Abstract
Objective.
Mesenchymal (MES) subtype of high-grade serous ovarian cancer (HGSOC) is associated with worse outcomes including survival and resectability compared with other molecular subtypes. Molecular subtypes have historically been derived from ‘tumor’, consisting of both cancer and stromal cells. We sought to determine the origins of multiple MES subtype gene signatures in HGSOC.
Methods.
Fifteen patients with MES subtype of HGSOC diagnosed between 2010 and 2013 were identified. Formalin-fixed paraffin-embedded (FFPE) blocks from primary surgery were sectioned for immunohistochemistry (IHC) staining of relevant proteins. Eight genes (ACTA2, COL5A1, COL11A1, FAP, POSTN, VCAN, ZEB1 and p-SMAD2) were selected for IHC staining based on their differential expression in MES vs. non-MES subtypes of HGSOC. Slides were scored for intensity and localization and simple statistics were used to compare expression results in cancer vs. stroma and between primary and metastatic sites.
Results.
COL5A1, VCAN, FAP, and ZEB1 proteins were almost exclusively expressed by stroma as opposed to cancer cells. In addition, stromal expression was dominant for ACTA2, COL11A1, POSTN and p-SMAD2. In general there were minimal differences in expression of proteins between primary and metastatic sites, exceptions being COL5A1 (reduced in metastases) and COL11A1 (increased in metastases). Nuclear p-SMAD2 expression was more common in metastatic stroma.
Conclusions.
The existing molecular classification of HGSOC MES subtype reflects a significant stromal contribution, suggesting an important role in HGSOC behavior and thus stroma may be a relevant therapeutic target. Specific patterns of expression indicate that collagens and TGF-β signaling are involved in the metastatic process.
Keywords: High-grade serous ovarian cancer (HGSOC), mesenchymal (MES), stroma, primary, metastatic
1. Introduction
In the United States high-grade serous ovarian cancer (HGSOC) accounts for 70% of ovarian cancer cases, most of these are advanced stages [1]. Typical first-line treatment includes cytoreductive surgery followed by chemotherapy. The majority of patients experience recurrence and the 5-year survival rate is less than 50% illustrating the need for novel therapies [1, 2].
Molecular subtypes of HGSOC have been defined by using tumor mRNA profiling to classify, and hopefully better understand, the biologic basis of tumor behavior [3–5]. Such developments should lead to more personalized therapeutic strategies instead of a ‘one size fits all’ approach. Tothill et al. initially clustered high-grade serous and endometrioid OC samples into 4 molecular subtypes: C1, C2, C4, and C5 [3]. The 4 molecular subtypes were subsequently validated by The Cancer Genome Atlas (TCGA) network and termed: mesenchymal (MES), immunoreactive (IMM), differentiated (DIF), and proliferative (PRO) subtypes [4].
We and others have shown that cases classified as MES subtype have the lowest rates of complete resection and have the poorest prognosis amongst HGSOC [5–8]. In our own cohort we observed that MES subtype was an independent predictor of overall survival (OS) in HGSOC (34.2 vs. 44.6 months; p=0.009, compared to non-MES subtype) [7]. We also reported that MES subtype is associated with higher rates of miliary and upper abdominal disease patterns suggesting a more invasive and metastatic phenotype. Clinically, these disease patterns are associated with dense stromal reaction and commonly contain areas of clinical fibrosis [7]. These results support the oft-quoted statement regarding HGSOC that the underlying biology of the disease impacts both phenotype (spread pattern) and outcomes.
Classification systems based on global transcriptional profiling have typically been considered to represent the expression specifically from cancer cells. However, whole-tumor samples contain both cancer cells and stroma, with stroma contributing a median of 50% of the OC tumor [9]. In the Tothill study, 40% of tumors within the C1 (MES) subgroup had a high percentage (defined as >50%) stromal component [3]. In TCGA analysis, the stromal content of samples was set at <30% but was not further described [4]. Relevant to the contribution from stroma to these gene signatures is the observation that specific genes (i.e., ACTA2, FAP, POSTN, ZEB1, and COL11A1) associated with worse outcomes in cancer are generally thought to arise from stroma not epithelial cells. By using IHC staining Tothill et al. observed that ACTA2 expression was strong in areas of stromal desmoplasia surrounding tumor, and suggested that this stromal contribution to the genetic signature was important [3]. Calon et al. reported that FAP showed higher expression in stroma and POSTN was exclusively expressed in stroma in colorectal tumors [10]. Isella et al. also reported that ZEB1 showed stronger staining in stroma compared with cancer cells in colorectal cancer [11]. Liu et al. found that COL11A1 was associated with stroma in HGSOC [6]. Many of these same genes are also significantly overexpressed in MES subtype of HGSOC [3–6, 12, 13]. It was also reported that stromal activation provides a favorable tumor microenvironment and stimulates OC metastasis [5, 8, 15]. Defining the cells of origin has significant implications for our understanding of events related to metastasis and clinical behavior of the cancer. Taken together, these observations support the concept that expression of MES subtype gene signatures is derived from both cancer and stromal populations and commonly from stromal compartment.
In this study, our primary hypothesis is that MES subtype gene signatures partially arise from cancer-associated stroma. We performed IHC to study the origin of several MES subtype gene signatures in tumor samples. An important strength of our study is the inclusion of both primary tumor and metastatic sites. This approach allowed us to ask if the stromal reaction was different at metastatic implantation sites or varied across tissue types.
2. Methods
2.1. Patients and slides
We studied 15 patients diagnosed with HGSOC between 2010 and 2013. Institutional Review Board approval was obtained for all studies and a written informed consent was obtained from all patients for use of data and biospecimens for research. Molecular subtype analysis categorized these 15 patients into MES subtype. A total of 45 formalin-fixed paraffin-embedded (FFPE) blocks (1 primary and 2 metastatic sites per patient) from primary surgery were selected to make slides.
2.2. Gene signatures
From the literature we selected 8 genes/proteins associated with the MES subtype. Table 2 describes the genes selected for this study and evidence from the literature of expression pattern in MES subtype in comparison with other molecular subtypes.
Table 2.
Gene selected in this study
| Gene symbol | Full name | Information | References |
|---|---|---|---|
| ACTA2 | Alpha-smooth muscle | Overexpressed in Cl subtype of OC | [3] |
| Overexpressed in MES subtype of OC | [5] | ||
| COL5A1 | Collagen type V, alpha 1 | Overexpressed in MES subtype of OC | [4,5] |
| Associated with poor OS in HGSOC | [24] | ||
| COL11A1 | Collage type XI, alpha 1 | Overexpressed in MES subtype of OC | [5] [4] |
| Associated with poor OS in HGSOC | [24] | ||
| FAP | Fibroblast activation protein | Overexpressed in Cl subtype of OC | [3] |
| Overexpressed in MES subtype of OC | [3, 4, 5] | ||
| POSTN | Periostin | Overexpressed in MES subtype of OC | [4, 5, 6] |
| Associated with poor OS in HGSOC | [24] | ||
| p-SMAD2 | Phosphorylated SMAD2 | TGF-p signaling significantly activated in MES subtype of OC | [6] |
| TGF-p signaling related to poor OS in HGSOC | [24] | ||
| VCAN | Versican | Overexpressed in MES subtype of OC | [5,6] |
| Associated with poor OS in HGSOC | [24] | ||
| Upregulated in cancer-associated fibroblasts | [13] | ||
| ZEB1 | Zinc finger E-box binding homeobox 1 |
Overexpressed in MES subtype of OC | [5, 6, 10] |
2.3. IHC staining
Five micrometer-thick paraffin sections were mounted on Superfrost Adhesion Slides and dried at room temperature for overnight. The sections were deparaffinized in xylene and hydrated and rehydrated in graded solutions of ethanol. The endogenous peroxidase was blocked with Peroxidazed 1 (Biocare Medical, Pacheco, CA, USA) for 5 mins. Antigen retrieval procedure was performed by using water bath heating in pH=8.0, 0.001M EDTA buffer or pH=6.0, 0.01M citrate buffer (Newcomer Supply, Middleton, WI, USA). Sections were incubated with DAKO Protein block serum-free ready-to-use solution (Agilent, Santa Clara, CA, USA) for 1 hour at room temperature to reduce the nonspecific binding. Primary antibodies were diluted with DAKO Antibody diluent (Agilent, Santa Clara, CA, USA) and incubated at 4°C for overnight. After washing, the sections were then incubated with Signal Stain Boost IHC Detection Solution (Cell signaling, Danvers, MA, USA) at room temperature for 30 mins. The slides were developed with Betazoid Diaminobenzidine (DAB) Chromogen (Agilent, Santa Clara, CA, USA), counterstained with haematoxylin, dehydrated in ethanol and xylene, and finally mounted. The stained slides then scanned with Desktop Scanner (Objective Imaging, Kansasville, WI, USA). Primary antibodies ACTA2 (ab5694), COL5A1 (ab7046), FAP (ab207178), POSTN (ab79946), and VCAN (ab177480) purchased from Abcam (Cambridge, UK); COL11A1 (PA5–36227) and p-SMAD2 (44–244G) from Thermo Fisher Scientific (Waltham, MA, USA); and ZEB1 (HPA027524) from Sigma-Aldrich (St. Louis, MO, USA).
2.4. IHC score
Two researchers independently, in a blinded manner, evaluated the intensity and distribution of positive staining of each slide. Slides and scores were reviewed together using a dual-headed scope to reach a final consensus score for any specimens that were discordant. A standard 4-point scale scoring system as 0 (absent), 1 (weak), 2 (moderate), and 3 (strong) was used to score intensity to study origin (stroma vs. cancer) and gene expression alteration (primary vs. metastatic sites). In expression alteration analysis, the average of 2 scores of metastatic sites was compared with the score of primary site.
2.5. Statistical analysis
The analysis of protein expression was performed using GraphPad Prism 7 software and statistical differences were evaluated using t-test.
3. Results
3.1. Patient demographics
Patients’ characteristics are shown in Table 1. All patients had MES subtype of HGSOC, and underwent cytoreductive surgery. The vast majority of patients had either high or intermediate complexity surgery. All patients had less than 0.5 cm of gross residual disease at the conclusion of surgery with 40% having complete gross resection. Most patients were either platinum sensitive (47%) or resistant (47%) showing disease progression within 6 months of completion of chemotherapy.
Table 1.
Patient demographics.
| Characteristic | N(%) |
|---|---|
| Age at surgery (years, mean ±SD) | 67 ±9 |
| Tumor stage | |
| IIC | 6 (40.0%) |
| IV | 9 (60.0%) |
| Surgical complex | |
| Low | 2(13.3%) |
| Intermediate | 6 (40.0%) |
| High | 7 (46.7%) |
| Residual disease (%of 323) | |
| 0 cm | 6 (40.0%) |
| 0.1–0.5 cm | 9 (60.0%) |
| Sensitivity to platinum | |
| Sensitive (Recurrence > 12 months) | 7 (46.7%) |
| Refractory (Recurrence = 6–12 months) | 1 (6.7%) |
| Resistant (Recurrence < 6 months) | 7 (46.7%) |
3.2. Gene signatures
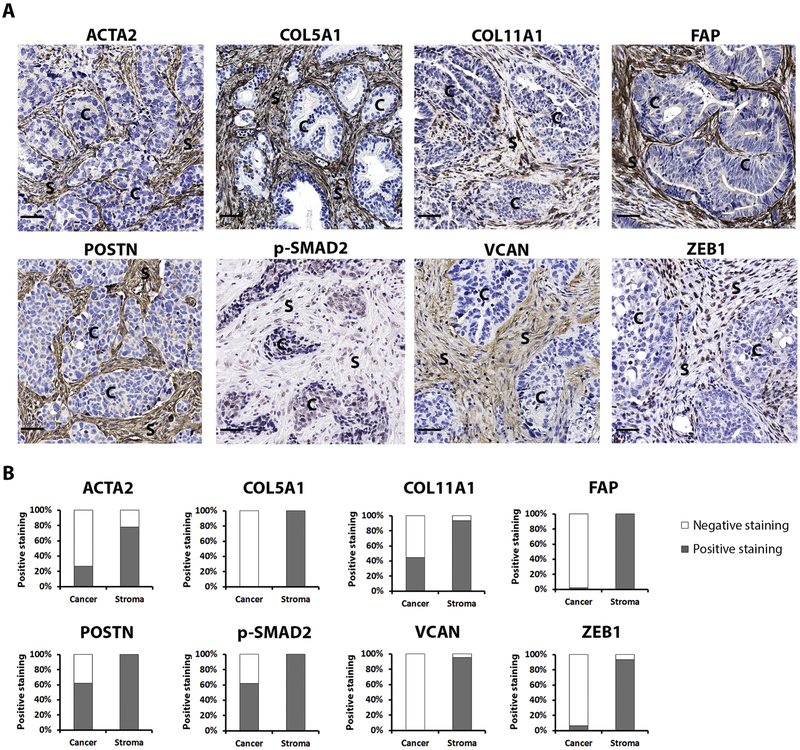
Based on existing literature, an initial candidate list selected proteins that are recognized to be highly expressed in MES vs. non-MES subtypes. We then narrowed this candidate list to focus on those with established antibodies that further performed well in testing in FFPE samples to arrive at the 8 proteins selected. Published reports of microarray analyses show correlation with overexpression and prognosis for many of these genes (Table 2). These proteins include alpha-smooth muscle (ACTA2), collagen type V alpha 1 (COL5A1), collagen type XI alpha 1 (COL11A1), fibroblast activation protein (FAP), periostin (POSTN), phosphorylated-SMAD2 (p-SMAD2), versican (VCAN), and zinc finger E-box binding homeobox 1 (ZEB1). The representative images of IHC staining of these proteins are shown in Fig. 1A.
Figure 1.
Protein expression in MES subtype of HGSOC.
3.3. Protein localization
The staining intensity was classified as: absent, weak, moderate, or strong. We used the broader 4-score grading system to help discern even low levels of protein expression which may be important in cancer vs. stroma of 45 samples (Fig. 1B). Three proteins (COL5A1, FAP, and VCAN) were expressed exclusively in stroma and they were expressed in all or the majority of 45 samples (COL5A1: 45/45, 100%; FAP: 45/45, 100%; VCAN: 43/45, 95.6%). For the 5 remaining proteins (ACTA2, COL11A1, POSTN, p-SMAD2 and ZEB1), stromal expression was the most commonly observed pattern (Fig. 1B). These data confirm that the molecular description of the MES subtype of HGSOC reflects a significant stromal contribution.
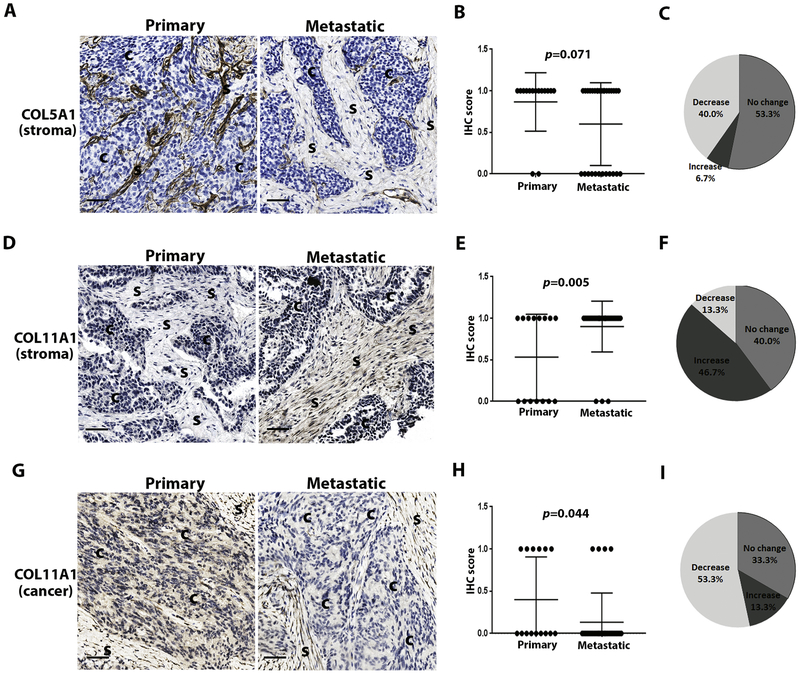
3.4. COL5A1 and COL11A1
We next investigated the differences in expression between primary and metastatic disease sites. We used the 2-score grading system of expression (negative = absent or weak, and positive = moderate or strong) to minimize the risk of over-interpretation of minor changes in protein expression across specimens. Two collagen proteins demonstrated different expression levels between the primary and metastatic sites within a given patient (Fig. 2). COL5A1 expression overall, was reduced in 40% (6/15) of cases in the metastatic vs. primary tumor (Fig. 2A, C). More specifically, COL5A1 expression was absent in 12/30 (40%) metastatic sites, compared with absent expression in only 2/15 (13.3%) of the primary sites (Fig. 2A & B, Supplementary Table 1). This trend did not reach statistical significance (p=0.071, Fig. 2B), though this likely reflects small sample size.
Figure 2.
COL5A1 and COL11A1 protein expression in primary vs. metastatic MES subtype of HGSOC.
In contrast, COL11A1 demonstrated increased expression in the stroma of metastatic vs. primary sites (p=0.005, Fig. 2D & E). In stroma, staining intensity was increased in 7/15 (46.7%) patients, no change in 6/15 (40%), and decreased in 2/15 (13.3%) patients (Fig. 2F). The relationship was reversed when considering expression from cancer cells instead of stroma: COL11A1 expression was reduced in metastatic vs. primary sites (p=0.044, Fig. 2G & H). Staining intensity in cancer cells was also decreased in 8/15 (53.3%) patients, no change in 5/15 (33.3%), and increased in 2/15 (13.3%) patients (Fig. 2I).
Collectively these results suggest that collagen proteins, as the major component of stroma play important but different roles in cancer metastasis.
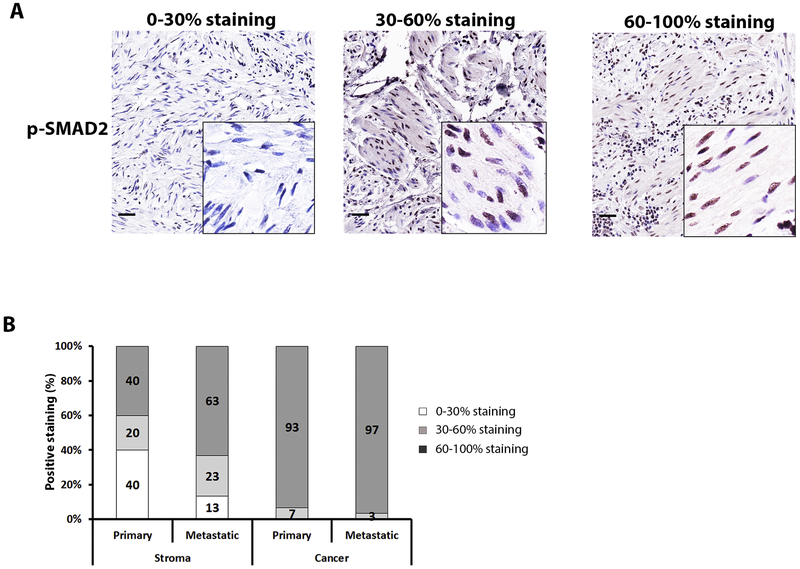
3.5. p-SMAD2
P-SMAD2 was expressed in the nucleus of both cancer cells and stroma (Fig. 1). We observed that nearly all cancer nuclei expressed p-SMAD2 (Fig. 3B & Supplementary Table 1). In contrast there was wider variation in nuclear expression of p-SMAD2 in stromal cells. Specifically, when evaluating the primary cancer, 40% of tumors demonstrated only rare (0–30%) nuclear expression – this decreased in the metastatic sites (Fig. 3B & Supplementary Table 1). We did not characterize the specific stromal cell type to be able to determine if this decrease in stromal expression was confined to a specific cell type. These results support that TGF-β signaling is important in stromal activation associated with disease metastases.
Figure 3.
P-SMAD2 expression in metastatic vs. primary MES subtype of HGSOC.
4. Discussion
The MES subtype of HGSOC is associated with several high risk clinical features including worse overall survival (OS), higher intraperitoneal disease dissemination patterns, and low rates of resection [7]. Molecularly, MES subtype is characterized by the overexpression of many genes which have been associated with activated stroma [3, 4], though the stromal component to the MES classification has not been well characterized. The current study demonstrates that multiple MES subtype gene signatures are primarily expressed in stroma rather than cancer cells (Fig. 1). In addition, several proteins appear differentially expressed between primary and metastatic sites including COL5A1, COL11A1 and p-SMAD2 (Fig. 2 & 3). These results support the hypothesis that stroma significantly contributes to what is currently considered the MES subtype of HGSOC. Stromal activation may play a role in the behaviors of these aggressive cancers.
It is acknowledged that cancer-associated stroma not only serves as a scaffold for tissue organization and integrity but also affects tumor microenvironment to promote cancer initiation, angiogenesis, invasion, and metastasis [14, 15]. The stimulatory contribution of stroma in tumor progression, coupled with the fact that stroma is more genetically stable compared with cancer cells, has led to stroma increasingly being recognized as a therapeutic target [16, 17]. In support of this, we recently published that inhibition of TGF-β signaling, which suppresses the cancer-stroma interplay, can delay tumor growth and ascites development in MES subtype HGSOC patient-derived xenografts [18]. In comparison with the advances in our understanding of cancer cell biology, our knowledge of the nature and roles of stromal contribution has lagged. Our findings suggest that molecular classifications are at least partially comprised of stroma signatures: whether this represents signal or response to tumor cells will need to be determined. Improved understanding of the complicated stromal biology will accelerate the development of effective therapeutic strategies for HGSOC, especially for MES HGSOC. Stroma contains both a cellular component (e.g., fibroblasts, immune cells) and a non-cellular component (e.g., extracellular matrix) [17, 19]. How each constituent affects cancer progression is still largely unknown.
Metastasis occurs in the vast majority of high-grade epithelial ovarian cancer, making presentation at advanced stage common [20]. After initial surgery, most patients will be left with relatively large volume of microscopic cancer cells, even after complete gross resection. This so-called ‘minimal residual’ model of widespread intraperitoneal disease after initial surgery influences recurrence patterns as these sites often harbor dormant cells. Inhibiting metastatic potential or halting the growth and invasiveness of established metastasis should improve progression free survival. Cancer-associated stroma has been shown to promote tumor progression [16, 21]. Our preliminary results demonstrate the collagen proteins (COL5A1 and COL11A1) and TGF-β signaling protein (p-SMAD2) may be differentially expressed in primary vs. metastatic sites.
Collagen is the most abundant constituent of extracellular matrix (ECM). Collagens degrading, re-depositing, cross-linking and stiffening are the characteristics of stroma activation to promote cancer metastasis [19, 22]. Increases and decreases of collagen are coordinated reciprocally to promote tumor metastasis and invasion [22]. Microarray data analysis from whole tumors demonstrates that both COL11A1 and COL5A1 are enriched in metastatic vs. primary tumors in OC [23]. Though limited in sample size, we confirm these observations showing that COL11A1 is increased in metastatic vs. primary tumors (Fig. 2D–F). Contrary to the increase of COL11A1 in stroma, COL5A1 appears reduced in stroma associated with metastases (Fig. 2A–C). Until now only a few studies have been published on COL5A1. Previous research suggested that high expression of COL5A1 mRNA from whole tumors was associated with metastasis and/or poor survival and that COL5A1 knockdown in vitro in cancer cell lines inhibited cell migration and invasion in lung adenocarcinoma, gastric cancer and OC [23–25]. Our results are based on small numbers and differences in techniques, primary cancers vs. cell lines, and the focus on cancer cells vs. stroma likely play a role in explaining these differences.
TGF-β signaling plays an important role in cancer-stromal crosstalk and is an important driver of fibroblast activation [10, 23, 26, 27]. P-SMAD2 expression is an indicator of active canonical TGF-β signaling and this occurs in both cancer and stromal cells [28]. We observed that an increasing number of stromal cells exhibit p-SMAD2 expression in metastatic vs. primary sites (Fig. 3) implicating activation of TGF-β signaling in OC metastasis. Supporting the proposed importance of TGF-β in metastases, we have demonstrated that inhibition of TGF-β signaling delays tumor growth and suppresses ascites development in OC xenograft models [18]. Collectively these observations demonstrate the potential of TGF-β signaling targeting as a therapeutic strategy in HGSOC.
An important strength of our study is that we begin to compare gene expression between primary and metastatic sites and this highlights the significant heterogeneity across tumor sites. Understanding this heterogeneity within individual patients is important as it will be a limitation of triage or therapy based biopsy strategy. Our study has some important limitations. The study was performed on 45 patient samples from only 15 patients and assessing subtle difference in expression between sites is somewhat subjective. A larger study will be required to validate these findings and to investigate the clinical significance of patterns of expression. We demonstrated that all tested gene signatures mainly arise from stroma rather than cancer cells but the specific impact of these proteins in OC behavior is not known. Further functional study of these genes is required.
In conclusion, this is the first study to clearly show that the origins of MES subtype gene signatures derive substantially from stroma rather than cancer cells in OC. This observation supports the value of stroma targeting in treatment of MES subtype HGSOC patients.
Supplementary Material
Highlights.
Stroma significantly contributes to the molecular classification of mesenchymal subtype of high-grade serous ovarian cancer.
Collagen expression is associated with metastases of mesenchymal subtype of high-grade serous ovarian cancer but with different roles.
Activated TGF-β signaling is associated with metastases of mesenchymal subtype of high-grade serous ovarian cancer.
Funding:
This research was supported by the Mayo Clinic Specialized Program in Research Excellence (SPORE) grant CA136393 and the Minnesota Ovarian Cancer Alliance (MOCA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest
None of the authors has any conflicts of interest to declare.
References
- 1.Siegel RL, Miller KD, and Jemal A, Cancer statistics, 2018. CA Cancer J Clin, 2018. 68(1): p. 7–30. [DOI] [PubMed] [Google Scholar]
- 2.Matulonis UA, et al. , Ovarian cancer. Nat Rev Dis Primers, 2016. 2: p. 16061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tothill RW, et al. , Novel molecular subtypes of serous and endometrioid ovarian cancer linked to clinical outcome. Clin Cancer Res, 2008. 14(16): p. 5198–208. [DOI] [PubMed] [Google Scholar]
- 4.Cancer Genome Atlas Research, N., Integrated genomic analyses of ovarian carcinoma. Nature, 2011. 474(7353): p. 609–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang C, et al. , Pooled Clustering of High-Grade Serous Ovarian Cancer Gene Expression Leads to Novel Consensus Subtypes Associated with Survival and Surgical Outcomes. Clin Cancer Res, 2017. 23(15): p. 4077–4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu Z, et al. , Suboptimal cytoreduction in ovarian carcinoma is associated with molecular pathways characteristic of increased stromal activation. Gynecol Oncol, 2015. 139(3): p. 394–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Torres D, et al. , Factors that influence survival in high-grade serous ovarian cancer: A complex relationship between molecular subtype, disease dissemination, and operability. Gynecol Oncol, 2018. 150(2): p. 227–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang S, et al. , Stroma-associated master regulators of molecular subtypes predict patient prognosis in ovarian cancer. Sci Rep, 2015. 5: p. 16066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Labiche A, et al. , Stromal compartment as a survival prognostic factor in advanced ovarian carcinoma. Int J Gynecol Cancer, 2010. 20(1): p. 28–33. [DOI] [PubMed] [Google Scholar]
- 10.Calon A, et al. , Stromal gene expression defines poor-prognosis subtypes in colorectal cancer. Nat Genet, 2015. 47(4): p. 320–9. [DOI] [PubMed] [Google Scholar]
- 11.Isella C, et al. , Stromal contribution to the colorectal cancer transcriptome. Nat Genet, 2015. 47(4): p. 312–9. [DOI] [PubMed] [Google Scholar]
- 12.Konecny GE, et al. , Prognostic and therapeutic relevance of molecular subtypes in high-grade serous ovarian cancer. J Natl Cancer Inst, 2014. 106(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yeung TL, et al. , TGF-beta modulates ovarian cancer invasion by upregulating CAF-derived versican in the tumor microenvironment. Cancer Res, 2013. 73(16): p. 5016–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Egeblad M, Rasch MG, and Weaver VM, Dynamic interplay between the collagen scaffold and tumor evolution. Curr Opin Cell Biol, 2010. 22(5): p. 697–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davidson B, Trope CG, and Reich R, The role of the tumor stroma in ovarian cancer. Front Oncol, 2014. 4: p. 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yeung TL, et al. , Targeting Stromal-Cancer Cell Crosstalk Networks in Ovarian Cancer Treatment. Biomolecules, 2016. 6(1): p. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Valkenburg KC, de Groot AE, and Pienta KJ, Targeting the tumour stroma to improve cancer therapy. Nat Rev Clin Oncol, 2018. 15(6): p. 366–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Q, et al. , LY2157299 Monohydrate, a TGF-betaR1 Inhibitor, Suppresses Tumor Growth and Ascites Development in Ovarian Cancer. Cancers (Basel), 2018. 10(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shoulders MD and Raines RT, Collagen structure and stability. Annu Rev Biochem, 2009. 78: p. 929–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lengyel E, Ovarian cancer development and metastasis. Am J Pathol, 2010. 177(3): p. 1053–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hansen JM, Coleman RL, and Sood AK, Targeting the tumour microenvironment in ovarian cancer. Eur J Cancer, 2016. 56: p. 131–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fang M, et al. , Collagen as a double-edged sword in tumor progression. Tumour Biol, 2014. 35(4): p. 2871–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheon DJ, et al. , A collagen-remodeling gene signature regulated by TGF-beta signaling is associated with metastasis and poor survival in serous ovarian cancer. Clin Cancer Res, 2014. 20(3): p. 711–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu W, et al. , COL5A1 may contribute the metastasis of lung adenocarcinoma. Gene, 2018. 665: p. 57–66. [DOI] [PubMed] [Google Scholar]
- 25.Zhao X, et al. , Discovery of signature genes in gastric cancer associated with prognosis. Neoplasma, 2016. 63(2): p. 239–45. [DOI] [PubMed] [Google Scholar]
- 26.Karlan BY, et al. , POSTN/TGFBI-associated stromal signature predicts poor prognosis in serous epithelial ovarian cancer. Gynecol Oncol, 2014. 132(2): p. 334–42. [DOI] [PubMed] [Google Scholar]
- 27.Calon A, et al. , Dependency of colorectal cancer on a TGF-beta-driven program in stromal cells for metastasis initiation. Cancer Cell, 2012. 22(5): p. 571–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Massague J, TGFbeta signalling in context. Nat Rev Mol Cell Biol, 2012. 13(10): p. 616–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.