Live attenuated simian immunodeficiency virus (SIV) vaccine protects rhesus macaques from high-dose vaginal challenge by pathogenic SIV strain mac251 by maintaining epithelial integrity in the female genital tract to prevent virus entry at the mucosa.
Keywords: SIV, female reproductive tract, epithelium, SIVΔnef, epithelium integrity
Abstract
To identify the mechanisms by which human immunodeficiency virus type 1 (HIV-1) might penetrate the epithelial barrier during sexual transmission to women and the mechanisms of vaccine-associated protection against entry, we characterized early epithelial responses to vaginal inoculation of simian immunodeficiency virus strain mac251 (SIVmac251) in naive or SIVmac239Δnef-vaccinated rhesus macaques. Vaginal inoculation induced an early stress response in the cervicovaginal epithelium, which was associated with impaired epithelial integrity, damaged barrier function, and virus and bacterial translocation. In vaccinated animals, early stress responses were suppressed, and the maintenance of epithelial barrier integrity correlated with prevention of virus entry. These vaccine-protective effects were associated with a previously described mucosal system for locally producing and concentrating trimeric gp41 antibodies at the mucosal interface and with formation of SIV-specific immune complexes that block the stress responses via binding to the epithelial receptor FCGR2B and subsequent inhibitory signaling. Thus, blocking virus entry may be one protective mechanism by which locally concentrated non-neutralizing Ab might prevent HIV sexual transmission to women.
Antiretroviral treatment (ART) has greatly improved life expectancy and quality of life for the >35 million people currently infected with human immunodeficiency virus (HIV) [1, 2]. However, ART alone cannot end the pandemic [3] because of continuing new infections currently in close to 2 million people [1], mainly via sexual contact [4]. Women comprise 60% of these new infections [5], and young women in sub-Saharan Africa are at particularly high risk of acquiring HIV [5]. Thus, an effective HIV vaccine that would protect these vulnerable populations is urgently needed.
To that end, we have used the simian immunodeficiency virus (SIV)–rhesus macaque model to investigate HIV-1 heterosexual transmission to women, to gain a better understanding of the mechanisms of mucosal transmission and thereby guide vaccine design. In this model, we have shown that virus replication in CD4+ T cells in the cervicovaginal mucosa is a critical event during mucosal transmission [6, 7] because it establishes founder populations that then expand locally, continuously seeding virus at distal sites, particularly lymphoid tissues, which then serve as the major reservoir for virus production and persistence [8, 9]. We have also shown that the robust protection by SIVΔnef vaccination against high-dose vaginal challenge in the SIV–rhesus macaque model [10] correlates with a vaccine-induced, 2-component immune response involving local production of immunoglobulin G antibodies (Abs), predominantly to gp41t in the virion envelope; and concentration of these gp41t Abs by the neonatal Fc receptor in cervicovaginal epithelium. The extraordinarily high concentration of these nonneutralizing but virus-binding Abs at the mucosal interface with virus might block transmission in 2 ways: (1) by restricting viral access to CD4+ T cells in the submucosa, to thereby prevent establishment of founder populations of infected cells at the portal of virus entry, and (2) by preventing expansion of these founder populations by blocking CD4+ T-cell recruitment through an inhibitory mechanism mediated by immune complex–FCGR2B receptor interactions [6, 10–16].
In thinking about how concentrated antibodies might restrict access to CD4+ T-cell targets in the mucosa, we had originally been thinking in general terms about how Ab binding interferes with 3 mechanisms by which virus is proposed to cross the epithelial barrier to reach target cells in the submucosa [17]: (1) Langerhans cell–facilitated transport [18–21], (2) transcytosis through epithelial cells [22], and (3) preexisting breaches in the epithelium [23]. While these mechanisms for virus entry are plausible, they are unlikely to account for observations such as the consistent finding of virus replication in the endocervix, where Langerhans cells are rare [7, 14]. Moreover, transcytosis models are generally based on experiments in cell lines or gut explants [22, 24], with necessarily uncertain relevance to transmission events in vivo, and direct evidence of entry through preexisting breaches in the female reproductive tract epithelium is relatively scanty [6]. We were thus motivated to further investigate other potential mechanisms of virus entry that might be targeted by concentrated nonneutralizing antibodies in vaccinated animals.
In this study, we extended our unbiased approach to identifying potential mechanisms of virus entry by combining microarray analysis and immunohistochemical (IHC) tissue mapping of the early events in the cervicovaginal epithelium of rhesus macaques between 1 and 3 days after vaginal inoculation. In unvaccinated animals, we found that the cervicovaginal epithelium quickly launched stress responses to vaginal inoculation, which was associated with impaired epithelial integrity, damaged barrier functions, and translocation of bacteria and viruses across the epithelium. Thus, early host responses to vaginal inoculation in cervicovaginal epithelium facilitated mucosal transmission by opening a paracellular pathway for virus to cross the epithelial barrier. By contrast, the robust protection elicited by the live attenuated SIVmac239Δnef vaccine against vaginal challenge correlated with suppression of early stress responses and maintenance of mucosal epithelial integrity and barrier function. We further linked these vaccine-protective effects to concentrated Abs at the mucosal interface, where formation of SIV-specific immune complexes blocked the stress responses through binding to the inhibitory receptor FCGR2B in the epithelium.
MATERIALS AND METHODS
Animals, Vaccination, and Vaginal Challenge
We examined archived necropsy tissue specimens obtained from the female genital tract during previous longitudinal studies of mucosal transmission following high-dose vaginal inoculation of pathogenic SIV strain mac251 (SIVmac251) in unvaccinated [7, 25] and SIVmac239Δnef-vaccinated [26] rhesus macaques. The animals had been housed in accordance with the regulations of the American Association for the Accreditation of Laboratory Animal Care and the standards of the Association for the Assessment and Accreditation of Laboratory Animal Care International at the New England Primate Research Center and the California National Primate Research Center. Unvaccinated animals had been atraumatically inoculated intravaginally with pathogenic SIVmac251 (supplied by Dr Christopher Miller) twice in a single day, with a 4-hour interval between inoculations. Each inoculation contained 1 mL of virus stock of 105 median tissue culture infective doses. Animals were then euthanized and necropsied on day 1 (n = 8) or day 3 (n = 11) after vaginal infection; 4 animals served as uninfected controls. For vaccination, animals were inoculated intravenously with SIVmac239Δnef (supplied by Dr Ronald C. Desrosiers). At week 20 after vaccination, vaccinated animals were vaginally challenged using the same protocol as that for unvaccinated animals. Necropsy tissue specimens were collected on day 4 (n = 3) or day 5 (n = 2) after challenge; specimens from vaccinated but unchallenged controls were collected during week 20 (n = 4).
Tissue Specimen Collection and Processing
At the time of euthanasia, tissue specimens were collected and fixed in 4% paraformaldehyde or SafeFix II (Fisher Scientific, Kalamazoo, MI) and embedded in paraffin for later sectioning. To examine the critical anatomical niches in the female reproductive tract, the uterus, cervix, and vagina were dissected en bloc. The relevant region of cervix was dissected away from most of the uterus and vagina and then further divided into 4 quadrants. Tissue pieces from each quadrant were either snap frozen, fixed as described, or used unfixed for other assays.
Microarray Analysis
The global transcription analysis was performed on the Affymetrix Rhesus Gene 1.0 ST Array platform (Affymetrix, Santa Clara, CA). After statistical analysis, genes were selected based on their associated P values (<.05), false-positive cutoff q values (<0.2), and fold changes in expression (>1.2). See the detailed description in the Supplementary Materials.
IHC and Quantitative Image Analyses
The primary Abs used in this study are summarized in Supplementary Table 1. In brief [14, 27], tissue sections were deparaffinized in xylene and rehydrated in phosphate-buffered saline. After blocking in Background Sniper (Biocare Medical, Concord, CA), sections were incubated with primary Abs at 4°C overnight. Then, signals were amplified with biotinylated secondary Abs and the ABC system (Vector Lab, Burlingame, CA). Nuclei were counterstained with hematoxylin (Invitrogen, Eugene, OR). For each tissue block, 2–3 sections at various depth were cut and stained for specific IHC markers. Images were taken on Olympus BX60 microscope (Olympus, Center Valley, PA). For quantitative image analysis, stained tissue sections were scanned by the Aperio ScanScope System (Leica, Buffalo Grove, IL). The entire endocervical tissue sections/regions were then analyzed and quantified by use of ImageScope software (Leica) to measure the pixel intensities, numbers of positive cells and nuclei, and tissue areas, using the pixel intensity, membrane functions, and nuclei counting functions provided by the software. We did not select particular areas for quantification in this work. For a specific IHC marker, specimens from all animals were stained, quantified, and included in the plots. Each dot in quantification plots of specific IHC markers represents the mean value for all sections from an individual animal. Owing to spatial limitation, only representative images of IHC staining are shown in the figures.
In Situ Hybridization and Tyramide Signaling Amplification for Detection of Virions
Virions were detected in tissue sections by viral RNA in situ hybridization/tyramide signal amplification/enzyme-linked fluorescence. Briefly, sections were deparaffinized, rehydrated, treated with protease, acetylated, and then hybridized to digoxigenin-labeled SIV-specific RNA probes. Bound digoxigenin-labeled RNA probes were detected by 3 rounds of tyramide signal amplification (Fisher Scientific, Waltham, MA) combined with avidin–biotinylated peroxidase (ABC Elite; Vector Labs). Then, slides were stained with enzyme-linked fluorescence 97 substrate (Molecular Probes, Waltham) and Hoechst 33342. See the detailed description in the Supplementary Materials.
Ex Vivo Organ Culture
The entire fresh cervix from a healthy, uninfected, young adult rhesus macaque was equally split into small pieces (5–10 mm in diameter by 5–10 mm in thickness) and embedded in agarose gel, leaving only the epithelium surfaces exposed upward. Medium (containing uninfected control sera of rhesus macaques), SIV, SIV and immune complexes, or SIV, immune complexes, and FCGR2B-blocking Ab were topically applied onto the epithelium surface. After incubation for 24 hours at 37°C/5% CO2, the tissue specimens were fixed or frozen and then compared pairwise across tissue specimens from the same animal for detection of immune complex–induced changes in cervical mucosa. For immune complex formation, 1.2 × 105 infectious units of SIVmac251 were combined with excess Ab (approximately 7 µg; gp41 monoclonal Ab 4.9C or serum from SIVmac239ΔNef-vaccinated animals) and incubated for 60 minutes at room temperature before application to explants. For blocking FCGR2B, explants were preincubated with 5 µg of FCGR2B-blocking antibodies (rabbit monoclonal Ab, lot GR32165-2 [product code ab45143; Abcam]; and mouse polyclonal Ab, lot 08338 WULZ [catalog no. H00002213-B01P; Abnova]) for 30 minutes at 37°C in 5% CO2 before addition of immune complexes.
Statistics
The nonparametric Wilcoxon signed rank test was used to compare the variations in expression of IHC markers across different groups over the course of infection. Statistical analyses were performed using Graph Pad Prism 4 software.
RESULTS
Vaginal Inoculation Induces Stress Responses in Female Reproductive Tract Epithelium in Unvaccinated Animals but Not Vaccinated Animals
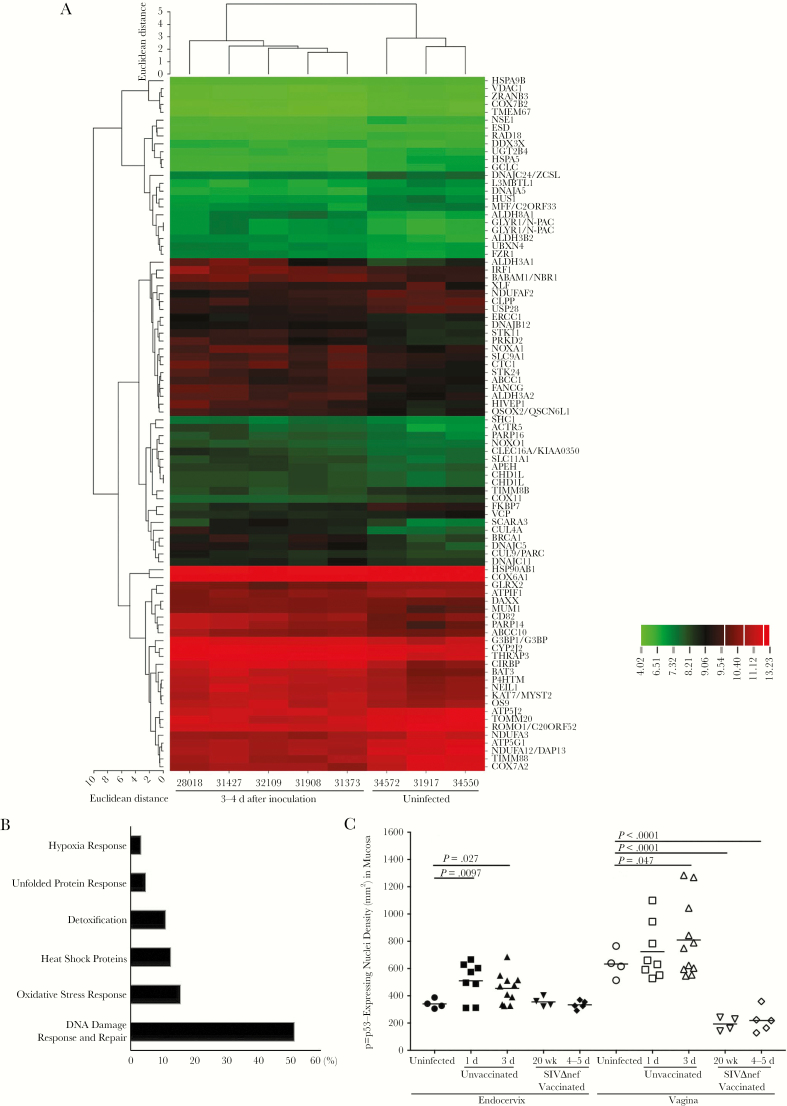
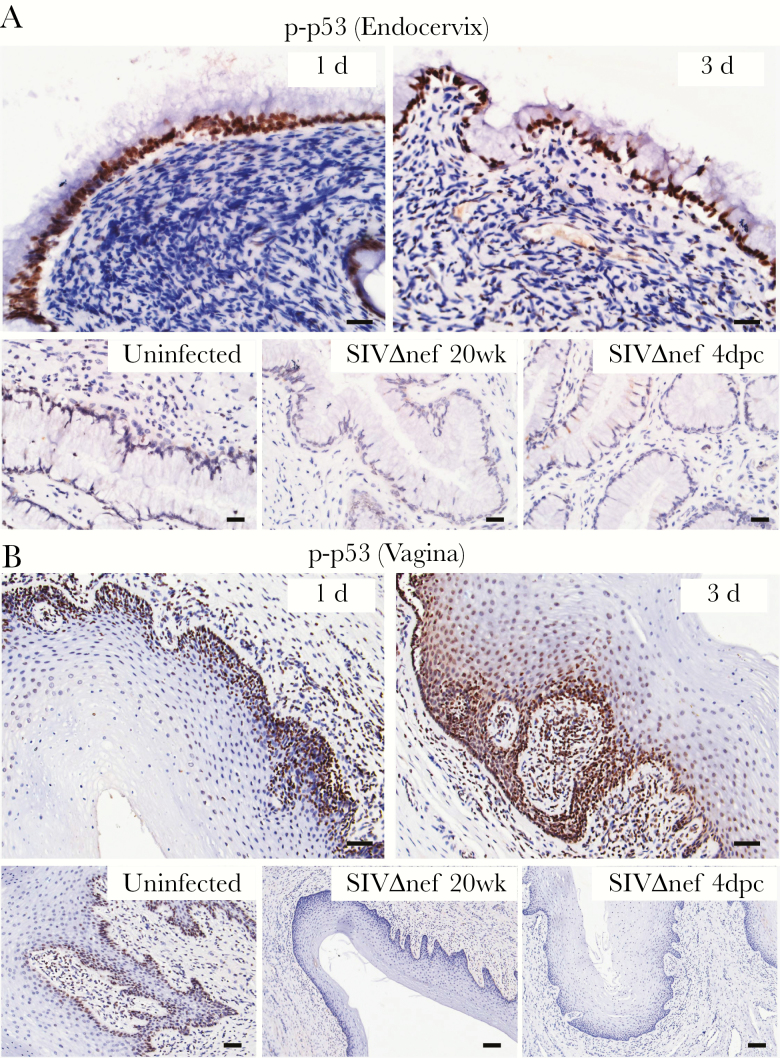
In our approach to identifying novel mechanisms of mucosal transmission and protection, we first compared the global transcriptional profiles of cervical tissue specimens from uninfected rhesus macaques to profiles after exposure intravaginally to high doses of SIV. In previous studies using this approach [15], we discovered proinflammatory responses in early infection in the cervical mucosa that contribute to recruitment of CD4+ T cells and local expansion of virus replication [14, 15]. Since stress responses often lead to inflammatory responses and because the virus inoculum contains a variety of stimulating components and viral proteins that are toxic to epithelial cells in vitro [28], we hypothesized that virus inoculation might induce stress responses in mucosal tissues. We therefore reexamined the microarray data for evidence of stress-induced genes and found that expression of stress response genes increased after vaginal inoculation (Figure 1A). The majority of these genes are related to the p53-associated DNA damage response and DNA repair pathways (Figure 1B). Thus, we chose nuclear expression of phosphorylated p53 (p-p53) as the marker in IHC analysis to profile these stress responses in the tissues. The densities of p-p53–expressing nuclei in both cervical and vaginal tissues significantly increased within 1–3 days after inoculation (Figure 1C). In the endocervix (Figure 2A), p-p53–expressing nuclei were almost exclusively located in columnar epithelial cells. In the vagina (Figure 2B), we observed expression of p-p53 in the basal layer of epithelium in uninfected tissues, which quickly expanded into adjoining epithelium and submucosa, with high levels of expression within 1–3 days after inoculation. By contrast, stress responses and epithelial p-p53 expression in the SIVmac239Δnef-vaccinated animals were equivalent to those in unchallenged animals (Figure 2A and 2B). These results demonstrate that vaginal exposure to the SIV inoculum quickly induced stress responses in the female reproductive tract mucosa, particularly in the epithelium, in naive animals but not in vaccinated animals, consistent with the previously described early proinflammatory activation in the mucosa and the rapid production of inflammatory chemokines by the epithelium in naive animals but not in vaccinated animals [6, 14, 15].
Figure 1.
Vaginal inoculation induces cervical stress responses in unvaccinated rhesus macaques. A, Microarray analysis of the cervical tissues from uninfected and vaginally inoculated rhesus macaques (3–4 days after inoculation). The messenger RNA in the 1–2-mm surface layers of cervical tissues was extracted and subjected to microarray analysis. Genes were selected on the basis of their associated P values (<.05), false-positive cutoff q values (<0.2), and fold changes in expression (>1.2). Selected genes were then analyzed through Ingenuity Pathway analysis and grouped on the basis of their putative functions. Vaginal exposure to simian immunodeficiency virus (SIV) induced increased expression of genes related to a variety of stress responses. Fold changes in gene expression are indicated in the color scale bar. B, More than 50% of these stress response genes are associated with the DNA damage response and repair pathways. Genes were grouped on the basis of their known functions and associated signaling pathways. The percentages (x-axis) were calculated by dividing the number of genes in each group by the total number of genes. C, The densities of the p-p53–expressing nuclei in the endocervix significantly increased within 1 day after inoculation in unvaccinated animals but remained at baseline levels in SIVmac239Δnef-vaccinated animals before (20 weeks after vaccination) and after (4–5 days after challenge) vaginal challenge. Vaginal tissues of unvaccinated animals have a higher basal level of p-p53–expressing nuclei than the endocervix. The increases in p-p53–expressing nuclei were minimal 1 day after inoculation and then became significant 3 days after inoculation. However, in vaginal tissue specimens obtained from SIVmac239Δnef-vaccinated animals before and after vaginal challenge, the levels of p-p53–expressing nuclei were even lower than those at baseline. The nonparametric Wilcoxon signed rank test was used to compare the variations in expression of immunohistochemical markers across different groups over the course of infection. The bars represent median values in each group.
Figure 2.
Vaginal inoculation induces stress responses in the cervicovaginal mucosa of unvaccinated rhesus macaques but not SIVmac239Δnef-vaccinated animals. Immunohistochemical staining demonstrates widespread increases in p-p53–expressing nuclei, mainly located in the epithelium in unvaccinated animals but not in vaccinated. A, In the endocervix, the p-p53–expressing nuclei were predominantly located in the endocervical epithelium after vaginal inoculation. B, In the vagina, p-p53–expressing nuclei were located in the basal layer of epithelium in uninfected animals and then quickly expanded into the adjoining epithelium and submucosa within 3 days after inoculation. In the SIVmac239Δnef-vaccinated animals, both the endocervical and vaginal epithelium were negative for p-p53 staining before and after vaginal challenge. All scale bars denote 50 µm.
Vaginal SIV Exposure Impairs Female Reproductive Tract Epithelial Homeostasis in Unvaccinated Animals but Not Vaccinated Animals
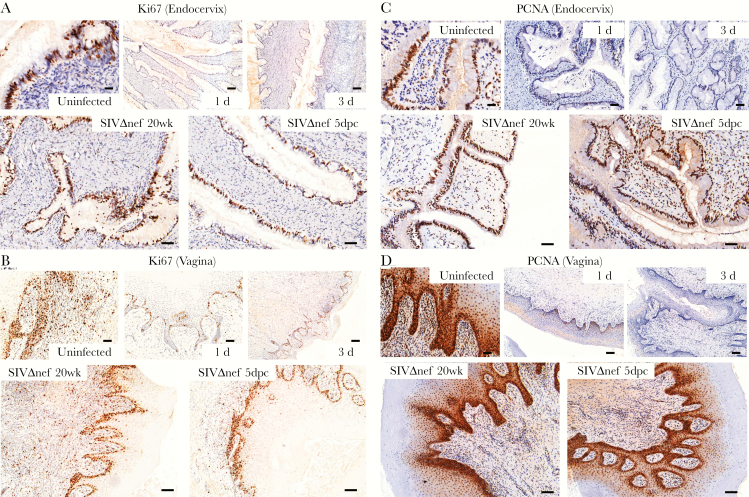
Since the p53 signaling pathway induces cell cycle arrest [29, 30], we further hypothesized that early stress responses triggered by vaginal inoculation might disturb the homeostatic replacement of female reproductive tract epithelium. We first examined the proliferative capacity in the female reproductive tract, using nuclear antigens Ki67 and PCNA as IHC markers. Both markers were abundant in the cervical and vaginal tissues of uninfected animals, but levels decreased rapidly within 1 day after inoculation (Figure 3A–D and Supplementary Figure 1A and 1B). The Ki67-expressing nuclei were mainly located in the endocervical epithelium, the basal layers of vaginal epithelium, and the vaginal submucosa of uninfected animals (Figure 3A and 3B). At 1 day after inoculation, Ki67-expressing nuclei were nearly undetectable in the endocervical epithelium or vaginal submucosa, and only a few Ki67-expressing cells remained in the basal layer of vaginal epithelium (Figure 3A and 3B). Compared with Ki67, the PCNA marker was expressed by more nuclei in the cervical and vaginal tissues of uninfected animals, but expression consistently decreased to nearly undetectable levels at 1 day after inoculation, as well (Figure 3C and 3D). By contrast, both Ki67 and PCNA epithelial expression in vaccinated animals was equivalent to that in unchallenged controls (Figure 3A–D and Supplementary Figure 1A and 1B).
Figure 3.
Decreased proliferation and impaired epithelial homeostasis following vaginal inoculation in unvaccinated but not SIVmac239Δnef-vaccinated rhesus macaques. A and B, Immunohistochemical staining of the nuclear antigens Ki67 and PCNA were used as markers of proliferation. In uninfected animals, the Ki67-expressing nuclei were mainly located in the endocervical epithelium, the basal layers of vaginal epithelium, and the vaginal submucosa. Vaginal inoculation of simian immunodeficiency virus (SIV) was associated with dramatically reduced the numbers of positive nuclei to nearly undetectable levels in the endocervix and vaginal submucosa. In SIVmac239Δnef-vaccinated animals, the cervicovaginal epithelium still has strong expression of Ki67 after vaginal challenge. C and D, Similarly, the PCNA-expressing nuclei in the endocervix and vagina decreased quickly to nearly undetectable levels within 3 days after inoculation in unvaccinated animals but remained at a high level in SIVmac239Δnef-vaccinated animals before and after vaginal inoculation. The tissue distribution of PCNA-expressing nuclei is similar to that of Ki67-expressing nuclei, but PCNA was expressed in more nuclei than Ki67. All scale bars denote 50 µm.
Vaginal exposure to SIV was additionally associated with evidence of impaired epithelial differentiation. Using the IHC markers EpCAM (a proliferative marker in all female reproductive tract epithelia), cytokeratin 8/18 (CK8/18; a differentiation marker in the endocervical epithelium), and CK13 (a differentiation marker in the vaginal epithelium) [31, 32], we found reduced expression of EpCAM in both cervical and vaginal epithelia as early as 1 day after inoculation, with further reduction 3 days after inoculation (Supplementary Figures 1C and 1D and 2A and 2B), and reduced expression of endocervical CK8/18 and vaginal CK13 within 3 days after inoculation in unvaccinated but not vaccinated animals (Supplementary Figures 1C and 1D and 2C and 2D). Therefore, in unvaccinated animals but not in vaccinated animals, vaginal inoculation rapidly and dramatically reduced the homeostatic proliferation and differentiation that maintains cervicovaginal epithelial integrity.
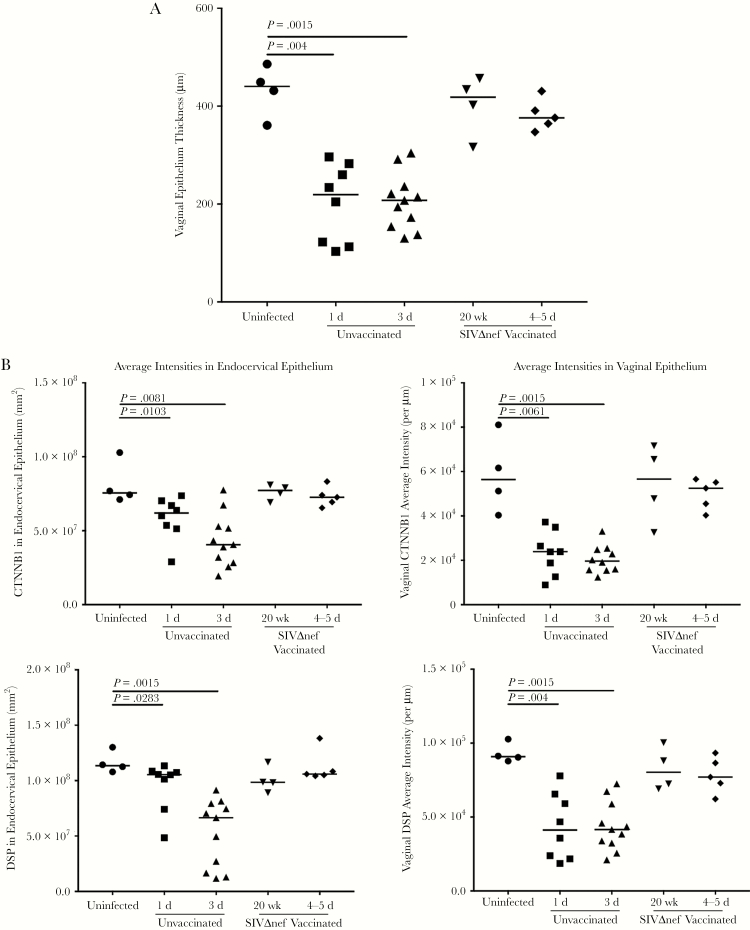
Vaginal Inoculation Reduces Epithelial Integrity and Barrier Function in Unvaccinated Animals but Not Vaccinated Animals
The disruption of homeostatic mechanisms to replace damaged epithelial surfaces thus suggested, as we soon found, that vaginal exposure to SIV quickly disrupted epithelial integrity and barrier function. Thinning of vaginal epithelium was evident within 1 day after inoculation (Figure 4A), and, at 3 days after inoculation, some regions of vaginal submucosa were only covered by 1–2 layers of epithelial cells (Supplementary Figures 3–5). To examine the integrity of cell-cell junctions in female reproductive tract epithelium, we used IHC staining with Abs to occludin, to assess tight junctions; Abs to β-catenin, to assess adhesion junctions; and Abs to desmoplakin, to assess desmosomes. Levels of all 3 cell-cell junction markers decreased following vaginal inoculation in both cervical and vaginal epithelium within 3 days after inoculation (Figures 4B and 5A and 5B). In the endocervix of uninfected animals prior to exposure to SIV, the epithelium was fully covered by a continuous layer of cells, with visible cell-cell junctions (Supplementary Figures 3–5). By 1 day after inoculation, breaches in the epithelium that lacked cell-cell junctions became visible; by 3 days after inoculation, epithelial layers with intact cell-cell junctions in the endocervical epithelium were rare (Supplementary Figures 3–5). In the vagina, thinning of cell-cell junction layers was apparent at 1 day after inoculation; by 3 days after inoculation, some vaginal submucosa was only covered a very thin layer of epithelium, without any cell-cell junctions (Supplementary Figures 3–5). Again, epithelial integrity, as evaluated by all these measures, was comparable between vaccinated animals and unchallenged controls (Supplementary Figures 3–5).
Figure 4.
Vaginal inoculation impairs the integrity of cervicovaginal epithelium in unvaccinated rhesus macaques but not vaccinated animals. A, Vaginal epithelium thinning within 1 day after inoculation in unvaccinated animals but not in SIVmac239Δnef-vaccinated animals after vaginal infection. First, the entire vaginal tissue section was scanned, and then the entire area of the vaginal epithelium and the length of the epithelium were quantified in ImageScope software. The average thickness of epithelial layer was acquired by dividing the epithelium area by the epithelium length. B, Decreased expression of β-catenin (CTNNB1) and desmoplakin (DSP) in the endocervix and vagina in unvaccinated animals but not in vaccinated animals after vaginal challenge. The nonparametric Wilcoxon signed rank test was used to compare the variations in expression of immunohistochemical markers across different groups over the course of infection. The bars represent median values in each group.
Figure 5.
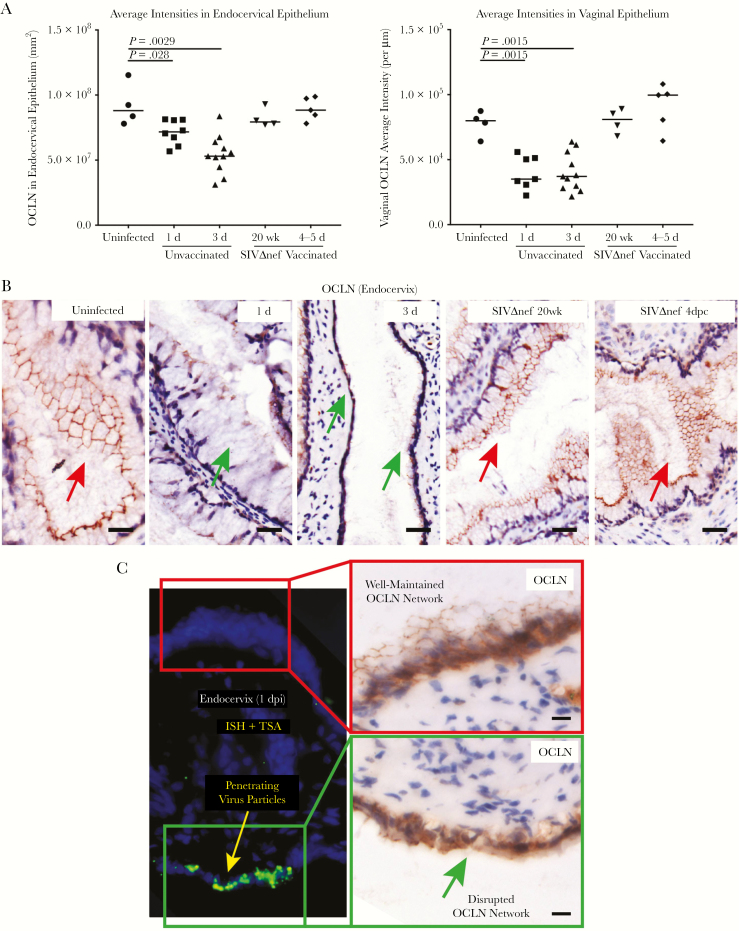
Virus penetration of damaged cervicovaginal epithelium in unvaccinated rhesus macaques but not vaccinated animals. A, Decreased expression of occludin (OCLN) in the endocervix and vagina of unvaccinated but not SIVmac239Δnef-vaccinated animals. The nonparametric Wilcoxon signed rank test was used to compare the variations in expression of immunohistochemical markers across different groups over the course of infection. The bars represent median values in each group. B, The OCLN network was continuous in uninfected and vaccinated animals in endocervical epithelium (red arrows), whereas, following vaginal inoculation, epithelial expression was discontinuous (green arrows) within 1 day after inoculation. Three days after inoculation, a continuous layer of OCLN-expressing endocervical epithelium was barely visible. The OCLN network was well maintained in the SIVmac239Δnef-vaccinated animals before and after vaginal challenge. C, The disruption of the OCLN network structure was spatially correlated with green virus particles and aggregates that penetrated the epithelial barrier. All scale bars denote 50 µm.
Microbial and Viral Translocation Across Damaged Epithelium
As further evidence of impaired epithelial integrity, we found an increase in bacterial translocation in the endocervical mucosa within 3 days after inoculation (Figure 6A and 6B). In addition, we documented penetration of virus particles through the epithelium into mucosal tissues that was spatially correlated with disruption of the tight junction network at the epithelial surface (Figure 5C and Supplementary Figure 6). Again, we found no evidence of bacterial or viral translocation in the vaccinated animals. Thus, vaginal exposure to SIV rapidly damages the integrity of female reproductive tract epithelium and its barrier functions to enable virus entry and access to target cells in the submucosa in unvaccinated animals but not in vaccinated animals.
Figure 6.
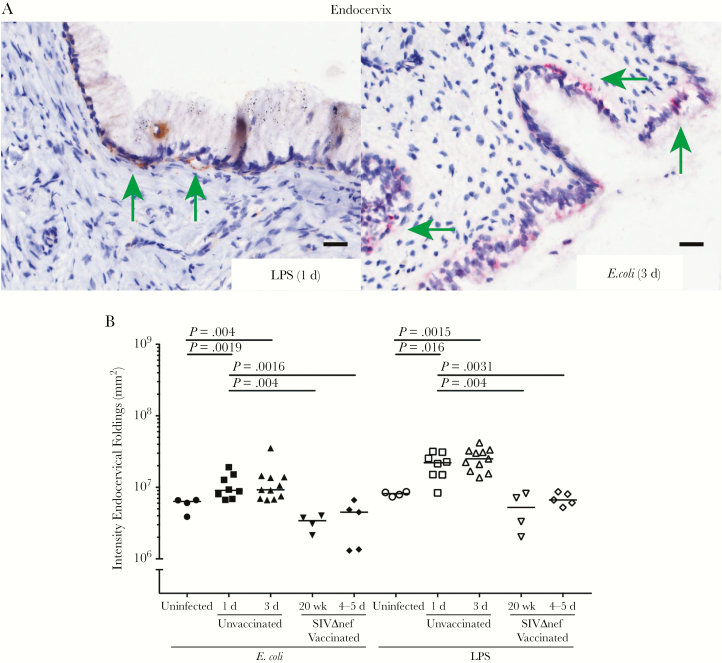
Increased bacterial translocation in unvaccinated but not vaccinated rhesus macaques. A, Immunohistochemical (IHC) staining with antibodies to lipopolysaccharide (LPS) and Escherichia coli detect bacteria underneath the columnar epithelium in the endocervix within 3 days after inoculation (green arrows; quantified in B), but not in the vaginal tissues. Bacteria and LPS were not detected in the submucosa of SIVmac239Δnef-vaccinated animals. All scale bars denote 50 µm. The nonparametric Wilcoxon signed rank test was used to compare the variations in expression of IHC markers across different groups over the course of infection. The bars represent median values in each group.
Immune Complex–FCGR2B Interactions Block Stress Responses in the Epithelium
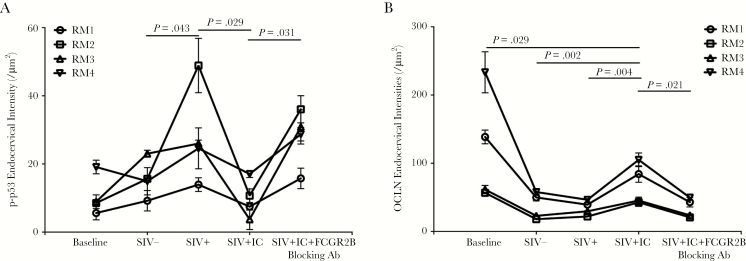
How might SIVΔnef vaccination preserve the homeostasis and integrity of cervicovaginal epithelium to prevent transmission-facilitating stress responses to viral exposure? We had previously found that the locally concentrated gp41t Ab immediately formed immune complexes with SIV inoculum following high-dose vaginal challenge. These immune complexes interacted with the inhibitory FCGR2B receptor in the mucosal epithelium, and this interaction blocked early proinflammatory signaling and the CD4+ T-cell recruitment that fuels expansion of virus infection at the portal of virus entry [14, 15]. In a logical extension of this mechanism for suppressing transmission-facilitating responses to virus exposure, we similarly hypothesized that the immune complexes and FCGR2B receptor might block the stress responses and associated injury to epithelial integrity. We tested this hypothesis in an ex vivo explant system in which we pretreated fresh cervical tissues of rhesus macaques with SIV-specific immune complexes before topical SIV inoculation. While this investigation was not ideal because of the stress inherent in establishing the culture system, we nonetheless were able to document significant suppression of p-p53 expression in the cervicovaginal epithelium by pretreatment with SIV-gp41t immune complexes (Figure 7A) and increased expression of occludin, compared with findings in untreated tissues, after SIV inoculation (Figure 7B). These effects were abrogated by FCGR2B-blocking Ab (Figure 7A and 7B). Thus, high levels of Ab concentrated at the mucosal interface through the formation of immune complexes and FCGR2B inhibitory signaling can moderate early stress responses in the epithelium, thereby contributing to the protection conferred by SIVΔnef vaccination.
Figure 7.
Immune complex-FCGR2B inhibitory signaling suppresses the stress responses to simian immunodeficiency virus (SIV) exposure in cervical tissues and maintains expression of cell-cell junction proteins in the epithelium. Pretreating fresh cervical explants of rhesus macaque ex vivo with SIV-specific immune complexes (ICs) before topical application of SIV suppressed the increases of p-p53–expressing nuclei (A) in the mucosa, and maintained to some degree the expression of occludin (OCLN; B) in the epithelium. These inhibitory effects were abrogated when the binding of ICs to FCGR2B was interrupted by blocking antibody (Ab). The nonparametric Wilcoxon signed rank test was used to compare the variations in expression of immunohistochemical markers across different treatment groups. The bars represent standard deviations among repeats in each group.
DISCUSSION
In our current understanding of HIV-1 sexual transmission to women, based on studies in the SIV–rhesus macaque animal model, the establishment of infected founder populations at the portal of entry and the expansion of those populations to disseminate infection systemically play critical roles in the mucosal transmission of HIV-1 and thus are logical targets for vaccines or other preventive strategies. Previously, we have shown a cascade of cytokine and chemokine production in cervicovaginal mucosa after vaginal inoculation that is initiated in the epithelium and amplified through infiltrating macrophages and pDCs and then leads to massive recruitment of CD4+ T cells as the target cell of virus replication [6, 14]. Here, we explored the initiating events and mechanisms by which virus might cross epithelial barriers to gain access to CD4+ T-cell targets underlying the epithelium, and we showed that the stress response to the virus inoculum induces epithelial damage and disrupts the barrier to provide a path for virus entry. We further showed that SIVmac239Δnef vaccination moderates this stress response to maintain mucosal barrier integrity as one correlate of protection.
Current models of how HIV and SIV might cross the epithelial barrier invoke host-mediated processes such as Langerhans cell–mediated transport into the ectocervical and vaginal epithelium [18, 20, 21] and transcytosis through epithelium [23]. Our in vivo studies point to alternative active pathways induced by the virus itself, with antecedent examples in paracellular pathways opened by HIV-1 in primary female reproductive tract epithelial cell and intestinal cell cultures [33]. In these in vitro studies, exposure to HIV-1 gp120 reduced transepithelial cell resistance and increased permeability by disrupting tight junctions to allow viral and bacterial translocation across epithelial monolayers without affecting the viability of the cells. In vivo, we showed that the stress response elicited by vaginal exposure to the virus inoculum is associated with broad effects on epithelial integrity, including inhibition of proliferation, differentiation to replace damaged epithelium, and, at levels sufficient to allow bacteria and virus particles to translocate into the submucosa, disruption of tight junctions and physical thinning and loss of epithelial barriers.
Further studies are needed to understand the extent to which these host responses are strictly virus induced, virus related, or independent. Viral components have been shown to elicit inflammatory cytokine production and trigger damage in cell-cell junctions in the cervicovaginal epithelium [33, 34]. The challenge stocks also contain a variety of nonspecific stimulating factors [28], such as tumor necrosis factor α [35], that are known to induce inflammatory reactions and epithelial damages, which could contribute to the early host responses in the epithelium. However, semen contains a plethora of factors to which the cervicovaginal mucosa will be exposed in unprotected heterosexual intercourse that also can induce inflammatory activation and stress responses in the epithelium [36–40]. Thus, transmission-facilitating host responses could be elicited independent of virus exposure or virus dose in exposure.
The local mechanisms by which vaccination with the live attenuated virus SIVmac239Δnef protects against vaginal challenge are increasingly seen as both direct and indirect [6, 14, 15]. Certainly, the production of antibodies to trimeric gp41 (gp41t) and the concentration of those antibodies on the path of virus entry by the neonatal Fc receptor in cervicovaginal epithelium could bind to virus and directly block establishment of founder populations of infected cells. These concentrated antibodies can also indirectly impact the expansion of founder populations by forming immune complexes with virus that interact with FCGR2B to engage inhibitory pathways that block recruitment of target cells to fuel that expansion [6, 14–16]. Here, we showed that vaccination is further associated with unresponsiveness to SIV after vaginal exposure, because it moderates the stress response that disrupts epithelial homeostasis, integrity, and barrier functions, blocking virus entry. SIVΔnef vaccination has previously been shown to suppress proinflammatory recruitment of CD4+ T cells via immune complex–FCGR2B inhibitory signaling. Although this investigation was restricted to archived tissue specimens from limited time points, we showed that a similar pathway suppresses damage in the mucosal epithelium of vaccinated animals. Thus, vaccine strategies aimed at inducing high-level local Ab production in the mucosa and concentration of these Ab at the mucosal border can protect against infection by suppressing 2 critical transmission-facilitating host responses at the portal of entry. In addition, mimicking the protective correlate of “mucosal epithelial quiescence” and downstream target recruitment with safer alternatives to the SIVmac239Δnef vaccine, microbicides such as glycerol monolaurate [6] and newly developed agents will advance effective prevention strategies against HIV-1 sexual transmission to women.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank R. Desrosiers and C. Miller, for virus stocks; C. Miller, for tissue samples from uninfected animals and from unvaccinated and infected animals; A. Carville, for expert veterinary care; E. Curran and A. Miller, for assistance with tissue processing and analysis; and C. O’Neill and T. Leonard, for help in preparing the manuscript and figures.
L. S. and A. T. H. designed this study; L. S. and A. J. S. performed microarray analysis and ex vivo explant experiments; L. S., L. D., K. E. P., and S. W. performed IHC analysis; M. Z. processed tissue samples; P. J. S. helped with ex vivo explant experiments; R. P. J. designed and supervised animal experiments; and J. V. C. helped write the manuscript.
Financial support. This work was supported by the International AIDS Vaccine Initiative, the National Institutes of Health (grants AI071306, AI102625, P51 OD011103, and P51 OD011132), and the National Cancer Institute, National Institutes of Health (contract HHSN261200800001E).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. UN Joint Programme on HIV/AIDS (UNAIDS). Global Report: UNAIDS Report on the Global AIDS Epidemic. Geneva, Switzerland: UNAIDS, World Health Organization; 2014. [Google Scholar]
- 2. Fauci AS. 25 years of HIV. Nature 2008; 453:289–90. [DOI] [PubMed] [Google Scholar]
- 3. UN Joint Programme on HIV/AIDS (UNAIDS). Global Report: UNAIDS Report on the Global AIDS Epidemic. Geneva, Switzerland: UNAIDS, World Health Organization; 2013. [Google Scholar]
- 4. Morison L. The global epidemiology of HIV/AIDS. Br Med Bull 2001; 58:7–18. [DOI] [PubMed] [Google Scholar]
- 5. Quinn TC, Overbaugh J. HIV/AIDS in women: an expanding epidemic. Science 2005; 308:1582–3. [DOI] [PubMed] [Google Scholar]
- 6. Li Q, Estes JD, Schlievert PM et al. Glycerol monolaurate prevents mucosal SIV transmission. Nature 2009; 458:1034–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Miller CJ, Li Q, Abel K et al. Propagation and dissemination of infection after vaginal transmission of simian immunodeficiency virus. J Virol 2005; 79:9217–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cavert W, Notermans DW, Staskus K et al. Kinetics of response in lymphoid tissues to antiretroviral therapy of HIV-1 infection. Science 1997; 276:960–4. [DOI] [PubMed] [Google Scholar]
- 9. Haase AT, Henry K, Zupancic M et al. Quantitative image analysis of HIV-1 infection in lymphoid tissue. Science 1996; 274:985–9. [DOI] [PubMed] [Google Scholar]
- 10. Li Q, Zeng M, Duan L et al. Live simian immunodeficiency virus vaccine correlate of protection: local antibody production and concentration on the path of virus entry. J Immunol 2014; 193:3113–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Adnan S, Reeves RK, Gillis J et al. Persistent low-level replication of SIVΔnef drives maturation of antibody and CD8 T cell responses to induce protective immunity against vaginal SIV infection. PLoS Pathog 2016; 12:e1006104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Haase AT. Targeting early infection to prevent HIV-1 mucosal transmission. Nature 2010; 464:217–23. [DOI] [PubMed] [Google Scholar]
- 13. Haase AT. Early events in sexual transmission of HIV and SIV and opportunities for interventions. Annu Rev Med 2011; 62:127–39. [DOI] [PubMed] [Google Scholar]
- 14. Shang L, Duan L, Perkey KE et al. Epithelium-innate immune cell axis in mucosal responses to SIV. Mucosal Immunol 2017; 10:508–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Smith AJ, Wietgrefe SW, Shang L et al. Live simian immunodeficiency virus vaccine correlate of protection: immune complex-inhibitory Fc receptor interactions that reduce target cell availability. J Immunol 2014; 193:3126–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zeng M, Smith AJ, Shang L et al. Mucosal humoral immune response to SIVmac239∆nef vaccination and vaginal challenge. J Immunol 2016; 196:2809–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Greenhead P, Hayes P, Watts PS, Laing KG, Griffin GE, Shattock RJ. Parameters of human immunodeficiency virus infection of human cervical tissue and inhibition by vaginal virucides. J Virol 2000; 74:5577–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ballweber L, Robinson B, Kreger A et al. Vaginal langerhans cells nonproductively transporting HIV-1 mediate infection of T cells. J Virol 2011; 85:13443–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hladik F, Sakchalathorn P, Ballweber L et al. Initial events in establishing vaginal entry and infection by human immunodeficiency virus type-1. Immunity 2007; 26:257–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Miller CJ. Localization of Simian immunodeficiency virus-infected cells in the genital tract of male and female Rhesus macaques. J Reprod Immunol 1998; 41:331–9. [DOI] [PubMed] [Google Scholar]
- 21. Miller CJ, Hu J. T cell-tropic simian immunodeficiency virus (SIV) and simian-human immunodeficiency viruses are readily transmitted by vaginal inoculation of rhesus macaques, and Langerhans’ cells of the female genital tract are infected with SIV. J Infect Dis 1999; 179(Suppl 3):S413–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bomsel M. Transcytosis of infectious human immunodeficiency virus across a tight human epithelial cell line barrier. Nat Med 1997; 3:42–7. [DOI] [PubMed] [Google Scholar]
- 23. Shattock RJ, Moore JP. Inhibiting sexual transmission of HIV-1 infection. Nat Rev Microbiol 2003; 1:25–34. [DOI] [PubMed] [Google Scholar]
- 24. Bomsel M, Heyman M, Hocini H et al. Intracellular neutralization of HIV transcytosis across tight epithelial barriers by anti-HIV envelope protein dIgA or IgM. Immunity 1998; 9:277–87. [DOI] [PubMed] [Google Scholar]
- 25. Johnson RP, Lifson JD, Czajak SC et al. Highly attenuated vaccine strains of simian immunodeficiency virus protect against vaginal challenge: inverse relationship of degree of protection with level of attenuation. J Virol 1999; 73:4952–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Smith AJ, Schacker TW, Reilly CS, Haase AT. A role for syndecan-1 and claudin-2 in microbial translocation during HIV-1 infection. J Acquir Immune Defic Syndr 2010; 55:306–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shang L, Smith AJ, Duan L et al. NK cell responses to simian immunodeficiency virus vaginal exposure in naive and vaccinated rhesus macaques. J Immunol 2014; 193:277–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Del Prete GQ, Scarlotta M, Newman L et al. Comparative characterization of transfection- and infection-derived simian immunodeficiency virus challenge stocks for in vivo nonhuman primate studies. J Virol 2013; 87:4584–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Aylon Y, Oren M. Living with p53, dying of p53. Cell 2007; 130:597–600. [DOI] [PubMed] [Google Scholar]
- 30. Levine AJ, Oren M. The first 30 years of p53: growing ever more complex. Nat Rev Cancer 2009; 9:749–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Litvinov SV, van Driel W, van Rhijn CM et al. Expression of Ep-CAM in cervical squamous epithelia correlates with an increased proliferation and the disappearance of markers for terminal differentiation. Am J Pathol 1996; 148:865–75. [PMC free article] [PubMed] [Google Scholar]
- 32. Purkis PE, Steel JB, Mackenzie IC, Nathrath WB, Leigh IM, Lane EB. Antibody markers of basal cells in complex epithelia. J Cell Sci 1990; 97(Pt 1):39–50. [DOI] [PubMed] [Google Scholar]
- 33. Nazli A, Chan O, Dobson-Belaire WN et al. Exposure to HIV-1 directly impairs mucosal epithelial barrier integrity allowing microbial translocation. PLoS Pathog 2010; 6:e1000852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sankapal S, Gupta P, Ratner D et al. HIV exposure to the epithelia in ectocervical and colon tissues induces inflammatory cytokines without tight junction disruption. AIDS Res Hum Retroviruses 2016; 32:1054–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nazli A, Kafka JK, Ferreira VH et al. HIV-1 gp120 induces TLR2- and TLR4-mediated innate immune activation in human female genital epithelium. J Immunol 2013; 191:4246–58. [DOI] [PubMed] [Google Scholar]
- 36. Ferreira VH, Kafka JK, Kaushic C. Influence of common mucosal co-factors on HIV infection in the female genital tract. Am J Reprod Immunol 2014; 71:543–54. [DOI] [PubMed] [Google Scholar]
- 37. Jeremias JC, Bongiovanni AM, Witkin SS. Induction of heat shock protein expression in cervical epithelial cells by human semen. Infect Dis Obstet Gynecol 1999; 7:17–22. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 38. Hearps AC, Tyssen D, Srbinovski D et al. Vaginal lactic acid elicits an anti-inflammatory response from human cervicovaginal epithelial cells and inhibits production of pro-inflammatory mediators associated with HIV acquisition. Mucosal Immunol 2017; 10:1480–90. [DOI] [PubMed] [Google Scholar]
- 39. Robertson SA. Seminal plasma and male factor signalling in the female reproductive tract. Cell Tissue Res 2005; 322:43–52. [DOI] [PubMed] [Google Scholar]
- 40. Sharkey DJ, Macpherson AM, Tremellen KP, Robertson SA. Seminal plasma differentially regulates inflammatory cytokine gene expression in human cervical and vaginal epithelial cells. Mol Hum Reprod 2007; 13:491–501. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.