Abstract
Introduction:
Renal transplant outcomes result from a combination of factors. Traditionally, donor factors were summarized by classifying kidneys as extended criteria or standard criteria. In 2014, the nomenclature changed to describe donor factors with the kidney donor profile index (KDPI). We aim to evaluate the relationship between KDPI and delayed graft function (DGF), and the impact KDPI on transplant outcomes for both donor after cardiac death (DCD) and donor after brain death (DBD).
Methods:
An IRB-approved single-center retrospective chart review was performed from January 1999 to July 2013. The patients were divided into six groups: DBD KDPI ≤60, DBD KPDI 61–84, DBD KDPI ≥85, DCD KDPI ≤60, DCD KPDI 61–84, and DCD KDPI ≥85. Rates of DGF, patient survival, and graft survival were examined among groups.
Results:
A total of 2161 kidney transplants were included. DGF rates increased, and graft and patient survival decreased with increasing KDPI (P < .001). DCD kidneys had higher DGF rates than their DBD counterparts (P < .001). In DCD kidneys, a higher KDPI score did not significantly affect the DGF rates (P > .302). There was no significant difference in graft or patient survival in all-comers when comparing DCD and DBD kidneys with equivalent KDPIs (P > .317). Patients with DGF across all categories demonstrated worse graft half-lives.
Conclusion:
The KDPI system is an accurate predictor of donor contributions to transplant outcomes. Recipients of DBD kidneys experience an increase in the rate of DGF as their KDPI increases. DCD kidneys have higher DGF rates than their DBD counterparts with similar KDPIs. Patients with documented post-transplant DGF had between 3- and 5-year shorter graft half-lives when compared to recipients that did not experience DGF. Initiatives to reduce the rate of DGF could provide a significant impact on graft survival and result in a reduction in the number of patients requiring retransplant.
Keywords: classification systems, delayed graft function, donors and donation: deceased, graft survival, DGF, KDPI, kidney donor profile index, kidney transplant
1 |. INTRODUCTION
In 2012, there were approximately 93 000 renal patients on the organ waitlist. Unfortunately, given the shortage of suitable organs for transplantation, 5200 renal patients die each year awaiting organs.1 One solution to our organ shortage is the utilization of lower quality donor kidneys.
Historically, donor kidney quality was denoted by a dichotomous classification system whereby a kidney was identified as either standard criteria donor (SCD) or extended criteria donor (ECD). ECD kidneys were defined as those coming from a donor ≥60 years old or a donor ≥50 years in which the donor met at least two of the following criteria: serum creatinine >1.5, death by cerebrovascular accident, or history of hypertension.2 To improve upon the ECD/SCD classification, a new kidney donor profile index (KDPI) system was created in 2014 to give more precise information on donor kidney quality. This system is based on a Cox proportional hazards regression model from Organ Procurement and Transplantation Network (OPTN) data.2 The kidney donor risk index (KDRI) assigns each kidney a continuous risk score between 1 and 100 based on 10 donor characteristics: age, height, weight, ethnicity, history of hypertension or diabetes, cause of death, serum creatinine, hepatitis C virus (HCV), and donation after cardiac death (DCD).2 This number is then converted to a percentage called KDPI. Kidneys with KDPIs ≥85 have been characterized in the literature as having similar donor characteristics as an ECD kidney.3 Although the purpose of the KDPI system was to give precise information regarding potential for graft failure,4 little in the literature is known about the impact of KDPI on rates of delayed graft function (DGF) or the impact of DGF on graft survival for kidneys of varying KDPIs.
When designing the study, we divided the subgroups not simply by KDPI, but also with regard to donor type. Even though DCD status is a component of the KDPI equation, many clinicians discuss DCD status as a risk factor separate from KPDI classification. By understanding transplant outcomes based on both donor type and KDPI, we can accurately advise patients on their individual long-term results. The aim of our study was to better understand how increasing KDPI affects transplant outcomes, specifically, rates of DGF, graft survival for both DGF and non-DGF kidneys, patient survival, and graft half-life.
2 |. METHODS
2.1 |. Patients
Following institutional review board approval, a retrospective chart review was conducted of 2161 patients receiving kidney transplantation at University of Wisconsin between 1999 and 2013. Eligibility requirements included: recipient age >18 and single organ kidney transplantation from a deceased donor. Patients were excluded if they received living donor transplants, kidney-pancreas transplants, and dual kidney transplants or if KDPI could not be calculated based on donor information available.
2.2 |. KDPI calculations
All KDPI scores were calculated using the OPTN calculation formula.5 In this calculation, 10 donor characteristics (age, height, weight, ethnicity, history of hypertension, history of diabetes, cause of death, creatinine, HCV status, and DCD status) are multiplied by their beta coefficient (Table 1) and summed to determine a XBETA. The KDRI_RAO is calculated by taking the exp(XBETA). The KDRI_ MEDIAN is calculated by dividing by the KDRI_RAO by a scaling factor provided by the OPTN based on the kidney donors recovered from the previous year. Finally, using the KDRI to KDPI mapping table provided by the OPTN, all KDPIs were derived from the calculated KDRIs.
TABLE 1.
KDRI donor factors and model coefficients
| Donor characteristic | Applies to: | KDRI coefficient (“Beta”) | KDRI “XBETA” component |
|---|---|---|---|
| Age (y) | All donors | .0128 | .0128*(age-40) |
| Donors with age ≤18 | −.0194 | −.0194*(age-18) | |
| Donors with age >50 | .0107 | .0107*(age-50) | |
| Height (cm) | All donors | −.0464 | −.0464*(hgt-170)/10 |
| Weight (kg) | All donors w/weight ≤80 kg | −.0199 | −.0199*(wgt-80)/5 |
| Ethnicity | African American donors | .1790 | .1790 |
| History of hypertension | Hypertensive donors | .1260 | .1260 |
| History of diabetes | Diabetic donors | .1300 | .1300 |
| Serum creatinine | All donors | .2200 | .2200*(creat-1) |
| Donors with creat >1.5 mg/dL | −.2090 | −.2090*(creat-1.5) | |
| HCV status | HCV-positive donors | .2400 | .2400 |
| Donor after cardiac death status | DCD donors | .1330 | .1330 |
DCD, donor after cardiac death; HCV, hepatitis C virus; KDRI, kidney donor risk index.
2.3 |. Study groups
Once KDPIs were determined for all patients, they were divided into 3 groups based on presumed risk. Low-risk, high-quality kidneys were defined as KDPIs ≤60. The moderate-risk, moderate-quality group consisted of kidneys with KDPIs between 61 and 84. The high-risk, poor-quality kidney group consisted of kidneys with KDPIs ≥85. Clinically, it is well documented that differences exist between a donor after brain death (DBD) and donor after cardiac death (DCD) kidney related to warm ischemia time.6,7 As a result, our three groups were further subdivided by type of deceased donor. Our final six comparison groups consisted of 1) DBD kidneys with KDPI ≤60, 2) DBD kidneys with KDPI 61–84, 3) DBD kidneys with KDPI ≥85, 4) DCD kidneys with KDPI ≤60, 5) DCD kidneys with KDPI 61–84, and 6) DCD kidneys with KDPI ≥85.
2.4 |. Primary and secondary outcomes
The primary outcome measured was rates of DGF and the effect of DGF on graft survival for kidneys of differing KDPIs. In addition to determining the frequency of DGF in each group, we also assessed severity by determining the number of hemodialysis (HD) treatments in the post-transplant period. Our secondary outcomes were overall death-censored graft survival and patient survival using Kaplan-Meier survival analysis. Additionally, Cox proportional hazards regression analysis was performed to determine the association between death-censored graft survival in our respective six groups after controlling for transplant number, cold time, recipient and donor sex, pretransplant dialysis, induction, and recipient age.
All transplant outcomes were first analyzed between all six groups to determine trends. Next, the groups were compared to each other. First, all DBD kidneys with varying KDPIs were examined. Second, all DCD kidneys with varying KDPIs were examined. Third, DCD and DBD kidneys with equivalent KDPIs were analyzed. Using Cox proportional hazards regression and logistic regression analysis, odds ratio (OR) and hazard ratio (HR) for DGF and death-censored graft failure were calculated comparing each group to the most common kidney transplanted (DBD KDPI ≤60). In these models, transplant number, cold time, recipient and donor sex, pretransplant dialysis, induction, and recipient age were controlled for, given that we saw statistically significant differences in these factors when recipient and donor demographics were examined. We did not control for donor age, BMI, race, and cause of death in our models as these were components of the KDPI equation. We finally compare the impact of DGF on the graft half-life of the transplanted kidney among the same donor types with similar KDPIs. To determine graft half-life, graft loss was defined according to patient death, graft removal, or return to dialysis based on actuarial rates.
2.5 |. Statistics
Statistical analysis was performed with SAS software, and P-values ≤ .05 were considered significant. Univariate analysis was performed to identify potential risk factors leading to poor transplant outcomes. Factors found to be significant in univariate analysis were used to construct a multivariate model. Group comparisons were performed using Student’s t tests and chi-squared analysis. Patient and graft survival were estimated by Kaplan-Meier analysis.
3 |. RESULTS
A total of 2161 kidney transplant patients were included in our analysis. A little over half (N = 1118) of the organs transplanted were DBD KDPI 0–60 kidneys. The next most common subgroups were DBD KDPI 61–84 (N = 328) and DCD KDPI 0–60 (N-328). The DBD KDPI ≥85 and DCD KDPI 61–84 groups consisted of 130 and 163 patients, respectively. The least utilized kidneys were from DCD donors with KDPI ≥85 (N = 38).
3.1 |. Recipient demographics
When examining recipient demographics (Table 2), there was no statistical difference between the BMI, race, or number of months of pretransplant dialysis of the patients receiving kidneys from different groups. Recipient patient age was found to be statistically different (P ≤ .001) with the DBD and DCD kidneys with KDPIs ≤60 being allocated to the youngest patients at 48.4 ± 13.0 years and 50.2 ± 12.2 years, respectively. In contrast, the DBD and DCD kidneys with KDPI ≥85 were allocated to the oldest patients with mean ages of 60.6 ± 9.0 years and 61.0 ± 8.7 years. There were also differences in recipient gender, type of induction therapy, and etiology of chronic kidney disease (CKD) among the groups. The percentage of patients on dialysis prior to transplant was highest in the DCD KDPI 61–84 and DCD KDPI ≥85 groups at 90.8% and 94.7%. The number of prior transplants differed with the DBD KDPI ≤60 and DBD KDPI 61–84 groups having the highest number of patients receiving their third or greater kidney transplant. Warm ischemia time decreased with increasing KDPI in DCD kidney recipients (P = .04). Finally, the cold ischemia time differed (P ≤ .001) with the DBD kidneys averaging between 18.7 and 19.4 hours and the DCD kidneys averaging between 14.5 and 15.4 hours.
TABLE 2.
Recipient demographics
| DBD KDPI 0–60 N = 1118 (%) |
DBD KDPI 61–84 N = 384 (%) |
DBD KDPI >85 N = 130 (%) |
DCD KDPI 0–60 N = 328 (%) |
DCD KDPI 61–84 N = 163 (%) |
DCD KDPI >85 N = 38 (%) |
P value | |
|---|---|---|---|---|---|---|---|
| Patient age (y) | 48.4 ± 13.0 | 55.1 ± 11.7 | 60.6 ± 9.0 | 50.2 ± 12.2 | 54.1 ± 11.1 | 61.0 ± 8.7 | ≤.001 |
| Patient BMI | 27.6 ± 5.3 | 27.8 ± 5.4 | 26.5 ± 4.6 | 28.1 ± 5.3 | 27.3 ± 5.5 | 27.3 ± 5.5 | .079 |
| Recipient gender | |||||||
| Female | 470 (42) | 125 (33) | 51 (39) | 140 (43) | 52 (32) | 18 (47) | .004 |
| Male | 648 (58) | 259 (67) | 79 (61) | 188 (57) | 111 (68) | 20 (53) | |
| Recipient race | |||||||
| Native American | 20 (1.8) | 5 (1.3) | 0 (0) | 6 (1.8) | 3 (3.4) | 0 (0) | .358 |
| Asian | 82 (7.3) | 17 (4.4) | 9 (6.9) | 35 (10.7) | 15 (9.2) | 4 (10.5) | |
| Black | 142 (12.8) | 42 (10.9) | 13 (10) | 49 (14.9) | 20 (12.3) | 4 (10.5) | |
| Unknown | 4 (0.4) | 0 (0) | 0 (0) | 1 (0.3) | 0 (0) | 0 (0) | |
| White | 869 (77.8) | 320 (83.3) | 108 (83.1) | 237 (72.3) | 122 (74.9) | 30 (79) | |
| Etiology of chronic kidney disease | |||||||
| Obstructive nephropathy | 56 (5.0) | 19 (4.9) | 3 (2.3) | 12 (3.7) | 0 (0) | 2 (5.3) | ≤.001 |
| Drug toxin | 30 (2.7) | 18 (4.7) | 4 (3.1) | 13 (4.0) | 4 (2.5) | 0 (0) | |
| Glomerulonephritis | 123 (11.0) | 42 (10.9) | 14 (10.8) | 17 (5.2) | 8 (4.9) | 1 (2.6) | |
| Glomerulosclerosis | 89 (8) | 19 (4.9) | 11 (8.5) | 28 (8.5) | 10 (6.1) | 4 (10.5) | |
| Diabetes | 269 (24.1) | 109 (28.4) | 30 (23.1) | 74 (22.6) | 53 (32.5) | 11 (28.9) | |
| Hypertension | 132 (11.8) | 61 (15.9) | 21 (16.2) | 40 (12.2) | 20 (12.3) | 4 (10.5) | |
| IgA nephropathy | 55 (4.9) | 11 (2.9) | 4 (3.1) | 17 (5.2) | 8 (4.9) | 3 (7.9) | |
| Cancer | 5 (0.4) | 3 (0.8) | 3 (2.3) | 4 (1.2) | 1 (0.6) | 0 (0) | |
| Cystic kidney disease | 125 (11.2) | 37 (9.6) | 18 (13.8) | 54 (16.5) | 26 (16.0) | 5 (13.2) | |
| Lupus | 50 (4.5) | 13 (3.4) | 2 (1.5) | 12 (3.7) | 4 (2.5) | 1 (2.6) | |
| Other | 184 (16.5) | 52 (13.5) | 20 (15.4) | 57 (17.4) | 29 (17.8) | 7 (18.4) | |
| Pretransplant dialysis | |||||||
| Yes | 937 (83.8) | 345 (89.8) | 112 (86.2) | 280 (85.4) | 148 (90.8) | 36 (94.7) | .011 |
| No | 181 (16.2) | 39 (10.2) | 18 (13.8) | 48 (14.6) | 15 (9.2) | 2 (5.3) | |
| Pretransplant dialysis (mo) | 28.1 ± 29.3 | 28.1 ± 24.7 | 26.9 ± 23.1 | 28.8 ± 27.3 | 29.7 ± 30.2 | 29.7 ± 21.9 | .963 |
| Transplant number | |||||||
| 1 | 813 (72.7) | 321 (83.6) | 116 (89.2) | 273 (83.2) | 132 (81.0) | 32 (84.2) | .004 |
| 2 | 245 (21.9) | 50 (13.0) | 14 (10.8) | 46 (14.0) | 25 (15.3) | 5 (13.2) | |
| 3 | 50 (4.5) | 10 (2.6) | 0 (0) | 9 (2.7) | 6 (3.7) | 1 (2.6) | |
| 4 | 7 (0.6) | 3 (0.8) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| 5 | 3 (0.3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Induction therapy | |||||||
| ATG | 17 (1.6) | 7 (1.9) | 1 (0.8) | 0 (0) | 0 (0) | 0 (0) | .007 |
| Campath | 227 (20.9) | 97 (25.7) | 36 (28.1) | 48 (14.7) | 34 (20.9) | 14 (36.8) | |
| OKT3 | 3 (0.3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Rituximab | 9 (0.8) | 1 (0.3) | 2 (1.6) | 2 (0.6) | 0 (0) | 0 (0) | |
| Simulect | 643 (59.2) | 212 (56.2) | 75 (58.6) | 221 (67.6) | 96 (58.9) | 22 (57.9) | |
| Thymoglobulin | 187 (17.2) | 60 (15.9) | 13 (10.2) | 56 (17.3) | 33 (20.3) | 2 (5.3) | |
| Zenpax | 0 (0) | 0 (0) | 1 (0.8) | 0 (0) | 0 (0) | 0 (0) | |
| Cold time (h) | 19.4 ± 6.3 | 18.7 ± 7.2 | 19.4 ± 6.1 | 15.4 ± 6.4 | 15.1 ± 6.6 | 14.5 ± 5.7 | ≤.001 |
| Warm ischemia time (min) | n/a | n/a | n/a | 34.2 ± 25.2 | 30.45 ± 18.1 | 25.95 ± 10.1 | .04 |
DBD, donor after brain death; DCD, donor after cardiac death; KDPI, kidney donor profile index.
3.2 |. Donor demographics
We noted statistically significant differences in age, BMI, race, and cause of death among our groups. We also noted differences in donor gender, cytomegalovirus status, and Epstein-Barr virus status (Table 3).
TABLE 3.
Donor demographics
| DBD KDPI 0–60 N = 1118 (%) |
DBD KDPI 61–84 N = 384 (%) |
DBD KDPI >85 N = 130 (%) |
DCD KDPI 0–60 N = 328 (%) |
DCD KDPI 61–84 N = 163 (%) |
DCD KDPI >85 N = 38 (%) |
P value | |
|---|---|---|---|---|---|---|---|
| Donor age (y) | 35.1 ± 13.6 | 56.4 ± 8.8 | 65.1 ± 5.6 | 38.6 ± 11.3 | 54.0 ± 6.9 | 62.8 ± 3.5 | ≤.001 |
| Donor BMI | 27.4 ± 7.0 | 29.1 ± 6.7 | 27.5 ± 6.3 | 28.0 ± 7.1 | 30.5 ± 8.1 | 29.8 ± 6.0 | ≤.001 |
| Donor gender | |||||||
| Female | 397 (36) | 207 (54) | 70 (54) | 87 (27) | 51 (31) | 21 (55) | ≤.001 |
| Male | 721 (64) | 177 (46) | 60 (46) | 241 (73) | 112 (69) | 17 (45) | |
| Donor race | |||||||
| Native American | 5 (0.5) | 0 (0) | 0 (0) | 4 (1.2) | 0 (0) | 0 (0) | ≤.001 |
| Asian | 8 (0.7) | 2 (0.5) | 6 (4.6) | 4 (1.2) | 0 (0) | 0 (0) | |
| Black | 29 (2.6) | 19 (5) | 12 (9.2) | 0 (0) | 1 (0.6) | 0 (0) | |
| Hispanic | 44 (3.9) | 6 (1.5) | 2 (1.5) | 4 (1.2) | 0 (0) | 0 (0) | |
| Multiracial | 2 (0.2) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| White | 1030 (92) | 357 (93) | 110 (84.6) | 316 (96.3) | 162 (99.4) | 38 (100) | |
| Donor cause of death | |||||||
| Trauma/Burns | 550 (49) | 63 (16.4) | 9 (6.9) | 133 (40.5) | 35 (21.4) | 2 (5.3) | ≤.001 |
| Anoxic injury | 245 (22) | 40 (10.4) | 11 (4.5) | 146 (44.5) | 52 (31.9) | 8 (2.1) | |
| Respiratory failure | 0 (0) | 0 (0) | 0 (0) | 2 (0.6) | 3 (1.2) | 0 (0) | |
| CNS infection | 17 (1.5) | 1 (0.3) | 0 (0) | 0 (0) | 2 (1.2) | 0 (0) | |
| Stroke | 290 (26) | 276 (71.9) | 110 (84.6) | 42 12.8) | 52 (31.9) | 28 (73.7) | |
| CNS tumor | 13 (1.2) | 4 (10.4) | 0 (0) | 5 (1.5) | 4 (2.5) | 0 (0) | |
| Encephalopathy | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 3 (1.8) | 0 (0) | |
| Other | 3 (0.3) | 0 (0) | 0 (0) | 0 (0) | 13 (8.0) | 0 (0) | |
| CMV donor | |||||||
| Negative | 466 (41.6) | 161 (41.9) | 49 (37.7) | 128 (39.0) | 71 (43.6) | 12 (31.6) | .741 |
| Positive | 649 (58.1) | 223 (58.1) | 81 (62.3) | 198 (60.4) | 1 (0.6) | 0 (0) | |
| Unknown | 3 (0.3) | 0 (0) | 0 (0) | 2 (0.6) | 91 (55.8) | 26 (68.4) | |
| EBV donor | |||||||
| Negative | 1 (4.6) | 18 (4.7) | 5 (3.9) | 15 (4.6) | 7 (4.3) | 3 (7.9) | .136 |
| Positive | 1032 (92.3) | 358 (93.2) | 4 (3) | 298 (90.8) | 152 (93.3) | 34 (89.5) | |
| Unknown | 35 (3.1) | 8 (2.08) | 121 (93.1) | 15 (4.6) | 4 (2.4) | 1 (2.6) | |
DBD, donor after brain death; DCD, donor after cardiac death; KDPI, kidney donor profile index.
3.3 |. Transplant outcomes
All transplant outcomes were compared first between all six groups (Table 4) and then groups were compared to each other (Table 5). Using Cox proportional hazards regression and logistic regression analysis, OR and HR for DGF and graft failure were calculated comparing each group to DBD KDPI ≤60 kidneys (Table 6).
TABLE 4.
Outcomes: transplant outcomes by DBD vs DCD status and KDPI
| DBD KDPI 0–60 N = 1118 |
DBD KDPI 61–84 N = 384 |
DBD KDPI >85 N = 130 |
DCD KDPI 0–60 N = 328 |
DCD KDPI 61–84 N = 163 |
DCD KDPI >85 N = 38 |
P value | |
|---|---|---|---|---|---|---|---|
| Post-transplant DGF (%) | 209 (18.7) | 111 (29) | 41 (31.5) | 148 (45.1) | 80 (49.1) | 21 (55.3) | ≤.001 |
| Graft survival at 5 y, % | 74.6 | 62.7 | 48.6 | 77.7 | 61.1 | 54.2 | ≤.001 |
| Patient survival at 5 y, % | 85.8 | 76.3 | 63.4 | 86.4 | 75.0 | 59.5 | ≤.001 |
DBD, donor after brain death; DCD, donor after cardiac death; DGF, delayed graft function; KDPI, kidney donor profile index.
TABLE 5.
Outcomes: transplant outcomes by KDPI, group-wise comparison
| DBD KDPI 0–60 N = 1118 |
DBD KDPI 61–84 N = 384 |
P value | DBD KDPI 61–84 N = 163 |
DBD KDPI ≥85 N = 130 |
P value | DBD KDPI 0–60 N = 1118 |
DBD KDPI ≥85 N = 130 |
P value | |
|---|---|---|---|---|---|---|---|---|---|
| DGF, % | 18.7 | 29 | ≤.001 | 29 | 31.5 | .580 | 18.7 | 31.5 | ≤.001 |
| Graft survival at 5 y, % | 74.6 | 62.7 | ≤.001 | 62.7 | 48.6 | .005 | 74.6 | 48.6 | ≤.001 |
| Patient survival at 5 y, % | 85.8 | 76.3 | ≤.001 | 76.3 | 63.4 | .030 | 85.8 | 63.4 | ≤.001 |
| DCD KDPI 0–60 N = 328 |
DCD KDPI 61–84 N = 163 |
P value | DCD KDPI 61– 84 N = 163 |
DCD KDPI ≥85 N = 38 |
P value | DCD KDPI 0–60 N = 328 |
DCD KDPI ≥85 N = 38 |
P value | |
| DGF, % | 45.1 | 49.1 | .442 | 49.1 | 55.3 | .302 | 45.1 | 55.3 | .590 |
| Graft survival at 5 y, % | 77.7 | 61.1 | ≤.001 | 61.1 | 54.2 | .509 | 77.7 | 54.2 | .002 |
| Patient survival at 5 y, % | 86.4 | 75.0 | .003 | 75.0 | 59.5 | .177 | 86.4 | 59.5 | .002 |
| DBD KDPI 0–60 N = 1118 |
DCD KDPI 0–60 N = 328 |
P value | DBD KDPI 61–84 N = 384 |
DCD KDPI 61–84 N = 163 |
P value | DBD KDPI ≥85 N = 130 |
DCD KDPI ≥85 N = 38 |
P value | |
| DGF, % | 18.7 | 45.1 | ≤.001 | 29 | 49.1 | ≤.001 | 31.5 | 55.3 | .007 |
| Graft survival at 5 y, % | 74.6 | 77.7 | .317 | 62.7 | 61.1 | .982 | 48.6 | 54.2 | .407 |
| Patient survival at 5 y, % | 85.8 | 86.4 | .604 | 76.3 | 75.0 | .819 | 63.4 | 59.5 | .987 |
DBD, donor after brain death; DCD, donor after cardiac death; DGF, delayed graft function; KDPI, kidney donor profile index.
TABLE 6.
Outcomes: transplant outcomes logistic regression model
| Group | DGF |
Graft failure |
||||
|---|---|---|---|---|---|---|
| Odds ratio | 95% CI | P value | Hazard ratio | 95% CI | P value | |
| DBD KDPI 0–60 | 1 | n/a | n/a | 1 | n/a | n/a |
| DBD KDPI 61–84 | 1.806 | 1.380–2.364 | ≤.0001 | 1.588 | 1.356–1.860 | ≤.0001 |
| DBD KDPI ≥85 | 2.050 | 1.372–3.063 | ≤.0001 | 2.270 | 1.822–2.828 | ≤.0001 |
| DCD KDPI 0–60 | 4.115 | 3.126–5.418 | .0005 | 0.935 | 0.754–1.161 | .5447 |
| DCD KDPI 61–84 | 4.834 | 3.401–6.871 | ≤.0001 | 1.636 | 1.297–2.063 | ≤.0001 |
| DCD KDPI ≥85 | 6.313 | 3.248–12.270 | ≤.0001 | 1.881 | 1.234–2.868 | .0033 |
DBD, donor after brain death; DDC, donor after cardiac death; DGF, delayed graft function; KDPI, kidney donor profile index.
3.4 |. Primary outcomes: impact of KDPI on DGF
3.4.1 |. Post-transplant rates of DGF
The primary outcome analyzed was post-transplantation rates of DGF. The highest rates of DGF were in the DCD KDPI ≥85 kidneys (55.3%), followed by the DCD KDPI 61–84 kidneys (49.1%), DCD KDPI ≤60 kidneys (45.1%), DBD KDPI ≥85 (31.5%), DBD KDPI 61–84 (29%), and finally DBD KDPI ≤60 (18.7%) (P < .001) (Table 4). We noted that 18 patients who met DGF criteria (requiring dialysis within the first week post-transplant) were preemptive transplant patients who had not previously been on dialysis.
When analyzing rates of DGF in DBD kidneys with varying KDPIs, there were statistically significant differences between the KDPI ≤60 and both KDPI 61–84 and KDPI ≥85 groups (P = .001) (Table 5). There was no difference in the rates of DGF between DBD kidneys with KDPI 61–84 and ≥85. When analyzing all DCD kidneys with varying KDPIs, there were no statistically significant differences between rates of DGF. When comparing DCD and DBD kidneys with equivalent KDPIs, there were significant differences between DGF rates at all levels of KDPI (P ≤ .007).
Utilizing a logistic regression model after controlling for transplant number, cold time, recipient and donor sex, pretransplant dialysis, induction, and recipient age, we demonstrated an increased OR for the development of DGF in all kidneys when compared to DBD kidneys with KDPI ≤60 (P ≤ .0005) (Table 6). For DBD kidneys with KDPI 61–84 and KDPI ≥85, the OR was approximately 2 (61–84 OR = 1.806, ≥85 OR = 2.050). For DCD kidneys with KDPI ≤60 and KDPI 61–84, the OR was 4.115 and 4.834, respectively. DCD KDPI ≥85 kidneys had an OR for the development of DGF of 6.313 when compared to DBD KDPI ≤60 kidneys.
Next, the severity of DGF was assessed by analyzing the number of HD treatments needed in the postoperative period. There was not sufficient information in the chart review to determine a total number of dialysis treatments in 265 of the DGF patients. For the remaining patients, there was a statistically significant (P < .001) difference in post-op dialysis duration between the groups, with DCD patients requiring dialysis for longer periods of time. To illustrate, 33% of DBD KDPI 0–60 required only one dialysis treatment and 18.8% required >4 HD treatments. In contrast, 9.8% of DCD KDPI 0–60 patients required only one dialysis treatment and 34% required >4 HD treatments. Similarly, 35.5% DBD KDPI 61–84 patients required one HD treatment and 17.8% required >4 HD treatments. In comparison, 8.3% DCD KDPI 61–84 patients required only one HD treatment post-transplant and 43.8% required >4 HD treatments.
3.4.2 |. Graft half-life by DGF status
Next, we examined the impact DGF status had on graft half-life for each subgroup (Table 7). In DBD kidneys, graft half-life decreased with increasing KDPI (KDPI ≤60 half-life 12.50 years, KDPI ≥85 half-life 7.25 years, P value < .001). This phenomenon held true when subdivided out into patients who had confirmed DGF (KDPI ≤60 half-life 8.50 years, KDPI ≥85 half-life 4.25 years, P value < .001) and those who did not undergo DGF (KDPI ≤60 half-life 13.50 years, KDPI ≥85 half-life 8.25 years, P value < .001). In DCD kidneys, graft half-life decreased with increasing KDPI once the groups were sub-divided into patients with DGF and those without. We were unable to calculate a graft half-life for the DCD, KDPI >85, non-DGF group due to the small sample size. Consistently, DCD kidneys had longer half-lives than DBD kidneys with equivalent KDPI and DGF status. In both the DBD and DCD populations, patients with similar KDPIs with DGF have shorter half-lives than those patients who did not have DGF. In the KDPI ≤60 group, the half-life of a DBD kidney with DGF was 8.5 years, compared to 13.50 years without DGF. In DCD kidneys, the half-life was 12 years in those with DGF, compared to 15 years without DGF. In the KDPI 60–84 group, the half-life of a DBD kidney with DGF was 6.25 years, compared to 9.50 years in those without DGF, and in DCD kidneys, the half-life was 6.50 years in those with DGF, compared to 10.5 years for those without DGF.
TABLE 7.
Graft half-life within groups
| DBD KDPI 0–60 N = 1118 |
DBD KDPI 61–84 N = 384 |
DBD KDPI >85 N = 130 |
DCD KDPI 0–60 N = 328 |
DCD KDPI 61–84 N = 163 |
DCD KDPI >85 N = 38 |
P value | |
|---|---|---|---|---|---|---|---|
| Graft half-life (y)—all comers | 12.50 | 8.50 | 7.25 | 13.00 | 8.25 | 10.00 | ≤.001 |
| Graft half-life (y)—DGF patients | 8.50 | 6.25 | 4.25 | 12.00 | 6.50 | 4.50 | ≤.001 |
| Graft half-life (y)—non-DGF patients | 13.50 | 9.50 | 8.25 | 15.00 | 10.50 | n/a | ≤.001 |
DBD, donor after brain death; DCD, donor after cardiac death; DGF, delayed graft function; KDPI, kidney donor profile index.
3.5 |. Secondary outcomes: graft and patient survival
3.5.1 |. Graft survival and graft half-life
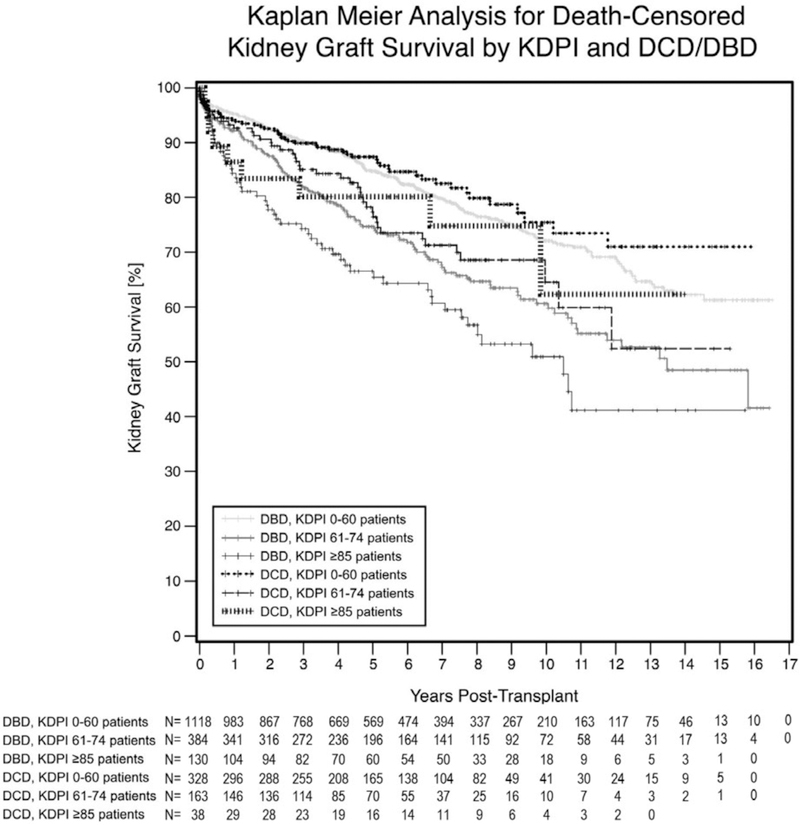
There was a statistically significant difference in renal graft survival (P ≤ .001) among the six groups displayed on the Kaplan-Meier curve in Figure 1. The graft survival at 5 years was highest in the DCD kidneys with KDPI ≤60 (87.4%), followed by the DBD kidneys with KDPI ≤60 (84.8%). Conversely, the poorest 5-year graft survival was noted in DBD KDPI ≥85 kidneys (66.6%). DCD and DBD kidneys with KDPI 61–84 had 5-year graft survivals of 77.3% and 74.6%. Graft half-life corresponded similarly. The longest half-life occurred in DCD KDPI ≤60 kidneys (13 years), followed by DCD KPDI ≤60 (12.5 years), then DBD and DCD kidneys with KDPI 61–85 (8.5 years and 8.25 years, respectively), and finally DCD ≥85 (7.25 years).
FIGURE 1.
Graft survival: This Kaplan-Meier curve illustrates the kidney graft survival based on both DCD vs DBD and KDPI status with kidneys with higher KDPIs having poorer graft survival. DBD, donor after brain death; DCD, donor after cardiac death; KDPI, kidney donor profile index
Next, we performed our group-wise comparison between all DBD kidneys with varying KDPIs, all DCD kidneys with varying KDPIs, and DCD and DBD kidneys with the same KDPIs (Table 5). When analyzing DBD kidneys with varying KDPIs, there were statistically significant differences in 5-year death-censored graft survival between all groups of increasing KDPI (P ≤ .035). Similarly, in DCD kidneys with varying KDPIs, there were significant differences between the DCD KDPI ≤60 group and both DCD KDPI 61–84 (P < .015). There were no statistically significant differences between graft survival when comparing the DCD KDPI ≤60 and KDPI ≥85 groups (P = .261) and the DCD KDPI 61–84 and DCD KDPI ≥85 groups (P = .888). When comparing DCD and DBD kidneys with equivalent KDPIs, there were no statistically significant differences.
After utilizing a Cox proportional hazards regression model and controlling for transplant number, cold time, recipient and donor sex, pretransplant dialysis, induction, and recipient age, there was no difference between DCD and DBD kidneys with KDPI ≤60 (Table 6). The HR was approximately 1.5 for kidneys with KDPI 61–84 (1.588 for DBD and 1.636 for DCD) when compared to a DBD KDPI ≤60 kidney. In kidneys with KDPI ≥85, the HR was approximately 2 (2.270 for DBD and 1.881 for DCD kidneys).
3.5.2 |. Patient survival
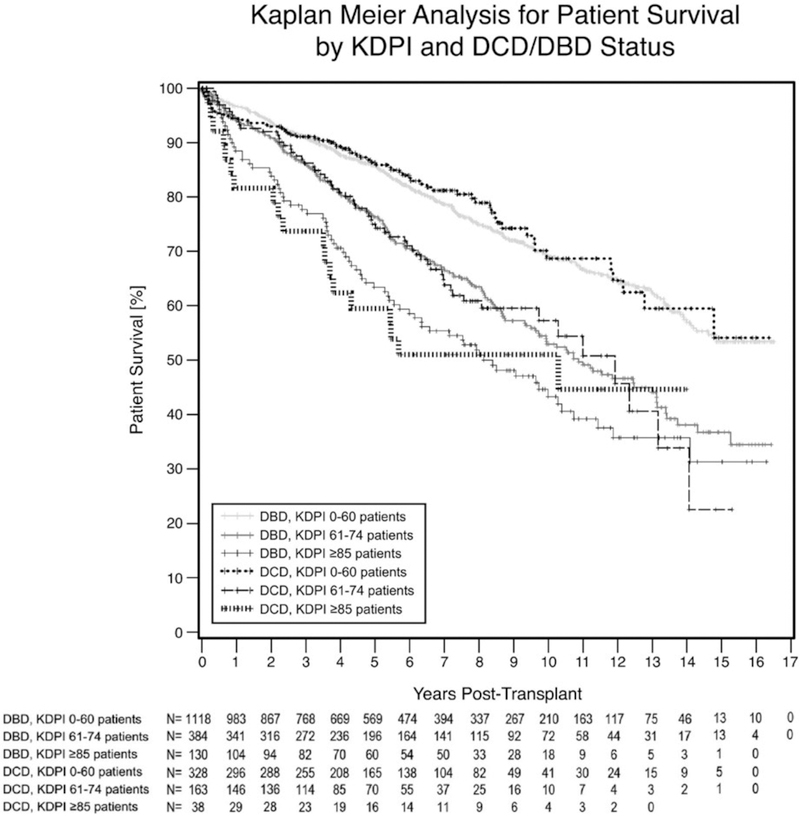
There was a significant difference in patient survival (P ≤ .001) among the six groups (displayed on the Kaplan-Meier curve in Figure 2). The highest 5-year patient survival was the DCD kidneys with KDPI ≤60 (86.7%), followed by the DBD kidneys with KDPI ≤60 (85.8%). The worst 5-year patient survival was in DCD KDPI ≥85 kidneys (59.5%), followed by DBD KDPI ≥85 (63.4%). DCD and DBD kidneys with KDPI 61–84 had 5-year patient survivals of 75% and 76.3%.
FIGURE 2.
Patient survival: This Kaplan-Meier curve illustrates the kidney graft survival based on both DCD vs DBD and KDPI status with kidneys with higher KDPIs having poorer patient survival. DBD, donor after brain death; DCD, donor after cardiac death; KDPI, kidney donor profile index
In DBD kidneys with varying KDPIs, there were significant differences in 5-year patient survival between all groups of increasing KDPI (P ≤ .030) (Table 5). Similarly, when analyzing all DCD kidneys with varying KDPIs, we noted significant differences between the DCD KDPI ≤60 group and those with DCD KDPI 61–84 and KDPI ≥85 (P ≤ .003). Again, we did not note a difference between patient survival in DCD KDPI 61–84 and DCD KDPI ≥85 groups (P = .177). When comparing DCD and DBD kidneys with the same KDPIs, there were no significant differences in any the group-wise comparisons.
4 |. DISCUSSION
This study demonstrates that the KDPI system is an accurate predictor of donor contributions to transplant outcomes. DCD kidneys have higher DGF rates than their DBD counterparts with similar KDPIs and patients with documented post-transplant DGF had poorer graft half-lives. Despite higher rates of DGF, the DCD kidneys did not show increased graft failure or patient mortality when compared to DBD kidneys of equivalent KDPI.
When designing this study, the authors decided to divide the sub-groups not simply by KDPI, but also with regard to type of donor (DCD vs. DBD) despite this factor being a component of the KDPI equation for several reasons. It has been our experience that when discussing kidney quality and risks, many clinicians discuss DCD status as a risk factor separate from ECD/KPDI classification. In fact, some transplant programs do not consider DCD kidneys for transplant in select patients based on the presumption that these kidneys are considered an expanded resource and associated with a high failure rate. Our results directly contradict this conclusion by demonstrating the recipients of DCD kidneys have improved graft survival when compared to recipients of DBD kidneys of equivalent KDPI score. In addition, by categorizing transplant outcomes based on both donor type and KDPI score, we are able to better anticipate resources needed to care for these patients and can more accurately advise patients on their individual long-term results. Furthermore, clinical and basic science research aimed at improving transplant outcomes are better able to identify and target specific donor groups where improvement is most needed.
When examining the outcomes in terms of recipient demographics, there were many interesting trends. One of the goals of the adaptation of the KDPI scoring system was to better match patients with longer estimated post-transplant longevity with lower KDPI kidneys.5 This goal correlated with our observations that both DBD and DCD kidneys with KDPIs ≤60 were being allocated to the youngest patients in our study. Furthermore, a study by Massie et al8 demonstrated that patients who are >50 years old and at centers with >33 month transplant wait times benefit most from high-KDPI kidney transplantation. In our data, the oldest patients and populations with higher pretransplant dialysis rates received DBD and DCD kidneys with KDPI ≥85.
Another interesting trend from our recipient demographics data was that patients undergoing retransplant were more likely to have a KDPI ≤60 or KDPI 61–84. Although there is no literature to date on outcomes of retransplant in high-KDPI patients, a study done by Sellers et al9 did find the relative risk of death-censored graft loss was 1.58 in the ECD group compared to SCD. It stands to reason KDPI ≥85 kidneys will have similar outcomes, and it is therefore appropriate that they are not currently being allocated to retransplant patients in great numbers.
The differences in cold time between kidney recipients in our study were likely an institutional bias due to an internal policy requiring DCD kidneys to have an OR time within 12 hours of procurement. As a result, our mean cold ischemia times for all groups were <20 hours and for DCD groups were <15.5 hours, which is lower than other authors have documented.10,11 That being said, a recent analysis of nation kidney cold ischemia times concluded from 2003 to 2011, the average cold ischemia time was 17 hours, which is comparable to our results.12 Given that DCD status, and not KDPI, was found to be the driving factor in the development of DGF post-transplant, our findings support this practice. The relationship between cold ischemia time and graft survival in high-KDPI kidneys has not been studied. That being said, a paper by Kayler et al13 showed in ECD kidneys, although the likelihood of DGF increases as cold ischemia time increases, there is no significant effect on overall graft survival.
When examining death-censored graft and patient survival rates, our data validate the original hypothesis that KDPI can be used to estimate all-cause allograft survival.4,14 We noted that graft and patient 5-year survival was highest in the kidneys with KDPI ≤60 and lowest in those with KDPIs ≥85. Furthermore, we demonstrated that corrected HRs increased with worsening KDPI. The DCD kidneys showed a slightly higher graft and patient survival compared to DBD kidneys with similar KDPIs. This is likely due to the fact that DCD status is a component of the KDPI equation. As a result, a DCD kidney with that same KDPI as a DBD kidney has better nephron quality with regard to long-term damage accrued from other KDPI factors like obesity, hypertension, diabetes, or HCV to offset the increased score from DCD status. When directly comparing DBD and DCD kidneys of the same KDPI status, we saw no statistical differences in graft or patient survival. In addition, while DCD KDPI ≤60 kidneys showed longer patient survival than DCD KDPI ≥85, there was little difference between the DCD KDPI 61–84 and DCD KDPI ≥85 groups. Unfortunately, a major limitation in our data is that our group of DCD KDPI ≥85 patients was limited to only 38 people. As a result, this group was underpowered. Although we saw interesting clinical trends in a group-wise comparisons of patient and death-censored graft survival, we were only able to note statistically significant differences in patient survival between DCD KDPI ≤60 and DCD KDPI ≥85. This pattern did not hold true in DBD donors where there was a significant improvement in graft and patient survival with each decrease of KDPI. Possible explanations for this discrepancy include the fact that as DCD is a component of the KDPI equation, we may see less impact on outcomes once KDPI >61 or due to an unknown effect of warm ischemia on high-KDPI kidneys.
Next, we closely examined the rates of DGF among our groups. DCD status is the primary risk factor for the development of DGF, not KDPI. This finding is supported by research by Nagaraja et al15 showing higher rates of DGF in DCD kidney recipients. That being said, within the DBD and DCD subgroups, we found that higher KDPIs correlated to marginal increases in DGF rates. These differences only met statistical significance when comparing DBD KDPI ≤60 kidneys to both DBD KDPI 61–84 and DBD KDPI ≥85 groups. As a result, novel clinical and experimental trials aimed at decreasing the incidence of DGF would be best targeted in patients receiving DCD kidneys than those with high KDPIs. Although the DGF rates were much higher in the DCD groups, long-term patient survival and graft survival showed little impact. Studies have showed that despite the higher rates of DGF, DCD kidneys are still cost-effective in comparison with remaining on dialysis and provide overall survival benefit when compared to waiting for a DBD.16,17 In addition to noting the fact that DCD patients had higher overall rates of DGF, we also found DCD to have more severe DGF requiring longer post-transplant HD treatments. This finding has not been documented in the literature to date.
Finally, we noted interesting trends when examining graft half-lives. DBD patients had an expected decrease in graft half-life with increasing KDPI. In addition, we demonstrated the subset of patients within each KDPI group who underwent DGF had between 3.25- and 5-year shorter graft half-lives when compared to those who did not. This phenomenon has been demonstrated in previous studies linking DGF to worse transplant outcomes.18–20 To illustrate, Humar et al18 found patients with DGF had a 47% incidence of acute rejection in the first 6 months, compared to only 30% in the non-DGF group. Furthermore, Zeraati et al20 found DGF patients had worse graft survival at 6 months and 3 years when compared to patients without DGF. Our data on the effect of DGF on graft half-life further emphasize the long-term consequences of DGF post-transplant. The literature regarding the effect of DGF on a DCD kidney has been mixed. A study out of Austria and New Zealand demonstrated DCD patients with DGF have higher rates of graft loss than those without DGF.21 In contrast, a study conducted by Le Dinh et al22 demonstrated no significant decrease in graft survival rates for patients with and without DGF. In our study, DCD patients with KDPIs <84% who suffered from DGF had between 3- and 4-year shorter half-lives than those with similar KDPIs who did not experience DGF. We were unable to calculate the half-life difference in the DCD, KDPI ≥85 group due to our small sample size, which is a limitation of our study. When examining all-comer DCD kidneys, we noted the graft half-life for a DCD KDPI ≥85 kidney was 10 years compared to 8.25 years in KDPI 61–84 kidneys. This phenomenon is likely explained by institutional biases. It is common practice in our hospital to prioritize operating room time for our higher risk DCD kidney and limit their cold ischemia time. A study out of the UK demonstrated increased graft survival with DCD kidneys in which the cold ischemia time was <12 hours.23
Our study had several limitations. First, given this is a retrospective analysis of a single center’s kidney transplant outcomes, our data can only suggest a correlation between KDPI and patient out-comes, and not a cause and effect relationship. As stated above, the sample size for our DCD KDPI >85% group is small, and therefore it is difficult to draw definitive conclusions of comparisons made with this group. Furthermore, our study spans an extended period of time in which the nomenclature changed from ECD/SCD to KDPI. As a result, all of the KDPIs prior to 2014 were calculated based on the 2014 kidney allocation pool, not the allocation pool of the year that the kidneys were transplanted. Finally, given our center has relatively short cold ischemia times (especially in the case of DCD kidneys), routinely pumps our kidneys and transplants a predominately Caucasian recipient population with lower KDPI kidneys, there is an inherent selection bias to our data and our results may not be completely generalizable to other centers with different practice patterns and patient populations.
5 |. CONCLUSION
The new KDPI system is an accurate predictor of donor contributions to transplant outcomes as rates of DGF and graft failure increase with increasing KDPI in all patients. When examining patients at risk for DGF, DCD status is the strongest factor contributing to the development of DGF. For those kidneys that did undergo DGF, there was a consistent trend of decreased graft half-life when compared to similar kidneys in terms of KDPI and DCD/DBD status which did not undergo DGF. When determining risk of patient or graft survival, increasing KDPI is a more powerful predictor than DCD vs DBD status given there are no differences between DBD and DCD kidneys with similar KDPIs.
ACKNOWLEDGEMENTS
We are indebted to the transplant recipients and donors for their participation in this study. Special thanks to the UW Organ Procurement Organization and all OPOs.
Funding information Foundation for the National Institutes of Health; American Society of Transplant Surgeons
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- 1.Israni AK, Zaun D, Rosendale JD, Snyder JJ, Kasiske BL. OPTN/SRTR 2012 Annual Data Report: deceased organ donation. Am J Transplant 2014;14(Suppl 1):167–183. [DOI] [PubMed] [Google Scholar]
- 2.Lee AP, Abramowicz D. Is the kidney donor risk index a step forward in the assessment of deceased donor kidney quality? Nephrol Dial Transplant 2015;30:1285–1290. [DOI] [PubMed] [Google Scholar]
- 3.Bae S, Massie AB, Luo X, Anjum S, Desai NM, Segev DL. Changes in discard rate after the introduction of the kidney donor profile index (KDPI). Am J Transplant 2016;16:2202–2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rao PS, Schaubel DE, Guidinger MK, et al. A comprehensive risk quantification score for deceased donor kidneys: the kidney donor risk index. Transplantation 2009;88:231–236. [DOI] [PubMed] [Google Scholar]
- 5.Network OPaT. A Guide to Calculating and Interpreting the Kidney Donor Profile Index (KDPI) Available at: https://optn.transplant.hrsa.gov/media/1512/guide_to_calculating_interpreting_kdpi.pdf: Network OPaT; 2014. [Google Scholar]
- 6.Kokkinos C, Antcliffe D, Nanidis T, Darzi AW, Tekkis P, Papalois V. Outcome of kidney transplantation from nonheart-beating versus heart-beating cadaveric donors. Transplantation 2007;83:1193–1199. [DOI] [PubMed] [Google Scholar]
- 7.Akoh JA, Rana TA. Impact of donor age on outcome of kidney transplantation from controlled donation after cardiac death. Saudi J Kidney Dis Transpl 2013;24:673–681. [DOI] [PubMed] [Google Scholar]
- 8.Massie AB, Luo X, Chow EK, Alejo JL, Desai NM, Segev DL. Survival benefit of primary deceased donor transplantation with high-KDPI kidneys. Am J Transplant 2014;14:2310–2316. [DOI] [PubMed] [Google Scholar]
- 9.Sellers MT, Velidedeoglu E, Bloom RD, et al. Expanded-criteria donor kidneys: a single-center clinical and short-term financial analysis–cause for concern in retransplantation. Transplantation 2004;78:1670–1675. [DOI] [PubMed] [Google Scholar]
- 10.Salahudeen AK, May W. Reduction in cold ischemia time of renal allografts in the United States over the last decade. Transplant Proc 2008;40:1285–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Debout A, Foucher Y, Trébern-Launay K, et al. Each additional hour of cold ischemia time significantly increases the risk of graft failure and mortality following renal transplantation. Kidney Int 2015;87:343–349. [DOI] [PubMed] [Google Scholar]
- 12.Koizumi N, DasGupta D, Patel AV, et al. Geographic variation in cold ischemia time: kidney versus liver transplantation in the United States, 2003 to 2011. Transplantation Direct 2015;1:e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kayler LK, Magliocca J, Zendejas I, Srinivas TR, Schold JD. Impact of cold ischemia time on graft survival among ECD transplant recipients: a paired kidney analysis. Am J Transplant 2011;11:2647–2656. [DOI] [PubMed] [Google Scholar]
- 14.Gupta A, Chen G, Kaplan B. KDPI and donor selection. Am J Transplant 2014;14:2444–2445. [DOI] [PubMed] [Google Scholar]
- 15.Nagaraja P, Roberts GW, Stephens M, et al. Influence of delayed graft function and acute rejection on outcomes after kidney transplantation from donors after cardiac death. Transplantation 2012;94:1218–1223. [DOI] [PubMed] [Google Scholar]
- 16.Snyder RA, Moore DR, Moore DE. More donors or more delayed graft function? A cost-effectiveness analysis of DCD kidney transplantation. Clin Transplant 2013;27:289–296. [DOI] [PubMed] [Google Scholar]
- 17.Snoeijs MG, Schaubel DE, Hené R, et al. Kidneys from donors after cardiac death provide survival benefit. J Am Soc Nephrol 2010;21:1015–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Humar A, Johnson EM, Payne WD, et al. Effect of initial slow graft function on renal allograft rejection and survival. Clin Transplant 1997;11:623–627. [PubMed] [Google Scholar]
- 19.Tapiawala SN, Tinckam KJ, Cardella CJ, et al. Delayed graft function and the risk for death with a functioning graft. J Am Soc Nephrol 2010;21:153–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zeraati AA, Naghibi M, Kianoush S, Kianoosh S, Ashraf H. Impact of slow and delayed graft function on kidney graft survival between various subgroups among renal transplant patients. Transplant Proc 2009;41:2777–2780. [DOI] [PubMed] [Google Scholar]
- 21.Lim WH, McDonald SP, Russ GR, et al. Association between delayed graft function and graft loss in donation after cardiac death kidney transplants – a paired kidney registry analysis. Transplantation 2017;101:1139–1143. [DOI] [PubMed] [Google Scholar]
- 22.Le Dinh H, Weekers L, Bonvoisin C, et al. Delayed graft function does not harm the future of donation-after-cardiac death in kidney transplantation. Transplant Proc 2012;44:2795–2802. [DOI] [PubMed] [Google Scholar]
- 23.Summers DM, Watson CJ, Pettigrew GJ, et al. Kidney donation after circulatory death (DCD): state of the art. Kidney Int 2015;88:241–249. [DOI] [PubMed] [Google Scholar]