar]
- 30.Standl M, Thiering E, Demmelmair H, Koletzko B, Heinrich J. Age-dependent effects of cord blood long-chain PUFA composition on BMI during the first 10 years of life. Br J Nutr. 2014, 1–8. [DOI] [PubMed] [Google Scholar]
- 31.Massiera F, Barbry P, Guesnet P, Joly A, Luquet S, Moreilhon-Brest C, Mohsen-Kanson T, Amri EZ, Ailhaud G. A Western-like fat diet is sufficient to induce a gradual enhancement in fat mass over generations. J Lipid Res. 2010, 51, 2352–2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hellmuth C, Demmelmair H, Schmitt I, Peissner W, Bluher M, Koletzko B. Association between Plasma Nonesterified Fatty Acids Species and Adipose Tissue Fatty Acid Composition. PLoS One. 2013, 8, e74927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.da Silva TF, Sousa VF, Malheiro AR, Brites P. The importance of ether-phospholipids: a view from the perspective of mouse models. Biochim Biophys Acta. 2012, 1822, 1501–1508. [DOI] [PubMed] [Google Scholar]
- 34.Braverman NE, Moser AB. Functions of plasmalogen lipids in health and disease. Biochim Biophys Acta. 2012, 1822, 1442–1452. [DOI] [PubMed] [Google Scholar]
- 35.Elias SL, Innis SM. Infant plasma trans, n-6, and n-3 fatty acids and conjugated linoleic acids are related to maternal plasma fatty acids, length of gestation, and birth weight and length. Am J Clin Nutr. 2001, 73, 807–814. [DOI] [PubMed] [Google Scholar]
- 36.Rump P, Mensink RP, Kester AD, Hornstra G. Essential fatty acid composition of plasma phospholipids and birth weight: a study in term neonates. Am J Clin Nutr. 2001, 73, 797–806. [DOI] [PubMed] [Google Scholar]
- 37.van Eijsden M, Hornstra G, van der Wal MF, Vrijkotte TG, Bonsel GJ. Maternal n-3, n-6, and trans fatty acid profile early in pregnancy and term birth weight: a prospective cohort study. Am J Clin Nutr. 2008, 87, 887–895. [DOI] [PubMed] [Google Scholar]
- 38.Starling AP, Brinton JT, Glueck DH, Shapiro AL, Harrod CS, Lynch AM, Siega-Riz AM, Dabelea D. Associations of maternal BMI and gestational weight gain with neonatal adiposity in the Healthy Start study. Am J Clin Nutr. 2015, 101, 302–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McCarthy FP, Khashan AS, Murray D, Kiely M, Hourihane JO, Pasupathy D, Kenny LC; SCOPE Ireland Cohort study and the Cork BASELINE Birth Cohort Study. Parental physical and lifestyle factors and their association with newborn body composition. Br J Obstet Gynecol. 2016, 123, 1824–1829. [DOI] [PubMed] [Google Scholar]
- 40.Breij LM, Steegers-Theunissen RP, Briceno D, Hokken-Koelega AC. Maternal and Fetal Determinants of Neonatal Body Composition. Horm Res Paediatr. 2015, 84, 388–395. [DOI] [PubMed] [Google Scholar]
- 41.Logan KM, Gale C, Hyde MJ, Santhakumaran S, Modi N. Diabetes in pregnancy and infant adiposity: systematic review and meta-analysis. Arch Dis Child Fetal Neonatal Ed. 2017, 102, F65–F72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.