Abstract
Purpose:
To identify patterns of locoregional recurrence in patients treated with surgery and preoperative or postoperative radiotherapy or chemoradiation for rectal cancer.
Methods and Materials:
Between November 1989 and October 2001, 554 patients with rectal cancer were treated with surgery and preoperative (85%) or postoperative (15%) radiotherapy, with 95% receiving concurrent chemotherapy. Among these patients, 46 had locoregional recurrence as the first site of failure. CT images showing the site of recurrence and radiotherapy simulation films were available for 36 of the 46 patients. CT images were used to identify the sites of recurrence and correlate the sites to radiotherapy fields in these 36 patients.
Results:
The estimated 5-year locoregional control rate was 91%. The 36 patients in the study had locoregional recurrences at 43 sites. There were 28 (65%) in-field, 7 (16%) marginal, and 8 (19%) out-of-field recurrences. Among the in-field recurrences, 15 (56%) occurred in the low pelvis, 6 (22%) in the presacral region, 4 (15%) in the mid-pelvis and 2 (7%) in the high pelvis. Clinical T stage, pathologic T stage and pathologic N stage were significantly associated with the risk of in-field locoregional recurrence. The median survival after locoregional recurrence was 24.6 months.
Conclusions:
Patients treated with surgery and radiotherapy or chemoradiation for rectal cancer had a low risk of locoregional recurrence, with the majority of recurrences occurring within the radiation field. Since 78% of in-field recurrences occur in the low pelvic and presacral regions, consideration should be given to including the low pelvic and presacral regions in the radiotherapy boost field, especially in patients at high risk of recurrence.
Keywords: Rectal cancer, recurrence, radiation therapy, surgery, patterns of failure
INTRODUCTION
Preoperative or postoperative radiotherapy with concurrent chemotherapy is a current standard of care for patients with stage II and III rectal cancer1–7. Knowledge about patterns of relapse is essential for appropriate radiation therapy field design in rectal cancer patients. More than three decades ago, Gunderson and Sosin reported patterns of failure in rectal cancer patients treated with surgery8. Subsequently, other studies have characterized patterns of relapse in rectal patients treated with surgery without radiotherapy9–12. However, there is limited information about patterns of relapse in rectal patients treated with surgery and radiotherapy or chemoradiation. Such information may help determine whether modifications in radiotherapy dose or field design are warranted for rectal cancer patients.
The goal of this study was to identify sites of locoregional recurrence among over five hundred and fifty patients treated with surgery and radiotherapy, and to correlate the sites of locoregional recurrence to radiotherapy fields. To our knowledge, this represents the first effort to associate sites of relapse on computed tomography (CT) images with radiotherapy simulation films in rectal cancer patients.
METHODS AND MATERIALS
Between November 1989 and November 2001, 554 patients with newly diagnosed rectal cancer (located at ≤ 12 cm from the anal verge) were treated with mesorectal or local excision, and either preoperative (85%) or postoperative (15%) radiation therapy, with (95%) or without (5%) concurrent chemotherapy, at M. D. Anderson Cancer Center. Among these patients, 46 patients had a locoregional recurrence as the first site of failure, including those with a simultaneous distant metastasis (N=9). Patients who had a locoregional recurrence after the development of distant metastasis were excluded from this study. CT images documenting the site of locoregional recurrence and radiotherapy simulation films were available for 36 of the 46 patients. The study comprised these 36 patients. The clinical characteristics of the 36 patients and the full cohort of 554 patients are shown in Table 1. The M. D. Anderson Institutional Review Board approved this study.
Table 1:
Clinical and Treatment Characteristics
| Characteristic | All Patients (N=554) Number of Patients (%) | Study Patients (N=36) Number of Patients (%) |
|---|---|---|
| Age (years) | ||
| Median (Range) | 58 (20–88) | 55 (24–86) |
| Sex | ||
| Male | 336 (61%) | 14 (39%) |
| Female | 218 (39%) | 22 (61%) |
| Clinical T stage | ||
| ≤T2 | 61 (11%) | 4 (11%) |
| T3 | 414 (75%) | 21 (58%) |
| T4 | 41 (7%) | 7 (19%) |
| Unknown | 38 (7%) | 4 (11%) |
| Clinical N stage | ||
| N0 | 260 (47%) | 16 (44%) |
| N1–2 | 278 (50%) | 18 (50%) |
| Unknown | 16 (3%) | 2 (6%) |
| Pathologic T stage | ||
| ≤T2 | 311 (56%) | 14 (39%) |
| T3 | 8%) | 15 (42%) |
| T4 | 22 (4%) | 4 (11%) |
| Unknown | 8 (1%) | 3 (8%) |
| Pathologic N stage | ||
| N0 | 331(60%) | 14(39%) |
| N1–2 | 148 (27%) | 15 (42%) |
| Unknown | 75 (13%) | 7 (19%) |
| M stage | ||
| M0 | 522 (94%) | 34 (94%) |
| M1 | 32 (6%) | 2 (6%) |
| Distance from anal verge (cm) | ||
| Median (Range) | 5 (0–12) | 5 (0–12) |
| Radiotherapy Type | ||
| Preoperative | 473 (85%) | 28 (78%) |
| Postoperative | 81 (15%) | 8 (22%) |
| Radiotherapy Dose (cGy) | ||
| Median (Range) | 4500 (1440–6300) | 4500 (4500–5300) |
| Concurrent chemotherapy | 529 (95%) | 34 (94%) |
| Type of Surgery | ||
| Local Excision | 56 (10%) | 8 (22%) |
| Low Anterior Resection | 214 (39%) | 12 (33%) |
| Proctectomy/ coloanal anastomosis | 103 (19%) | 4 (11%) |
| Abdominoperineal resection | 156 (28%) | 9 (25%) |
| Pelvic Exenteration | 17 (3%) | 2 (6%) |
| Others | 8 (1%) | 1 (3%) |
Treatment
Of the 36 patients, 28 (78%) had received preoperative radiotherapy and 8 (22%) had received postoperative radiotherapy. The median radiation dose was 45 Gy (range 45–53 Gy). All 36 patients were treated in the prone position, using an open tabletop (belly board) device for bowel exclusion. Radiation therapy was delivered with a 3-field technique (posterior and two lateral fields) in 35 of the 36 patients. Patients were treated with standardized fields, based on departmental guidelines, with the superior border at the L5-S1 interspace, the inferior border at least 3 cm below the inferior extent of the tumor, lateral borders 1.5–2 cm outside the pelvic brim, anterior border 3 cm in front of the sacral promontory, and posterior border 1–1.5 cm behind the sacrum. However, there was some individual variation in field design, based on patient characteristics and physician preference. Radiation therapy was delivered by 18 MV photons with customized blocking. The pelvis was treated to a dose of 45 Gy in 25 fractions in all patients, along with a boost of 7.5–8 Gy in 10 patients. One patient received intra-operative radiotherapy (IORT) with an additional dose of 15 Gy. Thirty-four (94%) patients received concurrent chemotherapy, with 33 receiving concurrent protracted infusional 5-fluorouracil. Local excision was performed in 8 (22%), low anterior resection in 12 (33%), proctectomy with coloanal anastomosis in 4 (11%), abdominoperineal resection in 9 (25%), and pelvic exenteration in 2 (6%) patients. The treatment details of the full cohort of 554 patients are shown in Table 1.
Follow-up
Follow-up information was obtained from hospital records, radiation therapy department records and radiology reports. Follow-up information was also obtained from the M. D. Anderson Tumor Registry, which collects information on patients annually through letters, phone calls, and Bureau of Vital Statistics records. The median follow-up interval was 64 months (range 2–172 months).
Characterization of Locoregional Recurrences
Two radiologists (PRB and RBI) reviewed CT images on the 36 patients to identify the precise site of locoregional recurrence for each patient. Some patients had more than one site of locoregional recurrence, and the different sites were considered separately. With the aid of the radiologists, the sites of locoregional recurrence were marked on the radiotherapy simulation films for each patient. Each locoregional recurrence was classified into one of three categories: (1) In-field: Within the radiation field by more than 1 cm from the field border, (2) Marginal: Within 1 cm inside or outside the field border, and (3) Out-of-field: More than 1 cm from the field border. Each in-field recurrence was further classified into one of four categories: (1) Low pelvis: Below the top of the acetabulum, (2) Mid pelvis: From the top of the acetabulum to the bottom of the sacroiliac joint, (3) High pelvis: Above the bottom of the sacroiliac joint, and (4) Presacral: Within 2 cm anterior to the sacrum.
Statistical Analysis
Fisher’s exact test and Pearson’s chi-square test were used to evaluate association between categorical variables. Locoregional control, in-field control and overall survival were estimated by Kaplan-Meier methods.13 Log rank tests were performed to identify predictors of in-field recurrence. Cox proportional hazards regression analysis was then performed to identify significant multivariate predictors of recurrence. All univariate significant variables were entered into a multivariate model. In a backward stepwise fashion, the univariate significant variable with the least significance was eliminated from the multivariate model. This was continued until only significant variables remained. A p-value less than 0.05 was considered significant.
RESULTS
Among the entire cohort of 554 patients, 46 patients had locoregional recurrence as the first site of failure, including 9 patients who had simultaneous distant metastasis. The estimated 5-year rate of locoregional control was 91% (95% confidence interval, C.I. 88%−94%). CT images and radiotherapy simulation films were available for 36 of the 46 patients. These 36 patients had locoregional recurrences at 43 separate sites, and results on these sites of recurrence are presented below.
Patterns of Locoregional Recurrence
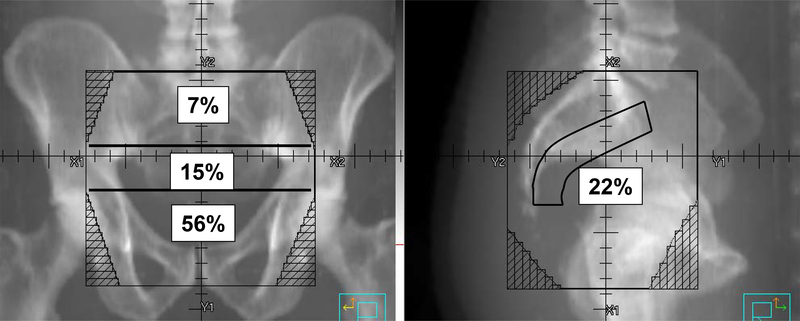
Among the 43 sites of locoregional recurrence, 28 (65%) were in-field recurrences, 7 (16%) were marginal recurrences, and 8 (19%) were out-of-field recurrences (Table 2). Among the in-field recurrences, 15 (56%) occurred in the low pelvis, 6 (22%) in the presacral region, 4 (15%) in the mid-pelvis, and 2 (7%) in the high pelvis (Figure 1). One in-field recurrence extended across multiple areas and could not be classified.
Table 2:
Patterns of Locoregional Recurrence
| Region | No. of Recurrences (%) |
|---|---|
| In-Field | 28 (65%) |
| Marginal | 7 (16%) |
| Out-of-Field | 8 (19%) |
Figure 1:
Distribution of In-Field Recurrences. The numbers denote the percentage of in-field recurrences at each region.
The patterns of locoregional recurrence were evaluated separately for patients treated with preoperative and postoperative radiotherapy. There were 33 sites of locoregional recurrence in the 28 patients treated with preoperative radiotherapy, of which 21 (64%) were in-field recurrences, 5 (15%) were marginal recurrences, and 7 (21%) were out-of-field recurrences. There were 10 sites of locoregional recurrence in the 8 patients treated with postoperative radiotherapy, of which 7 (70%) were in-field recurrences, 2 (20%) were marginal recurrences, and 1 (10%) was an out-of-field recurrence. Among the in-field recurrences in patients treated with preoperative radiotherapy, 12 (60%) occurred in the low pelvis, 5 (25%) in the presacral region, and 3 (15%) in the mid-pelvis. Among the in-field recurrences in patients treated with postoperative radiotherapy, 3 (43%) occurred in the low pelvis, 1 (14%) in the presacral region, 1 (14%) in the mid-pelvis, and 2 (29%) in the high pelvis.
Univariate analysis showed that clinical tumor stage T4 (p=0.043, hazard ratio, HR 2.94), pathologic tumor stage T4 (p=0.019, HR 3.87), and pathologic nodal stage N1–2 (p=0.008, HR 3.19) were significantly associated with a higher risk of in-field locoregional recurrence. Clinical N stage, distance from the anal verge, extent of surgery (local excision vs. mesorectal resection), positive radial margin, and radiotherapy dose were not significantly associated with the risk of in-field locoregional recurrence. Among patients treated with preoperative chemoradiation, T stage downstaging was not significantly associated with the risk of in-field locoregional recurrence. There was no significant association between tumor distance from the anal verge and the site of in-field recurrence. Multivariate Cox proportional hazards analysis showed that pathologic nodal stage N1–2 (p=0.026, HR 2.95) independently predicted for the risk of in-field locoregional recurrence.
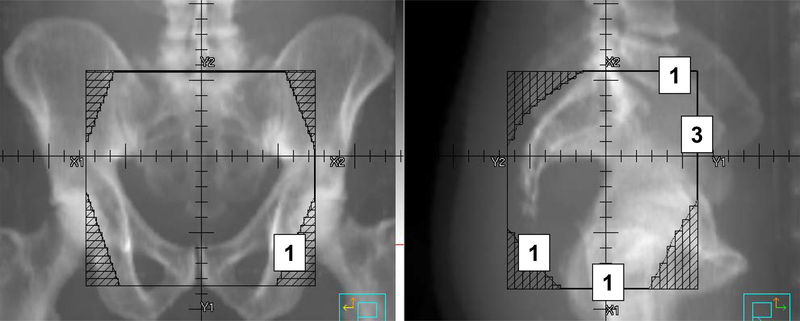
The locations of the marginal recurrences are shown in Figure 2. Two marginal recurrences occurred at the inferior field border, 3 at the anterior border, 1 at the superior border, and 1 at the lateral and inferior borders. Four of the 7 marginal recurrences occurred in areas that should be included in standard rectal radiotherapy fields, but were not included for those patients. Among the out-of-field recurrences, 2 occurred in the inguinal region, 4 occurred beyond the superior field border, 1 occurred beyond the anterior field border, and 1 occurred beyond the anterior and lateral field borders.
Figure 2:
Location of Marginal Recurrences. The numbers denote the number of marginal recurrences at each site.
Outcomes after Locoregional Recurrence
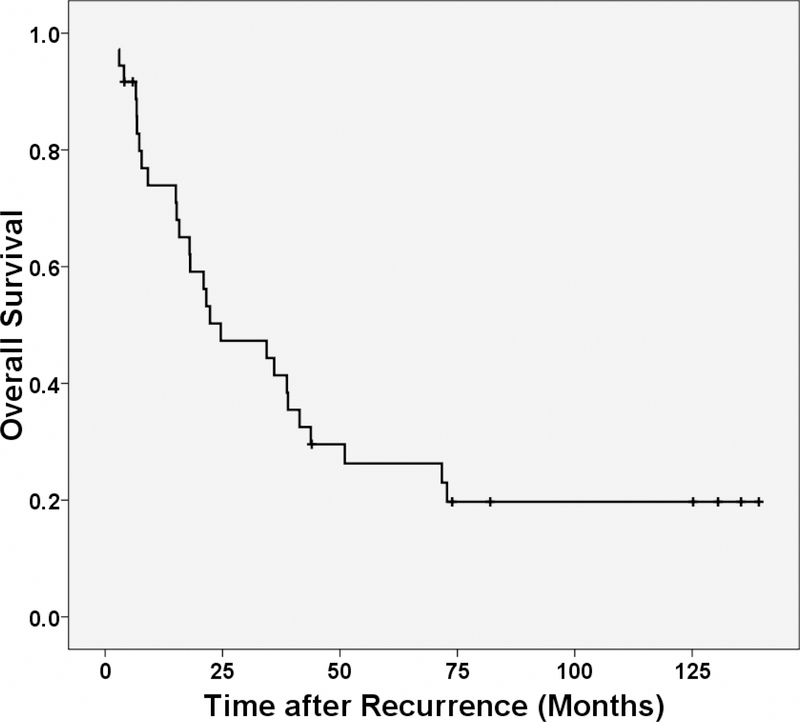
Among the 36 patients in this study, 27 (75%) died after having locoregional recurrence. Twenty-six of the deaths were from rectal cancer and 1 was from other causes. The median overall survival after locoregional recurrence was 24.6 months. The estimated 1-year, 3-year and 5-year rates of overall survival after locoregional recurrence were 74%, 41% and 26%, respectively. The Kaplan- Meier curve for overall survival after locoregional recurrence is shown in Figure 3.
Figure 3:
Kaplan-Meier estimates of overall survival after locoregional recurrence.
DISCUSSION
This represents the first study to correlate CT images and radiotherapy simulation films, and thereby identify patterns of locoregional recurrence in rectal cancer patients treated with surgery and radiotherapy or chemoradiation. The study shows that the combination of surgery and radiotherapy achieved good locoregional control rates in these patients, with an estimated 5-year locoregional control rate of 91%. Among the locoregional recurrences, around two-thirds were in-field recurrences, and only one-third were marginal or out-of-field recurrences. Of the in-field recurrences, nearly 80% occurred in the low pelvic and presacral regions. Hence, attempts should be made to improve locoregional control in the low pelvic and presacral regions.
A possible option to improve locoregional control in the low pelvic and presacral regions would be to include the entire low pelvic and presacral region in the radiotherapy boost field. However, it is unlikely that this approach can be studied prospectively. Since the risk of locoregional recurrence is low in patients treated with rectal cancer and surgery, a prohibitively large number of patients would be necessary to determine whether boosting the presacral and low pelvic regions improves locoregional control. At present, there is no consensus about the extent of the boost field for rectal cancer patients. Some radiation oncologists include just the rectal primary tumor with a margin, while others use a more generous field. At the authors’ institution, we have now adopted a boost field that includes the primary tumor and involved nodes, as well as the adjacent low pelvic region and presacral region. We believe that this approach could potentially improve locoregional control in the low pelvic and presacral regions, without significantly increasing toxicity from chemoradiation. The Radiation Therapy Oncology Group (RTOG) has also advocated including the entire sacral hollow in the boost field, in recent prospective trials on chemoradiation for rectal cancer14, 15. Another possible approach to enhancing locoregional control in the low pelvic and presacral regions could be the use of IORT in appropriate patients16–18. We must acknowledge that although in-field failures occur in the low pelvic and presacral regions, boosting these regions or administering IORT may not necessarily improve locoregional control.
Patients with clinical tumor stage T4, pathologic tumor stage T4 and pathologic nodal stage N1–2 had a higher risk of in-field recurrence on univariate analysis, while patients with pathologic nodal stage N1–2 had a higher risk of in-field recurrence on multivariate analysis. These factors could be used to identify patients that have a higher risk of in-field recurrence. Such patients could particularly benefit from a larger boost field that includes the low pelvic and presacral regions. These patients could also be targeted in trials of radiotherapy dose escalation, or trials of new radiosensitization agents. Moreover, these patients may benefit from more aggressive adjuvant chemotherapy regimens that could potentially help reduce the risk of locoregional recurrence. The recently published European Organization for Research and Treatment of Cancer (EORTC) randomized trial showed that chemotherapy improves local control, whether given concurrently with radiation, or in the adjuvant setting after surgery19.
Only a third of the locoregional recurrences were classified as marginal or out-of-field recurrences. Because of the small number of marginal and out-of-field recurrences, it was not feasible to investigate factors that may predict marginal or out-of-field recurrences. The marginal and out-of-field recurrences were scattered over a variety of areas. Some marginal recurrences occurred in patients in whom standard field borders were not used. We were not able to identify any discernible pattern of marginal or out-of-field failures that could be reduced by a modification of standard field borders. The relatively low number of marginal and out-of-field recurrences indicates that commonly used standard pelvic radiotherapy fields are appropriate for most rectal cancer patients.
Patients who had locoregional recurrence had a poor prognosis. The median overall survival was just over two years from the time of recurrence, and the estimated 3-year survival rate was 41%. The poor prognosis in these patients underscores the importance of optimizing locoregional control in rectal cancer patients.
Recently, studies have started investigating the use of new radiotherapy techniques such as intensity modulated radiation therapy (IMRT) for rectal cancer20–23. A thorough understanding of patterns of failure will be important as we develop and evaluate IMRT for rectal cancer. Investigators must ensure that pelvic nodal regions and potential sites of failure are appropriately covered in IMRT treatment plans. The results of this study could, therefore, inform IMRT field design.
This retrospective study has a number of limitations. Since complete information was not available for all patients, we may have underestimated the rate of locoregional recurrence. The study included only those patients who had CT images and radiotherapy simulation films available; hence, the results may have been affected by selection bias. The majority of patients in this study were treated at a time when CT simulation was not available. CT simulation could potentially help in more accurate delineation of the rectal tumor and any involved nodes, leading to more accurate and more customized field design, which could potentially result in enhanced locoregional control and decreased marginal and out-of-field failures. Therefore, patients treated using CT simulation may not have the same rate of locoregional recurrence or patterns of failure, as reported in this study. Most patients in this study were treated with standard pelvic radiotherapy fields, as described above. The patterns of failure seen in this study may not be applicable to patients treated with more limited fields, such as those used in the recent EORTC trial24. A radiotherapy boost was used in only 10 of the 36 patients in this study, and the patterns of failure may be different in patients treated routinely with a boost. The use of a boost could also potentially lower the risk of locoregional recurrence, and hence, for the past few years, the use of a boost has been adopted as a routine practice at the authors’ institution.
We recently reported that female sex, clinical T stage, pathologic T and N stage, and positive radial margin predicted for the overall risk of locoregional recurrence, in a similar cohort of patients25. In the present study, we found that only some of these factors (clinical T stage, pathologic T stage and pathologic N stage) affected the risk of in-field recurrence. However, since the number of events was much lower for in-field locoregional recurrences than all locoregional recurrences, we may have had limited power to evaluate the impact of different predictors.
In conclusion, rectal cancer patients treated with surgery and preoperative or postoperative radiotherapy or chemoradiation had low rates of locoregional recurrence. There were only a limited number of marginal and out-of-field failures, indicating that standard pelvic radiotherapy fields are appropriate for most rectal cancer patients. Since a large proportion of locoregional failures occurred within the radiotherapy field in the low pelvic and presacral regions, consideration should be given to including the low pelvic and presacral regions in the radiotherapy boost field, especially in patients at high risk of locoregional recurrence.
Acknowledgments
Supported in part by grant CA06294 from the National Cancer Institute, Department of Health and Human Services.
Footnotes
CONFLICT OF INTEREST NOTIFICATION:
Actual and potential conflicts of interest do not exist.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Anonymous. Prolongation of the disease-free interval in surgically treated rectal carcinoma. Gastrointestinal Tumor Study Group. N Engl J Med 1985;312:1465–1472. [DOI] [PubMed] [Google Scholar]
- 2.Krook JE, Moertel CG, Gunderson LL, et al. Effective surgical adjuvant therapy for high-risk rectal carcinoma. N Engl J Med 1991;324:709–715. [DOI] [PubMed] [Google Scholar]
- 3.O’ Connell M, Martenson JA, Wieand HS, et al. Improving adjuvant therapy for rectal cancer by combining protracted-infusion fluorouracil with radiation therapy after curative surgery. N Engl J Med 1994;331:502–507. [DOI] [PubMed] [Google Scholar]
- 4.Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 2004;351:1731–1740. [DOI] [PubMed] [Google Scholar]
- 5.Wolmark N, Wieand HS, Hyams DM, et al. Randomized trial of postoperative adjuvant chemotherapy with or without radiotherapy for carcinoma of the rectum: National Surgical Adjuvant Breast and Bowel Project Protocol R-02. J Natl Cancer Inst 2000;92:388–396. [DOI] [PubMed] [Google Scholar]
- 6.Folkesson J, Birgisson H, Pahlman L, et al. Swedish Rectal Cancer Trial: long lasting benefits from radiotherapy on survival and local recurrence rate. J Clin Oncol 2005;23:5644–5650. [DOI] [PubMed] [Google Scholar]
- 7.Kapiteijn E, Marijnen CA, Nagtegaal ID, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med 2001;345:638–646. [DOI] [PubMed] [Google Scholar]
- 8.Gunderson LL, Sosin H. Areas of failure found at reoperation (second or symptomatic look) following “curative surgery” for adenocarcinoma of the rectum. Clinicopathologic correlation and implications for adjuvant therapy. Cancer 1974;34:1278–1292. [DOI] [PubMed] [Google Scholar]
- 9.Minsky BD, Mies C, Recht A, et al. Resectable adenocarcinoma of the rectosigmoid and rectum. I. Patterns of failure and survival. Cancer 1988;61:1408–1416. [DOI] [PubMed] [Google Scholar]
- 10.Pilipshen SJ, Heilweil M, Quan SH, et al. Patterns of pelvic recurrence following definitive resections of rectal cancer. Cancer 1984;53:1354–1362. [DOI] [PubMed] [Google Scholar]
- 11.Hruby G, Barton M, Miles S, et al. Sites of local recurrence after surgery, with or without chemotherapy, for rectal cancer: implications for radiotherapy field design. Int J Radiat Oncol Biol Phys 2003;55:138–143. [DOI] [PubMed] [Google Scholar]
- 12.Hocht S, Hammad R, Thiel HJ, et al. Recurrent rectal cancer within the pelvis. A multicenter analysis of 123 patients and recommendations for adjuvant radiotherapy. Strahlenther Onkol 2004;180:15–20. [DOI] [PubMed] [Google Scholar]
- 13.Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958;53:457–481. [Google Scholar]
- 14.Radiation Therapy Oncology Group. RTOG R-0012 Randomized phase II trial of preoperative combined modality chemoradiation for distal rectal cancer. www.rtog.org Accessed June 28, 2007.
- 15.Radiation Therapy Oncology Group. RTOG 0247 Randomized phase II trial of neoadjuvant combined modality therapy for locally advanced rectal cancer. www.rtog.org Accessed June 28, 2007.
- 16.Nuyttens JJ, Kolkman-Deurloo IK, Vermaas M, et al. High-dose-rate intraoperative radiotherapy for close or positive margins in patients with locally advanced or recurrent rectal cancer. Int J Radiat Oncol Biol Phys 2004;58:106–112. [DOI] [PubMed] [Google Scholar]
- 17.Willett CG, Czito BG, Tyler DS. Intraoperative radiation therapy. J Clin Oncol 2007;25:971–977. [DOI] [PubMed] [Google Scholar]
- 18.Roeder F, Treiber M, Oertel S, et al. Patterns of failure and local control after intraoperative electron boost radiotherapy to the presacral space in combination with total mesorectal excision in patients with locally advanced rectal cancer. Int J Radiat Oncol Biol Phys 2007;67:1381–1388. [DOI] [PubMed] [Google Scholar]
- 19.Bosset JF, Collette L, Calais G, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med 2006;355:1114–1123. [DOI] [PubMed] [Google Scholar]
- 20.Meyer J, Czito B, Yin FF, et al. Advanced radiation therapy technologies in the treatment of rectal and anal cancer: intensity-modulated photon therapy and proton therapy. Clin Colorectal Cancer 2007;6:348–356. [DOI] [PubMed] [Google Scholar]
- 21.Freedman GM, Meropol NJ, Sigurdson ER, et al. Phase I trial of preoperative hypofractionated intensity-modulated radiotherapy with incorporated boost and oral capecitabine in locally advanced rectal cancer. Int J Radiat Oncol Biol Phys 2007;67:1389–1393. [DOI] [PubMed] [Google Scholar]
- 22.Kim JY, Kim DY, Kim TH, et al. Intensity-modulated radiotherapy with a belly board for rectal cancer. Int J Colorectal Dis 2007;22:373–379. [DOI] [PubMed] [Google Scholar]
- 23.Guerrero Urbano MT, Henrys AJ, Adams EJ, et al. Intensity-modulated radiotherapy in patients with locally advanced rectal cancer reduces volume of bowel treated to high dose levels. Int J Radiat Oncol Biol Phys 2006;65:907–916. [DOI] [PubMed] [Google Scholar]
- 24.Bosset JF, Calais G, Mineur L, et al. Enhanced tumorocidal effect of chemotherapy with preoperative radiotherapy for rectal cancer: preliminary results--EORTC 22921. J Clin Oncol 2005;23:5620–5627. [DOI] [PubMed] [Google Scholar]
- 25.Das P, Skibber JM, Rodriguez-Bigas MA, et al. Clinical and pathologic predictors of locoregional recurrence, distant metastasis, and overall survival in patients treated with chemoradiation and mesorectal excision for rectal cancer. Am J Clin Oncol 2006;29:219–224. [DOI] [PubMed] [Google Scholar]