Abstract
Background and Objectives
The present cross-sectional study examines gender differences in three major health measures among older adults in India and in China, and investigates whether these differences can be explained by major sociodemographic and health risk characteristics.
Research Design and Methods
The study included 7,150 individuals in India and 13,367 individuals in China aged 50-plus who participated in the WHO Study on Global AGEing and Adult Health in 2007–2010. Logistic regression models for self-reported health (SRH) and ordinary least square regression models for grip strength and cognitive function were used to investigate gender differences in health.
Results
A consistent female disadvantage was found in India and in China for all three health measures. Compared to their male counterparts, women in the Indian and the Chinese samples had, respectively, 38% (95% confidence interval [CI]: 1.22, 1.56) and 36% (95% CI: 1.25, 1.48) higher risk of reporting poor SRH, 9.56 kg (95% CI: 9.91, 9.22) and 11.95 kg (95% CI: 12.29, 11.62) lower grip strength, and 3.64 (95% CI: 3.96, 3.32) and 1.99 (95% CI: 2.28, 1.71) lower cognitive scores. The magnitude of the female disadvantage in poor SRH and in grip strength changed very little when adjustments were made for marital status, education, place of residence, smoking status, height, and number of chronic conditions; but these characteristics accounted for about 50% of the gender gap in cognitive function.
Discussion and Implications
In these study populations, major sociodemographic and health risk characteristics accounted for very small parts of the gender differences in health, except in cognition.
Keywords: China, Cognition, Cross-country comparison, Gender difference, Grip strength, Health, India, Older age, Self-rated health
Background and Objectives
Research evidence suggests that although women are currently expected to live longer than men worldwide, women tend to report being in worse health than men (Barford, Dorling, Smith, & Shaw, 2006; Oksuzyan, Juel, Vaupel, & Christensen, 2008; UN, 2015). Comparisons of developed countries have consistently found a female disadvantage in levels of physical disability, depression, and physical performance; while gender differences in morbidity levels have been shown to vary depending on the chronic condition (Crimmins, Kim, & Solé-Auró, 2011; Oksuzyan et al., 2010; Van de Velde, Bracke, & Levecque, 2010). Research findings from low- and middle-income countries have suggested that relative to men, women have worse general health (Ng et al., 2010), lower physical functioning levels (Yount & Agree, 2005), a substantial disadvantage in physical performance levels (Zunzunegui, Alvarado, Béland, & Vissandjee, 2009), and lower scores on various cognition measures (Weir, Lay, & Langa, 2014).
The existing research evidence regarding the underlying determinants of gender differences in health in India and in China is scarce and mixed. Some findings suggest that the female disadvantage in health persists even after controlling for socioeconomic characteristics, whereas others indicate that such an adjustment may reverse the direction of the gender gap (Pandey & Ladusingh, 2015; Saikia, Bora, Jasilionis, & Shkolnikov, 2016). In the present study, we analyze the gender differences in three major health measures among older urban and rural subpopulations in India and in China, and examine whether these differences can be explained by major sociodemographic characteristics and health risk factors.
In India, temporary life expectancy (LE, between exact ages 0 and 60) among women did not exceed temporary LE among men until the early 1990s in urban areas, and until the mid-2000s in rural areas (Saikia, Jasilionis, Ram, & Shkolnikov, 2011). Substantial reductions in child and maternal mortality in India from the mid-1970s to the mid-2000s contributed to improved survival, especially among women (Saikia et al., 2011). The postponement of deaths to older ages is likely to result in a growing population of disabled older people—a development that will pose huge challenges for both India and China. According to India’s 2011 census, there were 27 million disabled people in India, and the burdens of poor health and disability were unequally distributed across men and women, urban and rural areas, and geographic regions (Saikia et al., 2016). Projections indicate that the numbers of disabled people at older ages will increase substantially in the near future. Moreover, the gender gap in health is becoming an important public health issue. This gap has been attributed to persisting gender disparities in educational and occupational opportunities, income, and access to health services.
India and China share certain cultural characteristics, including the prevalence of patrilineal and patrilocal kinship systems (Dummer & Cook, 2008) and of institutionalized forms of discrimination against women in many aspects of life (Das Gupta et al., 2003). Although they are similar in some respects, India and China have different histories and different political, economic, and health care systems (Wolf et al., 2011), which might have influenced the differences in levels of social and gender inequality observed in these countries (Hannum & Xie, 1994). According to recent estimates for India, 33% of women aged 15+ participate in the labor market (2009) (Wolf et al., 2011) and the female adult literacy rate is 65% (2010–11) (World Bank, 2011); while the respective estimates for China are 67% and 93%.
Compared to India, China performs better on a broad range of health measures, has superior systems of health care service delivery and health insurance coverage (Dummer & Cook, 2008; Yip & Mahal, 2008), and has lower levels of gender inequality in education and in formal employment. Thus, we hypothesized that the female disadvantage in health is greater in India than in China, and that the gender differences are more pronounced in rural areas than in urban areas in both countries.
Design and Methods
Study Population
For the study, we used data from Wave 1 of the WHO Study on Global AGEing and Adult Health (SAGE), a multicountry study conducted to monitor the health and well-being of adult populations in China, Ghana, India, Mexico, the Russian Federation, and South Africa (Kowal et al., 2012). Multistage cluster sampling strategies were used in all of these countries. The response rates among the age groups 50–59 and 60+ were, respectively, 92% and 93% in China and 90% and 85% in India. The survey materials and the interviewer training and were standardized across the countries. The present analysis is based on responses from 7,150 individuals in India and 13,367 individuals in China who were aged 50 or older.
Health Outcomes
Self-reported general health (SRH) was assessed through a single question: “In general, how would you rate your health today?” The five possible response options—very good, good, moderate, bad, and very bad—were collapsed into two categories to allow us to examine the prevalence of poor self-rated health (very bad and bad response options). Epidemiological studies have shown that the self-assessment of general health is a valid measure of individual health status, and an important predictor of functional ability and survival (Idler & Benyamini, 1997).
Handgrip strength (in kilograms) was measured twice in each hand using a Smedley Hand Dynamometer (Scandidact Aps, Denmark), and the highest of the four measurements was used in the present study. A number of scholars have suggested that handgrip strength is a better marker of frailty than chronological age (Syddall, Cooper, Martin, Briggs, & Aihie Sayer, 2003); and this indicator has been shown to predict all-cause and cause-specific mortality (Fujita et al., 1995; Leong et al., 2015), disability at older ages (Rantanen et al., 1999), cognitive decline (Alfaro-Acha et al., 2006), and hospitalization (Cawthon et al., 2009). There is compelling evidence that men outperform women on handgrip tests at all ages and across all continents (Bohannon, Peolsson, Massy-Westropp, Desrosiers, & Bear-Lehman, 2006; Leong et al., 2015).
Five cognition tests were used to generate a Cognitive Composite Score (CCS) in both countries. These tests examined the participants’ immediate verbal recall (number of words immediately recalled from a list of 12 nouns), delayed verbal recall (number of words recalled from the same 12-item list after a delay of around 10 min), forward digit span (number of correct responses out of a possible 14 on the digits forward task), backward digit span (number of correct responses out of a possible 14 on the digits backward task), and verbal fluency (number of animals named in one minute). The CCS was based on the combined results of the five individual cognitive tests (Lee, Shih, Feeney, & Langa, 2014). Sex- and age-specific (10-year age intervals) means were used to replace missing scores on an individual cognitive test. If two or more items were missing, the CCS was coded as missing.
Covariates
Sociodemographic characteristics (age, marital status, education, and place of residence), lifestyle behavior (tobacco use), and health risk characteristics (height and weight for grip strength and number of chronic conditions) were included in the analyses as potential confounders. The participants’ ages were included in 5-year age groups, from 50–54 to 80+; and each participant’s marital status was included as married or another status. Education was measured as the highest completed level with three categories: less than primary, primary, and secondary or higher. The participants’ tobacco use (smoke, sniff, or chew) was categorized as never, former, and current use. The morbidity measure was composed of a total of eight self-reported physician-diagnosed chronic conditions: angina, arthritis, asthma, stroke, diabetes, depression, chronic lung disease, and hypertension. The participants were further categorized as having no, one, or two or more chronic conditions. Additionally, height was included in the analysis of the CCS, as height is influenced by food intake and the burden of infectious diseases in childhood, is associated with late-life cognitive abilities, and is often used as a proxy for conditions in childhood (Case & Paxson, 2008; Guven & Lee, 2013). Height and weight were measured using standard procedures previously described elsewhere (Agrawal & Agrawal, 2016).
Analytical Strategy
The descriptive statistics for the analysis variables were weighted using individual poststratification weights that adjust for differences in locality, gender, and age relative to the 2006 projected population estimates in India, and to the 2008 population projections in China (Arokiasamy, Parasuraman, Sekher, & Lhungdim, 2013). Direct age standardization relative to the World Standard Population was applied to compare the prevalence of poor SRH and the means of grip strength and the CCS in the Indian and the Chinese total study populations.
Logistic regression analysis was applied to investigate the gender differences in poor SRH, and ordinary least squares linear (OLS) regression was used to examine the gender differences in grip strength and the CCS. The regression models were country-specific, and were based on unweighted data. Three models were estimated to examine gender differences in health when additional covariates were included. Model 1 includes gender and age, while Model 2 also includes marital status, education, place of residence, tobacco use, height (for grip strength and the CCS), weight (for grip strength), and number of chronic conditions. To examine whether education and place of residence had different effects on men and women, the interactions of gender with education and residency area were included in Model 3. Since the results of a descriptive analysis suggest that the age-related decline in grip strength may differ by gender, Model 3 also includes the interaction between age and gender. In response to previous findings showing that health and mortality patterns differed by place of residence in India and in China (Saikia et al., 2011; Weir et al., 2014), we conducted additional analyses of gender differences in health for urban and rural subsamples separately. The model specifications were similar to those used in the analyses of the total country-specific samples. All of the analyses were performed using Intercooled Stata 14 (StataCorp, L, 2015).
Results
Descriptive Results
The Chinese male and female study participants (63.2 years SD = 9.4 and 63.1 years, SD = 9.5, respectively) were older than their same-sex counterparts in India (62.3 years, SD = 9.0 for men and 61.4 years, SD = 9.1 for women). The shares of Chinese men and women who were married, had at least primary education, were living in an urban area, and had never used tobacco were higher than those of their Indian counterparts. The shares of participants who had no chronic conditions were similar in the two male populations, but were higher among Indian women than among Chinese women (Table 1). The gender differences in marital status and education were larger in India than in China. Tobacco use was very low among Chinese women, and was lower among Indian women than among Indian men. The age-standardized prevalence of poor SRH was higher among Indian and Chinese women than among their male counterparts (Table 2), and the gender differences were similar in the two countries (−5.2 in India vs. −4.8 in China). The age-standardized means of grip strength and the CCS were higher among men than among women in both countries, but the male advantage was larger in China than in India for grip strength (9.5 in India vs. 11.9 in China), and was smaller in China than in India for cognitive function (3.6 in India vs. 1.9 in China).
Table 1.
Characteristics of Study Populations in WHO-SAGE India and China, 2007–2010
| India | China | |||||
|---|---|---|---|---|---|---|
| N | Men | Women | N | Men | Women | |
| Age | ||||||
| 50–54 | 1,662 | 21.8 | 24.8 | 2,880 | 21.1 | 21.9 |
| 55–59 | 1,517 | 20.3 | 22.1 | 2,927 | 21.7 | 22.1 |
| 60–64 | 1,310 | 18.2 | 18.5 | 2,139 | 16.5 | 15.6 |
| 65–69 | 1,146 | 17.3 | 14.7 | 1,829 | 13.7 | 13.7 |
| 70–74 | 776 | 11.5 | 10.2 | 1,652 | 12.5 | 12.2 |
| 75–79 | 372 | 5.8 | 4.6 | 1,150 | 8.8 | 8.4 |
| 80+ | 367 | 5.1 | 5.2 | 790 | 5.7 | 6.1 |
| Current marital status | ||||||
| Nonmarried | 1,845 | 12.3 | 39.7 | 2,264 | 10.8 | 22.4 |
| Married | 5,305 | 87.6 | 60.3 | 11,093 | 89.3 | 77.6 |
| Education | ||||||
| Never attended | 4,111 | 46.6 | 79.0 | 5,877 | 33.5 | 53.2 |
| Primary | 929 | 17.6 | 10.7 | 2,595 | 22.9 | 16.3 |
| Secondary and above | 1,520 | 35.9 | 10.3 | 4,895 | 43.6 | 30.5 |
| Place of residence | ||||||
| Urban | 1,861 | 24.6 | 27.5 | 6,567 | 46.6 | 51.4 |
| Rural | 5,289 | 75.4 | 72.5 | 6,800 | 53.4 | 48.7 |
| Tobacco consumption | ||||||
| Never used | 3,109 | 27.3 | 67.8 | 8,643 | 34.5 | 95.3 |
| Former user | 344 | 8.5 | 1.9 | 797 | 12.1 | 1.1 |
| Current user | 3,104 | 64.2 | 30.2 | 3,482 | 53.5 | 3.7 |
| Chronic health condition | ||||||
| None | 3,260 | 50.4 | 49.0 | 6,124 | 50.8 | 44.1 |
| 1 | 1,880 | 27.9 | 29.4 | 3,840 | 28.8 | 30.4 |
| 2+ | 1,419 | 21.6 | 21.7 | 2,995 | 20.4 | 25.5 |
| Total sample | 7,150 | 13,367 | ||||
Note: Subtotals may not be equal to total sample due to missing cases.
Table 2.
Age-standardized Prevalencea of Poor Self-rated Health and Age-standardized Means of Grip Strength and Cognitive Function
| India | China | |||||||
|---|---|---|---|---|---|---|---|---|
| Men | Women | Men | Women | |||||
| %/Mean | SE | %/Mean | SE | %/Mean | SE | %/Mean | SE | |
| Poor SRHb | 18.71 | 0.01 | 23.95 | 0.007 | 17.76 | 0.005 | 22.58 | 0.005 |
| Grip strength | 28.26 | 0.14 | 18.77 | 0.106 | 34.82 | 0.129 | 22.87 | 0.110 |
| CCS | 27.41 | 0.11 | 23.81 | 0.117 | 33.68 | 0.109 | 31.80 | 0.099 |
Note: aStandardized to the WHO Standard world population. bSRH = Self-reported health; SE = Standard error; CCS = Cognitive composite score.
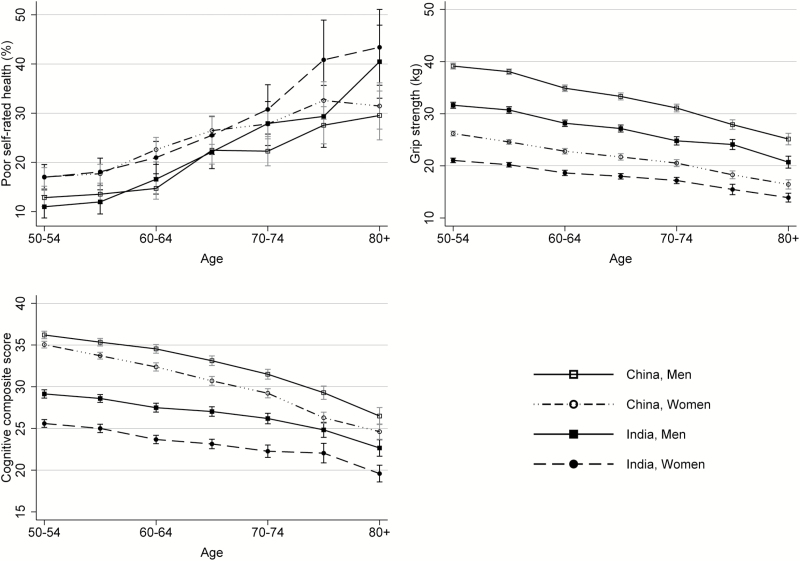
Figure 1 illustrates that the prevalence of poor SRH was higher among Indian and Chinese women than among their opposite-sex counterparts, and that the magnitude of the gender differences in overall health between the two countries varied across age groups. The men had higher grip strength levels and cognitive scores than the women in both countries. Chinese men and women performed better than their Indian counterparts on grip strength and cognitive tests; and Chinese women had higher cognitive scores than Indian men.
Figure 1.
Cross-sectional age trajectories of poor self-rated health, grip strength, and cognitive function in India and in China, WHO-SAGE 2007–2010.
Regression Results for Gender Differences in SRH
The risk of reporting poor SRH was 38% (odds ratio [OR] = 1.38, 95% CI: 1.22, 1.56) higher among Indian women and 36% (OR = 1.36, 95% CI: 1.25, 1.48) higher among Chinese women than it was among their male counterparts in the model adjusted for age only, and it changed very little when additional covariates were adjusted for (Table 3). In the Indian sample, place of residence and education did not affect the risk of reporting poor SRH differently among men and women; whereas in the Chinese sample, the risk of reporting poor SRH was lower among women with at least primary education (Supplementary Table 1). However, when the interactions were included in the model, there was no improvement in the model fit in either the Indian or the Chinese sample (likelihood-ratio [LR] test, chi-square (df) = 2.37 (3), p = .499 in India and LR test, chi-square (df) = 6.12 (3), p = .106 in China).
Table 3.
Gender Differences in the Risk of Reporting Poor Self-reported Health, Grip Strength, and Cognitive Function in India and in China
| India | China | |||||||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p value | OR | 95% CI | p value | |||
| Poor SRHa | (n = 6,546) | (n = 12,877) | ||||||
| Model 1b | 1.38 | 1.22 | 1.56 | <.001 | 1.36 | 1.25 | 1.48 | <.001 |
| Model 2 | 1.34 | 1.15 | 1.57 | <.001 | 1.33 | 1.17 | 1.51 | <.001 |
| Model 3 | 1.10 | 0.79 | 1.54 | .556 | 1.43 | 1.14 | 1.79 | .002 |
| Coeff. | 95% CI | p value | Coeff. | 95% CI | p value | |||
| Grip strength | (n = 6,221) | (n = 12,120) | ||||||
| Model 1 | −9.56 | −9.91 | −9.22 | <.001 | −11.95 | −12.29 | −11.62 | <.001 |
| Model 2 | −8.27 | −8.68 | −7.85 | <.001 | −11.34 | −11.80 | −10.88 | <.001 |
| Model 3 | −8.93 | −9.96 | −7.90 | <.001 | −11.37 | −12.54 | −10.21 | <.001 |
| CCS | (n = 6,356) | (n = 12,927) | ||||||
| Model 1 | −3.64 | −3.96 | −3.32 | <.001 | −1.99 | −2.28 | −1.71 | <.001 |
| Model 2 | −1.88 | −2.24 | −1.52 | <.001 | −0.95 | −1.31 | −0.59 | <.001 |
| Model 3 | −1.94 | −2.66 | −1.22 | <.001 | −0.74 | −1.40 | −0.09 | .026 |
Note: aSRH = Self-reported health; CI = Confidence interval; CCS = Cognitive composite score. bModel 1: gender and age; Model 2: Model 1 + marital status, education, place of residence, smoking status, height (grip strength and CCS), and number of chronic conditions; Model 3: Model 2 + interactions of gender with education and place of residence, Model 3 for grip strength: Model 2 + interactions of gender with age, education, and place of residence.
An additional analysis of gender differences in poor SRH by place of residence showed that the risk of reporting poor health was substantially higher among women than it was among men in both rural and urban areas of India and China (Table 4). However, after adjustments for additional covariates were made, the female disadvantage in SRH was apparent in rural areas only in both countries. These patterns remained unchanged in Model 3, which included interactions between gender and education (Supplementary Table 4). Including these interactions did not change the levels of poor SRH in the urban or in the rural subsamples in India or in China.
Table 4.
Gender Differences in the Risk of Reporting Poor Self-reported Health, Grip Strength, and Cognitive Function by Place of Residence in India and in China
| India | China | |||||||
|---|---|---|---|---|---|---|---|---|
| Rural area | OR/Coeff. | 95% CI | p value | OR/Coeff. | 95% CI | p value | ||
| Poor SRHa | (n = 4,872) | (n = 6,541) | ||||||
| Model 1b | 1.41 | 1.23 | 1.62 | <.001 | 1.48 | 1.32 | 1.66 | <.001 |
| Model 2 | 1.35 | 1.14 | 1.61 | .001 | 1.42 | 1.18 | 1.69 | <.001 |
| Model 3 | 1.26 | 1.04 | 1.52 | .017 | 1.48 | 1.21 | 1.80 | <.001 |
| Grip strength | (n = 4,641) | (n = 6,293) | ||||||
| Model 1 | −9.45 | −9.85 | −9.05 | <.001 | −11.89 | −12.30 | −11.48 | <.001 |
| Model 2 | −7.98 | −8.46 | −7.50 | <.001 | −10.86 | −11.46 | −10.27 | <.001 |
| Model 3 | −9.34 | −10.22 | −8.46 | <.001 | −11.17 | −12.24 | −10.10 | <.001 |
| CCS | (n = 4,749) | (n = 6,362) | ||||||
| Model 1 | −3.97 | −4.33 | −3.62 | <.001 | −2.73 | −3.08 | −2.38 | <.001 |
| Model 2 | −2.11 | −2.52 | −1.70 | <.001 | −1.03 | −1.53 | −0.53 | <.001 |
| Model 3 | −2.45 | −2.90 | −1.99 | <.001 | −0.90 | −1.47 | −0.32 | .002 |
| Urban area | OR/Coeff. | 95% CI | p value | OR/Coeff. | 95% CI | p value | ||
| Poor SRH | (n = 1,674) | (n = 6,336) | ||||||
| Model 1 | 1.41 | 1.07 | 1.85 | .015 | 1.31 | 1.14 | 1.50 | <.001 |
| Model 2 | 1.35 | 0.94 | 1.93 | .105 | 1.19 | 0.98 | 1.43 | .075 |
| Model 3 | 1.37 | 0.85 | 2.20 | .193 | 1.52 | 1.11 | 2.10 | .010 |
| Grip strength | (n = 1,580) | (n = 5,827) | ||||||
| Model 1 | −9.89 | −10.57 | −9.20 | <.001 | −12.43 | −12.96 | −11.91 | <.001 |
| Model 2 | −9.09 | −9.93 | −8.25 | <.001 | −11.86 | −12.56 | −11.16 | <.001 |
| Model 3 | −8.68 | −10.42 | −6.93 | <.001 | −12.09 | −13.96 | −10.21 | <.001 |
| CCS | (n = 1,607) | (n = 5,935) | ||||||
| Model 1 | −3.06 | −3.72 | −2.40 | <.001 | −1.76 | −2.18 | −1.34 | <.001 |
| Model 2 | −1.17 | −1.89 | −0.46 | .001 | −0.72 | −1.24 | −0.20 | .006 |
| Model 3 | −3.48 | −4.56 | −2.41 | <.001 | −1.47 | −2.47 | −0.47 | .004 |
aSRH = Self-reported health; CI = Confidence interval; CCS = Cognitive composite score. bModel 1: gender and age; Model 2: Model 1 + marital status, education, smoking status, height (grip strength and CCS), and number of chronic conditions; Model 3: Model 2 + interactions between gender and education, Model 3 for grip strength: interactions of gender with age and education.
Regression Results for Gender Differences in Grip Strength
Grip strength was about 9.5 kg (95% CI: −9.9, −9.2) lower among Indian women and 11.9 kg (95% CI: −12.3, −11.6) lower among Chinese women than it was among their male counterparts (Table 3); and the male advantage in grip strength was greater in China than in India. The male advantage diminished only slightly when additional covariates were included in the model. Further analysis showed that in both countries, levels of age-related decline in grip strength were steeper among men than among women, and that women with higher educational levels tended to have lower grip strength (Supplementary Table 2). The association between place of residence and grip strength was similar among men and women in both countries.
As in the analyses of the total country-specific samples, grip strength was found to be lower among Indian and Chinese women than among their male counterparts in both rural and urban areas (Table 4). The male advantage diminished only slightly when additional covariates were included in the models, and the gender differences were larger in China than in India, irrespective of place of residence. The decline in grip strength was steeper among Indian and Chinese men in rural areas than among their female counterparts, but this pattern was less apparent in urban areas (Supplementary Table 5). Education had similar effects on grip strength among men and women in the Indian and in the Chinese rural–urban subsamples.
Regression Results for Gender Differences in Cognitive Function
Men performed better than women on cognitive tests in both countries, but the female disadvantage was greater in India (β = −3.64, 95% CI: −3.96, −3.32) than in China (β = −1.99, 95% CI: −2.28, −1.71) (Table 3). Adjustments for additional covariates substantially reduced these gender differences, but women still had lower CCSs than men in both countries (Model 2). The association between education and the CCS was similar among Chinese men and women, while having at least primary education was more beneficial for women than for men in India (Supplementary Table 3). Furthermore, in both India and China, women who were living in rural areas had lower levels of cognitive function than their male counterparts. Levels of age-related decline in the CCS did not differ by gender in either country.
Gender differences in cognitive function were apparent in both rural and urban areas of India and China in the base model, and after adjustment for additional covariates (Table 4). The female disadvantage in cognition was greater in India than in China, irrespective of the place of residence. The effect of education on cognition was similar among Chinese men and women residing in both rural and urban areas, whereas in India the interaction of education with gender was statistically significant (Supplementary Table 6).
Discussion and Implications
The present cross-sectional study of Indian and Chinese populations provided evidence that women had lower self-rated health, grip strength, and cognitive function at older ages than their male counterparts. These female disadvantages were observed in both rural and urban areas, and our finding that gender differences in poor SRH and in the CCS were greater in rural than in urban subsamples partially supports our initial hypothesis. Marital status, education, place of residence, smoking status, height, and the number of chronic conditions were found to account for very small shares of the gender differences in SRH and grip strength, but for about 50% of the total gender gap in cognitive function. Further analysis indicated that the gender differences in cognition were primarily attributable to differences in education.
In line with comparative research evidence from developed countries (Crimmins et al., 2010; Oksuzyan et al., 2008), we found that women had worse self-reported health and lower grip strength than men in India and in China. The similarities in the gender differences in SRH found in India and in China can be partially explained by differences in the reporting patterns in these two countries. A recent study has shown that older Indians tend to have relatively positive perceptions of their overall health, but perform worse at the level of objective health measures (Cramm, Bornscheuer, Selivanova, & Lee, 2015). Our findings for both India and China that the gender differences in SRH were greater in rural than in urban areas support the results of previous research indicating that individuals who were living in areas with better health facilities were able to evaluate their health more accurately than their counterparts who were living in less advantaged areas, mainly due to differences in their respective levels of awareness of treatable conditions (Sen, 2002).
Like many previous studies (Bohannon et al., 2006; Ramlagan, Peltzer, & Phaswana-Mafuya, 2014), we observed that Indian and Chinese men had higher grip strength levels than their female counterparts. The male advantage was greater in China than in India, in both the total samples and in the rural and the urban subpopulations. Research evidence suggests that early life circumstances affect physical performance in later life (Strand, Cooper, Hardy, Kuh, & Guralnik, 2011). Most of the cohorts included in the SAGE Wave 1 grew up during periods in which poverty and infectious diseases were widespread, and food supplies and health care resources were limited. During the war with Japan in the 1940s and the Great Leap Forward Famine in 1959–1962, China experienced extreme food shortages, and Chinese families had to make hard choices about which family members to sustain (Ashton, Hill, Piazza, & Zeitz, 1992; Yi et al., 1993). India was less affected by wars, but experienced the Bengal famine in 1943 and the partitioning of the country in 1947, which was accompanied by riots and one of the largest mass migrations in human history (Mari Bhat, 1989; Sen, 1981). The strong economic incentives for families to provide boys with better education, nutrition, and health care than girls have been well-documented for China and India (Mishra, Roy, & Retherford, 2004; Watson, 1991). Thus, the harsh conditions that characterized these periods of war, famine, and political upheaval likely affected girls more than boys, and may have caused women who grew up during these periods to experience worse health than their male counterparts at older ages in India, and especially in China.
Like other studies conducted in low- and middle-income countries (Lee et al., 2014; Maurer, 2010), we observed a female disadvantage in cognitive function in India and in China. This finding is in contrast to evidence on the direction of gender differences in high-income countries, where women are commonly found to perform at the same or at higher levels than men (Langa et al., 2008; Oksuzyan et al., 2010). Weber and colleagues showed that both men and women in less advantaged regions (with relatively low gross domestic products, high levels of mortality, large family sizes, and low educational levels) have worse cognitive abilities than their counterparts in more advantaged regions, but that women tend to have even worse cognitive abilities than men (Weber, Skirbekk, Freund, & Herlitz, 2014). This pattern could partly explain the contrasting directions of gender differences in cognition found in India and China on the one hand, and high-income countries on the other.
As we initially hypothesized, our results show that the gender differences in cognition were larger in India than in China. It has been reported that education explains about one-quarter of the gender gap in various cognitive measures in the Chinese population (Lee et al., 2014). The fact that much larger shares of women in India than in China never attended school (79% vs. 34%, respectively) may partially explain our finding that the gender differences in cognition were larger in India than in China. Moreover, because of the traditional distribution of gender roles, the women of these cohorts may have been confined to household activities that restricted their opportunities for social engagement and for working outside the home. Since social engagement has been shown to be protective of cognitive function (Yeh & Liu, 2003), a relative lack of social engagement among the women studied could help to explain the female disadvantage in cognitive health observed in these two countries. Our finding of a greater female disadvantage in cognitive health in rural than in urban areas in both countries can be explained by the larger proportions of women with no education in rural than in urban areas in India (87% vs. 58%) and in China (75% vs. 32%).
The study also revealed that while education had different effects on the grip strength levels of women and men, these differences disappeared in the analyses of rural-urban subsamples. These unexpected findings contradict the results of previous research conducted in European settings (Mohd Hairi, Mackenbach, Andersen-Ranberg, & Avendano, 2010), and thus require further investigation.
The present study has some limitations. Although the same methodology was applied across all six WHO SAGE countries, cross-country comparisons of health should be made with caution, especially given that the measures of health used were self-reported. It is, however, likely that the comparison of gender differences across countries was less sensitive to methodological differences. Furthermore, the sample sizes were small given the enormous regional, socioeconomic, ethnic, and religious diversity of India and China. The samples were also too small to allow us to identify the most vulnerable groups within the respective national populations. An additional analysis that controlled for caste and religion in the Indian sample generated similar results, but much larger samples are needed to examine gender differences across castes. These issues should be taken into account when planning future surveys seeking to examine social and spatial diversities in health and mortality in countries where nationwide registers are not available.
Another limitation is that no data on individual income were collected in these surveys. Although information on household wealth is available in the survey data, previous studies from developing countries have shown that household asset-based wealth is often unrelated to individual health status, which can vary depending on which member of the household owns the assets (Smith & Goldman, 2007). It has been reported that less than 15% of Indian women are designated as the head of household, and that nearly 20% of women with earned income have no decision-making power (Kishor & Gupta, 2009).
To conclude, the present study adds to previous empirical evidence that women tend to have worse health in all countries, regardless of their economic development level. The female disadvantage in cognitive function was found to be larger in India than in China, while the opposite relationship was observed for grip strength, and gender differences of similar magnitudes were found for self-perceived health. A female disadvantage was observed for all health outcomes except for SRH in urban and rural areas. The results further showed that selected major socioeconomic characteristics and risk factors accounted for only very small shares of the differences in self-perceived health and grip strength, but contributed substantially to the gender gaps in cognitive function. In light of previous research findings (Weber et al., 2014), it is likely that as educational opportunities and living conditions improve in India and in China, women will make more gains in cognitive performance levels than men, and that the direction of gender differences in cognitive abilities in these two countries will be similar to those found in high-income countries. To better explain the gender differences in health in these two countries, future studies should focus on other individual-level characteristics, such as the role of social networks and women’s levels of decision-making autonomy; and community characteristics, such as health care infrastructure. There is also an urgent need to collect longitudinal data on subjective and objective health measures that would enable us to investigate the development of gender differences in health in low- and middle-income countries.
Supplementary Material
Supplementary data are available at The Gerontologist online.
Funding
The work was supported by the U.S. National Institute of Health (P01AG031719, R01AG026786, and 2P01AG031719), the VELUX Foundation, and the Max Planck India Mobility Grant.
Conflict of Interest
None reported.
Supplementary Material
Acknowledgments
The authors are grateful to Dr Vladimir Shkolnikov and Dr Jutta Gampe at the Max Planck Institute for Demographic Research for their comments on an earlier version of the manuscript, and to Torsten Sauer for help in preparing the figures and the tables.
References
- Agrawal S., & Agrawal P. K (2016). Association between body mass index and prevalence of multimorbidity in low-and middle-income countries: A Cross-Sectional Study. International Journal of Medicine and Public Health, 6, 73–83. doi:10.5530/ijmedph.2016.2.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfaro-Acha A., Al Snih S., Raji M. A., Kuo Y. F., Markides K. S., Ottenbacher K. J. (2006). Handgrip strength and cognitive decline in older Mexican Americans. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences, 61, 859–865. doi:10.1093/gerona/61.8.859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arokiasamy P. Parasuraman S. Sekher T. V., & Lhungdim H (2013). Study on global AGEing and adult health (SAGE) Wave 1 India. National Report. In P., Rebecca, W., Russell (Eds.). Geneva: WHO. [Google Scholar]
- Ashton B. Hill K. Piazza A., & Zeitz R (1984). Famine in China, 1958–61. Population and Development Review, 10(4), 613–645. doi:10.2307/1973284 [Google Scholar]
- Barford A. Dorling D. Smith G. D., & Shaw M (2006). Life expectancy: Women now on top everywhere. BMJ, 332, 808. doi:10.1136/bmj.332.7545.808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat, N. M. (1989). Mortality and fertility in India, 1881–1961: A reassessment. In T. Dyson (Ed.), India’s Historical Demography (pp. 73–118), London: Curzon.
- Bohannon R. W. Peolsson A. Massy-Westropp N. Desrosiers J., & Bear-Lehman J (2006). Reference values for adult grip strength measured with a Jamar dynamometer: A descriptive meta-analysis. Physiotherapy, 92, 11–15. doi:10.1016/j.physio.2005.05.003 [Google Scholar]
- Case A., Paxson C. (2008). Height, health, and cognitive function at older ages. The American Economic Review, 98, 463–467. doi:10.1257/aer.98.2.463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawthon P. M., Fox K. M., Gandra S. R., Delmonico M. J., Chiou C. F., Anthony M. S., Harris T. B; Health, Aging and Body Composition Study (2009). Do muscle mass, muscle density, strength, and physical function similarly influence risk of hospitalization in older adults?Journal of the American Geriatrics Society, 57, 1411–1419. doi:10.1111/j.1532-5415.2009.02366.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramm J. M., Bornscheuer L., Selivanova A., Lee J. (2015). The health of India’s elderly population: A comparative assessment using subjective and objective health outcomes. Journal of Population Ageing, 8, 245–259. doi:10.1007/s12062-015-9122-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crimmins E. M., Kim J. K., Solé-Auró A. (2011). Gender differences in health: Results from SHARE, ELSA and HRS. European Journal of Public Health, 21, 81–91. doi:10.1093/eurpub/ckq022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das Gupta M. Zhenghua J. Bohua L. Zhenming X. Chung W., & Hwa-Ok B (2003). Why is son preference so persistent in East and South Asia? A cross-country study of China, India and the Republic of Korea. The Journal of Development Studies, 40, 153–187. doi:10.1080/00220380412331293807 [Google Scholar]
- Dummer T. J., Cook I. G. (2008). Health in China and India: A cross-country comparison in a context of rapid globalisation. Social Science & Medicine (1982), 67, 590–605. doi:10.1016/j.socscimed.2008.04.019 [DOI] [PubMed] [Google Scholar]
- Fujita Y., Nakamura Y., Hiraoka J., Kobayashi K., Sakata K., Nagai M., Yanagawa H. (1995). Physical-strength tests and mortality among visitors to health-promotion centers in Japan. Journal of Clinical Epidemiology, 48, 1349–1359. doi:10.1016/0895-4356(95)00533-1 [DOI] [PubMed] [Google Scholar]
- Guven C., Lee W. S. (2013). Height and cognitive function at older ages: Is height a useful summary measure of early childhood experiences?Health Economics, 22, 224–233. doi:10.1002/hec.1827 [DOI] [PubMed] [Google Scholar]
- Hannum E., & Xie Y (1994). Trends in educational gender inequality in China: 1949–1985. Research in Social Statification and Mobility, 13, 73–98. [Google Scholar]
- Idler E. L., Benyamini Y. (1997). Self-rated health and mortality: A review of twenty-seven community studies. Journal of Health and Social Behavior, 38, 21–37. [PubMed] [Google Scholar]
- Kishor S., & Gupta K (2009). Gender equality and womens empowerment in India. National Family Health Survey (NFHS-3) India 2005–06. Mumbai: International Institute for Population Sciences; Calverton, Maryland, USA: ICF Macro. [Google Scholar]
- Kowal P. Chatterji S. Naidoo N. Biritwum R. Fan W. Ridaura R. L., & Williams S (2012). Data resource profile: The World Health Organization Study on global AGEing and adult health (SAGE). International Journal of Epidemiology, 41, 1639–1649. doi:10.1093/ije/dys210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langa K. M. Larson E. B. Karlawish J. H. Cutler D. M. Kabeto M. U. Kim S. Y., & Rosen A. B (2008). Trends in the prevalence and mortality of cognitive impairment in the United States: Is there evidence of a compression of cognitive morbidity?Alzheimer’s & Dementia, 4, 134–144. doi:10.1016/j.jalz.2008.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., Shih R., Feeney K., Langa K. M. (2014). Gender disparity in late-life cognitive functioning in India: Findings from the longitudinal aging study in India. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 69, 603–611. doi:10.1093/geronb/gbu017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong D. P. Teo K. K. Rangarajan S. Lopez-Jaramillo P. Avezum A. Jr Orlandini A., & Yusuf S (2015). Prognostic value of grip strength: Findings from the Prospective Urban Rural Epidemiology (PURE) study. The Lancet, 386, 266–273. doi: 10.1016/S0140-6736(14)62000–6 [DOI] [PubMed] [Google Scholar]
- Maurer J. (2010). Height, education and later-life cognition in Latin America and the Caribbean. Economics & Human Biology, 8, 168–176. doi:doi:10.1016/j.ehb.2010.05.013 [DOI] [PubMed] [Google Scholar]
- Mishra V. Roy T. K., & Retherford R. D (2004). Sex differentials in childhood feeding, health care, and nutritional status in India. Population and Development Review, 30(2), 269–295. [Google Scholar]
- Mohd Hairi F., Mackenbach J. P., Andersen-Ranberg K., Avendano M. (2010). Does socio-economic status predict grip strength in older Europeans? Results from the SHARE study in non-institutionalised men and women aged 50+. Journal of Epidemiology and Community Health, 64, 829–837. doi:10.1136/jech.2009.088476 [DOI] [PubMed] [Google Scholar]
- Ng N. Kowal P. Kahn K. Naidoo N. Abdullah S. Bawah A.,…Chatterji S (2010). Health inequalities among older men and women in Africa and Asia: Evidence from eight Health and Demographic Surveillance System sites in the INDEPTH WHO-SAGE study. Global Health Action, 3. doi:10.3402/gha.v3i0.5420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oksuzyan A., Crimmins E., Saito Y., O’Rand A., Vaupel J. W., Christensen K. (2010). Cross-national comparison of sex differences in health and mortality in Denmark, Japan and the US. European Journal of Epidemiology, 25, 471–480. doi:10.1007/s10654-010-9460-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oksuzyan A., Juel K., Vaupel J. W., Christensen K. (2008). Men: Good health and high mortality. Sex differences in health and aging. Aging Clinical and Experimental Research, 20, 91–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey A., Ladusingh L. (2015). Socioeconomic correlates of gender differential in poor health status among older adults in India. Journal of Applied Gerontology: The Official Journal of the Southern Gerontological Society, 34, 879–905. doi:10.1177/0733464813481850 [DOI] [PubMed] [Google Scholar]
- Ramlagan S., Peltzer K., Phaswana-Mafuya N. (2014). Hand grip strength and associated factors in non-institutionalised men and women 50 years and older in South Africa. BMC Research Notes, 7, 8. doi:10.1186/1756-0500-7-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rantanen T., Guralnik J. M., Foley D., Masaki K., Leveille S., Curb J. D., White L. (1999). Midlife hand grip strength as a predictor of old age disability. JAMA, 281, 558–560. doi:10.1001/jama.281.6.558 [DOI] [PubMed] [Google Scholar]
- Saikia N., Bora J. K., Jasilionis D., Shkolnikov V. M. (2016). Disability divides in India: Evidence from the 2011 Census. PLOS ONE, 11, e0159809. doi:10.1371/journal.pone.0159809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saikia N., Jasilionis D., Ram F., Shkolnikov V. M. (2011). Trends and geographic differentials in mortality under age 60 in India. Population Studies, 65, 73–89. doi:10.1080/00324728.2010.534642 [DOI] [PubMed] [Google Scholar]
- Sen A. (1981). Poverty and famines: An essay on entitlement and deprivation. Oxford: Oxford University Press. [Google Scholar]
- Sen A. (2002). Health: Perception versus observation: Self reported morbidity has severe limitations and can be extremely misleading. BMJ: British Medical Journal, 324, 860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K. V., Goldman N. (2007). Socioeconomic differences in health among older adults in Mexico. Social Science & Medicine (1982), 65, 1372–1385. doi:10.1016/j.socscimed.2007.05.023 [DOI] [PubMed] [Google Scholar]
- StataCorp, L (2015). Stata Statistical Software: Release 14. College Station, TX: Stata-Corp LP. [Google Scholar]
- Strand B. H., Cooper R., Hardy R., Kuh D., Guralnik J. (2011). Lifelong socioeconomic position and physical performance in midlife: Results from the British 1946 birth cohort. European Journal of Epidemiology, 26, 475–483. doi:10.1007/s10654-011-9562-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syddall H., Cooper C., Martin F., Briggs R., Aihie Sayer A. (2003). Is grip strength a useful single marker of frailty?Age and Ageing, 32, 650–656. doi:10.1093/ageing/afg111 [DOI] [PubMed] [Google Scholar]
- UN (2015). United Nations Department of Economic and Social Affairs. World Population Prospects: The 2015 Revision, Volume II: Demographic Profiles (Vol. ST/ESA/SER.A/380). New York: Department of Economic and Social Affairs, Population Division, United Nations. [Google Scholar]
- Van de Velde S. Bracke P., & Levecque K (2010). Gender differences in depression in 23 European countries. Cross-national variation in the gender gap in depression. Social Science & Medicine, 71, 305–313. doi:10.1016/j.socscimed.2010.03.035 [DOI] [PubMed] [Google Scholar]
- Watson R. S. (1991). Afterword: Marriage and gender inequality. In R. S. Watson & P. B. Ebrey (Eds), Marriage and Inequality in Chinese Society (pp. 347–368). Berkeley: University of California Press. [Google Scholar]
- Weber D. Skirbekk V. Freund I., & Herlitz A (2014). The changing face of cognitive gender differences in Europe. Proceedings of the National Academy of Sciences, 111, 11673–11678. doi:10.1073/pnas.1319538111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir D. Lay M., & Langa K (2014). Economic development and gender inequality in cognition: A comparison of China and India, and of SAGE and the HRS sister studies. The Journal of the Economics of Ageing, 4, 114–125. doi:10.1016/j.jeoa.2014.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf C. Jr Dalal S. DaVanzo J. Larson E. V. Akhmedjonov A. Dogo H., & Montoya S (2011). China and India, 2025: A comparative assessment. Santa Monica, CA: RAND Corporation, National Defense Research Institute.
- World Bank (2011). World Bank Country Indicators. Retrieved December 6, 2015. http://data.worldbank.org/indicator.
- Yeh S. C., Liu Y. Y. (2003). Influence of social support on cognitive function in the elderly. BMC Health Services Research, 3, 9. doi:10.1186/1472-6963-3-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi Z. Ping T. Baochang G. Yi X. Bohua L., & Yongpiing L (1993). Causes and implications of the recent increase in the reported sex ratio at birth in China. Population and Development Review, 19(2), 283–302. [Google Scholar]
- Yip W., Mahal A. (2008). The health care systems of China and India: Performance and future challenges. Health Affairs (Project Hope), 27, 921–932. doi:10.1377/hlthaff.27.4.921 [DOI] [PubMed] [Google Scholar]
- Yount K. M., Agree E. M. (2005). Differences in disability among older women and men in Egypt and Tunisia. Demography, 42, 169–187. [DOI] [PubMed] [Google Scholar]
- Zunzunegui M.-V. Alvarado B.-E. Béland F., & Vissandjee B (2009). Explaining health differences between men and women in later life: A cross-city comparison in Latin America and the Caribbean. Social Science & Medicine, 68, 235–242. doi:10.1016/j.socscimed.2008.10.031 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.