Abstract
Objective:
Studies have documented disparities in exposure to endocrine disrupting chemicals (EDC), but no studies have investigated potential implications for racial/ethnic disparities in chronic disease and associated costs. Our objective was to examine EDC levels in the US population according to race/ethnicity and to quantify disease burden and associated costs.
Study Design and Setting:
EDC exposure levels in 2007-2010 were obtained from the National Health and Nutrition Examination Surveys. The associated disease burden and costs for twelve exposure-response relationships were determined for Non-Hispanic Whites, Non-Hispanic Blacks, Mexican-Americans, Other Hispanics, and Other/Multicultural.
Results:
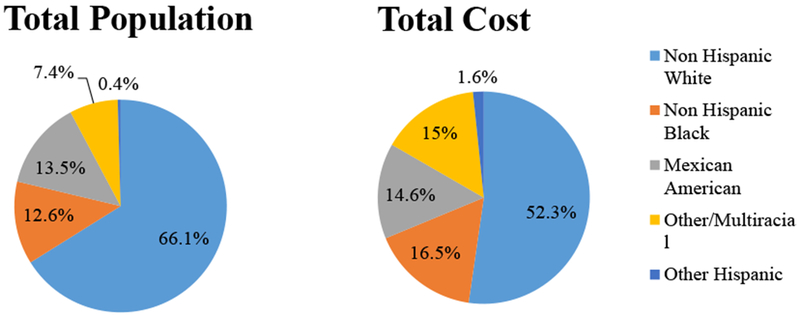
EDC exposure levels and associated burden of disease and costs were higher in Non-Hispanic Blacks ($56.8 billion; 16.5% of total costs) and Mexican-Americans ($50.1 billion; 14.6%) compared to their proportion of the total population (12.6% and 13.5%, respectively). Associated costs among Non-Hispanic Whites comprised 52.3% of total costs ($179.8 billion), though they comprise 66.1% of the US population. These disparities are driven by generally higher exposure to persistent pesticides and flame retardants among Non-Hispanic Blacks and Mexican-Americans.
Conclusion:
Our estimates suggest that racial/ethnic disparities in chronic diseases in the US may be due to chemical exposures, and are an important tool to inform policies that address such disparities.
Keywords: endocrine disrupting chemicals, disease burden, economic costs, obesity, neurodevelopment, reproductive health
Introduction
Since the publication of the first Endocrine Society Scientific Statement report on endocrine disrupting chemicals (EDCs) [1], evidence has increasingly confirmed that EDCs contribute to disease and disability across the lifespan [2, 3]. The Endocrine Society has defined EDCs as chemicals that interfere with hormonal function, resulting in adverse health outcomes. EDCs include industrial solvents, such as flame retardants (polychlorinated and polyborminated biphenyls, dioxins); plasticizers (phthalates); persistent pesticides (dichlorodiphenyldichloroethylene); plastics (Bisphenol A); and pharmaceutical compounds (diethylstilbestrol). Potential adverse health outcomes associated with exposure to EDCs include diabetes, overweight/obesity in childhood and adulthood, breast and prostate cancer, male and female reproductive dysfunction, cardiovascular and pulmonary disease, and learning impairments attributable to neurobehavioral dysfunction[1].
Previous reports have documented a substantial health and economic burden due to EDCs in Europe and the US, with annual costs totaling €163 billion and $340 billion, respectively, while at the same time highlighting the importance of the regulatory environment in addressing preventable exposures [4, 5]. Racial and ethnic disparities in chronic conditions are well documented in the US [6–8], and which are even more apparent among children [9]. Race/ethnicity can affect health care delivery and quality through a number of pathways, which are often connected, including health care affordability, geographic access, transportation, education, literacy, health beliefs, and provider bias [10].
Disproportionate environmental exposures, including exposure to EDCs, among racial/ethnic minorities have been described as considerable risk factors for adverse health outcomes [11]. For example, significantly higher exposure to diabetogenic EDCs, such as Bisphenol A (BPA) and phthalates has been observed in Latinos and African Americans in the US [12]. James-Todd and colleagues reported that non-Whites seem to have higher exposure to EDCs, such as phthalates, which may disproportionately increase the incidence of endometriosis, among other female reproductive health outcomes[13]. Factors driving the disproportionate exposure to several EDCs includes differences in food consumption, usage of consumer products, as well as built environmental conditions [2] driven at least in part by socioeconomic status [14].
Reducing health disparities in the US has been one of the main goals of Healthy People from its inception. Healthy People is an initiative of the Department of Health and Human Services that represents a framework for public health prevention priorities and actions in the US. One of the overarching goals of Healthy People 2020 is to “achieve health equity, eliminate disparities, and improve the health of all groups” [15]. Though studies have identified potential implications of differences in EDC exposures, particularly among women and in relationship to reproductive health outcomes [13, 16–19], to our knowledge, no studies have quantified potential population-level differences in burden of disease and economic cost associated with EDCs exposures. To this end, the main objective of this analysis was to examine levels of EDCs in the US population according to race/ethnicity and to quantify the disease burden and associated economic costs.
Materials and methods
1.1. General Description
We leveraged human biomonitoring data from the US Centers for Disease Control and Prevention’s National Health and Nutrition Examination Surveys (NHANES), which measures EDCs in nationally representative samples, permitting precise estimates and distribution of EDC exposure by race/ethnicity subgroups. We then used previously described models [4] to quantify disease burden among non-Hispanic Whites, non-Hispanic Blacks, Mexican Americans and other Hispanics, and Other/Multicultural. We present an overview of this modeling here, and refer the reader to the Appendixi to allow a complete understanding of the present exercise without referring to previous publications. We also applied a similar approach to that previously described for the entire US population [4] to calculate associated economic costs for each disease and disability examined.
We applied the model first used by the Institute of Medicine [20] to estimate the cost of environmentally mediated disease, described by the equations below:
| (1) |
| (2) |
The environmentally attributable fraction of a risk factor can be defined as the proportional decrease in the number of cases of ill health or deaths as a result of reducing the risk factor [21] and can be estimated by the following equation:
| (3) |
where RR represent the relative risk of morbidity associated with the specific exposure.
Cost per case was derived from previously published estimates of per case direct or indirect costs and used to calculate overall costs (adjusted to reflect 2010 dollars using the Medical Care Consumer Price Index [22] where necessary), according to the incidence or prevalence of a disease and the size of the population at risk. Data from the Centers for Disease Control and Prevention (CDC) Wonder database were used for conversion of prevalence/incidence to the appropriate population size according to race/ethnicity [23].
1.2. Approach to exposure-outcome relationships
The EDCs and health outcomes investigated in this study have been the subject of previous work in which the burden of disease and costs associated with EDC exposure were assessed in the general US and European population. In these previous studies, the strength of the epidemiological and toxicological evidence was evaluated and ranges for probability of causation were assigned accordingly in order to determine specifically which EDCs and their associated health outcomes to explore [4, 24]. Accordingly, the exposure-response relationships examined in this analysis are: IQ loss and consequent intellectual disability, obesity (adult and childhood obesity), adult diabetes, cryptorchidism, testis cancer, early cardiovascular mortality due to reduced testosterone, leiomyomas and endometriosis [24, 25]. For autism, attention deficit hyperactivity disorder (ADHD) and male factor infertility, we were not able to obtain data that would permit precision in estimating race/ethnicity-specific disease burden. In these cases, we multiplied our previous estimates for the entire population by the appropriate proportion from US Census data. Burden of disease estimates relied on exposure-response relationships, which are summarized in Appendixi Table A1.
1.3. Approach to modeling economic estimates
To estimate total costs due to EDCs, we relied on a cost of illness approach [26] that aggregated total costs incurred due to each disease/condition, encompassing direct and indirect costs. For our calculations, we followed the guidelines provided by the Panel on Cost Effectiveness and Medicine [27] and used US data sources and previously published cost estimates.
For each exposure-outcome relationship, expert panels had previously identified a range for probability of causation. To aggregate costs across all the exposure-outcome relationships while accounting for probability of causation, Monte Carlo simulations were performed to generate realistic ranges of aggregate cost estimates across all the exposure-outcome relationships according to race/ethnicity. Monte Carlo simulations were performed identically to previous work [28], with one exception: in the present analysis, we used only the median of the probability range produced by the expert panel. Estimates for median EDC-related disease costs for other and multiracial subpopulations were derived by subtracting all of the other racial/ethnic subgroups from the total national cost estimates. To quantitate the economic cost disparities, we performed a counterfactual model in which we assumed that chemical exposure was equally distributed. We calculated the expected economic costs incurred by each race/ethnicity group according to their respective proportion of the total population. We, then, determined the total costs of each exposure-associated health outcome across all race/ethnicity groups, and subsequently multiplied the total cost by each group’s proportion of the total population (Table 4).
Table 4.
Distribution of crude annual costs (unadjusted for probability of causation) expected according to the respective proportion of each race/ethnicity category within the total population (base case estimates)
| Exposure and related outcome | Cost ($) | ||||
|---|---|---|---|---|---|
| Non-Hispanic White (66.1% of population) | Non-Hispanic Black (12.6% of population) | Mexican American (13.5% of population) | Other Hispanic (0.4% of population) | Other/Multira cial (7.4% of population) | |
| Polybrominateddiphenyl ethers - (PBDE) IQ Loss and Intellectual Disability cases | 161.1 billion | 30.7 billion | 32.9 billion | 974.8 million | 18.0 billion |
| Organophosphate pesticides (OP)- IQ Loss and Intellectual Disability cases | 31.2 billion | 5.9 billion | 6.4 billion | 188.8 million | 3.5 billion |
| Dichlorodiphenyldichloro ethylene (DDE) - Childhood overweight | 46.1 million | 8.8 million | 9.4 million | 278,800 | 5.2 million |
| Dichlorodiphenyldichloro ethylene (DDE) – Adult diabetes | 1.8 billion | 350.1 million | 375.1 million | 11.1 million | 205.6 million |
| Di-2-ethylhexylphthalate (DEHP)-Adult obesity | 1.2 billion | 221 million | 236.8 million | 7.0 million | 129.8 million |
| Di-2-ethylhexylphthalate (DEHP)-Adult diabetes | 179 million | 34.1 million | 36.6 million | 1.1 million | 20.0 million |
| Bisphenol A - Childhood obesity | 1.6 billion | 312.1 million | 334.4 million | 9.9 million | 183.3 million |
| Polybrominateddiphenyl ethers (PBDE) - Testicular cancer | 53.9 million | 10.3 million | 11.0 million | 326,400 | 6.1 million |
| Polybrominateddiphenyl ethers (PBDE) - Cryptorchidism | 22.9 million | 4.4 million | 4.7 million | 138,800 | 2.6 million |
| Phthalates - Low testosterone, Resulting in Increased Early Mortality | 5.9 billion | 1.1 billion | 1.2 billion | 35.8 million | 663.1 million |
| Dichlorodiphenyldichloro ethylene (DDE) - Fibroids | 388.7 million | 74.1 million | 79.4 million | 2.4 million | 43.5 million |
| Di-2-ethylhexylphthalate (DEHP) - Endometriosis | 31.0 billion | 5.9 billion | 6.3 billion | 187.6 million | 3.5 billion |
1.4. Data source
NHANES is a continuous, multicomponent, nationally representative survey of the noninstitutionalized US population administered by the National Centers for Health Statistics (NCHS) of the Centers for Disease Control and Prevention (CDC). Biomarker data were derived from the 2007-08 survey for polybrominated diphenyl ethers (PBDEs), dichlorodiphenyldichloroethylene (DDE) and organophosphate pesticides (OPs), and from the 2009-10 survey for bisphenol A (BPA) and phthalates. The values were separated into quantiles (0–9th, 10th–24th, 25th–49th, 50th–74th, 75th–89th, and 90th–99th), and stratified by race/ethnicity categories. Categories represented in the NHANES were: Mexican-American, Other Hispanic, Non-Hispanic White, Non-Hispanic Black, Other/Multiracial. For PBDEs and DDE, since these were analyzed in group samples, race/ethnicity is reported as divided in four categories (the Other Hispanic category is not reported). The exposure-response relationships considered in this analysis (IQ loss and consequent intellectual disability, obesity, adult diabetes, cryptorchidism, testis cancer, early cardiovascular mortality due to reduced testosterone, leiomyomas and endometriosis) were then applied to these race/ethnicity categories. For each of the chemicals examined, a standard error (SE) value less than 30% of estimates was used as a cut-off to confirm precision of estimates for each quantile. Additional details for the chemicals examined are provided in the Appendixi.
1.5. Institutional Review Board
Dr. Trasande signed a letter of attestation developed by the New York University School of Medicine Institutional Review Board for non-human subjects research, which this work represents.
Results
For the exposure-disease relationships examined in our analysis, crude total annual costs (unadjusted for probability of causation) associated with exposure to EDC reached $179.8 billion (52.3% of total costs) in Non-Hispanic White, corresponding to 66.1% of the target population (respective race/ethnicity subset of the total population examined); $56.8 billion (16.5% of total) in Non-Hispanic Black, corresponding to 12.6% of the target population; $50.1 billion (14.6% of total) in Mexican-American, corresponding to 13.5% of the target population; $5.7 billion (1.6% of total) in the category Other Hispanic, corresponding to 0.4% of the target population; and $51.7 billion (15.0% of total) in the category Other/Multiracial, corresponding to 7.4% of the target population.
With few exceptions, exposure levels and associated burden of disease and crude costs were higher in racial/ethnic minorities in proportion to their respective populations, as shown in Table 1–3, and Figure 1. Table 1 presents selected examples of exposure-outcome relationships showing how differences in exposures produce differences in burden of disease, resulting in disproportionate percentages when compared to the respective populations.
Table 1.
Selected examples of distribution of burden of disease for each percentile of exposure according to race/ethnicity (base case estimates)
| Exposure and related outcome | Percentiles of exposure and associath burden of disease | ||||
|---|---|---|---|---|---|
| Polybrominateddiphenyl ethers -(PBDE) IQ Point Loss Intellectual Disability | P10 (ng/g) IQ loss/ID cases | P25 (ng/g) IQ loss/ID cases | P50 (ng/g) IQ loss/ID cases | P75 (ng/g) IQ loss/ID cases | P90 (ng/g) IQ loss/ID cases |
| --Non-Hispanic White | 10.7 325,794/1,254 | 19.1 1,155,179/4,793 | 22.2 1,314,083/5,560 | 43.9 1,220,661/5,639 | 74.8 1,038,981/5,138 |
| --Non-Hispanic Black | 26 242,373/1,047 | 26 403,955/1,744 | 30.6 450,899/1,989 | 37.8 307,075/1,392 | 51 239,241/1,127 |
| --Mexican-American | 16.2 162,892/662 | 20 333,087/1,390 | 23.8 383,938/1,639 | 31 276,720/1,222 | 34.1 195,625/875 |
| --Other/multiracial | 15.8 171,896/696 | 20.7 372,128/1,560 | 20.7 372,128/1,560 | 53 402,105/1,903 | 68.5 300,601/1,470 |
| Organophosphate pesticides (OP)-IQ Point Loss Intellectual Disability | P10 (ng/g) IQ loss/ID cases | P25 (ng/g) IQ loss/ID cases | P50 (ng/g) IQ loss/ID cases | P75 (ng/g) IQ loss/ID cases | P90 (ng/g) IQ loss/ID cases |
| --Non-Hispanic White | 13.14 0 | 13.14 0 | 21.52 0 | 97.95 245,521/929 | 284.16 588,777/2,540 |
| --Non-Hispanic Black | 13.14 0 | 13.14 0 | 20.68 0 | 113.14 90,505/349 | 374.63 190,681/851 |
| --Mexican-American | 13.14 0 | 13.14 0 | 22.23 0 | 117.96 98,724/382 | 285.63 163,477/706 |
| --Other Hispanic | 13.14 0 | 15.12 0 | 27.36 0 | 208.46 111,916/465 | 427.55 120,597/547 |
| --Other/Multiracial | 16.53 0 | 18.22 0 | 75.79 0 | 233.36 106,871/450 | 1386.94 170,591/891 |
| Dichlorodiphenyldichloroethylene (DDE) – Adult diabetes | P10 (ng/g, wet weight) No. of cases | P25 (ng/g, wet weight) No. of cases | P50 (ng/g, wet weight) No. of cases | P75 (ng/g, wet weight) No. of cases | P90 (ng/g, wet weight) No. of cases |
| --Non-Hispanic White | 0.83 0 | 1.00 0 | 1.19 0 | 1.33 0 | 1.64 0 |
| --Non-Hispanic Black | 1.54 0 | 1.87 0 | 2.19 0 | 2.78 6,254 | 4.11 4,169 |
| --Mexican-American | 3.81 0 | 6.05 0 | 7.14 0 | 7.63 10,187 | 12.09 6,791 |
| --Other/multiracial | 2.29 0 | 4.00 0 | 5.87 0 | 7.88 7,139 | 14.12 4,759 |
Table 3.
Distribution of crude annual costs (unadjusted for probability of causation) associated with exposure to endocrine-disrupting chemicals in the US according to race/ethnicity (base case estimates)
| Cost ($) or percentage of total costs (%of target population) | |||||
|---|---|---|---|---|---|
| Exposure and related outcome | Non-Hispanic White | Non-Hispanic Black | Mexican American | Other Hispanic | Other/Multir acial |
| Polybrominateddiphenyl ethers - (PBDE) IQ Loss and Intellectual Disability cases | 127.5 billion or 52.3% (54.1) | 41.5 billion or 17% (14.7) | 33.8 billion or 13.9% (15.0) | 40.9 billion or 16.8% (16.2) | |
| Organophosphate pesticides (OP)- IQ Loss and Intellectual Disability cases | 20.7 billion or 43.9% (54.1) | 7.0 billion or 14.9% (14.7) | 6.5 billion or 13.8% (15.0) | 5.8 billion or 12.4% (8.7) | 7.2 billion or 15.1% (7.6) |
| Dichlorodiphenyldichloroet hylene (DDE) - Childhood overweight | 3.8 million or 5.3% (56.1) | 2.1 million or 3.0% (15.3) | 55.5 million or 79.1% (20.1) | 8.3 million or 12.6% (8.5) | |
| Dichlorodiphenyldichloroet hylene (DDE) – Adult diabetes | 0 (73.7) | 737.1 million or 26.5% (11.2) | 1.2 billion or 43.2% (8.8) | 841.3 million or 30.3% (6.2) | |
| Di-2-ethylhexylphthalate (DEHP)-Adult obesity | 1.2 billion or 68.3% (72.9) | 364.3 million or 20.9% (11.8) | 172.7 million or 9.9% (8.8) | 16.9 million or 1.0% (0.9) | 0 (5.6) |
| Di-2-ethylhexylphthalate (DEHP)-Adult diabetes | 33.1 million or 12.2% (72.9) | 201.7 million or 74.5% (11.8) | 32.6 million or 12.0% (8.8) | 3.4 million or 1.2% (0.9) | 0 (5.6) |
| Bisphenol A - Childhood obesity | 1.2 billion or 50.1% (53.8) | 565.9 million or 22.8% (15.0) | 495.8 million or 20.7% (22.0) | 96.8 million or 3.9% (2.9) | 118.5 million or 4.9% (6.3) |
| Polybrominateddiphenyl ethers (PBDE) - Testicular cancer | 52.9 million or 64.7% (64.7) | 10.1 million or 12.4% (12.4) | 12.4 million or 15.2% (15.2) | 6.2 million or 7.6% (7.6) | |
| Polybrominateddiphenyl ethers (PBDE) - Cryptorchidism | 23.0 million or 66.3% (54.2) | 1.8 million or 5.2% (14.7) | 1.6 million or4.7 % (14.9) | 8.3 million or 23.8% (16.2) | |
| Phthalates - Low testosterone, Resulting in Increased Early Mortality | 6.9 billion or 77.0% (76.2) | 589.6 million or 6.6% (10.1) | 789.3 million or 8.8% (8.0) | 48.1 million or 0.5% (0.7) | 633.3 million or 7.1% (4.9) |
| Dichlorodiphenyldichloroet hylene (DDE) - Fibroids | 203.3 million or 34.6% (62.0) | 108.7 million or 18.5% (13.9) | 186.9 million or 31.8% (15.3) | 89.2 million or 15.2% (8.7%) | |
| Di-2-ethylhexylphthalate (DEHP) - Endometriosis | 26.6 billion or 56.7% (59.8) | 7.3 billion or 15.6% (14.0) | 8.2 billion or 17.6% (16.7) | 1.0 billion or 2.2% (2.0) | 3.8 billion or 8.0% (7.5) |
| TOTAL | 179.8 billion or 52.3% (66.1) | 56.8 billion or 16.5% (12.6) | 50.1 billion or 14.6% (13.5) | 5.7 billion or 1.6% (0.4) | 51.7 billion or 15.0% (7.4) |
Figure 1.
Proportion of total disease burden and economic costs associated with exposure to endocrine-disrupting chemicals in the US according to race/ethnicity (base case estimates)
In Table 2, the total burden of disease associated with exposure to EDCs is shown. For example, exposure to PBDE and associated IQ loss and ID cases resulted in a cost of $127.5 billion or 52.3% of total costs in Non-Hispanic White, representing 54.1% of the target population, whereas the cost was $41.5 billion or 17% of total in Non-Hispanic Black, representing 14.7% of the target population. Exposure to OP and related IQ loss and ID cases resulted in a cost of $20.7 billion or 43.9% of the total in Non-Hispanic White, representing the majority of the target population (54.1%), whereas the cost was $7.0 billion or 14.9% in Non-Hispanic Black, higher than the respective target population (14.7%); similar findings were observed for the categories Other Hispanic and Other/Multiracial, with costs representing 12.4% and 15.1% of the total for 8.7% and 7.6% of the target populations, respectively.
Table 2.
Distribution of total disease burden associated with exposure to endocrine-disrupting chemicals in the US according to race/ethnicity (base case estimates)
| Burden of disease & percentage of total cases across all race/ethnicity categories | |||||
|---|---|---|---|---|---|
| Exposure and related outcome | Non-Hispanic White | Non-Hispanic Black | Mexican American | Other Hispanic | Other/Multiracial |
| Polybrominateddiphenyl ethers -(PBDE) IQ Point Loss and Intellectual Disability cases | IQ Loss: 5.1 million | IQ Loss: 1.6 million | IQ Loss: 1.4 million | IQ Loss: 1.6 million | |
| IQ cases: 22,400 (52.3%) | IQ cases: 7,300 (17.0%) | IQ cases: 5,800 (14.0%) | IQ cases: 7,200 (16.7%) | ||
| Organophosphate pesticides (OP)-IQ Point Loss and Intellectual Disability cases | IQ Loss: 834,300 | IQ Loss: 281,200 | IQ Loss: 262,200 | IQ Loss: 232,500 | IQ Loss: 277,500 |
| IQ cases: 3,470 (44.2%) | IQ cases: 1,200 (14.9%) | IQ cases: 1,100 (13.4%) | IQ cases: 1,000 (12.3%) | IQ cases: 1,340 (14.7%) | |
| Dichlorodiphenyldichloroethylene (DDE) - Childhood overweight | 108 (5.3%) | 61 (3.0%) | 1,600 (79.1%) | 255 (12.6%) | |
| Dichlorodiphenyldichloroethylene (DDE) – Adult diabetes | 0 (0%) | 10,400 (26.5%) | 16,980 (43.2%) | 11,900 (30.3%) | |
| Di-2-ethylhexylphthalate (DEHP)-Adult obesity | 4,180 (68.3%) | 1,280 (20.9%) | 604 (9.9%) | 59 (1.0%) | 0 (0%) |
| Di-2-ethylhexylphthalate (DEHP)-Adult diabetes | 468 (12.2%) | 2,850 (74.5%) | 461 (12.0%) | 47 (1.2%) | 0 (0%) |
| Bisphenol A - Childhood obesity | 16,400 (48.4%) | 7,730 (22.8%) | 6,780 (20.0%) | 1,322 (3.9%) | 1,620 (4.8%) |
| Polybrominateddiphenyl ethers (PBDE) - Testicular cancer | 2,320 (64.7%) | 445 (12.4%) | 546 (15.2%) | 274 (7.6%) | |
| Polybrominateddiphenyl ethers (PBDE) - Cryptorchidism | 2,750 (66.3%) | 217 (5.2%) | 194 (4.7%) | 990 (23.8%) | |
| Phthalates - Low testosterone, Resulting in Increased Early Mortality | 15,540 (77.3%) | 1,310 (6.5%) | 1,740 (8.7%) | 105 (0.5%) | 1,415 (7.0%) 13 |
| Dichlorodiphenyldichloroethylene (DDE) - Fibroids | 17,700 (34.6%) | 9,460 (18.5%) | 16,270 (31.8%) | 7,770 (15.2%) | |
| Di-2-ethylhexylphthalate (DEHP) - Endometriosis | 46,890 (56.7%) | 12,900 (15.6%) | 14,530 (17.6%) | 1,790 (2.2%) | 6,640 (8.0%) |
For exposure to DDE and adult diabetes, cost was estimated to be zero for Non-Hispanic White, representing 73.7% of the target population, whereas cost was estimated at $1.2 billion or 43.2% of the total in Mexican-American, representing a much smaller percentage of the target population (8.8%); similar findings were observed for the categories Non-Hispanic Black and Other/Multiracial, with costs representing 26.5% and 30.3% of the total cost for 11.2% and 6.2% of the target populations, respectively.
Similarly, for exposure to DEHP and adult diabetes, Non-Hispanic Black bore most of the cost ($201.7 million or 74.5% of the total), while representing only 11.8% of the target population (Table 3).
For comparison, the expected distribution of economic costs incurred by each race/ethnicity group according to their respective proportion of the total population is presented in Table 4. Monte Carlo simulations resulted in a median, adjusted cost of $340 billion, of which $175.5 billion (or 51.6%) was for Non-Hispanic White, $56.3 billion (or 16.6%) for Non-Hispanic Black, $48.5 billion (or 14.3%) for Mexican American, and $59.6 billion (or 17.5%) for the two other categories combined (Other Hispanic and Other/Multiracial). A detailed description of all the results of our analysis is provided in the Appendixi.
Discussion
The main aim of this study was to specifically examine EDC exposure according to race/ethnicity and related burden of disease and associated economic costs. Our findings suggest that exposure to EDC in the US population is not uniform but varies according to race/ethnicity groups. In turn, this leads to increased burden of disease and costs in the groups with higher burden of exposure, which disproportionately affects racial/ethnic minority groups. Specifically, Non-Hispanic Blacks and Mexican-Americans bore a disease burden and economic costs associated with EDC exposure that was disproportionate to their respective population size. Non-Hispanic Whites, on the other hand, exhibited the opposite pattern, in which their respective population size surpassed their proportion of EDC-associated disease burden and economic costs. Of all the EDCs examined in this analysis, disparities were largely driven by higher exposure to persistent pollutants and flame retardants.
One limitation of our analysis is that, while for PBDE, OP and DDE all the exposure estimates (with very few exceptions for the category Other/Multiracial) had SE less than 30%, for some of the urinary phthalate metabolites the SE was greater than 30%, especially for the race/ethnicity categories with the lowest number of observations (Other Hispanic and Other/Multiracial). This may have contributed to less precise estimates of burden of disease and costs for these specific racial/ethnic categories. Further, our study was limited in its use of NHANES data collected in 2007-2008 and 2009-2010. More recent chemical exposure data for certain chemcials, including organophosphates, has not yet been published by NHANES. However, in using the selected years, we were able to yield results that we could evaluate in the context of our previous work examining the burden of disease and associated costs of EDC exposure in the general US population. However, more recent data would have been optimal as changes in environmental exposures are dynamic across time and because later survey cycle years included a “Non-Hispanic Asian” race/ethnicity category, reflective of this growing U.S. subgroup [29]. An additional limitation of this analysis is that certain health outcomes, such as chronic childhood asthma [30], preterm birth and low birthweight [31], that have had historical race/ethnicity disparities were excluded. We restricted our analysis to those exposure-response relationships previously identified as having a substantial range of probability of causation based on the available epidemiologic and toxicological evidence [4, 24]. Our analysis was also limited in that we exclusively examined the exposure-specific contributions to disease disparity rather than other contributing factors. A final limitation of our study is that race/ethnicity disparities were compared qualitatively, and did not evaluate whether identifiable disparities were statistically significant between race/ethnicity groups as differences in chemical exposure between race/ethnicity groups has been previously published[32, 33].
Persistent health disparities have been extensively documented in the US, related to both medical and non-medical factors [6]. Access to care, insurance coverage, and ability to pay are among the more “conventional” factors contributing to health disparities in the US [34]. In addition, the Institute of Medicine released a report in 2003 showing that other factors contribute to racial/ethnic disparities in health, including culture, behavior, communication, substandard care, and health care quality issues [35]. For our analysis, the higher levels of exposure in racial/ethnic minorities together with disparities in the availability of resources considered to be protective factors, such as green spaces [23] or healthy food options [36], can have a cumulative effect, substantially contributing to racial/ethnic disparities in health.
Our results are consistent with existing evidence that racial/ethnic minorities may be disproportionately affected by the negative health effects of toxic environmental exposures. Hun and coll. documented that Hispanics had statistically higher cumulative cancer risks than did Whites because of differences in exposure to hazardous air pollutants [37]. More recently, Ruiz and coll. reviewed the available evidence supporting an association between unequal exposure to EDCs and disparities in diabetes mellitus in the US, and reported significantly higher exposures to diabetogenic EDCs, including bisphenol A, phthalates, organochlorine pesticides, among Latinos and African Americans[12]. James-Todd and coll. focused on chemical exposures and reproductive health outcomes in women, reporting that non-Whites seem to have higher exposures to EDCs compared to Whites and suggesting the potential for higher incidence of adverse reproductive health outcomes [13].
A more in-depth understanding of the factors that contribute to racial and ethnic differences in the development of several health conditions is essential to the design of targeted policies aimed at addressing inequalities in exposures to EDCs and overall disparities in health outcomes. Race/ethnicity is often associated with cultural behaviors and patterns of consumption that can contribute to explaining the differences in burden of exposure. A classic example can be seen in the use of consumer beauty products, which are a significant source of exposure to phthalates in women [38]. As highlighted by Zota and coll., patterns of use of these products varies according to race/ethnicity [39]. As such, identifying effective and targeted strategies to reduce chemical exposures may have substantial health benefits for the groups with higher burden of exposures. For example, at the individual level, consumers can make informed choices and buy products that are free of phthalates or BPA. This can significantly reduce personal exposure to these EDCs, as shown by Harley and coll. [40], who conducted an intervention study in Latina girls, in which participants avoided the use of personal care products containing phthalates and parabens for 3 days. Urinary metabolites of these chemicals were significantly reduced after the intervention, suggesting that this can be an effective strategy that contributes to reducing exposure at the individual level.
In addition to cultural behaviors and patterns of consumption, potential exposure from manufacturing and waste sites could contribute to these disparities, since hazardous waste sites and polluting factories tend to be located in minority and low-income neighborhoods [41, 42]. As such, effective strategies at the individual level need to be complemented by strategies that target the entire household as well as state and federal policies. A number of states have passed legislation to ban the use of flame retardant chemicals like PBDEs in a number of consumers’ products, especially children’s products [43]. In addition, in an attempt to avoid the issue of “regrettable substitution”[44] (replacement of one hazardous chemical with another) and promote the use of truly safer alternatives, some states like California have established a regulatory framework for companies to evaluate potential alternatives [45]. At the federal level, a recent update to the Toxic Substances Control Act (TSCA) could increase protections of endocrine function from EDCs, although it still falls short of providing the Environmental Protection Agency (EPA) with the adequate oversight power and funding necessary to protect public health in this area [46]. Additionally, the proposed Personal Care Products Safety Act would empower the Food and Drug Administration (FDA) with the authority to conduct mandatory reviews of chemicals in personal care products, though it has not yet been enacted into law[47]. Notably, none of these policies specifically target disparities in environmental exposures as major contributors to health disparities across race/ethnicity groups.
Investigations of the origins of health disparities has largely been limited to individual behavior and disparities in health service delivery, although the role played by both medical and nonmedical determinants is increasingly being recognized [34]. Here we encourage a paradigm shift when evaluating health disparities, focusing on disparities driven by different environmental exposures across race/ethnicity groups. We believe this shift may identify new opportunities for disease prevention in the demographic segments of the US population who need it most, as well as offer opportunities to devise social policies that specifically address environmental inequalities according to race/ethnicity groups.
Supplementary Material
Acknowledgments:
We thank all authors of seven previous studies that had assessed the economic costs of endocrine disrupting chemicals, on which we based this work: Anna Maria Andersson, Martine Bellanger, Bruce Blumberg, Jean-Pierre Bourguignon, Barbara Demeneix, Joseph DiGangi, Philippe Grandjean, Tony Fletcher, Paul A Fowler, Eva Govarts, Ulla Hass, Russ Hauser, Jerrold J Heindel, Patricia Hunt, Anders Juul, Juliette Legler, John Peterson Myers, Miquel Porta, Ruthann Rudel, Sheela Sathyanarayana, Niels E Skakkebaek, Jorma Toppari, and Thomas Zoeller.
Funding: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Abbreviations:
- AF
Attributable Fraction
- BPA
bisphenol A
- EDCs
endocrine disrupting chemicals
- DDE
dichlorodiphenyldichloroethylene
- PBDE
polybrominated diphenyl ethers
- PCBs
polychlorinated biphenyls
- TSCA
Toxic Substances Control Act
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Potential Conflicts of Interests: The authors have no conflicts of interest relevant to this article to disclose.
References
- 1.Diamanti-Kandarakis E, et al. , Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr Rev, 2009. 30(4): p. 293–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bergman Å, et al. , WHO (World Health Organization)/UNEP (United Nations Environment Programme) 2013. The State-of-the-Science of Endocrine Disrupting Chemicals – 2012. Geneva: UNEP/WHO; Available at http://www.who.int/ceh/publications/endocrine/en/ (accessed 14 December 2015). 2013. [Google Scholar]
- 3.Gore AC, et al. , EDC-2: The Endocrine Society’s Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocr Rev, 2015: p. er20151010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Attina TM, et al. , Exposure to endocrine-disrupting chemicals in the USA: a population-based disease burden and cost analysis. Lancet Diabetes Endocrinol, 2016. 4(12): p. 996–1003. [DOI] [PubMed] [Google Scholar]
- 5.Trasande L, et al. , Burden of disease and costs of exposure to endocrine disrupting chemicals in the European Union: an updated analysis. Andrology, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adler NE and Rehkopf DH, U.S. disparities in health: descriptions, causes, and mechanisms. Annu Rev Public Health, 2008. 29: p. 235–52. [DOI] [PubMed] [Google Scholar]
- 7.Williams DR, et al. , Race, socioeconomic status, and health: complexities, ongoing challenges, and research opportunities. Ann N Y Acad Sci, 2010. 1186: p. 69–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meyer PA, et al. , Conclusion and future directions: CDC Health Disparities and Inequalities Report - United States, 2013. MMWR Suppl, 2013. 62(3): p. 184–6. [PubMed] [Google Scholar]
- 9.Cheng TL, Goodman E, and Committee on Pediatric R, Race, ethnicity, and socioeconomic status in research on child health. Pediatrics, 2015. 135(1): p. e225–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fiscella K, et al. , Inequality in quality: addressing socioeconomic, racial, and ethnic disparities in health care. JAMA, 2000. 283(19): p. 2579–84. [DOI] [PubMed] [Google Scholar]
- 11.Morello-Frosch R, et al. , Understanding the cumulative impacts of inequalities in environmental health: implications for policy. Health Aff (Millwood), 2011. 30(5): p. 879–87. [DOI] [PubMed] [Google Scholar]
- 12.Ruiz D, et al. , Disparities in Environmental Exposures to Endocrine-Disrupting Chemicals and Diabetes Risk in Vulnerable Populations. Diabetes Care, 2018. 41(1): p. 193–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.James-Todd TM, Chiu YH, and Zota AR, Racial/ethnic disparities in environmental endocrine disrupting chemicals and women’s reproductive health outcomes: epidemiological examples across the life course. Curr Epidemiol Rep, 2016. 3(2): p. 161–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hicken MT, et al. , A novel look at racial health disparities: the interaction between social disadvantage and environmental health. Am J Public Health, 2012. 102(12): p. 2344–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Office of Disease Prevention and Health Promotion. Healthy People 2020. Available at https://www.healthypeople.gov/2020/About-Healthy-People (accessed 23 June 2017).
- 16.Nelson JW, et al. , Social disparities in exposures to bisphenol A and polyfluoroalkyl chemicals: a cross-sectional study within NHANES 2003-2006. Environ Health, 2012. 11: p. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ranjit N, Siefert K, and Padmanabhan V, Bisphenol-A and disparities in birth outcomes: a review and directions for future research. J Perinatol, 2010. 30(1): p. 2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stiel L, et al. , A review of hair product use on breast cancer risk in African American women. Cancer Med, 2016. 5(3): p. 597–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trasande L, et al. , Race/ethnicity-specific associations of urinary phthalates with childhood body mass in a nationally representative sample. Environ Health Perspect, 2013. 121(4): p. 501–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Institute of Medicine. Costs of Environment-Related Health Effects: A Plan for Continuing Study. Washington, DC:National Academy Press; 1981. [PubMed] [Google Scholar]
- 21.Smith KR, Corvalan CF, and Kjellstrom T, How much global ill health is attributable to environmental factors? Epidemiology, 1999. 10(5): p. 573–84. [PubMed] [Google Scholar]
- 22.Bureau of Labor Statistics, Consumer Price Index. Available at http://www.bls.gov/cpi/ (Accessed 21 Jan 2016). 2010.
- 23.Jennings V, et al. , Urban Green Space and the Pursuit of Health Equity in Parts of the United States. Int J Environ Res Public Health, 2017. 14(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trasande L, et al. , Estimating Burden and Disease Costs of Exposure to Endocrine-Disrupting Chemicals in the European Union. J Clin Endocrinol Metab, 2015. 100: p. 1245–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hunt P, et al. , Female Reproductive Disorders, Diseases and Costs of Exposure to Endocrind Disrupting Chemicals in the European Union. J Clin Endocrinol Metab, 2016. 101: p. 1562–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hodgson TA and Meiners MR, Cost-of-illness methodology: a guide to current practices and procedures. Milbank Mem Fund Q Health Soc, 1982. 60(3): p. 429–62. [PubMed] [Google Scholar]
- 27.Weinstein MC, et al. , Recommendations of the Panel on Cost-effectiveness in Health and Medicine. JAMA, 1996. 276(15): p. 1253–8. [PubMed] [Google Scholar]
- 28.Trasande L, et al. , Estimating burden and disease costs of exposure to endocrine-disrupting chemicals in the European union. J Clin Endocrinol Metab, 2015. 100(4): p. 1245–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bureau USC Demographic Turning Points for the United States: Population Projections for 2020 to 2060. 2018; Available from: https://www.census.gov/content/dam/Census/library/publications/2018/demo/P25_1144.pdf. [Google Scholar]
- 30.Boudreaux ED, et al. , Race/ethnicity and asthma among children presenting to the emergency department: differences in disease severity and management. Pediatrics, 2003. 111(5 Pt 1): p. e615–21. [DOI] [PubMed] [Google Scholar]
- 31.Reports NNVS, Births: Final Data for 2016. 2018. 67(Number 1). [PubMed] [Google Scholar]
- 32.Hendryx M and Luo J, Children’s environmental chemical exposures in the USA, NHANES 2003-2012. Environ Sci Pollut Res Int, 2018. 25(6): p. 5336–5343. [DOI] [PubMed] [Google Scholar]
- 33.Patel CJ, et al. , Systematic assessment of the correlations of household income with infectious, biochemical, physiological, and environmental factors in the United States, 1999-2006. Am J Epidemiol, 2015. 181(3): p. 171–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gibbons MC, A historical overview of health disparities and the potential of eHealth solutions. J Med Internet Res, 2005. 7(5): p. e50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Unequal Treatment: Confronting Racial and Ethnic Disparities in Healthcare. Institute of Medicine (US) Committee on Understanding and Eliminating Racial and Ethnic Disparities in Health Care. Smedley BD, Stith AY, Nelson AR, editors Washington (DC): National Academies Press (US) 2003. [PubMed] [Google Scholar]
- 36.Hilmers A, Hilmers DC, and Dave J, Neighborhood disparities in access to healthy foods and their effects on environmental justice. Am J Public Health, 2012. 102(9): p. 1644–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hun DE, et al. , Cancer risk disparities between Hispanic and non-hispanic white populations: the role of exposure to indoor air pollution. Environ Health Perspect, 2009. 117(12): p. 1925–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parlett LE, Calafat AM, and Swan SH, Women’s exposure tophthalates in relation to use of personal care products. J Expo Sci Environ Epidemiol, 2013. 23(2): p. 197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zota AR and Shamasunder B, The environmental injustice of beauty: framing chemical exposures from beauty products as a health disparities concern. Am J Obstet Gynecol, 2017. 217(4): p. 418 e1–418 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harley KG, et al. , Reducing Phthalate, Paraben, and Phenol Exposure from Personal Care Products in Adolescent Girls: Findings from the HERMOSA Intervention Study. Environ Health Perspect, 2016. 124(10): p. 1600–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Collins MB, Munoz I, and JaJa J, Linking ‘toxic outliers’ to environmental justice communities. Environmental Research Letters, 2016. 11(1). [Google Scholar]
- 42.Johnson R, Ramsey-White K, and Fuller CH, Socio-demographic Differences in Toxic Release Inventory Siting and Emissions in Metro Atlanta. Int J Environ Res Public Health, 2016. 13(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.SaferStates. Bill Tracker: Adopted Policy. Toxic Flame Retardants. Available at http://www.saferstates.com/bill-tracker/ (Accessed 26 December 2017). 2017.
- 44.Committee on the Design and Evaluation of Safer Chemical Substitutions A Framework to Guide Selection of Chemical Alternatives. National Academies Press (US), Washington (DC) Available at https://www.nap.edu/catalog/18872/a-framework-to-guide-selection-of-chemical-alternatives (Accessed 27 December 2017). 2014. [PubMed] [Google Scholar]
- 45.California Department of Toxic Substances Control. Draft Alternatives Analysis Guide. Avalable at http://www.dtsc.ca.gov/SCP/AlternativesAnalysisGuidance.cfm (accessed 27 December 2017). 2016.
- 46.Trasande L, Updating the Toxic Substances Control Act to Protect Human Health. JAMA, 2016. 315(15): p. 1565–6. [DOI] [PubMed] [Google Scholar]
- 47.Feinstein D and Collins S, The Personal Care Products Safety Act. JAMA Intern Med, 2018. 178(5): p. 601–602. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


