Abstract
Small vessel diseases (SVDs) are a group of disorders that result from pathological alteration of the small blood vessels in the brain, including the small arteries, capillaries and veins. Of the 35–36 million people that are estimated to suffer from dementia worldwide, up to 65% have an SVD component. Furthermore, SVD causes 20–25% of strokes, worsens outcome after stroke and is a leading cause of disability, cognitive impairment and poor mobility. Yet the underlying cause(s) of SVD are not fully understood. Magnetic resonance imaging has confirmed enlarged perivascular spaces (PVS) as a hallmark feature of SVD. In healthy tissue, these spaces are proposed to form part of a complex brain fluid drainage system which supports interstitial fluid exchange and may also facilitate clearance of waste products from the brain. The pathophysiological signature of PVS and what this infers about their function and interaction with cerebral microcirculation, plus subsequent downstream effects on lesion development in the brain has not been established. Here we discuss the potential of enlarged PVS to be a unique biomarker for SVD and related brain disorders with a vascular component. We propose that widening of PVS suggests presence of peri-vascular cell debris and other waste products that form part of a vicious cycle involving impaired cerebrovascular reactivity, blood-brain barrier dysfunction, perivascular inflammation and ultimately impaired clearance of waste proteins from the interstitial fluid space, leading to accumulation of toxins, hypoxia, and tissue damage. Here, we outline current knowledge, questions and hypotheses regarding understanding the brain fluid dynamics underpinning dementia and stroke through the common denominator of SVD.
Keywords: Stroke, Cerebrovascular, Dementia, Small vessel disease, Perivascular space
1. Introduction
The umbrella term ‘small vessel disease’ (SVD) refers to a heterogeneous group of vascular disorders resulting from the pathological impairment of the small vessels of the brain. It is responsible for a large proportion of the cases of stroke and dementia worldwide.1 SVD manifests in several different ways, showing various pathological, neuroimaging, and clinical presentations such as stroke, cognitive impairment, dementia, physical disability, and depression,2–7 may predispose to delirium, and worsen outcome after stroke.8 This multiplicity of clinical expressions has contributed to delays in recognizing the similarities between such patients and that small vessel damage is a common underlying pathophysiology. Importantly, the prevalence of SVD is increasing and effective disease-modifying interventions, including pharmacological treatments, are yet to be found. This presents a huge social and economic burden that needs to be urgently addressed.9
Pathological evidence of SVD has been recognised since the 1800s. Since then, further post-mortem studies and advanced imaging technologies have allowed the hallmarks of SVD to be studied in greater detail.10,11 Magnetic resonance imaging (MRI) images from patients with SVD show characteristic abnormalities, such as white matter hyperintensities (WMHs), cerebral microbleeds, lacunes and enlarged perivascular spaces (PVS).2,12,13 These individual imaging features of SVD are inter-related, contribute to a ‘total SVD burden’, and both the individual features and the total SVD burden are associated with increased exposure to vascular risk factors in adulthood (particularly hypertension and smoking), stroke risk, concurrent cognitive dysfunction plus early life factors such as lower educational attainment, lower socioeconomic status and low childhood IQ.13–20
Clinically ‘silent’ neuroimaging signs of SVD can appear during ageing, and markers of cerebrovascular disease are fairly common incidental findings on MRI performed for other reasons.21–24 Most cases of SVD occur sporadically, although a small proportion are caused by genetic mutations. These latter include cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL), the most common monogenic SVD, and others such as cerebral autosomal recessive arteriopathy with subcortical infarcts and leukoencephalopathy (CARASIL25–31), with more monogenic variants being identified all the time.32
Although these genetic variants show phenotypic similarities to sporadic cases, the underlying cause of most sporadic SVD remains unknown. WMH are highly heritable,33–36 making the limited genetic associations identified so far with sporadic SVD somewhat surprising. However, recent genome-wide association studies,28,33,37–39 and recent targeted studies, have identified some genetic variants associated with sporadic SVD,40,41 suggesting that genetic common variants with small effect sizes but with lifelong action could increase vulnerability to various exposures in later life, leading to accumulating small vessel damage and dysfunction.28,41 Since intelligence in part reflects white matter integrity, which in turn is partially determined genetically, this could partly explain the association between childhood IQ and the SVD burden seen in later life in any one individual, and the heritability of WMH.
Amongst several potential molecular mechanisms that could link genetic traits to pathogenesis, are advanced glycation endproducts (AGEs) and activation of the receptor for AGE (RAGE). AGEs accumulate during hypertension and ageing, leading to vascular stiffening and inflammation, both of which we discuss later as important known mechanisms in SVD. AGE accumulation is increased when inflammation is present, and in the presence of oxidative stress and diabetes42 indicating one of several ways that adverse effects of combinations of risk factors may be much worse than might be expected from adding together the effects of individual risk factors alone. RAGE activation leads to production of reactive oxygen species and altered gene expression.42,43 Furthermore RAGE is activated in animal models of hypertension, and inhibition of RAGE activation prevents amyloid deposition.44 RAGE activation is believed to be implicated in vascular diseases and neurodegenerative conditions such as Alzheimer’s disease (AD45)—e.g. RAGE expression is increased in cerebral blood vessels of animal AD models and transports amyloid β across the blood-brain barrier (BBB)46,47—but there is not yet specific evidence relating to SVD or PVS dysfunction, particularly not yet in humans.
Environmental influences, such as education and socioeconomic status, appear to modify the risk of both developing imaging features of SVD and of having a stroke, while factors such as cognitive reserve may protect cognitive function against a developing burden of SVD brain damage and contribute to variability in disease expression between patients.14,20,48,49 Further evidence for a complex ‘nature-nurture’ balance underlying SVD is that although genetic and vascular risk factors, particularly hypertension, diabetes, and smoking, increase the risk of developing SVD, these multiple common vascular risk factors combined explain only a small proportion of the variance in SVD imaging features; thus they may exacerbate a predisposition, rather than being the sole cause of SVD.2,50 This interpretation is further borne out by the disappointing results to date of clinical trials of vascular risk factor reduction therapies: these have not prevented recurrent lacunar stroke, cognitive decline, or made much impression on reducing SVD lesion progression (e.g. the SPS3 trial51), further suggesting that SVD-specific treatments will require other approaches.52,53
Research into the causes and pathophysiological mechanisms of SVD has been hampered by the difficulty in visualizing small vessels in the human brain during life and the fact that pathology at death is often not reflective of the early disease stages.2 Although specific lesions such as WMH or lacunes have received much research attention, features such as the PVS and its relevance to brain fluid balance have only been recognised more recently. Furthermore, while much clinical research and practice has focussed separately on ‘stroke’ and ‘dementia’, and thus overlooked until recently the common underlying importance of microvessels and their dysfunction, similarly, much laboratory research has focussed on either the blood vessels or the neurons/glia and thus overlooked the integration between microvessels and brain tissue and the importance of the PVS.
To this end, a Fondation Leducq Transatlantic Network of Excellence (TNE) is now focussed on understanding the role of PVS in SVD (Figure 1). Knowledge about PVS and brain fluid and waste drainage systems in health and disease is growing. How PVS become enlarged, at what stage in the progression of SVD this occurs, and what the downstream consequences are, remain unanswered questions. Below, we discuss the role of PVS in the healthy brain, the association between disease and enlarged PVS, and propose hypotheses for the potential involvement of these enlarged spaces in the pathogenesis of SVD which are being addressed as part of the Fondation Leducq TNE programme.
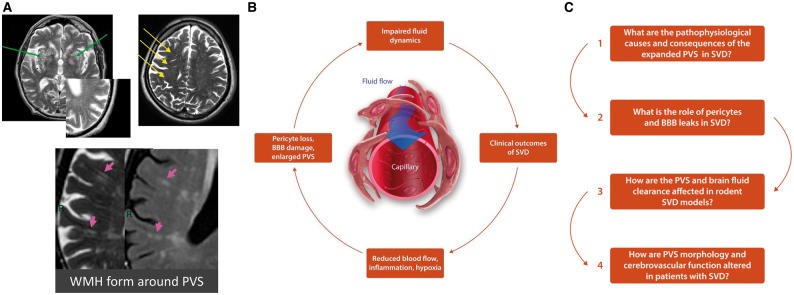
Figure 1.
(A) Enlarged PVS are key pathological features of SVD as shown on MRI from patients with sporadic SVD (Top panel, insert: PVS detected on T2 MRI), and associate with WMH (bottom panel). (B) The cycle of events we believe are involved in SVD pathogenesis and PVS dysfunction, including altered blood flow, BBB dysfunction and disrupted brain fluid flow. (C) Some of the key outstanding questions in SVD research, which also outline the scientific goals of the Fondation Leducq TNE ‘Understanding the role of the PVS in cerebral SVD’.
2. Perivascular spaces
PVS, also known as Virchow-Robin spaces, are fluid filled compartments surrounding the small blood vessels in the brain. PVS were originally named after Rudolf Virchow and Charles Philippe Robin, who individually provided detailed descriptions in the 1800s.54 Although historical descriptions of PVS and their function have been controversial,55,56 recent advances now recognise potentially important features such as architectural differences between PVS in different brain regions in humans.57
The current literature consensus is that PVS form a network of spaces around cerebral microvessels that act as a conduit for fluid transport, exchange between cerebrospinal fluid (CSF) and interstitial fluid (ISF) and clearance of waste products from the brain. Indeed, a central brain lymphatic-like system has been proposed since the 1700s.58 This clearance system has been identified in both animal models and humans and is varyingly referred to as ‘para-arteriolar’, ‘para-venular’, ‘paravascular’, or ‘glymphatic’—a term derived from the observed dependence on functional glial cells and its similarities to the lymphatic system elsewhere in the body.59,60
This proposed clearance pathway has most recently been explored in experiments involving the study of movement of fluorescent tracers in rodents.56,61–63 However, the system of fluid drainage is not completely understood. Efflux of ISF via PVS is proposed to facilitate waste clearance from the brain, while influx of CSF from the basal cisterns or superficial subarachnoid spaces into the periarteriolar spaces is thought to not only help flush out waste but also to deliver various signalling molecules and metabolic factors required for brain function.64,65 However, the precise routes of fluid clearance and whether these occur passively by diffusion or undergo periods of more active exchange by convection as a consequence of vascular pulsation, or both, are controversial.57 Fluid transport along this pathway is thought to be driven by cerebrovascular pulsatility60,64 (although there is conflicting evidence for this and others argue in favour of diffusion66). The rate and direction of fluid movement is also controversial.56,57,67–69 Many of these apparent differences in space function and fluid fluxes may reflect the effects of different experimental designs, closed vs. open craniotomy, temperature control, and anaesthetic agents to name but a few, on the delicate intracranial haemodynamics and PVS systems. In addition, the ability to capture beat-to-beat variations by real time imaging in the context of differentiating between convective vs. diffusion mass transport of CSF and solutes is currently limited.
The concept of this complex brain fluid and waste clearance system is controversial, as recently reviewed elsewhere.70–72 Nevertheless, this pathway appears to be important for the clearance of interstitial solutes from the brain, and is most likely vital for maintaining brain homeostasis. This view is supported by a reasonable body of human data demonstrating widening, and increasing visibility, of PVS in various presentations of SVD, stroke, dementia,73 systemic inflammation,74 and associations of PVS themselves with impaired cognition and poor blood pressure or glucose control.17,75,76 A recent small study in patients being investigated for hydrocephalus, who had gadolinium contrast injected into the CSF followed by serial brain MRI, also suggested that fluid uptake into PVS is more active in humans during the night.77 Compromised function (potentially indicated by widening of PVS, discussed below) may therefore have a negative impact on brain health78 and be involved in conditions such as AD, diabetes, increased risk of stroke and brain injury.79,80 If PVS can be measured accurately and dynamically, they present a potential biomarker and novel therapeutic target.81
2.1 Enlarged PVS in disease
Possibly the earliest description of dilated PVS pathologically was in the 1800s by Durant-Fardel.11,54 PVS become visible on MRI when enlarged, and though they may be detected on MRI in healthy individuals, widened PVS become more frequent during ageing and when associated with pathological alterations to the brain tissue such as with increasing burden of SVD lesions.73,82–86 Interestingly, as well as WMH being highly heritable, these enlarged PVS themselves are also highly heritable.87 Depending on the scan orientation these enlarged PVS will appear punctate or linear. PVS were defined in the STRIVE guidelines to aid the description of SVD pathophysiological features as having a diameter ‘<3 mm when imaged perpendicular to the course of the vessel’.88 Most are much smaller than 3 mm in diameter and there is acknowledged to be overlap between larger PVS and small lacunes about which more research is needed.
It may seem odd that enlargement of PVS, rather than shrinkage, should be abnormal, but as we discuss later, it appears likely that widening of PVS indicates obstruction by protein and cell debris and thus stagnation of fluid drainage. There is substantial evidence that enlarged PVS are abnormal. For example, they indicate increased stroke risk89 particularly with lacunar rather than large vessel stroke, and other SVD features particularly WMH.73,90 Their presence also correlates with vascular dementia, decreased performance in measures of cognitive function in healthy older men,17,91 hypertension,92–94 WMH73,74,76 and reduced von Willebrand factor suggesting reduced vessel elasticity86 in SVD. PVS enlargement can also be seen in cerebral amyloid angiopathy (CAA95,96), CADASIL,97,98 is a marker of SVD73,90,93,99,100 and is possibly associated with brain atrophy. Furthermore, enlarged PVS are associated with systemic inflammation,74 BBB dysfunction in SVD101 and with inflammatory exacerbations in multiple sclerosis (MS102,103).
2.2 Role of enlarged PVS in SVD
PVS are considered to play a role in normal brain homeostasis, while enlarged PVS are a feature of several diseases, and are associated with SVD. How PVS become enlarged in SVD and what the downstream effects of this are remains unclear. There are numerous potential ways in which PVS are likely to be involved in disease progression, and untangling the causes and consequences from the range of evidence in the literature is a challenge to be addressed. To investigate the factors that contribute to the enlargement of PVS, the consequences of these enlarged spaces, and the effect on clearance of waste products from the brain via the brain drainage system, four main areas of study can be identified and are discussed below and highlighted in Figure 1.
2.2.1 What are the pathophysiological causes and consequences of the expanded PVS in SVD?
We propose that expansion of PVS is likely to involve inflammation and that this in turn will result in increased oxygen consumption. Inflammatory markers are elevated in a range of vascular disorders, in ageing mice and in elderly people with cognitive decline.104–106 There is evidence for systemic inflammatory processes occurring in SVD.74,107,108 Interestingly, SVD burden is increased in lupus, an inflammatory disorder associated with increased stroke risk.109 Inflammatory markers are also associated with WMH, a hallmark feature of SVD.110 However, the role of inflammation in SVD is still to be fully elucidated111–113 and may lead to WMH development via triggering dysfunction of PVS.74 The triggers of inflammation are unknown, but potential factors could include salt intake114 and systemic inflammatory disorders such as rheumatoid arthritis.50,108,109,115
Inflammatory markers accumulate around cerebral blood vessels as shown pathologically in traumatic brain injury, intracerebral haemorrhage116 and MS.103 Pro-inflammatory markers are associated with enlarged PVS and inflammatory cells are known to accumulate in the PVS.117–121 Release of inflammatory cells can cause breakdown of the extracellular matrix and affect the integrity of the BBB, along with triggering demyelination.122 In the stroke-prone spontaneously hypertensive rat (SHRSP), a model of SVD, inflammation is associated with impaired myelin integrity and BBB dysfunction.123,124 Furthermore, perivascular macrophages are thought to be involved in AD, and may contribute to the neurovascular dysfunction seen in this disease.116,125
The precise interaction between inflammation, enlarged PVS and brain fluid dynamics is still to be determined. It is possible that aggregation of inflammatory cells in the PVS leads to remodelling and alterations in fluid clearance. Targeting inflammation may therefore present a therapeutic avenue for SVD. In fact, reducing perivascular macrophages in the SHRSP model improved endothelial function and remodelling of the middle cerebral artery.126 Depletion of perivascular macrophages also reduces oxidative stress, endothelial function and cognitive dysfunction.127 Further studies of inflammation in rodent models of SVD, with relation to the time course of vascular alterations and fluid movement, will help elucidate the role of inflammation in SVD.
Inflammation in SVD may be linked to reduced blood flow, hypoperfusion and hypoxia.128 It is traditionally thought that structural alterations in blood vessels and reduced blood flow are central mechanisms in SVD—in fact, hypoperfusion is used to model aspects of SVD in rodents.129,130 In Fisher’s seminal studies he described ‘segmental arteriolar disorganisation’ associated with lacunes, showing enlargement of the lumen and abnormalities in arterial architecture.131 It has since been proposed that dysfunction of the vessel endothelial cells leads to alterations to blood vessel architecture. These changes could lead to both enlargement and narrowing of the vessel lumen, along with vessel stiffening.2 Vascular smooth muscle cells are also involved in blood vessel remodelling,132 e.g. a narrowed lumen has also been associated with an increase in vascular smooth muscle cells in the SHR model.133 Further work is needed to confirm the cause and time course of vessel alterations, but multiple studies in both patients and animal models show that overall CBF is reduced, potentially as a result of these alterations. However, the exact role of reduced CBF in SVD pathogenesis is contentious due to the lack of longitudinal studies designed to illuminate causation.
Attenuated cerebrovascular reactivity (CVR) and CBF in CADASIL mouse models have been noted prior to other alterations in brain pathology, such as lacunes.134,135 Regions of normal-appearing WM in patients with WMH can show reduced CVR, suggesting that vascular alterations may precede WMH development136,137; however, direct evidence for this is surprisingly scarce. WM has fewer capillaries than the cortex,138 which along with slower blood flow and presence of a watershed area may contribute to greater susceptibility of WM to hypoperfusion than grey matter.139,140 However, while increased WMH are consistently associated with decreased CBF cross-sectionally, evidence to support the assumption that decreased CBF leads to white matter damage is somewhat lacking, as highlighted in a recent systematic review.141 This meta-analysis draws attention to the lack of convincing evidence that reduced CBF predates WMH in humans, the data suggesting that increased WMH precede decreased CBF rather than the opposite. Interestingly, recent studies in the CADASIL model suggest that reduced CBF and WML occur independently.142 Therefore, the temporal relationship between a reduction in blood flow and progressive damage to the brain, such as WMH, needs to be clarified143 as a priority.
Whether reduced CBF is cause or effect, there is strong evidence that it is a factor in SVD,141 and as such we can hypothesize that reduced blood flow could trigger hypoxia, neuronal death and other neuropathological alterations adding to worsening of the cerebral environment.122,128,144 These changes could occur alongside inflammation and demyelination because the resulting reduction in oxygen delivery could lead to activation of microglia and macrophages, triggering demyelination145 exacerbate dysfunction of the BBB122 and in turn encourage enlargement of PVS. We could further hypothesise that if fluid flow along the brain drainage pathway is driven by cerebral arterial pulsatility,146,147 then increased stiffness and loss of pulsatility could lead to reduced waste clearance.
Although it is likely that reduced CBF and vascular dysfunction are key events in SVD pathogenesis, the temporal association between reduced CBF and SVD pathogenesis, and its relationship with enlarged PVS remains unclear. This association can be examined using a range of techniques, including two-photon phosphorescence lifetime measurements of oxygen delivery,148 and histological measures of hypoxia,149 in rodent models of SVD.
2.2.2 The role of pericytes and BBB disruption in SVD
Endothelial dysfunction is a recognised contributor to SVD.2,150,151 BBB dysfunction increases with ageing, in vascular dementia, AD and with increased WM lesion load152–157 and has been found to precede the development of dementia.154 Furthermore, breakdown of the BBB is found in patients with lacunar stroke,101,158–160 in WMH on MRI,137,160–163 in vascular cognitive impairment and dementia152,153,164,165 and is associated with poor functional outcome after minor cortical or lacunar stroke.166
In patients with SVD, BBB leakage is also apparent in normal-appearing WM, increases together with increasing SVD-lesion burden, and appears to predict cognitive dysfunction,150 indicating an important role for BBB dysfunction in the pathogenesis and clinical expression of SVD. Patients with SVD have also been found to have elevated circulating levels of markers of endothelial activation and damage,167 elevated serum levels of homocysteine, an endothelial toxin and presumed risk factor for SVD,168,169 and there is evidence that the association between homocysteine levels and SVD risk is mediated by endothelial dysfunction.169
Interestingly, recent genome-wide association studies identified the Foxf2 gene region as a major risk locus for small vessel stroke.39 Foxf2 is expressed in brain vascular endothelial cells and pericytes,170,171 and mice deficient for Foxf2 develop prominent structural and functional abnormalities of endothelial cells along with a disruption of the BBB.171
The cause of the BBB dysfunction is unclear, but inflammation has been indicated as a causative factor that can trigger endothelial dysfunction (32see above). Another potential culprit is pericyte dysfunction. Pericytes are proposed to play a variety of roles within the neurovascular unit172 including control of the dilation of capillaries.173,174 Some controversy has arisen over the role of pericytes in capillary dilation175; however, this could potentially be explained by the presence of subclasses of pericytes and the correct labelling of pericytes vs. smooth muscle cells.176 Pericytes are also involved in the ischaemic response, contracting and causing capillary constriction, thereby presenting a potential therapeutic target.174,176 Animal models developed to have reduced numbers of pericytes show reduced CBF and CVR, BBB breakdown and neurodegeneration.155,177,178 More recently, pericyte loss has been demonstrated to underlie white matter damage, which is associated with both SVD and dementia.179 Animals with white matter damage resulting from pericyte loss were shown to develop axonal degeneration, enlarged PVS, and functional deficits in behavioural tests.
Pericyte dysfunction has been linked to several disorders, including AD and diabetic retinopathy179–181 and they are among a number of cells of the neurovascular unit that are affected in SVD.182 Pericyte cells have been proposed to be lost in rodent models of SVD such as the CADASIL mouse model,183,184 in models of cerebral hypoperfusion185 as well as in human post-mortem tissue,186 although an up-regulation of pericytes has been noted in some CADASIL patients.187 When pericytes are lost in SVD, nearby endothelial cells also show signs of dysfunction, and these changes together may contribute to alterations in CBF and BBB function.186
Markers of endothelial dysfunction correlate with enlarged PVS in SVD86,101 and in MS,102,103 indicating a relationship between enlarged PVS and BBB breakdown. We propose that in SVD, pericyte degeneration results in opening of the BBB and aggravates inflammation in PVS. These events may further compromise pericyte and PVS function triggering a vicious cycle of events.
Of course, it is not only pericytes that are affected in SVD. Oligodendrocytes,188 the basement membrane,189 and the extracellular matrix122,190 have all been proposed to play a role. The mechanisms underlying BBB dysfunction, and underlying the association between abnormal PVS and endothelial dysfunction are yet to be determined, and could be studied by cross-comparison between specific models of pericyte loss and models of other putative SVD mechanisms such as SHRSP. The therapeutic value of BBB preservation and how this would affect the appearance of enlarged PVS and the progression of SVD is also to be tested.
2.2.3 How are PVS and brain fluid clearance affected in rodent SVD models?
Enlarged PVS are evidently an imaging biomarker for cerebrovascular disease73,76,89 and could indicate dysfunction of fluid clearance due to excess accumulation (e.g. from BBB failure) or failure to drain the usual amount of ISF (e.g. from obstructed PVS), which may further result in impaired drainage of toxins and build-up of harmful waste products.191,192 Perivascular drainage is impaired in aged wildtype mice and in models of AD.193–195 PVS contribute to amyloid-β clearance from the brain. Normally, transvascular clearance across the BBB is thought to clear most amyloid-β from the brain (∼60–85%), whereas ISF flow across PVS removes the remaining smaller fraction (∼15–40%) of amyloid-β depending on whether the animal is awake196,197 or asleep192 and better amyloid-β clearance is also associated with physical activity.198–201 Faulty transvascular clearance of brain amyloid-β across the BBB is likely to play an important role in amyloid-β accumulation in the brain, both in human AD and animal models59,202 and might explain associations between reduced physical activity,203 poor sleep patterns and increased risk of cognitive decline and dementia.204,205 In CAA, AD, and other disorders, dysfunction of clearance via ISF flow additionally contribute to amyloid-β accumulation. Amyloid-β deposits are therefore suggestive of dysfunction of fluid clearance.193–195,206–210 In fact, amyloid-β clearance is reduced in aged mice, alongside reduced fluid transport,147 indicating a potentially key role for this process in the pathogenesis of AD and CAA.211
Fluid clearance is significantly impaired in models of stroke, multiple infarcts, diabetes and traumatic brain injury.79,212–215 Closure of PVS and impaired fluid transport has been shown in a model of migraine—commonly experienced in CADASIL.216 Ageing and brain injury may therefore impair function of the brain clearance pathway. Dysfunction of this system could contribute to interstitial oedema, accumulation of waste toxins, and trigger pathological events that could have a devastating impact on brain health. Enlarged PVS may therefore provide not only a biomarker for SVD but also indicate impaired fluid transport and waste clearance. However, it is not currently clear whether enlarged PVS result from impaired clearance of fluid, or conversely that impaired clearance occurs as a result of enlarged PVS. It is also possible to speculate that enlarged PVS may be a compensatory response, designed to improve fluid flow. While there is evidence for reduced fluid movement in models of AD, stroke and other disorders,79,195,212–215, interestingly, a recent report is suggestive of increased ISF flow in the hippocampus of the SHR model217—more long-term studies are needed since increased flow may predate reduced flow and failure of fluid clearance or vice versa.
Further studies of fluid dynamics in SVD and the role of enlarged PVS in this process are therefore warranted, and clarifying potential changes to flow in SVD presents an interesting avenue of research. It can be hypothesised that fluid transport is impaired in SVD, and in vivo MRI imaging and modelling in rodent models of SVD could be used to determine its role in disease pathophysiology.218–220 The timecourse of these alterations could be studied alongside investigations of the role of inflammation, hypoxia and vessel alterations to build a more complete picture of the pathophysiology of SVD, which the field is currently lacking.
2.2.4 Understanding PVS morphology and cerebrovascular function in patients with SVD
Currently, there is no gold-standard animal model for SVD. However, animal models do provide opportunities to examine certain aspects of SVD pathophysiology.221 Translational research is hugely important, and combining our knowledge of SVD at the cellular and network level in animal models with advanced imaging studies in both animals and humans will help us to develop a more complete picture of the pathophysiology of SVD (Figure 2).
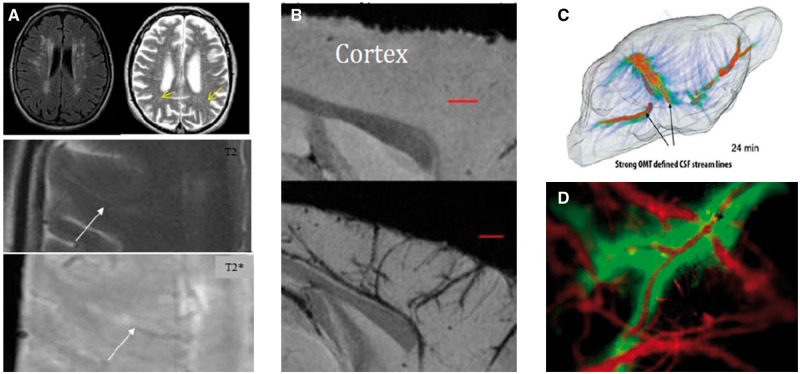
Figure 2.
Translation between preclinical and clinical findings will be facilitated by comparing and harmonising rodent and human imaging techniques, allowing the relationship between enlarged PVS on MRI (A and B) to fluid flow (C and D) to be determined. A. Clinical imaging. Top: FLAIR (L) and T2 (R) MRI shows WMH (L) form along the PVS (R, arrow). Below: T2 shows a PVS running inwards from cortex (arrow); T2* shows white matter venule (arrow) closely related to the PVS. (B) T2*-weighted MRI of a normotensive mouse (top panel) and hypertensive mouse showing cortical vessels associated with susceptibility contrast probably due to thickening of the vessel wall and/or altered PVSs (lower panel). (C) ‘Glymphatic’ transport pathways detected by T1 MRI and Optimal Mass Tomography analysis in a rodent brain following tracer infusion into the CSF. The timecourse of fluid flow from the cisterna magna throughout the brain can be revealed using this technique (D). Fluid transport in the PVS detected by macroscopic fluorescent optical imaging in the cortex following injection of fluorescent markers into the CSF and vasculature.
As discussed earlier, MRI abnormalities are recognised hallmarks of SVD.88 In addition to structural changes, such as WMH, functional abnormalities indicating impaired cerebrovascular function can also be detected. CVR and cardiac pulse transmission are both altered in SVD.222,223 PVS quantification in humans has been based on visual scoring to date, which has limitations. We have developed a computational method of analysing PVS structure,224,225 which can calculate centrum semiovale PVS volume and numbers meaning that we can now determine whether PVS morphology correlates with makers of cerebrovascular dysfunction, such as CVR, BBB dysfunction or serum markers in SVD. As clearance via PVS has been shown to occur diurnally, with enhanced clearance during sleep via an enlargement of the ISF space,219 it is also likely that sleep dysfunction will affect brain homeostasis via this pathway. A small convenience study in patients undergoing investigation of hydrocephalus provided some evidence that clearance increases during sleep in humans.77 Abnormal PVS have been observed in patients with sleep apnoea226 and sleep disruption occurs commonly in patients with AD and other neurodegenerative conditions.227 Sleep disruption also negatively impacts amyloid deposition in mice.228 Clearance during sleep may therefore provide a novel therapeutic avenue.78 We aim to address this by investigating the presence of SVD-related brain changes in patients with severe sleep apnoea, and determining whether treatment with continuous positive airways pressure therapy will affect the appearance of MRI and serum markers of cerebrovascular dysfunction.
As reviewed by Blair and colleagues,229 advanced imaging techniques have been essential in developing our understanding of SVD. Rating scales and improved analysis methods will aid assessment of PVS,73,86,224,225,230,231 while advances in imaging technology, such as 7 T MRI, will aid the study of the role of PVS in SVD and other disorders.85,232 Although current clinical evidence for dysfunction of the brain clearance pathway in disease is limited, newly developed techniques are being used to image fluid flow in PVS in patients.233,234 However, we need to reduce variability and cement reproducibility in MRI markers by harmonising protocols and quantification methods.12 Validation of advanced imaging techniques for use in multicentre studies provides promise for using new methods in clinical trials.235 Longitudinal clinical studies are needed to help us to fully understand disease progression.236,237
3. Conclusions
SVD has a complex pathophysiology with many contributing factors. A combination of vascular dysfunction, inflammation and BBB dysfunction are likely to underlie this disease and have devastating effects on brain health. However, the timing and contribution of these events to SVD pathophysiology is yet to be confirmed. MRI has provided imaging biomarkers for SVD,88 including enlarged PVS, which correlate with SVD burden. There is recent, and increasing, evidence for the association between abnormal PVS and SVD. However, how PVS come to be enlarged in SVD and their role in the pathogenesis of the disease is yet to be determined. Here we discussed potential pathways that could lead to enlarged PVS and the effects this may have on brain health, and present the work that is being addressed in the Fondation Leducq funded TNE. This network aims to tackle the problem of SVD using a cross-disciplinary approach, linking findings from animal studies (both in vivo and in vitro) to studies in patients with sporadic SVD. Furthermore, we aim to provide novel insight into related pathological mechanisms by studying SVD-related brain changes in sleep apnoea patients.
SVD is a pressing issue, with a global health impact. Enlarged PVS are just one factor in this complex disorder and there are many contributing factors that are not discussed here. Furthermore, there are many suppositions in the literature that remain to be proven. By looking at each of the processes that are known to occur in SVD in detail, and providing proof for some of the assumed knowledge we may further our understanding and uncover novel therapeutic avenues for SVD.
Funding
This work was supported by funding from the Fondation Leducq Transatlantic Networks of Excellence programme [16CVD05, Understanding the role of the perivascular space in cerebral small vessel disease].
Conflict of interest: none declared.
References
- 1. Kapasi A, DeCarli C, Schneider JA.. Impact of multiple pathologies on the threshold for clinically overt dementia. Acta Neuropathol 2017;134:171–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wardlaw JM, Smith C, Dichgans M.. Mechanisms of sporadic cerebral small vessel disease: insights from neuroimaging. Lancet Neurol 2013;12:483–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol 2010;9:689–701. [DOI] [PubMed] [Google Scholar]
- 4. Greenberg SM. Small vessels, big problems. N Engl J Med 2006;354:1451–1453. [DOI] [PubMed] [Google Scholar]
- 5. Munoz DG. Small vessel disease: neuropathology. Int Psychogeriatr 2003;15:67–69. [DOI] [PubMed] [Google Scholar]
- 6. Gouw AA, Seewann A, van der Flier WM, Barkhof F, Rozemuller AM, Scheltens P, Geurts JJ.. Heterogeneity of small vessel disease: a systematic review of MRI and histopathology correlations. J Neurol Neurosurg Psychiatry 2011;82:126–135. [DOI] [PubMed] [Google Scholar]
- 7. Dichgans M, Leys D.. Vascular Cognitive Impairment. Circ Res 2017;120:573–591. [DOI] [PubMed] [Google Scholar]
- 8. Sato S, Delcourt C, Heeley E, Arima H, Zhang S, Al-Shahi Salman R, Stapf C, Woo D, Flaherty ML, Vagal A, Levi C, Davies L, Wang J, Robinson T, Lavados PM, Lindley RI, Chalmers J, Anderson CS, Investigators I.. Significance of cerebral small-vessel disease in acute intracerebral hemorrhage. Stroke 2016;47:701–707. [DOI] [PubMed] [Google Scholar]
- 9. Iadecola C. The pathobiology of vascular dementia. Neuron 2013;80:844–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Norrving B. Evolving concept of small vessel disease through advanced brain imaging. J Stroke 2015;17:94–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bailey EL, Smith C, Sudlow CL, Wardlaw JM.. Pathology of lacunar ischemic stroke in humans–a systematic review. Brain Pathol 2012;22:583–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. De Guio F, Jouvent E, Biessels GJ, Black SE, Brayne C, Chen C, Cordonnier C, De Leeuw F-E, Dichgans M, Doubal F, Duering M, Dufouil C, Duzel E, Fazekas F, Hachinski V, Ikram MA, Linn J, Matthews PM, Mazoyer B, Mok V, Norrving B, O’brien JT, Pantoni L, Ropele S, Sachdev P, Schmidt R, Seshadri S, Smith EE, Sposato LA, Stephan B, Swartz RH, Tzourio C, van Buchem M, van der Lugt A, van Oostenbrugge R, Vernooij MW, Viswanathan A, Werring D, Wollenweber F, Wardlaw JM, Chabriat H.. Reproducibility and variability of quantitative magnetic resonance imaging markers in cerebral small vessel disease. J Cereb Blood Flow Metab 2016;36:1319–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Staals J, Booth T, Morris Z, Bastin ME, Gow AJ, Corley J, Redmond P, Starr JM, Deary IJ, Wardlaw JM.. Total MRI load of cerebral small vessel disease and cognitive ability in older people. Neurobiol Aging 2015;36:2806–2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Backhouse EV, McHutchison CA, Cvoro V, Shenkin SD, Wardlaw JM.. Early life risk factors for cerebrovascular disease: a systematic review and meta-analysis. Neurology 2017;88:976–984. [DOI] [PubMed] [Google Scholar]
- 15. de Groot JC, de Leeuw FE, Oudkerk M, van Gijn J, Hofman A, Jolles J, Breteler MM.. Cerebral white matter lesions and cognitive function: the Rotterdam Scan Study. Ann Neurol 2000;47:145–151. [DOI] [PubMed] [Google Scholar]
- 16. Pantoni L, Poggesi A, Inzitari D.. The relation between white-matter lesions and cognition. Curr Opin Neurol 2007;20:390–397. [DOI] [PubMed] [Google Scholar]
- 17. Maclullich AM, Wardlaw JM, Ferguson KJ, Starr JM, Seckl JR, Deary IJ.. Enlarged perivascular spaces are associated with cognitive function in healthy elderly men. J Neurol Neurosurg Psychiatry 2004;75:1519–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Huijts M, Duits A, van Oostenbrugge RJ, Kroon AA, de Leeuw PW, Staals J.. Accumulation of MRI markers of cerebral small vessel disease is associated with decreased cognitive function. A study in first-ever lacunar stroke and hypertensive patients. Front Aging Neurosci 2013;5:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Uiterwijk R, van Oostenbrugge RJ, Huijts M, De Leeuw PW, Kroon AA, Staals J.. Total cerebral small vessel disease MRI score is associated with cognitive decline in executive function in patients with hypertension. Front Aging Neurosci 2016;8:301.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Field TS, Doubal FN, Johnson W, Backhouse E, McHutchison C, Cox S, Corley J, Pattie A, Gow AJ, Shenkin S, Cvoro V, Morris Z, Staals J, Bastin M, Deary IJ, Wardlaw JM.. Early life characteristics and late life burden of cerebral small vessel disease in the Lothian Birth Cohort 1936. Aging 2016;8:2039–2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ylikoski A, Erkinjuntti T, Raininko R, Sarna S, Sulkava R, Tilvis R.. White matter hyperintensities on MRI in the neurologically nondiseased elderly. Analysis of cohorts of consecutive subjects aged 55 to 85 years living at home. Stroke 1995;26:1171–1177. [DOI] [PubMed] [Google Scholar]
- 22. Morris Z, Whiteley WN, Longstreth WT Jr, Weber F, Lee YC, Tsushima Y, Alphs H, Ladd SC, Warlow C, Wardlaw JM, Al-Shahi Salman R.. Incidental findings on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ 2009;339:b3016.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sandeman EM, Hernandez M. D C V, Morris Z, Bastin ME, Murray C, Gow AJ, Corley J, Henderson R, Deary IJ, Starr JM, Wardlaw JM.. Incidental findings on brain MR imaging in older community-dwelling subjects are common but serious medical consequences are rare: a cohort study. PLoS One 2013;8:e71467.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Debette S, Markus HS.. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ 2010;341:c3666.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ikram MA, Bersano A, Manso-Calderon R, Jia JP, Schmidt H, Middleton L, Nacmias B, Siddiqi S, Adams HH.. Genetics of vascular dementia–review from the ICVD working group. BMC Med 2017;15:48.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sondergaard CB, Nielsen JE, Hansen CK, Christensen H.. Hereditary cerebral small vessel disease and stroke. Clin Neurol Neurosurg 2017;155:45–57. [DOI] [PubMed] [Google Scholar]
- 27. Tikka S, Baumann M, Siitonen M, Pasanen P, Poyhonen M, Myllykangas L, Viitanen M, Fukutake T, Cognat E, Joutel A, Kalimo H.. CADASIL and CARASIL. Brain Pathol 2014;24:525–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Haffner C, Malik R, Dichgans M.. Genetic factors in cerebral small vessel disease and their impact on stroke and dementia. J Cereb Blood Flow Metab 2016;36:158–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Haffner C, Vinters HV.. CADASIL, CARASIL, CARASAL: the linguistic subtleties of cerebral small vessel disease. Neurology 2016;87:1752–1753. [DOI] [PubMed] [Google Scholar]
- 30. Chabriat H, Joutel A, Dichgans M, Tournier-Lasserve E, Bousser MG.. Cadasil. Lancet Neurol 2009;8:643–653. [DOI] [PubMed] [Google Scholar]
- 31. Joutel A, Corpechot C, Ducros A, Vahedi K, Chabriat H, Mouton P, Alamowitch S, Domenga V, Cecillion M, Marechal E, Maciazek J, Vayssiere C, Cruaud C, Cabanis EA, Ruchoux MM, Weissenbach J, Bach JF, Bousser MG, Tournier-Lasserve E.. Notch3 mutations in CADASIL, a hereditary adult-onset condition causing stroke and dementia. Nature 1996;383:707–710. [DOI] [PubMed] [Google Scholar]
- 32. Abbott NJ. Inflammatory mediators and modulation of blood-brain barrier permeability. Cell Mol Neurobiol 2000;20:131–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Verhaaren BFJ, Debette S, Bis JC, Smith JA, Ikram MK, Adams HH, Beecham AH, Rajan KB, Lopez LM, Barral S, van Buchem MA, van der Grond J, Smith AV, Hegenscheid K, Aggarwal NT, de Andrade M, Atkinson EJ, Beekman M, Beiser AS, Blanton SH, Boerwinkle E, Brickman AM, Bryan RN, Chauhan G, Chen CPLH, Chouraki V, de Craen AJM, Crivello F, Deary IJ, Deelen J, De Jager PL, Dufouil C, Elkind MSV, Evans DA, Freudenberger P, Gottesman RF, Guðnason V, Habes M, Heckbert SR, Heiss G, Hilal S, Hofer E, Hofman A, Ibrahim-Verbaas CA, Knopman DS, Lewis CE, Liao J, Liewald DCM, Luciano M, van der Lugt A, Martinez OO, Mayeux R, Mazoyer B, Nalls M, Nauck M, Niessen WJ, Oostra BA, Psaty BM, Rice KM, Rotter JI, von Sarnowski B, Schmidt H, Schreiner PJ, Schuur M, Sidney SS, Sigurdsson S, Slagboom PE, Stott DJM, van Swieten JC, Teumer A, Töglhofer AM, Traylor M, Trompet S, Turner ST, Tzourio C, Uh H-W, Uitterlinden AG, Vernooij MW, Wang JJ, Wong TY, Wardlaw JM, Windham BG, Wittfeld K, Wolf C, Wright CB, Yang Q, Zhao W, Zijdenbos A, Jukema JW, Sacco RL, Kardia SLR, Amouyel P, Mosley TH, Longstreth WT, DeCarli CC, van Duijn CM, Schmidt R, Launer LJ, Grabe HJ, Seshadri SS, Ikram MA, Fornage M.. Multiethnic genome-wide association study of cerebral white matter hyperintensities on MRI. Circ Cardiovasc Genet 2015;8:398–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Carmelli D, DeCarli C, Swan GE, Jack LM, Reed T, Wolf PA, Miller BL.. Evidence for genetic variance in white matter hyperintensity volume in normal elderly male twins. Stroke 1998;29:1177–1181. [DOI] [PubMed] [Google Scholar]
- 35. Atwood LD, Wolf PA, Heard-Costa NL, Massaro JM, Beiser A, D’Agostino RB, DeCarli C.. Genetic variation in white matter hyperintensity volume in the Framingham Study. Stroke 2004;35:1609–1613. [DOI] [PubMed] [Google Scholar]
- 36. DeStefano AL, Atwood LD, Massaro JM, Heard-Costa N, Beiser A, Au R, Wolf PA, DeCarli C.. Genome-wide scan for white matter hyperintensity: the Framingham Heart Study. Stroke 2006;37:77–81. [DOI] [PubMed] [Google Scholar]
- 37. Traylor M, Malik R, Nalls MA, Cotlarciuc I, Radmanesh F, Thorleifsson G, Hanscombe KB, Langefeld C, Saleheen D, Rost NS, Yet I, Spector TD, Bell JT, Hannon E, Mill J, Chauhan G, Debette S, Bis JC, Longstreth WT, Ikram MA, Launer LJ, Seshadri S, Hamilton-Bruce MA, Jimenez-Conde J, Cole JW, Schmidt R, Słowik A, Lemmens R, Lindgren A, Melander O, Grewal RP, Sacco RL, Rundek T, Rexrode K, Arnett DK, Johnson JA, Benavente OR, Wasssertheil-Smoller S, Lee J-M, Pulit SL, Wong Q, Rich SS, de Bakker PIW, McArdle PF, Woo D, Anderson CD, Xu H, Heitsch L, Fornage M, Jern C, Stefansson K, Thorsteinsdottir U, Gretarsdottir S, Lewis CM, Sharma P, Sudlow CLM, Rothwell PM, Boncoraglio GB, Thijs V, Levi C, Meschia JF, Rosand J, Kittner SJ, Mitchell BD, Dichgans M, Worrall BB, Markus HS.. Genetic variation at 16q24.2 is associated with small vessel stroke. Ann Neurol 2017;81:383–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rannikmae K, Sivakumaran V, Millar H, Malik R, Anderson CD, Chong M, Dave T, Falcone GJ, Fernandez-Cadenas I, Jimenez-Conde J, Lindgren A, Montaner J, O'Donnell M, Pare G, Radmanesh F, Rost NS, Slowik A, Soderholm M, Traylor M, Pulit SL, Seshadri S, Worrall BB, Woo D, Markus HS, Mitchell BD, Dichgans M, Rosand J, Sudlow CLM.. COL4A2 is associated with lacunar ischemic stroke and deep ICH: meta-analyses among 21, 500 cases and 40, 600 controls. Neurology 2017;89:1829–1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chauhan G, Arnold CR, Chu AY, Fornage M, Reyahi A, Bis JC, Havulinna AS, Sargurupremraj M, Smith AV, Adams HHH, Choi SH, Pulit SL, Trompet S, Garcia ME, Manichaikul A, Teumer A, Gustafsson S, Bartz TM, Bellenguez C, Vidal JS, Jian X, Kjartansson O, Wiggins KL, Satizabal CL, Xue F, Ripatti S, Liu Y, Deelen J, den Hoed M, Bevan S, Hopewell JC, Malik R, Heckbert SR, Rice K, Smith NL, Levi C, Sharma P, Sudlow CLM, Nik AM, Cole JW, Schmidt R, Meschia J, Thijs V, Lindgren A, Melander O, Grewal RP, Sacco RL, Rundek T, Rothwell PM, Arnett DK, Jern C, Johnson JA, Benavente OR, Wassertheil-Smoller S, Lee J-M, Wong Q, Aparicio HJ, Engelter ST, Kloss M, Leys D, Pezzini A, Buring JE, Ridker PM, Berr C, Dartigues J-F, Hamsten A, Magnusson PK, Traylor M, Pedersen NL, Lannfelt L, Lindgren L, Lindgren CM, Morris AP, Jimenez-Conde J, Montaner J, Radmanesh F, Slowik A, Woo D, Hofman A, Koudstaal PJ, Portegies MLP, Uitterlinden AG, de Craen AJM, Ford I, Jukema JW, Stott DJ, Allen NB, Sale MM, Johnson AD, Bennett DA, De Jager PL, White CC, Grabe HJ, Markus MRP, Schminke U, Boncoraglio GB, Clarke R, Kamatani Y, Dallongeville J, Lopez OL, Rotter JI, Nalls MA, Gottesman RF, Griswold ME, Knopman DS, Windham BG, Beiser A, Markus HS, Vartiainen E, French CR, Dichgans M, Pastinen T, Lathrop M, Gudnason V, Kurth T, Psaty BM, Harris TB, Rich SS, deStefano AL, Schmidt CO, Worrall BB, Rosand J, Salomaa V, Mosley TH, Ingelsson E, van Duijn CM, Tzourio C, Rexrode KM, Lehmann OJ, Launer LJ, Ikram MA, Carlsson P, Chasman DI, Childs SJ, Longstreth WT, Seshadri S, Debette S.. Identification of additional risk loci for stroke and small vessel disease: a meta-analysis of genome-wide association studies. Lancet Neurol 2016;15:695–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tan R, Traylor M, Rutten-Jacobs L, Markus H.. New insights into mechanisms of small vessel disease stroke from genetics. Clin Sci 2017;131:515–531. [DOI] [PubMed] [Google Scholar]
- 41. Lopez LM, Hill WD, Harris SE, Valdes Hernandez M, Munoz Maniega S, Bastin ME, Bailey E, Smith C, McBride M, McClure J, Graham D, Dominiczak A, Yang Q, Fornage M, Ikram MA, Debette S, Launer L, Bis JC, Schmidt R, Seshadri S, Porteous DJ, Starr J, Deary IJ, Wardlaw JM.. Genes from a translational analysis support a multifactorial nature of white matter hyperintensities. Stroke 2015;46:341–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Senatus LM, Schmidt AM.. The AGE-RAGE Axis: implications for age-associated arterial diseases. Front Genet 2017;8:187.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wautier MP, Chappey O, Corda S, Stern DM, Schmidt AM, Wautier JL.. Activation of NADPH oxidase by AGE links oxidant stress to altered gene expression via RAGE. Am J Physiol Endocrinol Metab 2001;280:E685–E694. [DOI] [PubMed] [Google Scholar]
- 44. Carnevale D, Mascio G, D'Andrea I, Fardella V, Bell RD, Branchi I, Pallante F, Zlokovic B, Yan SS, Lembo G.. Hypertension induces brain beta-amyloid accumulation, cognitive impairment, and memory deterioration through activation of receptor for advanced glycation end products in brain vasculature. Hypertension 2012;60:188–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zlokovic BV. New therapeutic targets in the neurovascular pathway in Alzheimer's disease. Neurotherapeutics 2008;5:409–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Deane R, Du Yan S, Submamaryan RK, LaRue B, Jovanovic S, Hogg E, Welch D, Manness L, Lin C, Yu J, Zhu H, Ghiso J, Frangione B, Stern A, Schmidt AM, Armstrong DL, Arnold B, Liliensiek B, Nawroth P, Hofman F, Kindy M, Stern D, Zlokovic B.. RAGE mediates amyloid-beta peptide transport across the blood-brain barrier and accumulation in brain. Nat Med 2003;9:907–913. [DOI] [PubMed] [Google Scholar]
- 47. Yan SD, Chen X, Fu J, Chen M, Zhu H, Roher A, Slattery T, Zhao L, Nagashima M, Morser J, Migheli A, Nawroth P, Stern D, Schmidt AM.. RAGE and amyloid-beta peptide neurotoxicity in Alzheimer's disease. Nature 1996;382:685–691. [DOI] [PubMed] [Google Scholar]
- 48. Jokinen H, Melkas S, Madureira S, Verdelho A, Ferro JM, Fazekas F, Schmidt R, Scheltens P, Barkhof F, Wardlaw JM, Inzitari D, Pantoni L, Erkinjuntti T.. Cognitive reserve moderates long-term cognitive and functional outcome in cerebral small vessel disease. J Neurol Neurosurg Psychiatry 2016;87:1296–1302. [DOI] [PubMed] [Google Scholar]
- 49. Zieren N, Duering M, Peters N, Reyes S, Jouvent E, Herve D, Gschwendtner A, Mewald Y, Opherk C, Chabriat H, Dichgans M.. Education modifies the relation of vascular pathology to cognitive function: cognitive reserve in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Neurobiol Aging 2013;34:400–407. [DOI] [PubMed] [Google Scholar]
- 50. Ihara M, Yamamoto Y.. Emerging evidence for pathogenesis of sporadic cerebral small vessel disease. Stroke 2016;47:554–560. [DOI] [PubMed] [Google Scholar]
- 51. Benavente OR, Hart RG, Pergola PE, Palacio S, Castro I, Farias A, Roldan A, Kase C, Gavras I, Lau H, Ogrodnik M, Allen N, Meissner I, Graves J, Herzig D, Covalt J, Meyer B, Jackson C, Gamble P, The SPS3I.. Blood-pressure targets in patients with recent lacunar stroke: the SPS3 randomised trial. Lancet 2013;382:507–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bath PM, Wardlaw JM.. Pharmacological treatment and prevention of cerebral small vessel disease: a review of potential interventions. Int J Stroke 2015;10:469–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mok V, Kim JS.. Prevention and management of cerebral small vessel disease. J Stroke 2015;17:111–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kwee RM, Kwee TC.. Virchow-Robin spaces at MR imaging. Radiographics 2007;27:1071–1086. [DOI] [PubMed] [Google Scholar]
- 55. Woollam DH, Millen JW.. The perivascular spaces of the mammalian central nervous system and their relation to the perineuronal and subarachnoid spaces. J Anat 1955;89:193–200. [PMC free article] [PubMed] [Google Scholar]
- 56. Abbott NJ. Evidence for bulk flow of brain interstitial fluid: significance for physiology and pathology. Neurochem Int 2004;45:545–552. [DOI] [PubMed] [Google Scholar]
- 57. Bakker EN, Bacskai BJ, Arbel-Ornath M, Aldea R, Bedussi B, Morris AW, Weller RO, Carare RO.. Lymphatic clearance of the brain: perivascular, paravascular and significance for neurodegenerative diseases. Cell Mol Neurobiol 2016;36:181–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bucchieri F, Farina F, Zummo G, Cappello F.. Lymphatic vessels of the dura mater: a new discovery? J Anat 2015;227:702–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sweeney MD, Sagare AP, Zlokovic BV.. Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat Rev Neurol 2018;14:133–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, Benveniste H, Vates GE, Deane R, Goldman SA, Nagelhus EA, Nedergaard M.. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci Transl Med 2012;4:147ra111.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hannocks MJ, Pizzo ME, Huppert J, Deshpande T, Abbott NJ, Thorne RG, Sorokin L.. Molecular characterization of perivascular drainage pathways in the murine brain. J Cereb Blood Flow Metab 2017;271678X17749689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Carare RO, Bernardes-Silva M, Newman TA, Page AM, Nicoll JA, Perry VH, Weller RO.. Solutes, but not cells, drain from the brain parenchyma along basement membranes of capillaries and arteries: significance for cerebral amyloid angiopathy and neuroimmunology. Neuropathol Appl Neurobiol 2008;34:131–144. [DOI] [PubMed] [Google Scholar]
- 63. Jessen NA, Munk AS, Lundgaard I, Nedergaard M.. The glymphatic system: a Beginner’s guide. Neurochem Res 2015;40:2583–2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Mestre H, Kostrikov S, Mehta RI, Nedergaard M.. Perivascular spaces, glymphatic dysfunction, and small vessel disease. Clin Sci 2017;131:2257–2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Nedergaard M. Neuroscience. Garbage truck of the brain. Science 2013;340:1529–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Asgari M, de Zelicourt D, Kurtcuoglu V.. Glymphatic solute transport does not require bulk flow. Sci Rep 2016;6:38635.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ichimura T, Fraser PA, Cserr HF.. Distribution of extracellular tracers in perivascular spaces of the rat brain. Brain Res 1991;545:103–113. [DOI] [PubMed] [Google Scholar]
- 68. Rennels ML, Gregory TF, Blaumanis OR, Fujimoto K, Grady PA.. Evidence for a ‘paravascular’ fluid circulation in the mammalian central nervous system, provided by the rapid distribution of tracer protein throughout the brain from the subarachnoid space. Brain Res 1985;326:47–63. [DOI] [PubMed] [Google Scholar]
- 69. Proescholdt MG, Hutto B, Brady LS, Herkenham M.. Studies of cerebrospinal fluid flow and penetration into brain following lateral ventricle and cisterna magna injections of the tracer [14C]inulin in rat. Neuroscience 1999;95:577–592. [DOI] [PubMed] [Google Scholar]
- 70. Smith AJ, Verkman AS.. The “glymphatic” mechanism for solute clearance in Alzheimer’s disease: game changer or unproven speculation? Faseb J 2018;32:543–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Bacyinski A, Xu M, Wang W, Hu J.. The paravascular pathway for brain waste clearance: current understanding, significance and controversy. Front Neuroanat 2017;11:101.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Abbott NJ, Pizzo ME, Preston JE, Janigro D, Thorne RG.. The role of brain barriers in fluid movement in the CNS: is there a ‘glymphatic’ system? Acta Neuropathol 2018;135:387–407. [DOI] [PubMed] [Google Scholar]
- 73. Potter GM, Doubal FN, Jackson CA, Chappell FM, Sudlow CL, Dennis MS, Wardlaw JM.. Enlarged perivascular spaces and cerebral small vessel disease. Int J Stroke 2015;10:376–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Aribisala BS, Wiseman S, Morris Z, Valdes-Hernandez MC, Royle NA, Maniega SM, Gow AJ, Corley J, Bastin ME, Starr J, Deary IJ, Wardlaw JM.. Circulating inflammatory markers are associated with magnetic resonance imaging-visible perivascular spaces but not directly with white matter hyperintensities. Stroke 2014;45:605–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Ferguson SC, Blane A, Perros P, McCrimmon RJ, Best JJ, Wardlaw J, Deary IJ, Frier BM.. Cognitive ability and brain structure in type 1 diabetes: relation to microangiopathy and preceding severe hypoglycemia. Diabetes 2003;52:149–156. [DOI] [PubMed] [Google Scholar]
- 76. Zhu YC, Tzourio C, Soumare A, Mazoyer B, Dufouil C, Chabriat H.. Severity of dilated Virchow-Robin spaces is associated with age, blood pressure, and MRI markers of small vessel disease: a population-based study. Stroke 2010;41:2483–2490. [DOI] [PubMed] [Google Scholar]
- 77. Ringstad G, Vatnehol SAS, Eide PK.. Glymphatic MRI in idiopathic normal pressure hydrocephalus. Brain 2017;140:2691–2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Ramirez J, Berezuk C, McNeely AA, Gao F, McLaurin J, Black SE.. Imaging the perivascular space as a potential biomarker of neurovascular and neurodegenerative diseases. Cell Mol Neurobiol 2016;36:289–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Jiang Q, Zhang L, Ding G, Davoodi-Bojd E, Li Q, Li L, Sadry N, Nedergaard M, Chopp M, Zhang Z.. Impairment of the glymphatic system after diabetes. J Cereb Blood Flow Metab 2017;37:1326–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Benveniste H, Lee H, Volkow ND.. The glymphatic pathway. Neuroscientist 2017;1073858417691030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Plog BA, Nedergaard M.. The glymphatic system in central nervous system health and disease: past, present, and future. Annu Rev Pathol 2018;13:379–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Groeschel S, Chong WK, Surtees R, Hanefeld F.. Virchow-Robin spaces on magnetic resonance images: normative data, their dilatation, and a review of the literature. Neuroradiology 2006;48:745–754. [DOI] [PubMed] [Google Scholar]
- 83. Zhu YC, Dufouil C, Mazoyer B, Soumare A, Ricolfi F, Tzourio C, Chabriat H.. Frequency and location of dilated Virchow-Robin spaces in elderly people: a population-based 3D MR imaging study. AJNR Am J Neuroradiol 2011;32:709–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Chen W, Song X, Zhang Y, Alzheimer’s D, Neuroimaging I.. Assessment of the Virchow-Robin Spaces in Alzheimer disease, mild cognitive impairment, and normal aging, using high-field MR imaging. Am J Neuroradiol 2011;32:1490–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Bouvy WH, Biessels GJ, Kuijf HJ, Kappelle LJ, Luijten PR, Zwanenburg JJ.. Visualization of perivascular spaces and perforating arteries with 7 T magnetic resonance imaging. Invest Radiol 2014;49:307–313. [DOI] [PubMed] [Google Scholar]
- 86. Wang X, Chappell FM, Valdes Hernandez M, Lowe G, Rumley A, Shuler K, Doubal F, Wardlaw JM.. Endothelial function, inflammation, thrombosis, and basal ganglia perivascular spaces in patients with stroke. J Stroke Cerebrovasc Dis 2016;25:2925–2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Duperron MG, Tzourio C, Sargurupremraj M, Mazoyer B, Soumare A, Schilling S, Amouyel P, Chauhan G, Zhu YC, Debette S.. Burden of dilated perivascular spaces, an emerging marker of cerebral small vessel disease, is highly heritable. Stroke 2018;49:282–287. [DOI] [PubMed] [Google Scholar]
- 88. Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, Lindley RI, O’Brien JT, Barkhof F, Benavente OR, Black SE, Brayne C, Breteler M, Chabriat H, Decarli C, de Leeuw FE, Doubal F, Duering M, Fox NC, Greenberg S, Hachinski V, Kilimann I, Mok V, Oostenbrugge R, Pantoni L, Speck O, Stephan BC, Teipel S, Viswanathan A, Werring D, Chen C, Smith C, van Buchem M, Norrving B, Gorelick PB, Dichgans M, nEuroimaging STfRVco.. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol 2013;12:822–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Selvarajah J, Scott M, Stivaros S, Hulme S, Georgiou R, Rothwell N, Tyrrell P, Jackson A.. Potential surrogate markers of cerebral microvascular angiopathy in asymptomatic subjects at risk of stroke. Eur Radiol 2009;19:1011–1018. [DOI] [PubMed] [Google Scholar]
- 90. Doubal FN, MacLullich AM, Ferguson KJ, Dennis MS, Wardlaw JM.. Enlarged perivascular spaces on MRI are a feature of cerebral small vessel disease. Stroke 2010;41:450–454. [DOI] [PubMed] [Google Scholar]
- 91. Patankar TF, Mitra D, Varma A, Snowden J, Neary D, Jackson A.. Dilatation of the Virchow-Robin space is a sensitive indicator of cerebral microvascular disease: study in elderly patients with dementia. Am J Neuroradiol 2005;26:1512–1520. [PMC free article] [PubMed] [Google Scholar]
- 92. Klarenbeek P, van Oostenbrugge RJ, Lodder J, Rouhl RP, Knottnerus IL, Staals J.. Higher ambulatory blood pressure relates to enlarged Virchow-Robin spaces in first-ever lacunar stroke patients. J Neurol 2013;260:115–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Rouhl RP, van Oostenbrugge RJ, Knottnerus IL, Staals JE, Lodder J.. Virchow-Robin spaces relate to cerebral small vessel disease severity. J Neurol 2008;255:692–696. [DOI] [PubMed] [Google Scholar]
- 94. Martinez-Ramirez S, Pontes-Neto OM, Dumas AP, Auriel E, Halpin A, Quimby M, Gurol ME, Greenberg SM, Viswanathan A.. Topography of dilated perivascular spaces in subjects from a memory clinic cohort. Neurology 2013;80:1551–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. van Veluw SJ, Biessels GJ, Bouvy WH, Spliet WG, Zwanenburg JJ, Luijten PR, Macklin EA, Rozemuller AJ, Gurol ME, Greenberg SM, Viswanathan A, Martinez-Ramirez S.. Cerebral amyloid angiopathy severity is linked to dilation of juxtacortical perivascular spaces. J Cereb Blood Flow Metab 2016;36:576–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Charidimou A, Boulouis G, Pasi M, Auriel E, van Etten ES, Haley K, Ayres A, Schwab KM, Martinez-Ramirez S, Goldstein JN, Rosand J, Viswanathan A, Greenberg SM, Gurol ME.. MRI-visible perivascular spaces in cerebral amyloid angiopathy and hypertensive arteriopathy. Neurology 2017;88:1157–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Cumurciuc R, Guichard JP, Reizine D, Gray F, Bousser MG, Chabriat H.. Dilation of Virchow-Robin spaces in CADASIL. Eur J Neurol 2006;13:187–190. [DOI] [PubMed] [Google Scholar]
- 98. Yao M, Herve D, Jouvent E, Duering M, Reyes S, Godin O, Guichard JP, Dichgans M, Chabriat H.. Dilated perivascular spaces in small-vessel disease: a study in CADASIL. Cerebrovasc Dis 2014;37:155–163. [DOI] [PubMed] [Google Scholar]
- 99. Hansen TP, Cain J, Thomas O, Jackson A.. Dilated perivascular spaces in the Basal Ganglia are a biomarker of small-vessel disease in a very elderly population with dementia. AJNR Am J Neuroradiol 2015;36:893–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Arba F, Quinn TJ, Hankey GJ, Lees KR, Wardlaw JM, Ali M, Inzitari D.. On behalf of the Vista Collaboration. Enlarged perivascular spaces and cognitive impairment after stroke and transient ischemic attack. Int J Stroke 2018;13:47–56. [DOI] [PubMed] [Google Scholar]
- 101. Wardlaw JM, Doubal F, Armitage P, Chappell F, Carpenter T, Munoz Maniega S, Farrall A, Sudlow C, Dennis M, Dhillon B.. Lacunar stroke is associated with diffuse blood-brain barrier dysfunction. Ann Neurol 2009;65:194–202. [DOI] [PubMed] [Google Scholar]
- 102. Wuerfel J, Haertle M, Waiczies H, Tysiak E, Bechmann I, Wernecke KD, Zipp F, Paul F.. Perivascular spaces–MRI marker of inflammatory activity in the brain? Brain 2008;131:2332–2340. [DOI] [PubMed] [Google Scholar]
- 103. Vos CM, Geurts JJ, Montagne L, van Haastert ES, Bo L, van der Valk P, Barkhof F, de Vries HE.. Blood-brain barrier alterations in both focal and diffuse abnormalities on postmortem MRI in multiple sclerosis. Neurobiol Dis 2005;20:953–960. [DOI] [PubMed] [Google Scholar]
- 104. Elahy M, Jackaman C, Mamo JC, Lam V, Dhaliwal SS, Giles C, Nelson D, Takechi R.. Blood-brain barrier dysfunction developed during normal aging is associated with inflammation and loss of tight junctions but not with leukocyte recruitment. Immun Ageing 2015;12:2.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Fagiolo U, Cossarizza A, Santacaterina S, Ortolani C, Monti D, Paganelli R, Franceschi C.. Increased cytokine production by peripheral blood mononuclear cells from healthy elderly people. Ann N Y Acad Sci 1992;663:490–493. [DOI] [PubMed] [Google Scholar]
- 106. Fagiolo U, Cossarizza A, Scala E, Fanales-Belasio E, Ortolani C, Cozzi E, Monti D, Franceschi C, Paganelli R.. Increased cytokine production in mononuclear cells of healthy elderly people. Eur J Immunol 1993;23:2375–2378. [DOI] [PubMed] [Google Scholar]
- 107. Rouhl RP, Damoiseaux JG, Lodder J, Theunissen RO, Knottnerus IL, Staals J, Henskens LH, Kroon AA, de Leeuw PW, Tervaert JW, van Oostenbrugge RJ.. Vascular inflammation in cerebral small vessel disease. Neurobiol Aging 2012;33:1800–1806. [DOI] [PubMed] [Google Scholar]
- 108. Wiseman S, Marlborough F, Doubal F, Webb DJ, Wardlaw J.. Blood markers of coagulation, fibrinolysis, endothelial dysfunction and inflammation in lacunar stroke versus non-lacunar stroke and non-stroke: systematic review and meta-analysis. Cerebrovasc Dis 2014;37:64–75. [DOI] [PubMed] [Google Scholar]
- 109. Wiseman SJ, Bastin ME, Jardine CL, Barclay G, Hamilton IF, Sandeman E, Hunt D, Amft EN, Thomson S, Belch JF, Ralston SH, Wardlaw JM.. Cerebral small vessel disease burden is increased in systemic lupus erythematosus. Stroke 2016;47:2722–2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Swardfager W, Yu D, Ramirez J, Cogo-Moreira H, Szilagyi G, Holmes MF, Scott CJ, Scola G, Chan PC, Chen J, Chan P, Sahlas DJ, Herrmann N, Lanctot KL, Andreazza AC, Pettersen JA, Black SE.. Peripheral inflammatory markers indicate microstructural damage within periventricular white matter hyperintensities in Alzheimer’s disease: a preliminary report. Alzheimers Dement (Amst) 2017;7:56–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Rosenberg GA. Matrix metalloproteinase-mediated neuroinflammation in vascular cognitive impairment of the binswanger type. Cell Mol Neurobiol 2016;36:195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. McAleese KE, Alafuzoff I, Charidimou A, De Reuck J, Grinberg LT, Hainsworth AH, Hortobagyi T, Ince P, Jellinger K, Gao J, Kalaria RN, Kovacs GG, Kovari E, Love S, Popovic M, Skrobot O, Taipa R, Thal DR, Werring D, Wharton SB, Attems J.. Post-mortem assessment in vascular dementia: advances and aspirations. BMC Med 2016;14:129.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Mitaki S, Nagai A, Oguro H, Yamaguchi S.. C-reactive protein levels are associated with cerebral small vessel-related lesions. Acta Neurol Scand 2016;133:68–74. [DOI] [PubMed] [Google Scholar]
- 114. Faraco G, Brea D, Garcia-Bonilla L, Wang G, Racchumi G, Chang H, Buendia I, Santisteban MM, Segarra SG, Koizumi K, Sugiyama Y, Murphy M, Voss H, Anrather J, Iadecola C.. Dietary salt promotes neurovascular and cognitive dysfunction through a gut-initiated TH17 response. Nat Neurosci 2018;21:240–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Wiseman SJ, Doubal FN, Chappell FM, Valdes-Hernandez MC, Wang X, Rumley A, Lowe GD, Dennis MS, Wardlaw JM.. Plasma biomarkers of inflammation, endothelial function and hemostasis in cerebral small vessel disease. Cerebrovasc Dis 2015;40:157–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Faraco G, Park L, Anrather J, Iadecola C.. Brain perivascular macrophages: characterization and functional roles in health and disease. J Mol Med 2017;95:1143–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Gareau PJ, Wymore AC, Cofer GP, Johnson GA.. Imaging inflammation: direct visualization of perivascular cuffing in EAE by magnetic resonance microscopy. J Magn Reson Imaging 2002;16:28–36. [DOI] [PubMed] [Google Scholar]
- 118. Maggi P, Macri SM, Gaitan MI, Leibovitch E, Wholer JE, Knight HL, Ellis M, Wu T, Silva AC, Massacesi L, Jacobson S, Westmoreland S, Reich DS.. The formation of inflammatory demyelinated lesions in cerebral white matter. Ann Neurol 2014;76:594–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Rouhl RP, van Oostenbrugge RJ, Theunissen RO, Knottnerus IL, Staals J, Henskens LH, Kroon AA, de Leeuw PW, Lodder J, Tervaert JW, Damoiseaux JG.. Autoantibodies against oxidized low-density lipoprotein in cerebral small vessel disease. Stroke 2010;41:2687–2689. [DOI] [PubMed] [Google Scholar]
- 120. Bechmann I, Priller J, Kovac A, Bontert M, Wehner T, Klett FF, Bohsung J, Stuschke M, Dirnagl U, Nitsch R.. Immune surveillance of mouse brain perivascular spaces by blood-borne macrophages. Eur J Neurosci 2001;14:1651–1658. [DOI] [PubMed] [Google Scholar]
- 121. Guillemin GJ, Brew BJ.. Microglia, macrophages, perivascular macrophages, and pericytes: a review of function and identification. J Leukoc Biol 2004;75:388–397. [DOI] [PubMed] [Google Scholar]
- 122. Rosenberg GA. Extracellular matrix inflammation in vascular cognitive impairment and dementia. Clin Sci 2017;131:425–437. [DOI] [PubMed] [Google Scholar]
- 123. Jalal FY, Yang Y, Thompson J, Lopez AC, Rosenberg GA.. Myelin loss associated with neuroinflammation in hypertensive rats. Stroke 2012;43:1115–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Kaiser D, Weise G, Moller K, Scheibe J, Posel C, Baasch S, Gawlitza M, Lobsien D, Diederich K, Minnerup J, Kranz A, Boltze J, Wagner DC.. Spontaneous white matter damage, cognitive decline and neuroinflammation in middle-aged hypertensive rats: an animal model of early-stage cerebral small vessel disease. Acta Neuropathol Commun 2014;2:169.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Park L, Uekawa K, Garcia-Bonilla L, Koizumi K, Murphy M, Pistik R, Younkin L, Younkin S, Zhou P, Carlson G, Anrather J, Iadecola C.. Brain perivascular macrophages initiate the neurovascular dysfunction of alzheimer abeta peptides. Circ Res 2017;121:258–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Pires PW, Girgla SS, McClain JL, Kaminski NE, van Rooijen N, Dorrance AM.. Improvement in middle cerebral artery structure and endothelial function in stroke-prone spontaneously hypertensive rats after macrophage depletion. Microcirculation 2013;20:650–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Faraco G, Sugiyama Y, Lane D, Garcia-Bonilla L, Chang H, Santisteban MM, Racchumi G, Murphy M, Van Rooijen N, Anrather J, Iadecola C.. Perivascular macrophages mediate the neurovascular and cognitive dysfunction associated with hypertension. J Clin Invest 2016;126:4674–4689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Martinez Sosa S, Smith KJ.. Understanding a role for hypoxia in lesion formation and location in the deep and periventricular white matter in small vessel disease and multiple sclerosis. Clin Sci 2017;131:2503–2524. [DOI] [PubMed] [Google Scholar]
- 129. Jiwa NS, Garrard P, Hainsworth AH.. Experimental models of vascular dementia and vascular cognitive impairment: a systematic review. J Neurochem 2010;115:814–828. [DOI] [PubMed] [Google Scholar]
- 130. Hainsworth AH, Allan SM, Boltze J, Cunningham C, Farris C, Head E, Ihara M, Isaacs JD, Kalaria RN, Lesnik Oberstein SA, Moss MB, Nitzsche B, Rosenberg GA, Rutten JW, Salkovic-Petrisic M, Troen AM.. Translational models for vascular cognitive impairment: a review including larger species. BMC Med 2017;15:16.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Fisher CM. The arterial lesions underlying lacunes. Acta Neuropathol 1968;12:1–15. [DOI] [PubMed] [Google Scholar]
- 132. Iadecola C, Davisson RL.. Hypertension and cerebrovascular dysfunction. Cell Metab 2008;7:476–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Mulvany MJ, Hansen OK, Aalkjaer C.. Direct evidence that the greater contractility of resistance vessels in spontaneously hypertensive rats is associated with a narrowed lumen, a thickened media, and an increased number of smooth muscle cell layers. Circ Res 1978;43:854–864. [DOI] [PubMed] [Google Scholar]
- 134. Joutel A, Monet-Lepretre M, Gosele C, Baron-Menguy C, Hammes A, Schmidt S, Lemaire-Carrette B, Domenga V, Schedl A, Lacombe P, Hubner N.. Cerebrovascular dysfunction and microcirculation rarefaction precede white matter lesions in a mouse genetic model of cerebral ischemic small vessel disease. J Clin Invest 2010;120:433–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Lacombe P, Oligo C, Domenga V, Tournier-Lasserve E, Joutel A.. Impaired cerebral vasoreactivity in a transgenic mouse model of cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy arteriopathy. Stroke 2005;36:1053–1058. [DOI] [PubMed] [Google Scholar]
- 136. Sam K, Crawley AP, Poublanc J, Conklin J, Sobczyk O, Mandell DM, Duffin J, Venkatraghavan L, Fisher JA, Black SE, Mikulis DJ.. Vascular dysfunction in leukoaraiosis. Am J Neuroradiol 2016;37:2258–2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Uh J, Yezhuvath U, Cheng Y, Lu H.. In vivo vascular hallmarks of diffuse leukoaraiosis. J Magn Reson Imaging 2010;32:184–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Nonaka H, Akima M, Hatori T, Nagayama T, Zhang Z, Ihara F.. The microvasculature of the cerebral white matter: arteries of the subcortical white matter. J Neuropathol Exp Neurol 2003;62:154–161. [DOI] [PubMed] [Google Scholar]
- 139. Varga AW, Johnson G, Babb JS, Herbert J, Grossman RI, Inglese M.. White matter hemodynamic abnormalities precede sub-cortical gray matter changes in multiple sclerosis. J Neurol Sci 2009;282:28–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Lin J, Wang D, Lan L, Fan Y.. Multiple factors involved in the pathogenesis of white matter lesions. Biomed Res Int 2017;2017:9372050.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Shi Y, Thrippleton MJ, Makin SD, Marshall I, Geerlings MI, de Craen AJ, van Buchem MA, Wardlaw JM.. Cerebral blood flow in small vessel disease: a systematic review and meta-analysis. J Cereb Blood Flow Metab 2016;36:1653–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Capone C, Cognat E, Ghezali L, Baron-Menguy C, Aubin D, Mesnard L, Stohr H, Domenga-Denier V, Nelson MT, Joutel A.. Reducing Timp3 or vitronectin ameliorates disease manifestations in CADASIL mice. Ann Neurol 2016;79:387–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Joutel A, Chabriat H.. Pathogenesis of white matter changes in cerebral small vessel diseases: beyond vessel-intrinsic mechanisms. Clin Sci 2017;131:635–651. [DOI] [PubMed] [Google Scholar]
- 144. Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat Rev Neurosci 2011;12:723–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Desai RA, Davies AL, Tachrount M, Kasti M, Laulund F, Golay X, Smith KJ.. Cause and prevention of demyelination in a model multiple sclerosis lesion. Ann Neurol 2016;79:591–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Iliff JJ, Wang M, Zeppenfeld DM, Venkataraman A, Plog BA, Liao Y, Deane R, Nedergaard M.. Cerebral arterial pulsation drives paravascular CSF-interstitial fluid exchange in the murine brain. J Neurosci 2013;33:18190–18199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Kress BT, Iliff JJ, Xia M, Wang M, Wei HS, Zeppenfeld D, Xie L, Kang H, Xu Q, Liew JA, Plog BA, Ding F, Deane R, Nedergaard M.. Impairment of paravascular clearance pathways in the aging brain. Ann Neurol 2014;76:845–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Lecoq J, Parpaleix A, Roussakis E, Ducros M, Goulam Houssen Y, Vinogradov SA, Charpak S.. Simultaneous two-photon imaging of oxygen and blood flow in deep cerebral vessels. Nat Med 2011;17:893–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Davies AL, Desai RA, Bloomfield PS, McIntosh PR, Chapple KJ, Linington C, Fairless R, Diem R, Kasti M, Murphy MP, Smith KJ.. Neurological deficits caused by tissue hypoxia in neuroinflammatory disease. Ann Neurol 2013;74:815–825. [DOI] [PubMed] [Google Scholar]
- 150. Wardlaw JM, Makin SJ, Valdés Hernández MC, Armitage PA, Heye AK, Chappell FM, Muñoz-Maniega S, Sakka E, Shuler K, Dennis MS, Thrippleton MJ.. Blood-brain barrier failure as a core mechanism in cerebral small vessel disease and dementia: evidence from a cohort study. Alzheimers Dement (Amst) 2017;13:634–643. [Google Scholar]
- 151. Wardlaw JM, Sandercock PAG, Dennis MS, Starr J, Kalimo H.. Is breakdown of the blood-brain barrier responsible for lacunar stroke, leukoaraiosis, and dementia? Stroke 2003;34:806–812. [DOI] [PubMed] [Google Scholar]
- 152. Farrall AJ, Wardlaw JM.. Blood-brain barrier: ageing and microvascular disease–systematic review and meta-analysis. Neurobiol Aging 2009;30:337–352. [DOI] [PubMed] [Google Scholar]
- 153. Raja R, Rosenberg GA, Caprihan A.. MRI measurements of Blood-Brain Barrier function in dementia: a review of recent studies. Neuropharmacology 2017;S0028-3908(17)30501-4. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Skoog I, Wallin A, Fredman P, Hesse C, Aevarsson O, Karlsson I, Gottfries CG, Blennow K.. A population study on blood-brain barrier function in 85-year-olds: relation to Alzheimer’s disease and vascular dementia. Neurology 1998;50:966–971. [DOI] [PubMed] [Google Scholar]
- 155. Montagne A, Barnes SR, Sweeney MD, Halliday MR, Sagare AP, Zhao Z, Toga AW, Jacobs RE, Liu CY, Amezcua L, Harrington MG, Chui HC, Law M, Zlokovic BV.. Blood-brain barrier breakdown in the aging human hippocampus. Neuron 2015;85:296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Munoz Maniega S, Chappell FM, Valdes Hernandez MC, Armitage PA, Makin SD, Heye AK, Thrippleton MJ, Sakka E, Shuler K, Dennis MS, Wardlaw JM.. Integrity of normal-appearing white matter: influence of age, visible lesion burden and hypertension in patients with small-vessel disease. J Cereb Blood Flow Metab 2017;37:644–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Rost NS, Cougo P, Lorenzano S, Li H, Cloonan L, Bouts MJ, Lauer A, Etherton MR, Karadeli HH, Musolino PL, Copen WA, Arai K, Lo EH, Feske SK, Furie KL, Wu O.. Diffuse microvascular dysfunction and loss of white matter integrity predict poor outcomes in patients with acute ischemic stroke. J Cereb Blood Flow Metab 2018;38:75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Topakian R, Barrick TR, Howe FA, Markus HS.. Blood-brain barrier permeability is increased in normal-appearing white matter in patients with lacunar stroke and leucoaraiosis. J Neurol Neurosurg Psychiatry 2010;81:192–197. [DOI] [PubMed] [Google Scholar]
- 159. Arba F, Leigh R, Inzitari D, Warach SJ, Luby M, Lees KR.. Blood-brain barrier leakage increases with small vessel disease in acute ischemic stroke. Neurology 2017;89:2143–2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Zhang CE, Wong SM, van de Haar HJ, Staals J, Jansen JF, Jeukens CR, Hofman PA, van Oostenbrugge RJ, Backes WH.. Blood-brain barrier leakage is more widespread in patients with cerebral small vessel disease. Neurology 2017;88:426–432. [DOI] [PubMed] [Google Scholar]
- 161. Simpson JE, Wharton SB, Cooper J, Gelsthorpe C, Baxter L, Forster G, Shaw PJ, Savva G, Matthews FE, Brayne C, Ince PG.. Alterations of the blood-brain barrier in cerebral white matter lesions in the ageing brain. Neurosci Lett 2010;486:246–251. [DOI] [PubMed] [Google Scholar]
- 162. Tomimoto H, Akiguchi I, Suenaga T, Nishimura M, Wakita H, Nakamura S, Kimura J.. Alterations of the blood-brain barrier and glial cells in white-matter lesions in cerebrovascular and Alzheimer's disease patients. Stroke 1996;27:2069–2074. [DOI] [PubMed] [Google Scholar]
- 163. Young VG, Halliday GM, Kril JJ.. Neuropathologic correlates of white matter hyperintensities. Neurology 2008;71:804–811. [DOI] [PubMed] [Google Scholar]
- 164. Huisa BN, Caprihan A, Thompson J, Prestopnik J, Qualls CR, Rosenberg GA.. Long-term blood-brain barrier permeability changes in binswanger disease. Stroke 2015;46:2413–2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Taheri S, Gasparovic C, Huisa BN, Adair JC, Edmonds E, Prestopnik J, Grossetete M, Shah NJ, Wills J, Qualls C, Rosenberg GA.. Blood-brain barrier permeability abnormalities in vascular cognitive impairment. Stroke 2011;42:2158–2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Wardlaw JM, Doubal FN, Valdes-Hernandez M, Wang X, Chappell FM, Shuler K, Armitage PA, Carpenter TC, Dennis MS.. Blood-brain barrier permeability and long-term clinical and imaging outcomes in cerebral small vessel disease. Stroke 2013;44:525–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Hassan A, Hunt BJ, O’Sullivan M, Parmar K, Bamford JM, Briley D, Brown MM, Thomas DJ, Markus HS.. Markers of endothelial dysfunction in lacunar infarction and ischaemic leukoaraiosis. Brain 2003;126:424–432. [DOI] [PubMed] [Google Scholar]
- 168. Fassbender K, Mielke O, Bertsch T, Nafe B, Froschen S, Hennerici M.. Homocysteine in cerebral macroangiography and microangiopathy. Lancet 1999;353:1586–1587. [DOI] [PubMed] [Google Scholar]
- 169. Hassan A, Hunt BJ, O’Sullivan M, Bell R, D’Souza R, Jeffery S, Bamford JM, Markus HS.. Homocysteine is a risk factor for cerebral small vessel disease, acting via endothelial dysfunction. Brain 2004;127:212–219. [DOI] [PubMed] [Google Scholar]
- 170. Hupe M, Li MX, Kneitz S, Davydova D, Yokota C, Kele-Olovsson J, Hot B, Stenman JM, Gessler M.. Gene expression profiles of brain endothelial cells during embryonic development at bulk and single-cell levels. Sci Signal 2017;10. [DOI] [PubMed] [Google Scholar]
- 171. Reyahi A, Nik AM, Ghiami M, Gritli-Linde A, Ponten F, Johansson BR, Carlsson P.. Foxf2 is required for brain pericyte differentiation and development and maintenance of the blood-brain barrier. Dev Cell 2015;34:19–32. [DOI] [PubMed] [Google Scholar]
- 172. Winkler EA, Bell RD, Zlokovic BV.. Central nervous system pericytes in health and disease. Nat Neurosci 2011;14:1398–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Peppiatt CM, Howarth C, Mobbs P, Attwell D.. Bidirectional control of CNS capillary diameter by pericytes. Nature 2006;443:700–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Hall CN, Reynell C, Gesslein B, Hamilton NB, Mishra A, Sutherland BA, O’farrell FM, Buchan AM, Lauritzen M, Attwell D.. Capillary pericytes regulate cerebral blood flow in health and disease. Nature 2014;508:55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Hill RA, Tong L, Yuan P, Murikinati S, Gupta S, Grutzendler J.. Regional blood flow in the normal and ischemic brain is controlled by arteriolar smooth muscle cell contractility and not by capillary pericytes. Neuron 2015;87:95–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Attwell D, Mishra A, Hall CN, O’Farrell FM, Dalkara T.. What is a pericyte? J Cereb Blood Flow Metab 2016;36:451–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Bell RD, Winkler EA, Sagare AP, Singh I, LaRue B, Deane R, Zlokovic BV.. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron 2010;68:409–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Kisler K, Nelson AR, Rege SV, Ramanathan A, Wang Y, Ahuja A, Lazic D, Tsai PS, Zhao Z, Zhou Y, Boas DA, Sakadzic S, Zlokovic BV.. Pericyte degeneration leads to neurovascular uncoupling and limits oxygen supply to brain. Nat Neurosci 2017;20:406–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Montagne A, Nikolakopoulou AM, Zhao Z, Sagare AP, Si G, Lazic D, Barnes SR, Daianu M, Ramanathan A, Go A, Lawson EJ, Wang Y, Mack WJ, Thompson PM, Schneider JA, Varkey J, Langen R, Mullins E, Jacobs RE, Zlokovic BV.. Pericyte degeneration causes white matter dysfunction in the mouse central nervous system. Nat Med 2018;24:326–337. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 180. Hamilton NB, Attwell D, Hall CN.. Pericyte-mediated regulation of capillary diameter: a component of neurovascular coupling in health and disease. Front Neuroenerg 2010;2:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Zhao Z, Nelson AR, Betsholtz C, Zlokovic BV.. Establishment and dysfunction of the blood-brain barrier. Cell 2015;163:1064–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Sengillo JD, Winkler EA, Walker CT, Sullivan JS, Johnson M, Zlokovic BV.. Deficiency in mural vascular cells coincides with blood-brain barrier disruption in Alzheimer’s disease. Brain Pathol 2013;23:303–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Gu X, Liu XY, Fagan A, Gonzalez-Toledo ME, Zhao LR.. Ultrastructural changes in cerebral capillary pericytes in aged Notch3 mutant transgenic mice. Ultrastruct Pathol 2012;36:48–55. [DOI] [PubMed] [Google Scholar]
- 184. Ghosh M, Balbi M, Hellal F, Dichgans M, Lindauer U, Plesnila N.. Pericytes are involved in the pathogenesis of cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Ann Neurol 2015;78:887–900. [DOI] [PubMed] [Google Scholar]
- 185. Liu Q, Radwanski R, Babadjouni R, Patel A, Hodis DM, Baumbacher P, Zhao Z, Zlokovic B, Mack WJ.. Experimental chronic cerebral hypoperfusion results in decreased pericyte coverage and increased blood-brain barrier permeability in the corpus callosum. J Cereb Blood Flow Metab 2017;271678X17743670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Dziewulska D, Lewandowska E.. Pericytes as a new target for pathological processes in CADASIL. Neuropathology 2012;32:515–521. [DOI] [PubMed] [Google Scholar]
- 187. Craggs LJ, Fenwick R, Oakley AE, Ihara M, Kalaria RN.. Immunolocalization of platelet-derived growth factor receptor-beta (PDGFR-beta) and pericytes in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL). Neuropathol Appl Neurobiol 2015;41:557–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Rajani RM, Williams A.. Endothelial cell-oligodendrocyte interactions in small vessel disease and aging. Clin Sci 2017;131:369–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Held F, Morris AWJ, Pirici D, Niklass S, Sharp MMG, Garz C, Assmann A, Heinze HJ, Schreiber F, Carare RO, Schreiber S.. Vascular basement membrane alterations and beta-amyloid accumulations in an animal model of cerebral small vessel disease. Clin Sci 2017;131:1001–1013. [DOI] [PubMed] [Google Scholar]
- 190. Joutel A, Haddad I, Ratelade J, Nelson MT.. Perturbations of the cerebrovascular matrisome: a convergent mechanism in small vessel disease of the brain? J Cereb Blood Flow Metab 2016;36:143–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Bedussi B, van Lier MG, Bartstra JW, de Vos J, Siebes M, VanBavel E, Bakker EN.. Clearance from the mouse brain by convection of interstitial fluid towards the ventricular system. Fluids Barriers CNS 2015;12:23.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Tarasoff-Conway JM, Carare RO, Osorio RS, Glodzik L, Butler T, Fieremans E, Axel L, Rusinek H, Nicholson C, Zlokovic BV, Frangione B, Blennow K, Menard J, Zetterberg H, Wisniewski T, de Leon MJ.. Clearance systems in the brain-implications for Alzheimer disease. Nat Rev Neurol 2015;11:457–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Hawkes CA, Jayakody N, Johnston DA, Bechmann I, Carare RO.. Failure of perivascular drainage of beta-amyloid in cerebral amyloid angiopathy. Brain Pathol 2014;24:396–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Hawkes CA, Sullivan PM, Hands S, Weller RO, Nicoll JA, Carare RO.. Disruption of arterial perivascular drainage of amyloid-beta from the brains of mice expressing the human APOE epsilon4 allele. PLoS One 2012;7:e41636.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Peng W, Achariyar TM, Li B, Liao Y, Mestre H, Hitomi E, Regan S, Kasper T, Peng S, Ding F, Benveniste H, Nedergaard M, Deane R.. Suppression of glymphatic fluid transport in a mouse model of Alzheimer’s disease. Neurobiol Dis 2016;93:215–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Shibata M, Yamada S, Kumar SR, Calero M, Bading J, Frangione B, Holtzman DM, Miller CA, Strickland DK, Ghiso J, Zlokovic BV.. Clearance of Alzheimer's amyloid-ss(1-40) peptide from brain by LDL receptor-related protein-1 at the blood-brain barrier. J Clin Invest 2000;106:1489–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Deane R, Wu Z, Sagare A, Davis J, Du Yan S, Hamm K, Xu F, Parisi M, LaRue B, Hu HW, Spijkers P, Guo H, Song X, Lenting PJ, Van Nostrand WE, Zlokovic BV.. LRP/amyloid beta-peptide interaction mediates differential brain efflux of Abeta isoforms. Neuron 2004;43:333–344. [DOI] [PubMed] [Google Scholar]
- 198. Lin TW, Shih YH, Chen SJ, Lien CH, Chang CY, Huang TY, Chen SH, Jen CJ, Kuo YM.. Running exercise delays neurodegeneration in amygdala and hippocampus of Alzheimer’s disease (APP/PS1) transgenic mice. Neurobiol Learn Mem 2015;118:189–197. [DOI] [PubMed] [Google Scholar]
- 199. Herring A, Yasin H, Ambree O, Sachser N, Paulus W, Keyvani K.. Environmental enrichment counteracts Alzheimer’s neurovascular dysfunction in TgCRND8 mice. Brain Pathol 2008;18:32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Richter H, Ambree O, Lewejohann L, Herring A, Keyvani K, Paulus W, Palme R, Touma C, Schabitz WR, Sachser N.. Wheel-running in a transgenic mouse model of Alzheimer’s disease: protection or symptom? Behav Brain Res 2008;190:74–84. [DOI] [PubMed] [Google Scholar]
- 201. He XF, Liu DX, Zhang Q, Liang FY, Dai GY, Zeng JS, Pei Z, Xu GQ, Lan Y.. Voluntary exercise promotes glymphatic clearance of amyloid beta and reduces the activation of astrocytes and microglia in aged mice. Front Mol Neurosci 2017;10:144.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Mawuenyega KG, Sigurdson W, Ovod V, Munsell L, Kasten T, Morris JC, Yarasheski KE, Bateman RJ.. Decreased clearance of CNS beta-amyloid in Alzheimer’s disease. Science 2010;330:1774.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Gow AJ, Bastin ME, Munoz Maniega S, Valdes Hernandez MC, Morris Z, Murray C, Royle NA, Starr JM, Deary IJ, Wardlaw JM.. Neuroprotective lifestyles and the aging brain: activity, atrophy, and white matter integrity. Neurology 2012;79:1802–1808. [DOI] [PubMed] [Google Scholar]
- 204. Picchioni D, Reith RM, Nadel JL, Smith CB.. Sleep, plasticity and the pathophysiology of neurodevelopmental disorders: the potential roles of protein synthesis and other cellular processes. Brain Sci 2014;4:150–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Spira AP, Gamaldo AA, An Y, Wu MN, Simonsick EM, Bilgel M, Zhou Y, Wong DF, Ferrucci L, Resnick SM.. Self-reported sleep and beta-amyloid deposition in community-dwelling older adults. JAMA Neurol 2013;70:1537–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Weller RO, Djuanda E, Yow HY, Carare RO.. Lymphatic drainage of the brain and the pathophysiology of neurological disease. Acta Neuropathol 2009;117:1–14. [DOI] [PubMed] [Google Scholar]
- 207. Weller RO, Subash M, Preston SD, Mazanti I, Carare RO.. Perivascular drainage of amyloid-beta peptides from the brain and its failure in cerebral amyloid angiopathy and Alzheimer’s disease. Brain Pathol 2007;18:253–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Keable A, Fenna K, Yuen HM, Johnston DA, Smyth NR, Smith C, Al-Shahi Salman R, Samarasekera N, Nicoll JA, Attems J, Kalaria RN, Weller RO, Carare RO.. Deposition of amyloid beta in the walls of human leptomeningeal arteries in relation to perivascular drainage pathways in cerebral amyloid angiopathy. Biochim Biophys Acta 2016;1862:1037–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Roher AE, Kuo YM, Esh C, Knebel C, Weiss N, Kalback W, Luehrs DC, Childress JL, Beach TG, Weller RO, Kokjohn TA.. Cortical and leptomeningeal cerebrovascular amyloid and white matter pathology in Alzheimer’s disease. Mol Med 2003;9:112–122. [PMC free article] [PubMed] [Google Scholar]
- 210. Huffman J, Phillips S, Taylor GT, Paul R.. The emerging field of perivascular flow dynamics: biological relevance and clinical applications. Tech Innov 2016;18:63–74. [Google Scholar]
- 211. Simon MJ, Iliff JJ.. Regulation of cerebrospinal fluid (CSF) flow in neurodegenerative, neurovascular and neuroinflammatory disease. Biochim Biophys Acta 2016;1862:442–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Gaberel T, Gakuba C, Goulay R, Martinez De Lizarrondo S, Hanouz JL, Emery E, Touze E, Vivien D, Gauberti M.. Impaired glymphatic perfusion after strokes revealed by contrast-enhanced MRI: a new target for fibrinolysis? Stroke 2014;45:3092–3096. [DOI] [PubMed] [Google Scholar]
- 213. Wang M, Ding F, Deng S, Guo X, Wang W, Iliff JJ, Nedergaard M.. Focal solute trapping and global glymphatic pathway impairment in a murine model of multiple microinfarcts. J Neurosci 2017;37:2870–2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Iliff JJ, Chen MJ, Plog BA, Zeppenfeld DM, Soltero M, Yang L, Singh I, Deane R, Nedergaard M.. Impairment of glymphatic pathway function promotes tau pathology after traumatic brain injury. J Neurosci 2014;34:16180–16193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Venkat P, Chopp M, Zacharek A, Cui C, Zhang L, Li Q, Lu M, Zhang T, Liu A, Chen J.. White matter damage and glymphatic dysfunction in a model of vascular dementia in rats with no prior vascular pathologies. Neurobiol Aging 2017;50:96–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Schain AJ, Melo-Carrillo A, Strassman AM, Burstein R.. Cortical spreading depression closes paravascular space and impairs glymphatic flow: implications for migraine headache. J Neurosci 2017;37:2904–2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Bedussi B, Naessens DM, de Vos J, Olde Engberink R, Wilhelmus MM, Richard E, Ten Hove M, vanBavel E, Bakker EN.. Enhanced interstitial fluid drainage in the hippocampus of spontaneously hypertensive rats. Sci Rep 2017;7:744.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Ratner V, Gao Y, Lee H, Elkin R, Nedergaard M, Benveniste H, Tannenbaum A.. Cerebrospinal and interstitial fluid transport via the glymphatic pathway modeled by optimal mass transport. Neuroimage 2017;152:530–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, O'Donnell J, Christensen DJ, Nicholson C, Iliff JJ, Takano T, Deane R, Nedergaard M.. Sleep drives metabolite clearance from the adult brain. Science 2013;342:373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Lee H, Mortensen K, Sanggaard S, Koch P, Brunner H, Quistorff B, Nedergaard M, Benveniste H.. Quantitative Gd-DOTA uptake from cerebrospinal fluid into rat brain using 3D VFA-SPGR at 9.4T. Magn Reson Med 2018;79:1568–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Bailey EL, McCulloch J, Sudlow C, Wardlaw JM.. Potential animal models of lacunar stroke: a systematic review. Stroke 2009;40:e451–e458. [DOI] [PubMed] [Google Scholar]
- 222. Stevenson SF, Doubal FN, Shuler K, Wardlaw JM.. A systematic review of dynamic cerebral and peripheral endothelial function in lacunar stroke versus controls. Stroke 2010;41:e434–e442. [DOI] [PubMed] [Google Scholar]
- 223. Makedonov I, Chen JJ, Masellis M, MacIntosh BJ.. Alzheimer’s Disease Neuroimaging I. Physiological fluctuations in white matter are increased in Alzheimer's disease and correlate with neuroimaging and cognitive biomarkers. Neurobiol Aging 2016;37:12–18. [DOI] [PubMed] [Google Scholar]
- 224. Ballerini L, Lovreglio R, Valdes Hernandez MDC, Ramirez J, MacIntosh BJ, Black SE, Wardlaw JM.. Perivascular spaces segmentation in brain MRI using optimal 3D filtering. Sci Rep 2018;8:2132.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Ramirez J, Berezuk C, McNeely AA, Scott CJ, Gao F, Black SE.. Visible Virchow-Robin spaces on magnetic resonance imaging of Alzheimer’s disease patients and normal elderly from the Sunnybrook Dementia Study. J Alzheimers Dis 2015;43:415–424. [DOI] [PubMed] [Google Scholar]
- 226. Berezuk C, Ramirez J, Gao F, Scott CJ, Huroy M, Swartz RH, Murray BJ, Black SE, Boulos MI.. Virchow-Robin spaces: correlations with polysomnography-derived sleep parameters. Sleep 2015;38:853–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Musiek ES, Holtzman DM.. Mechanisms linking circadian clocks, sleep, and neurodegeneration. Science 2016;354:1004–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Kang JE, Lim MM, Bateman RJ, Lee JJ, Smyth LP, Cirrito JR, Fujiki N, Nishino S, Holtzman DM.. Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science 2009;326:1005–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Blair GW, Hernandez MV, Thrippleton MJ, Doubal FN, Wardlaw JM.. Advanced neuroimaging of cerebral small vessel disease. Curr Treat Options Cardio Med 2017;19:56.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. del C. Valdés Hernández M, Piper RJ, Wang X, Deary IJ, Wardlaw JM.. Towards the automatic computational assessment of enlarged perivascular spaces on brain magnetic resonance images: a systematic review. J Magn Reson Imaging 2013;38:774–785. [DOI] [PubMed] [Google Scholar]
- 231. Gonzalez-Castro V, Valdes Hernandez MDC, Chappell FM, Armitage PA, Makin S, Wardlaw JM.. Reliability of an automatic classifier for brain enlarged perivascular spaces burden and comparison with human performance. Clin Sci 2017;131:1465–1481. [DOI] [PubMed] [Google Scholar]
- 232. Benjamin P, Viessmann O, MacKinnon AD, Jezzard P, Markus HS.. 7 Tesla MRI in cerebral small vessel disease. Int J Stroke 2015;10:659–664. [DOI] [PubMed] [Google Scholar]
- 233. Taoka T, Masutani Y, Kawai H, Nakane T, Matsuoka K, Yasuno F, Kishimoto T, Naganawa S.. Evaluation of glymphatic system activity with the diffusion MR technique: diffusion tensor image analysis along the perivascular space (DTI-ALPS) in Alzheimer’s disease cases. Jpn J Radiol 2017;35:172–178. [DOI] [PubMed] [Google Scholar]
- 234. de Leon MJ, Li Y, Okamura N, Tsui WH, Saint-Louis LA, Glodzik L, Osorio RS, Fortea J, Butler T, Pirraglia E, Fossati S, Kim HJ, Carare RO, Nedergaard M, Benveniste H, Rusinek H.. Cerebrospinal fluid clearance in Alzheimer disease measured with dynamic PET. J Nucl Med 2017;58:1471–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Croall ID, Lohner V, Moynihan B, Khan U, Hassan A, O’brien JT, Morris RG, Tozer DJ, Cambridge VC, Harkness K, Werring DJ, Blamire AM, Ford GA, Barrick TR, Markus HS.. Using DTI to assess white matter microstructure in cerebral small vessel disease (SVD) in multicentre studies. Clin Sci 2017;131:1361–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Biesbroek JM, Weaver NA, Biessels GJ.. Lesion location and cognitive impact of cerebral small vessel disease. Clin Sci 2017;131:715–728. [DOI] [PubMed] [Google Scholar]
- 237. Wren PB, Hill D, Lockhart A.. Mechanisms of vascular disease in dementia: what does industry want to know? Clin Sci 2017;131:799–802. [DOI] [PubMed] [Google Scholar]