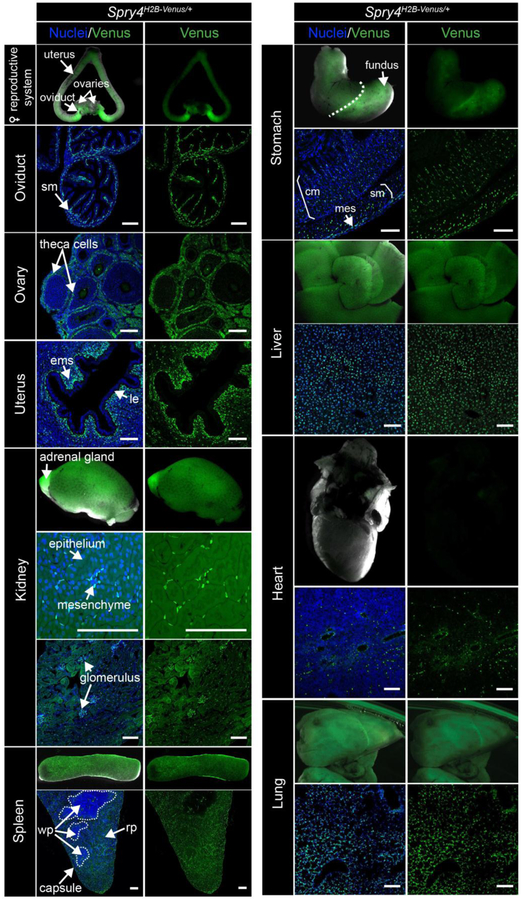
Abstract
The FGF/ERK signaling pathway is highly conserved throughout evolution and plays fundamental roles during embryonic development and in adult organisms. While a plethora of expression data exists for ligands, receptors and pathway regulators, we know little about the spatial organization or dynamics of signaling in individual cells within populations. To this end we developed a transcriptional readout of FGF/ERK activity by targeting a histone H2B-linked Venus fluorophore to the endogenous locus of Spry4, an early pathway target, and generated Spry4H2B-Venus embryonic stem cells (ESCs) and a derivative mouse line. The Spry4H2B-Venus reporter was heterogeneously expressed within ESC cultures and responded to FGF/ERK signaling manipulation. In vivo, the Spry4H2B-Venus reporter recapitulated the expression pattern of Spry4 and localized to sites of known FGF/ERK activity including the inner cell mass of the pre-implantation embryo and the limb buds, somites and isthmus of the post-implantation embryo. Additionally, we observed highly localized reporter expression within adult organs. Genetic and chemical disruption of FGF/ERK signaling, in vivo in pre- and post-implantation embryos, abrogated Venus expression establishing the reporter as an accurate signaling readout. This tool will provide new insights into the dynamics of the FGF/ERK signaling pathway during mammalian development.
Keywords: Sprouty4, FGF/ERK, FGF signaling, ESCs, mouse embryo, fluorescent reporter, live imaging, quantitative image analysis
1. Introduction
Fibroblast growth factors (FGF) are a family of 18 secreted ligands that bind to four receptor tyrosine kinase (RTK) FGF receptors (FGFR1–4). The interaction of FGFs with their cognate receptors results in the activation of a number of downstream signaling pathways, including the MAPK cascade which involves ERK [1, 2]. FGF signaling through ERK (referred to hereafter as FGF/ERK signaling) is evolutionarily conserved from primitive metazoans to mammals [3, 4] and plays a fundamental role in vital cellular processes encompassing proliferation, metabolism, migration, cell survival and differentiation. The FGF/ERK signaling axis is important throughout embryonic development, at post-natal stages for homeostatic regulation, and has been implicated in the progression of many diseases such as cancer and various neuropathies [5–11].
During early mouse development, FGF/ERK signaling is required to generate and/or maintain the three lineages of the pre-implantation embryo – the embryonic epiblast (Epi), and the extraembryonic primitive endoderm (PrE) and trophectoderm (TE). FGF/ERK regulates maturation of the pluripotent Epi, PrE specification [12–19] and TE proliferation [15, 20–23]. FGF is also essential for the isolation and maintenance of the in vitro counterpart of the post-implantation Epi, epiblast stem cells (EpiSCs) [24, 25] and of the TE, trophoblast stem (TS) cells [23, 26]. In both vertebrates and invertebrates [27–35], FGF signaling is required for efficient cell migration during gastrulation, when the three embryonic germ layers are specified [29, 33, 34, 36]. Later in development, FGF/ERK is employed in a variety of contexts including the regulation of somitogenesis, branching organogenesis (for example in the lung and kidney), as well as in limb, brain and tooth development [2].
Despite the critical and widespread roles of the FGF/ERK pathway, there is limited information on the pathway’s spatiotemporal signaling dynamics and how they correlate with functional outputs. The static distribution of ERK activity has been analyzed via immunoreactivity against its active di-phosphorylated form (ppERK) [37]. However, due to difficulties in capturing low-level or transient signaling activity, not all tissues with known FGF/ERK activity exhibit robust staining for ppERK, such as the inner cell mass (ICM) of the blastocyst and the primitive streak (PS) of the mouse gastrula [37]. Hence, sensitive tools that capture dynamics are required to monitor signaling in fixed and live samples at cellular resolution.
We found that, from a panel of known FGF/ERK pathway targets, Sprouty4 (Spry4) demonstrated the most rapid and robust transcriptional response to acute ERK activation in mouse embryonic stem cells (ESCs), the in vitro counterpart of the embryonic Epi. We therefore generated a fluorescent reporter ESC line and mouse line carrying an H2B-Venus fusion [38] knocked into the mouse Spry4 locus [39]. While we noted that the Spry4H2B-Venus reporter disrupted transcription at the endogenous locus, Spry4H2B-Venus/H2B-Venus mice were viable and fertile. The Spry4H2B-Venus reporter was expressed in known domains of ERK activity including the ICM of the blastocyst, the nascent mesoderm, somites and limb buds. We also observed Venus signal within the visceral endoderm (VE), a previously uncharacterized site of ERK activity, and noted highly localized expression within distinct cell types of adult organs. We validated the specificity of this reporter by inhibiting FGF/ERK, both genetically and pharmacologically, in ESCs and embryos. Both approaches significantly abrogated expression of Spry4H2B-Venus confirming that the reporter represents a bona fide readout for FGF/ERK activity. This reporter represents the first tool to study the transcriptional output of FGF/ERK signaling at single-cell resolution in live mice, and should yield novel insights into pathway regulation during embryonic development and in tissue homeostasis. As Sprouty family members are general RTK pathway regulators whose expression can be controlled by factors other than FGF [40–43], the reporter may also be utilized to study RTK signaling induced via a variety of ligands in different contexts.
2. Materials and methods
2.1. ESC culture and quantitative RT-PCR analysis
ESCs were maintained on gelatin-coated tissue culture dishes in GMEM (Gibco) supplemented with non-essential amino acids (NEAA, ThermoFisher Scientific), sodium pyruvate, GlutaMAX™, 50 μM β-mercaptoethanol (all from ThermoFisher Scientific), 10% fetal bovine serum (FBS, HyClone™ Defined FBS) and 10 ng/ml LIF. RNA isolation and quantitative RT-PCR (qRT-PCR) from ESCs were performed as previously described [44]. Primer details can be found in Table 3. Relative initial mRNA concentrations were estimated by curve fitting, and normalized to Ppia housekeeping gene mRNA levels in each sample.
Table 3.
List of qRT-PCR primers. List of forward and reverse primer sequences for primers used in this study.
| Gene | Forward primer sequence | Reverse primer sequence |
|---|---|---|
| Dusp1 | TTCTTCGCTTGCCTGTTTTT | GCAAACCTGCTCTGGGTCTA |
| Dusp4 | AGCATGTGTGTGCAGGAGTC | ACAGACCGCTGGAGAGAAAA |
| Dusp6 | GGGAGTGTCACCTCAAATGC | CACGAACATCATGGAGCAAG |
| Spry2 | ACAATTCAGCTAATGGAACCCG | TCTTCGCCTAGGAGTGTTGG |
| Spry4 | ATGGTGGATGTCGATCCTGT | GGAGGGGGAGCTACAGAGAC |
| Ppia | TTACCCATCAAACCATTCCTTCTG | AACCCAAAGAACTTCAGTGAGAGC |
2.2. Genetic modification of ESCs
To generate ESCs carrying a Spry4H2B-Venus reporter allele, we modified a EUCOMM knockout-first vector [45] with an H2B-Venus reporter [38] and Neomycin (NEO) selection cassette as previously described [46, 47]. This cassette was targeted to the 3’ end of intron 1 of the mouse Spry4 gene in ESCs of a 129Sv/C57BL6 F1 hybrid (V6.5) background. The ESC line used was a subclone of the previously described KH2 clone [48], in which an inducible Gata4-mCherry cDNA had been integrated into the Col1a1 locus [47]. The targeting construct was introduced into cells by electroporation, and correct targeting to the Spry4 locus was determined by long-range PCR amplification of the 5’ and 3’ arm junctions using the LongAmp Taq PCR Kit (NEB, #e5200) and the following primers: Spry4_GF3 (GACATGCTCACCTTCAACTTGGA) with R1RN_rev (TGATATCGTGGTATCGTTATGCGCCT) to detect the 5’ arm junction (correctly targeted: 6.7kb product); Spry4_GR3 (CTCCTGCTTAATGCGTCCAGATG) with LRPCR_neoR_fwd (GGGATCTCATGCTGGAGTTC) to detect the 3’ arm junction (correctly targeted: 7.5 kb product). Col1a1TetO-Gata4-mCherry/+;R26M2rtTA/+;Spry4H2B-Venus/+ ESCs were established on mitotically inactivated mouse embryonic fibroblasts (MEFs) in Knockout DMEM (ThermoFisher Scientific) supplemented with 15% FBS, 50 μM β-mercaptoethanol, GlutaMAX™, NEAA and 10 ng/ml LIF. After ESC line derivation, feeders were removed by serial passaging, and cells were maintained on gelatin coated dishes in GMEM-based serum/LIF medium as described above. ESCs were passaged every other day upon reaching approximately 80% confluence by washing with phosphate buffered saline (PBS), adding 0.05% Trypsin/EDTA (Life Technologies) for 3 minutes at 37°C and dissociating into a single cell suspension. Trypsin activity was neutralized with serum-containing medium. Cells were collected at 1300 rpm for 3 minutes and 1/5 of cells transferred to a new plate. The parental KH2 ESC line was used as a wild-type control in all ESC experiments.
Mutagenesis of the Fgf4 locus was achieved by lipofectamine-transfection of Col1a1TetO-Gata4-mCherry/+;R26M2rtTA/+;Spry4H2B-Venus/+ ESCs with the plasmid pX458 expressing the Cas9 nuclease, a GFP marker and a sgRNA complementary to the sequence immediately downstream of the Fgf4 start codon. A 100 nt single stranded oligonucleotide was co-transfected that served as repair template and introduced a stop codon, a frameshift and a MluI restriction site downstream of the Fgf4 start codon. Transfected cells were grown in PD0325901 (Stemgent) -containing medium and flow sorted for GFP expression, seeded at clonal density, and individual clones were expanded for DNA isolation. Successful homozygous mutagenesis of the Fgf4 locus was determined by complete MluI restriction digest of a PCR fragment of the Fgf4 locus that included the region around the start codon.
2.3. Flow cytometry
Cells for flow cytometry were recovered from flasks and dissociated to single cell suspensions with Trypsin/EDTA, spun down and resuspended in growth medium. Measurements were performed on a CyAn ADP (Beckman Coulter) or an LSRII (BD Biosciences) flow cytometer. Single cells were gated based on forward and side scatter, and Venus fluorescence recorded by excitation with a 488 nm Laser line and appropriate emission filters.
2.4. Immunoblotting
Cells were maintained for 3 days in 2i/LIF [49], followed by culture in N2B27 medium supplemented with CHIR99021 (Stemgent) and different FGF ligands for 24 hours. Lysates were prepared on ice using cell lysis buffer (Cell Signaling Technologies, 9803) supplemented with protease inhibitors (Roche, 04693159001) and phosphatase inhibitors (Sigma, P2850, P5726, P0044). 20 μg of total cell lysate per lane were separated on 12% bis-tris gels, and blotted onto PVDF membranes (Millipore, IPFL00010) in MOPS buffer (Invitrogen, B000102). Blots were blocked for 60 minutes (Li-Cor, 927–50000) at room temperature and incubated with primary antibodies overnight at 4°C. Washes were performed with PBS + 0.1% Tween-20 (MP Biomedicals). Secondary antibodies used were conjugated to 680 nm and 800 nm IR dyes (Li-Cor) compatible with the Odyssey (Li-Cor) immunoblot scanners. Quantification was performed in ImageJ (https://imagej.net).
2.5. ESC immunostaining and quantification
Cells were grown on ibidi μ-slides (ibidi, 80826) coated with 0.1% gelatin in GMEM-based medium supplemented with or without inhibitors for 24 hours, and then fixed using 4% paraformaldehyde (PFA) (28908, Thermo Fisher Scientific) for 15 minutes at room temperature. For immunostaining, samples were washed several times with PBS containing 0.1% TritonX-100 (Serva) and 1% Bovine serum albumin (BSA) (Sigma, A9418), followed by incubation with primary and then secondary antibodies. Nuclei were counterstained with DAPI, and samples were kept in 80% glycerol (Sigma, 191612) + 4% n-propyl gallate (Sigma, 02370) as a mounting medium. Imaging was carried out using a confocal Leica SP8 microscope, with consistent settings across samples. Quantification was performed in MATLAB using custom-written scripts. Primary antibody details can be found in the Key Resources Table. AlexaFluor® conjugated secondary antibodies (ThermoFisher Scientific) were used at a 1:500 dilution.
KEY RESOURCES TABLE
| Reagent or resource | Source | Identifier |
|---|---|---|
| Antibodies | ||
| anti-BRACHYURY (IF 1:200 dilution) | R&D: AF2085 | AB_2200235 |
| anti-tERK (WB 1:1000 dilution) | Abeam: ab36991 | AB_732206 |
| anti-pERK (WB 1:500 dilution) | Cell Signaling: 4370S | AB_2315112 |
| anti-FOXA2 (IF 1:500 dilution) | Abeam: ab108422 | AB_11157157 |
| anti-GATA6 (IF 1:100 dilution) | R&D: AF1700 | AB_2108901 |
| anti-GATA6 (IF 1:500 dilution) | Cell Signaling: 5851 | AB_10705521 |
| anti-GFP (IF 1:400 dilution) | Jackson Labs: GFP-1020 | AB_10000240 |
| anti-NANOG (IF 1:200 dilution) | ThermoFisher | AB_763613 |
| Scientific | ||
| anti-NANOG (IF 1:500 dilution) | Cosmo Bio: REC-RCAB0002PF | AB_567471 |
| anti-POU5F1 (IF: 1:200 dilution) | Santa Cruz: sc5279 | AB_628051 |
| anti-SOX2 (IF 1:100 dilution) | Abeam: ab97959 | AB_2341193 |
| anti-SOX17 (IF 1:100 dilution) | R&D:AF1924 | AB_355060 |
| anti-TUBULIN (WB 1:5000 dilution) | Sigma: T6074 | AB_477582 |
| Bacterial and Virus Strains | ||
| Biological Samples | ||
| Chemicals, Peptides, and Recombinant Proteins | ||
| rhFGF2 | R&D: 233-FB | |
| rhFGF4 | R&D: 235-F4 | |
| PD0325901 (MEK inhibitor) | Stemgent: 04–0006 | |
| AZD4547 (FGFRi inhibitor) | Santa Cruz: sc-364421 | |
| rhFGF2 | Cell GS: GFH146 | |
| rhFGF4 | Peprotech: 100–31 | |
| CHIR99021 (GSK3b inhibitor) | Stemgent: 04–0004 | |
| Critical Commercial Assays | ||
| Deposited Data | ||
| Experimental Models: Cell Lines | ||
| Spry4H2B-Venus/+ embryonic stem cell line | ||
| Spry4H2B-Venus/+; FGF4−/− embryonic stem cell line | ||
| Experimental Models: Organisms/Strains | ||
| Spry4H2B-Venus/+ mouse line | ||
| Oligonucleotides | ||
| Spry4H2B-Venus genotyping primer 1: | ||
| Spry4_genotyping_5’fwd_#2: | ||
| GGCTAGTCCCTCCTTGCTTCC | ||
| Spry4H2B-Venus genotyping primer 2: | ||
| LAR3 EN2: | ||
| CAACGGGTTCTTCTGTTAGTCC | ||
| Spry4H2B-Venus genotyping primer 2: | ||
| Spry4_genotyping_3’rev_#1: | ||
| GGCTGGAGGTCCTGAACTGC | ||
| Recombinant DNA | ||
| Software and Algorithms | ||
| Fiji | http://fiji.sc | SCR_002285 |
| GraphPad Prism | http://www.graphpad.com/ | SCR_002798 |
| MATLAB | http://www.mathworks.com/products/matlab/ | SCR_001622 |
| RStudio | http://www.rstudio.com/ | SCR_000432 |
| Other | ||
2.6. Mice
To generate chimeric mice, early passage Spry4 H2B-Venus/+ reporter ESCs were injected into C57BL6 blastocysts. Chimeric males were crossed with outbred MF1 wild-type females and screened for germline transmission of all three targeted alleles. The Spry4H2B-Venus targeted allele was bred away from the Col1a1TetO-Gata4-mCherry and R26M2rtTA alleles by further crosses with MF1 wild-type females. Genotyping of the modified Spry4 locus was performed by PCR with primers Spry4_genotyping_5’fwd_#2 (GGCTAGTCCCTCCTTGCTTCC), LAR3_EN2 (CAACGGGTTCTTCTGTTAGTCC) and Spry4_genotyping_3’rev_#1 (GGCTGGAGGTCCTGAACTGC) using the following PCR protocol: Step1 – 94°C for 5 seconds, Step 2 – 35x: 95°C for 30 seconds, 57°C for 30 seconds, 72°C for 1 minute, Step 3 – 72°C for 1 minute. With this primer combination, the wild-type allele results in a 532 bp band, while the targeted allele results in a 371 bp band. Genotyping of Col1a1 and R26 loci was performed as previously described [46].
All Spry4H2B-Venus/+ mice used in this study retained the NEO selection cassette, which has not yet been excised by crossing with Dre-expressing mice [50]. Spry4H2B-Venus mice were outbred to CD1 animals and maintained on a mixed bred CD-1/129Sv/C57BL6/C2J background in accordance with the guidelines of the Memorial Sloan Kettering Cancer Center (MSKCC) Institutional Animal Care and Use Committee (IACUC). All animal work within the UK was carried in accordance with UK and European legislation and in particular according to the regulations described in the Animals (Scientific Procedures) Act of 1986 (UK) and approved by the Animal Welfare and Ethical Review Body for the University of Cambridge. Mice were housed under a 12 hour light/dark cycle in a specific pathogen-free room in the designated facilities of MSKCC. Natural matings between Spry4H2B-Venus/+ studs and 4–6 weeks old virgin CD1 females were set up in the evening and mice were checked for copulation plugs the following morning. The date of vaginal plug was considered as E0.5. The Spry4H2B-Venus mouse line was maintained independently within the Wellcome Trust-Medical Research Council Centre for Stem Cell Research, University of Cambridge, UK, where the number of homozygous mice only were tracked, and at Memorial Sloan Kettering Cancer Center, New York, USA, where all genotypes were monitored. Genotyping was carried out at the time of weaning. No peri-natal lethality was observed for any genotype. The Spry4H2B-Venus are being made available from the Jackson Laboratory’s Mouse Mutant Resource Spry4<tm1(HIST1H2BB/Venus)Hadj>/J (JAX stock no. 032071).
2.7. Pre-implantation embryo collection and in vitro culture
Pre-implantation embryos were flushed from oviducts (morulae) or uterine horns (blastocyst stages) using M2 or FHM medium (Millipore) as described in [51]. For in vitro culture, embryos were placed in groups of 3–10 embryos and held within 10–15 μl microdrops of amino acid-supplemented Potassium Simplex Optimized Medium (KSOM-AA, Millipore), covered with mineral oil (Sigma), on 35 mm Petri dishes (Falcon) and allowed to develop at 37°C in a humidified 5% CO2 atmosphere. KSOM-AA was placed within the incubator at least 15 minutes prior to embryo culture to allow for pH equilibration. Morula-stage embryos were cultured within the Zona Pellucida until the time of cavitation (~24 hours into the culture), at which point the Zona was removed before continuing culture. All blastocyst-stage embryos were denuded before in vitro culture. Zona Pellucidae were removed by brief incubation in Acid Tyrode’s solution (Millipore) followed by washes in M2 or FHM.
2.8. Modulation of FGF/ERK activity in cells and pre-implantation embryos
For activation of the FGF/ERK pathway in ESCs, 10–100 ng/ml of recombinant human FGF2 (rhFGF2, CellGS) or FGF4 (rhFGF4, Peprotech) was added to the specified culture medium with 1 μg/ml of Heparin Sulfate (Sigma). To inhibit FGF/ERK activity in ESC cultures, cell culture medium was supplemented with the MEK inhibitor (MEKi) PD0325901 (Stemgent) at 15–1000 nM, as specified in the figures and figure legends.
For activation of the FGF/ERK pathway in pre-implantation embryos, 1 μg/ml of recombinant human FGF4 (rhFGF4, R&D Systems) and 1 μg/ml of Heparin Sulfate were diluted in culture medium (KSOM-AA) and embryos cultured as described above. Control embryos were cultured in KSOM-AA supplemented with 1 μg/ml of Heparin Sulfate. Similarly, for inhibition of FGF/ERK activity, either a MEK inhibitor (MEKi) PD0325901, or FGF receptor inhibitor (FGFRi) AZD4547 (Santa Cruz) were diluted to 1 μM in culture medium, and embryos grown as described for the periods indicated in the corresponding figures.
2.9. Ex vivo culture of post-implantation embryos
Embryos were dissected at E6.5 and Reichert’s membrane, including parietal endoderm, was removed, leaving the ectoplacental cone intact. Prior to culture, embryos expressing the Spry4H2B-Venus were identified using a fluorescence stereomicroscope and separated. Embryos were cultured ex vivo in organ culture dishes at 37°C, in an atmosphere of 5% CO2 and 90% relative humidity. Embryos were cultured for 24 hours in either control medium (50% DMEM F12 with GlutaMAX™ and 50% rat serum (Harlan)) or control medium with 2 μM of the FGF receptor inhibitor AZD4547 in combination with 1 μM PD0325901. Embryos were fixed and immunostained as described below.
2.10. Immunostaining of pre-implantation embryos
Immunostaining was carried out as previously described [52]. Embryos were washed in PBS after collection or in vitro culture, fixed for 10 minutes at room temperature in a 4% solution of PFA (Electron Microscopy Sciences) in PBS, and stored in PBS at 4°C until immunostaining was performed. Fixed embryos were washed in PBS with 0.1% Triton-X (PBX), then permeabilized in PBS with 0.5% Triton-X and 100 mM Glycine for 5 minutes at room temperature. Embryos were washed in PBX then blocked in PBS with 2% horse serum (Sigma) for 30–60 minutes at room temperature. Embryos were incubated in primary antibodies diluted in blocking solution overnight at 4°C. Details on antibodies used are provided in the Key Resources Table. The following day, embryos were washed 3× 5 minutes in PBX at room temperature. Embryos were then incubated in blocking solution for 30–60 minutes at room temperature before being incubated with secondary antibody diluted in blocking buffer for 60–75 minutes at 4°C. AlexaFluor® (AF) secondary antibodies (Thermo Fisher) were used at a 1:500 dilution. Embryos were washed 2× 5 minutes in PBX at room temperature before being placed in a 5 μg/ml solution of Hoechst (Thermo Fisher Scientific) in PBS for nuclear staining.
2.11. Immunostaining and RNA in situ hybridization of post-implantation embryos
For immunostaining of post-implantation embryos, embryos were isolated from deciduae and Reichert’s membrane was removed. Embryos were fixed for 30 minutes at room temperature in 4% PFA following by permeabilization with 0.5% Triton-X in PBS for 20 minutes at room temperature. Embryos were blocked overnight at 4°C in blocking buffer (PBX with 2% horse serum). Embryos were then washed 3x for 5 minutes at room temperature in PBX. Embryos were incubated overnight at 4°C in primary antibodies diluted in blocking buffer. The following day, embryos were washed 3x for 5 minutes at room temperature following by overnight incubation at 4°C in secondary antibodies diluted in blocking buffer. Embryos were washed the following day 3x for 5 minutes in PBX at room temperature. The final wash contained 5 μg/ml Hoechst. Details of primary antibodies can be found in the Key Resources Table. AlexaFluor® secondary antibodies were used at a 1:500 dilution. Riboprobes were generated and mRNA in situ hybridization was carried out as previously reported [53], using the probe described in [39].
2.12. Cryosectioning
Adult organs were dissected into smaller parts and embedded in OCT (Tissue-Tek). Pre-cooled 2-methyl butane on dry ice was used to snap-freeze samples in OCT. Samples were maintained for short periods at −80°C followed by cryosectioning using a Leica CM3050S cryostat. Sections of 10 μm were cut, stained with Hoechst and imaged using a confocal microscope as described.
2.13. Genotyping of pre-implantation embryos
All pre-implantation embryos analyzed were genotyped after imaging using the Spry4_genotyping_5’fwd_#2, LAR3_EN2 and Spry4_genotyping_3’rev_#1 primers indicated. Individual fixed, stained embryos were lysed after imaging in 10 μl lysis buffer (10 mM Tris pH 8.5, 50 mM KCl, 0.01% gelatin 300 μg/ml Proteinase K) described in [54] and 2 μl of the lysate were used as template for PCR amplification using the same cycling conditions used to genotype adult samples. Embryos where the genotype could not be determined by either PCR or visual inspection of fluorescence were excluded from the analysis.
2.14. Blastocyst qRT-PCR
E3.75 stage blastocysts were collected from intercrosses of Spry4H2B-Venus/+ mice, and zona pellucida removed by brief incubation in Acid Tyrode’s solution (Millipore). Embryos were subsequently incubated in 0.5% Trypsin-EDTA (Gibco) for 2 minutes at room temperature. Following incubation, embryos were transferred to flushing and holding medium (FHM, Millipore) for dissociation. Using a mouth pipette and pulled capillaries (Sutter Instruments) of diameter openings smaller than a blastocyst but larger than the ICM, 8–10 cells were removed from embryos by repeated pipetting. These cells were used for genotyping (see section 2.13), while the remaining blastocysts were transferred to individual tubes containing 5 μl of 2x Reaction Mix (Invitrogen, CellsDirect One-Step qRT-PCR Kit) and snap-frozen on dry ice and stored at −80°C until processing. Blastocyst lysates were used for cDNA preparation and target-specific pre-amplification as follows: 0.2 μl of SuperScript III RT/Platinum Taq mix (Invitrogen, CellDirect One-Step qRT-PCR Kit), 2.3 μl RNase-free H2O (Invitrogen), and 2.5 μl of 0.2x TaqMan assay mix (TaqMan assays – Spry4/Mm00442345_m1; Actb/Mm00607939_s1; Gapdh/Mm99999915_g1 – pooled to a final concentration of 0.2x, or 1:100) were added to each tube containing a blastocyst lysate. Cell lysis and cDNA synthesis were then performed at 50°C for 20 minutes and the SuperScript III RT subsequently inactivated and Platinum Taq activated at 95°C for 2 minutes. Pre-amplification of the specific targets was carried out with the following program: 95°C for 15 minutes followed by 18 cycles of 60°C incubation for 4 minutes. Pre-amplified blastocyst cDNA samples were diluted 1:10 in H2O for the qPCR reaction: 1.5 μl cDNA, 0.75 μl TaqMan Assay, 7.5 μl TaqMan Universal PCR Master Mix (Thermo Fisher Scientific), and 5.25 μl H2O. qPCR reactions were carried out in a CFX96 Real-Time PCR detection system (Bio-Rad) using the following program: 95°C for 5 minutes, 45x (95°C for 15 seconds, 60°C for 1 minute + plate read). Raw Ct values were processed using RStudio (v. 1.0.103) as an interactive developing environment for R (the code used for analysis is available in the GitHub repository indicated below). First, values of individual technical replicates were averaged. Mean Ct values were then normalized to the average of Actb and Gapdh values. Log normalized values were then converted to a linear scale by 2^(-value) and used for plotting. Statistical analysis of significance was assessed using Student’s t-test. Statistics were carried out on the average expression levels of the biological replicates per genotype.
2.15. Image acquisition and processing of post-implantation embryos and adult organs
Embryos were imaged on a Zeiss LSM880 laser scanning confocal microscope. Whole-mount embryos were imaged in glass-bottom dishes (MatTek) in PBS. Whole-embryo z-stacks of preimplantation embryos were acquired as described in [52]. To minimize experimental variability, laser output was measured prior to each imaging session and laser power adjusted to maintain a consistent output across experiments. The imaging parameters across experiments were otherwise identical. To position E5.5-E8.0 embryos for sagittal optical sections, glass-bottom dishes were coated with a thin layer of agarose into which small embryo-sized wells were generated using the thin end of a glass Pasteur pipette or a plastic pipette tip. Embryos were oriented with their distal tip facing down. Raw data was processed in ImageJ open source image processing software (Version: 2.0.0-rc-49/1.51d). Post-implantation stage embryos of E8.5 onwards and adult organs were imaged wholemount using a Leica M165 FC fluorescence stereomicroscope.
2.16. Quantitative analysis of reporter expression in pre-implantation embryos
Confocal z-stacks of whole-mount fixed embryos were segmented using the MINS software [55] to identify all nuclei in each embryo, and post-processed as described in [52, 55, 56]. Fluorescence decay along the z-axis, which is an artifact of the whole-mount imaging process, was corrected for each channel and embryo by fitting a linear regression model to the logarithm of fluorescence values as a function of the z-value, and further correcting the models’ slopes using an empirical Bayes approach, as described in [56]. All data analysis, including cell number calculations and fluorescence intensity analysis was performed in RStudio, a developing environment for R, version 3.2.2. Tables of both raw and corrected data for each embryo analyzed (as .csv tables), as well as original confocal microscopy images (as Zeiss .lsm files) and segmentation masks (as .tiff files) are freely available on Figshare with the identifier https://doi.org/10.6084/m9.figshare.c.4142081 [57]. All experimental reference tables, corrected data tables and annotated R scripts for all data transformations and calculations are available from http://github.com/nestorsaiz/morgani-et-al_2018.
2.17. Analysis of reporter expression in time-lapse movies of blastocysts
Fluorescence levels in time-lapse movies were analyzed using the Fiji distribution of ImageJ (NIH). The Venus channel was subject to noise reduction using the Despeckle filter. Fluorescent signal was subsequently segmented using thresholding and measured for all time frames and z-slices in each embryo. Thresholding levels were adjusted for each experiment but maintained for all embryos in each experiment and all images in each embryo. Data was then analyzed using RStudio.
To note, a number of factors introduce noise into the raw time-lapse data. These include a loss of fluorescent signal in the z-axis that is intrinsic to confocal image acquisition, movement of the ICM along the z-axis over the course of the movie, which is random and different for each embryo, and biological variability. To avoid altering any temporal change in the Venus signal due to inhibitor treatment, we opted for a minimal data transformation. Hence, the signal decrease along the z-axis was not corrected for, and thus the data exhibits a high degree of variance (see Fig. 5G). However, because the size of the z-stack is identical for all embryos within one experiment and the movement of the ICM along the z-axis during the movie is presumed to be random, we take this source of noise to affect all groups in the same way. We therefore calculated the average fluorescence values for each time frame and z-slice (time x z-axis) across the controls of each experiment. We then normalized the values for all embryos in each experiment (control and treated) by dividing them by the average of their corresponding time and z-axis position in that experiment’s controls. As a result, the expression value of control embryos is maintained around 1 over time (Fig. 5G), but the relative level in treated embryos decreases. When we normalized values against each group’s first time frame instead (dividing each value by the corresponding average at time = 1, i.e. 0 hours, of each group), we saw that expression in control embryos increases over time as observed at static timepoints (Fig. 3C,D and data not shown).
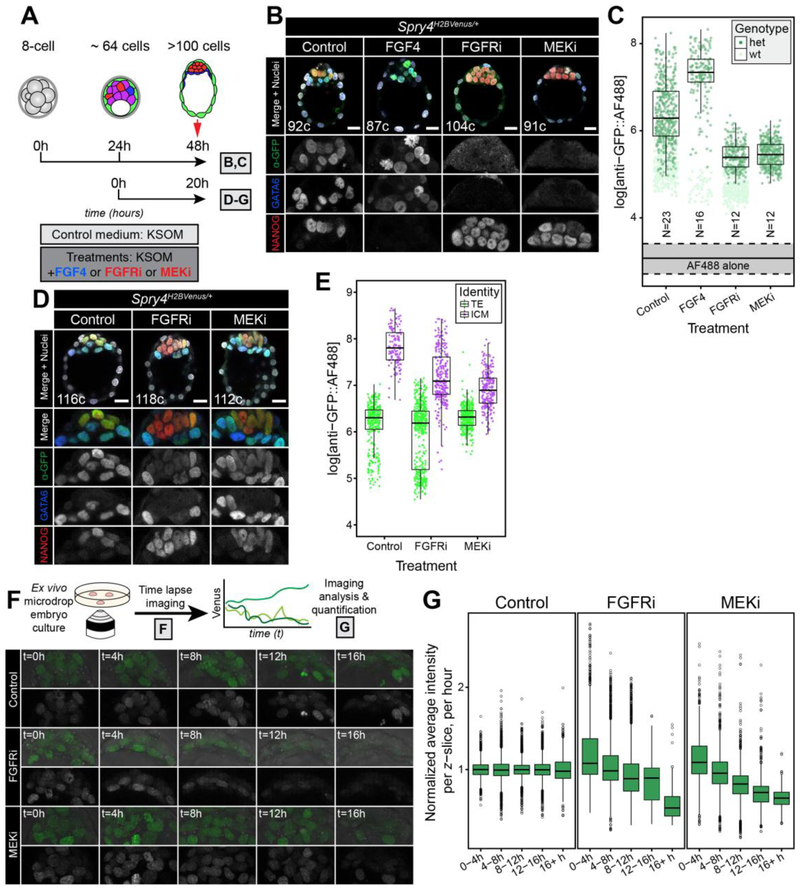
Figure 5. Spry4H2B-Venus is a faithful readout of FGF/ERK activity.
A. Schematic diagram showing the treatment regime with either 1000 μg/ml of FGF4 (+ 1 μg/ml Heparin), FGFRi (1 μM AZD4547) or MEKi (1 μM PD0325901), all diluted in KSOM culture media. Results of 48 hour (h) treatments from 8-cell stage are shown in panels B,C; 20h treatments from mid blastocyst (with FGFRi or MEKi only) are shown in panels (D-G), as indicated. B. Representative images showing 5 μm projections of confocal optical sections of immunostained embryos cultured from the 8-cell stage, for 48h, in the conditions indicated (see Materials and methods). All embryos were stained for NANOG and GATA6 to identify the Epi, PrE and double positive (DP) populations, as described [52, 55, 56] and with anti-GFP and an AF®488 coupled secondary antibody to detect H2B-Venus. Color-coding is indicated. Total cell number (c) for each individual embryo displayed is indicated. ICM magnifications for each channel are shown in grayscale. C. Boxplots showing Venus levels (as detected by immunostaining using anti-GFP + AF®488) in the ICM of Spry4H2B-Venus/+ embryos treated in each of the conditions indicated. Scattered dots represent fluorescence values of individual ICM cells from Spry4+/+ (wt) and Spry4H2B-Venus/+ (het), as indicated. Solid and dashed lines indicate the background signal in ICM cells of wild-type embryos stained only with secondary antibody, as shown in Fig. 3E (solid line, median value; dashed lines, inter-quartile range (IQR)). N indicates number of Spry4H2B-Venus/+ embryos analyzed for each group. D. Representative images showing 5 μm projections of confocal optical sections of immunostained embryos cultured as shown in panel A. All embryos were stained for NANOG and GATA6 to identify the Epi, PrE and double positive (DP) populations, as described [52, 55, 56] and with anti-GFP and an AlexaFluor®488-coupled secondary antibody to detect H2B-Venus. Color-coding is indicated. Total cell number (c) for each individual embryo displayed is indicated. ICM magnifications for each channel are shown in grayscale. E. Boxplots showing quantitation of Venus levels (detected by immunostaining using anti-GFP and an AlexaFluor® 488 coupled secondary antibody) in each condition, in ICM cells as well as TE cells, in which Spry4H2B-Venus is not expressed and signal represents Venus baseline levels as an internal negative control, in fixed and stained embryos shown in D. Scattered points represent measurements in individual nuclei. F. Upper panel is a schematic representation of the experimental procedure. Spry4H2B-Venus/+ embryos were isolated at the mid blastocyst stage (>64 cells) and cultured ex vivo either in control conditions or in the presence of small molecule inhibitors (as described in panel A) while being imaged by confocal microscopy for approximately 20 hours of development. Lower panels show still images of the ICM of embryos from the time-lapse microscopy in the conditions indicated. For each condition, top panel shows Venus signal overlaid on brightfield, bottom panel shows Venus signal alone in grayscale. G. Boxplots showing quantitation of Venus signal over time (shown in 4h bins) for the treatments indicated. Venus signal for each group and time point is normalized against the average of the corresponding control. Signal was not corrected for loss of fluorescence in the z-axis. In all boxplots, top and bottom edges of boxes represent third and first quartiles, respectively (interquartile range, IQR). Middle lines mark the median. Whiskers extend to 1.5 * IQR. Open circles represent outliers (values beyond 1.5 * IQR). Scale bars, 20 μm.
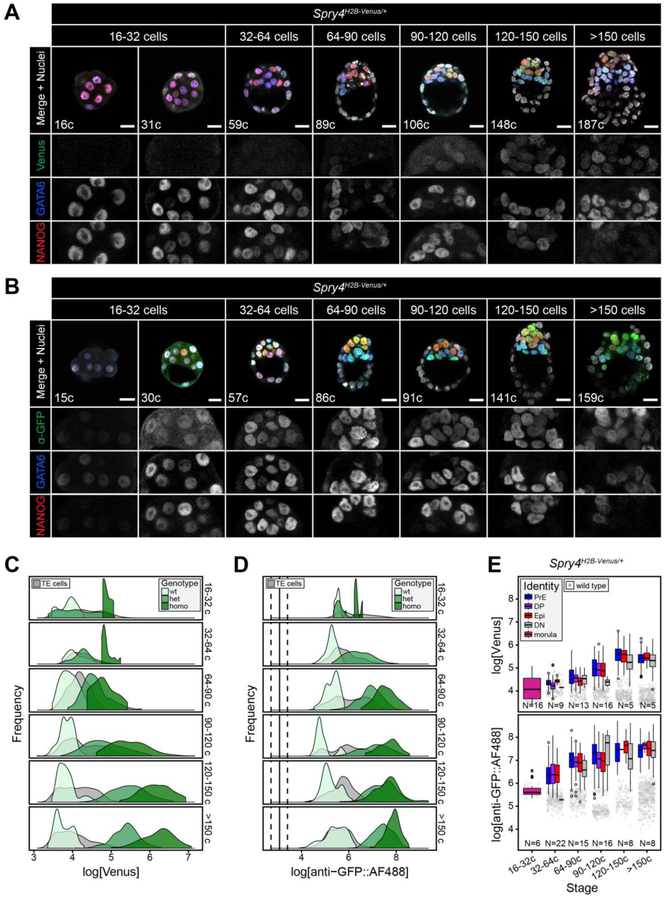
Figure 3. Spry4H2B-Venus is expressed specifically in the ICM at pre-implantation stages.
A, B. Representative images showing 5 μm projections of confocal optical sections of Spry4H2B-Venus/+ embryos fixed and immunostained at sequential stages of pre-implantation development, as indicated. Venus signal from the Spry4 locus was detected either directly (A) or by immunostaining using an anti-GFP antibody and an AlexaFluor® 488 coupled secondary antibody (B). All embryos were stained for NANOG and GATA6 to identify the Epi, PrE and double positive (DP) populations, as described [52, 55, 56]. Color-coding is indicated. Total cell number (c) for each individual embryo shown is indicated. ICM magnifications for each channel are shown in grayscale. C, D. Frequency distributions showing Spry4H2B-Venus levels in ICM cells, detected either directly (C) or by immunostaining using anti-GFP and an AlexaFluor® 488 coupled secondary antibody (D), for each stage and genotype (color-coded as indicated). Wt, wild-type; het, Spry4H2B-Venus/+; homo, Spry4H2B-Venus/H2B-Venus. Gray curves correspond to TE cells of all genotypes, in which Spry4H2B-Venus is not expressed and signal represents Venus baseline levels as an internal negative control. E. Boxplots indicating Spry4H2B-Venus levels in each ICM identity denomination, as indicated in the legend, for each sequential stage of development shown in A,B. For criteria used to assign lineage identities in the ICM see Fig. S2. Gray dots indicate green autofluorescence (top) or non-specific anti-GFP::AF®488 signal (bottom) in ICM cells of wild-type littermates at each stage, representing the baseline Venus signal. N indicates number of Spry4H2B-Venus/+ embryos analyzed. In all boxplots, top and bottom edges of boxes represent third and first quartiles, respectively (interquartile range, IQR). Middle lines mark the median. Whiskers extend to 1.5 * IQR. Open circles represent outliers (values beyond 1.5 * IQR). PrE, primitive endoderm (GATA6+); DP, double positive (NANOG+, GATA6+); Epi, epiblast (NANOG+); DN, double negative (NANOG−, GATA6−). Scale bars, 20 μm.
2.18. Quantitative analysis of reporter expression in post-implantation embryos
Confocal stacks of embryos were generated as described. Quantification of Spry4H2B-Venus/+ expression levels in cells within E6.5-E6.75 and E7.75-E8.0 gastrulating mouse embryos was carried out on transverse confocal optical sections. At E6.5–6.75, 3 distinct regions were identified based on positional information, epiblast (Epi) – the inner epithelial layer; endoderm (En) – the outer epithelial layer, primitive streak and mesoderm (PS/Meso) – mesenchymal cells that had exited the Epi, some of which had migrated bilaterally around the embryo. At E6.5–6.75 for each embryo, 3 z-planes were utilized. Individual cells were randomly chosen and, using Fiji (ImageJ) software, selected by manually drawing a boundary around the nucleus. For each plane, 5 cells were selected within each cell type (15 cells per group/embryo or 45 cells total/embryo) and the mean fluorescence intensity of Spry4H2B-Venus was measured in arbitrary units. Fluorescence intensity was z-corrected as described for pre-implantation embryos. Quantification was carried out on 5 embryos. At E7.75-E8.0, five distinct regions were identified for quantification based on both positional and marker information, Epi – inner embryonic epithelial cells expressing neither BRACHYURY nor GATA6, endoderm – outer epithelial layer, primitive streak (PS) – posterior cells leaving the Epi expressing high BRACHYURY, Mesoderm a (Ma) - cells close to the PS with intermediate BRACHYURY and GATA6, Mesoderm b (Mb) - cells further from the PS with high GATA6 and low BRACHYURY, Mesoderm c (Mc) – most anterior mesenchymal cells. For each embryo, 3 z-planes were utilized. Individual cells were randomly chosen and, using ImageJ software, selected by manually drawing a boundary around the nucleus. For each plane, 5 cells were selected within each cell type (15 cells per group/embryo or 75 cells total/embryo) and the mean fluorescence intensity of Spry4H2B-Venus, BRACHYURY and GATA6 were measured in arbitrary units. Fluorescence intensity was z-corrected for each channel as described for preimplantation embryos. Quantification was carried out on 3 embryos.
Quantification of cultured E6.5 Spry4H2B-Venus/+ embryos was carried out on sagittal optical sections of embryos using ImageJ software. For each embryo, 5 z-planes through the Epi were chosen. Individual cells were randomly chosen and, using ImageJ software, selected by manually drawing a boundary around the nucleus. For each plane, 10 cells were selected within the VE and 10 within the Epi (100 cells total/embryo) and the mean fluorescence intensity measured in arbitrary units. Fluorescence intensity was z-corrected for each channel as described for preimplantation embryos. Two independent experiments were carried out and quantification completed on 3 wild-type embryos (300 cells total), 5 Spry4H2B-Venus/+ embryos under control culture conditions (500 cells total) and 6 Spry4H2B-Venus/+ embryos treated with FGF signaling inhibitors (600 cells total).
For all post-implantation quantification, statistical analysis of significance was assessed using a One-way ANOVA followed by unpaired t-tests to compare particular groups (GraphPad Prism, GraphPad Software, Inc., Version 7.0a). Statistics were carried out on the average fluorescence levels per embryo.
2.19. Quantitative analysis of Spry4H2B-Venus limb bud gradient
Quantification of Spry4H2B-Venus fluorescence gradient in the limb buds of E10.5 embryos was carried out on 10 μm cryosections of E10.5 forelimb limb buds imaged by confocal microscopy. For each embryo, 5 distinct cryosections were imaged and quantified. Using ImageJ software, 4 lines were drawn either from the distal AER region to the proximal base of the limb bud (Lines 1–4) or from the anterior to posterior side of the limb bud, encompassing the region of Spry4H2B-Venus fluorescence (Lines A-D). Lines were drawn with approximate equal spacing across the limb bud and fluorescence intensity measured along each line. Hence, 4 proximal-distal and 4 anterior-posterior lines were measured per cryosection and 5 separate cryosections quantified per limb bud. The average fluorescence was then calculated per position (Line 1–4 and A-D), per limb bud. Five distinct limb buds were measured in this manner and the log fluorescence intensity of each limb plotted separately.
Further details of the tools and reagents used in this manuscript can be found in the Key Resources Table.
3. Results
3.1. Generation and characterization of Spry4H2B-Venus knock-in ESCs
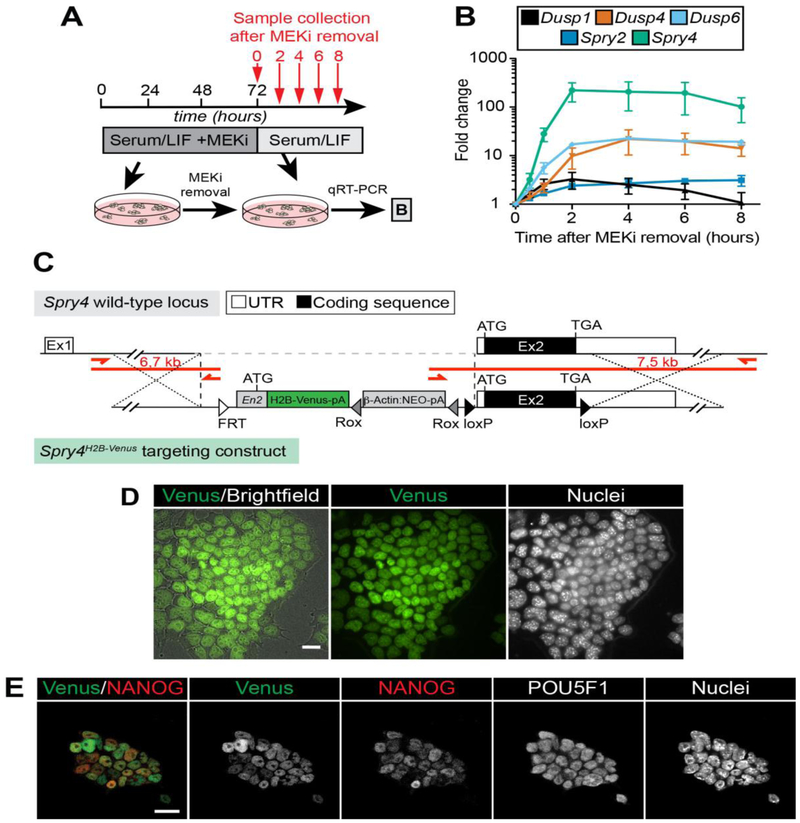
To identify candidate genes for a transcriptional reporter of ERK signaling activity, we investigated the expression dynamics of known pathway targets upon ERK activation in mouse ESCs. ERK is active in ESCs cultured in medium containing serum and leukemia inhibitor factor (serum/LIF). Therefore, in order to trigger ERK phosphorylation we first pre-cultured ESCs in serum/LIF supplemented with a MAPK/ERK kinase inhibitor, PD0325901 (MEKi), to block signaling activity and drive the system out of the steady state and subsequently switched to inhibitor-free conditions, to trigger full ERK activity [58]. The expression of a panel of ERK target genes was then analyzed over time (Fig. 1A). Of the genes analyzed, Spry4 mRNA was most rapidly and robustly upregulated (Fig. 1B), and hence represents the most dynamic and sensitive readout of FGF signaling through ERK (FGF/ERK) in ESCs. We therefore generated a knock-in fluorescent Spry4 transcriptional reporter in ESCs using a modified EUCOMM knockout-first construct [45, 46]. This construct encodes a histone H2B-linked nuclear-localized Venus fluorophore [38], to facilitate single-cell tracking and quantitative expression analysis, targeted immediately upstream of the second exon of the endogenous Spry4 locus (Fig. 1C). The resulting Spry4H2B-Venus/+ ESCs exhibited widespread heterogeneous Venus expression (Fig. 1D), in addition to expression of the pluripotency-associated genes POU5F1 (OCT3/4) and NANOG (Fig. 1E).
Figure 1. Developing a fluorescent reporter for the FGF/ERK target Spry4 in mouse ESCs.
A. Schematic diagram of ESC treatment regime for identifying FGF/ERK signaling targets. ESCs were grown for 3 days in serum/LIF medium with the MEK inhibitor PD0325901 (MEKi) and then released into inhibitor-free medium. Samples were collected 0, 2, 4, 6 and 8 hours after MEKi removal (indicated by red arrows) for qRT-PCR analysis. B. qRT-PCR data showing mRNA levels of FGF/ERK target genes in ESCs after treatment described in (A). Graph shows mean +/− SD of relative mRNA levels normalized to Ppia housekeeping gene expression from 2 (Spry2, Dusp6)or 3 (all other genes) independent experiments. C. Schematic representation of the Spry4H2B-Venus targeting strategy. A reporter cassette encoding the H2B-Venus fluorophore preceded by an Engrailed 2 (En2) intron and followed by a neomycin resistance cassette (NEO) was targeted immediately upstream of the second, ATG-containing exon of the Spry4 locus. Boxes denote exons (Ex1 and Ex2) with non-coding UTR shown as white boxes and open reading frame coding sequence as black boxes, triangles denote loxP sites (black) and Rox site (gray) that enable removal of coding exon and NEO, respectively. Open triangle indicates position of an FRT site from the original EUCOMM vector design. ATG, start codon; TAG, stop codon; pA, single polyadenylation sequences. Red bars and arrows indicate PCR amplicons and primers used to identify correctly targeted alleles. Distances within the diagram are not drawn to scale. D. Representative image of the Spry4H2B-Venus reporter imaged live in ESCs. Nuclei were stained with SiR-Hoechst (Spirochrome). E. Venus expression and POU5F1 (OCT3/4) and NANOG immunofluorescence staining of reporter-bearing ESCs grown in serum/LIF. Note the heterogeneous expression of NANOG and the Venus reporter, in contrast to the homogeneously expressed POU5F1. Scale bars, 20 μm.
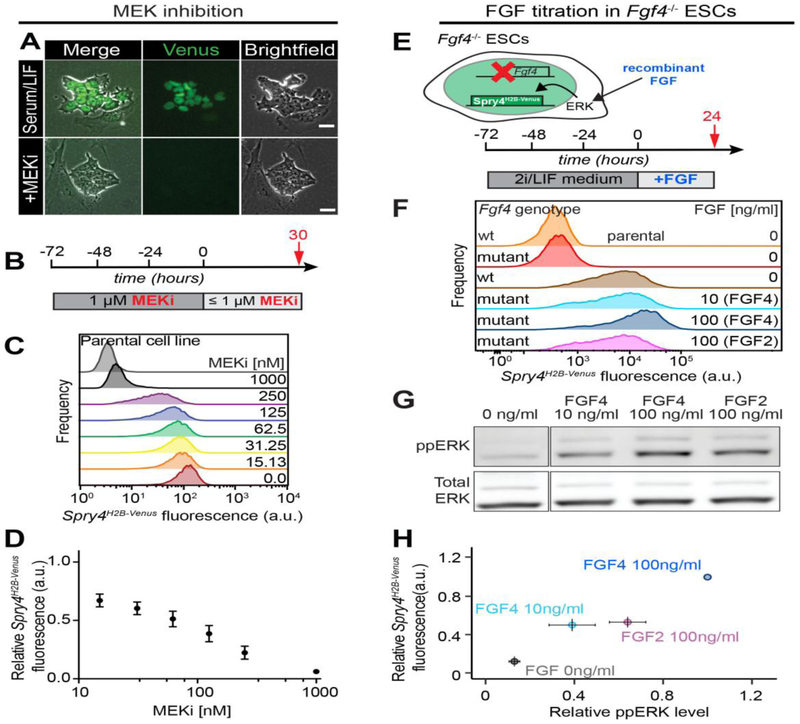
To validate the Spry4H2B-Venus reporter as a read-out of FGF/ERK activity, we analyzed the effect of chemically and genetically perturbing the pathway on Venus levels. Upon treatment with 1 μM MEKi, over the course of 72 hours, the Venus signal was abolished (Fig. 2A). Time-lapse microscopy of cells upon transfer into inhibitor-containing medium indicated an average half-life of the reporter protein of 9 hours in ESCs (Fig. S1A–C). When ESCs were transferred from MEK inhibition to medium containing reduced, subsaturating concentrations of the inhibitor (Fig. 2B), Venus increased as the degree of MEK inhibition decreased (Fig. 2C,D). Intriguingly, the Venus signal was most heterogeneous at intermediate levels of MEKi (125–250 nM, Fig. 2C), suggesting that individual ESCs have different sensitivity to FGF/ERK signaling.
Figure 2. Spry4H2B-Venus expression quantitatively reports FGF/ERK signaling in ESCs.
A. Brightfield (right panels) and Venus-fluorescence (middle) in Spry4H2B-Venus ESCs grown in serum/LIF medium (upper panels) or serum/LIF supplemented with 1 μM PD0325901 for 3 days (lower panels). Scale bar, 25 μm. B. Schematic diagram depicting experimental approach to determine ERK responsiveness of the reporter. Cells were cultured for 3 days in serum/LIF conditions supplemented with 1 μM PD0325901 to downregulate reporter expression, followed by transfer into subsaturating PD03 concentrations for one day. C. Flow cytometry analysis of Venus expression in Spry4H2B-Venus ESCs treated as indicated in (B). Uppermost histogram is from the Spry4 wild-type parental cell line. D. Quantification of results from (C). Expression of Venus in cells maintained in serum/LIF medium was normalized to 1, data points show average +/− SD from four independent experiments. E. Schematic of experimental approach to determine FGF responsiveness of the reporter. Wild-type and Fgf4 mutant cells were cultured in 2i/LIF conditions for three days followed by transfer into N2B27 medium supplemented with various doses of FGF4 or FGF2 for one day, or no FGF for parental, wild-type and mutant controls. F. Flow cytometry analysis of Venus expression in Fgf4 wild-type (wt) and Fgf4−/− mutant Spry4H2B-Venus ESCs treated as indicated in (E). The level of Venus expression in Fgf4 mutant cells is comparable to wt parental ESCs without the reporter, indicating that reporter expression in Fgf4 wt cells is mainly triggered by ESC-secreted FGF4. N = 3, one representative experiment shown. G. Total ERK and ppERK levels in Fgf4 mutant cells treated as indicated in (E) determined by immunoblotting. N = 3, one representative experiment shown. H. Relationship between ERK phosphorylation and Venus expression in Fgf4−/− mutant ESCs treated with different doses of FGF4 and FGF2 compiled from data in panels F and G. Data normalized to ppERK and Venus levels in cells treated with the highest dose of FGF4 (shown as 1). Data points indicate average +/− SD from two (Fgf4 mutant, 0 ng/ml FGF) or three (all other data points) independent experiments.
Expression of members of the Sprouty gene family can be induced by FGF but also by other ligands, such as PDGF or EGF [40–43]. To determine whether the Venus signal in ESC cultures was due solely to FGF signaling, we introduced a targeted frameshift and nonsense mutation in the Fgf4 locus in Spry4H2B-Venus ESCs, using the CRISPR/Cas9 system [59] (Fig. S1D). Disruption of Fgf4 reduced Venus levels in serum-free medium to baseline levels i.e. comparable to that of wild-type parental ESCs lacking the Spry4H2B-Venus reporter (Fig. 2E,F). This suggested that the signal was, indeed, largely dependent on paracrine and/or autocrine signaling stimulated by ESC-produced FGF4. Furthermore, in the Fgf4 mutant ESCs, Venus levels could be rescued to similar levels as that in Spry4H2B-Venus;Fgf4+/+ cells by supplementing medium with FGF4 or FGF2 (Fig. 2E–G). Increasing doses of FGF4 elevated ERK activity and Venus levels (Fig. 2F–H).
In ESC cultures maintained with serum/LIF medium, both NANOG [60, 61] and Venus were heterogeneously expressed (Fig. 1E, S1E). As FGF/ERK activity negatively regulates NANOG expression [62], we asked whether NANOG and Venus (indicating FGF/ERK activity) levels were inversely correlated. Average Venus levels decreased with MEK inhibition, and average NANOG levels increased with the level of MEK inhibition, consistent with previous reports [49, 63]. However, there was no correlation between the levels of these two markers within individual cells (Fig. S1E–G).
Together these data demonstrated that the Venus signal observed within serum/LIF ESC cultures was elicited by FGF/ERK activity, and that the Spry4H2B-Venus transcriptional reporter provides a quantitative read-out for FGF/ERK signaling in mouse ESCs.
3.2. Spry4H2B-Venus is expressed specifically in the ICM at pre-implantation stages
FGF signaling is critical for the specification of the Epi and PrE lineages within the ICM of the preimplantation mouse embryo [12–17, 64, 65]. Fgf4 is expressed from the 16-cell stage [65], when cells of the embryo are yet to become specified, and is the earliest known ligand to be asymmetrically expressed within ICM cells [64]. The FGF receptor Fgfr1 is expressed from embryonic day (E)3.25 (~32 cells) within all cells of the ICM [18, 19, 64]. Accordingly, Spry4 is expressed in all ICM cells from E3.5 [18, 64]. A second receptor, Fgfr2 is expressed only within PrE and TE cells [19, 64–66]. Absence of either the ligand or both receptors prevents the formation of the PrE lineage and impairs Epi maturation [14, 17–19].
To assess the utility of Spry4 expression as a readout of FGF/ERK signaling in the embryo, Spry4H2B-Venus/+ ESCs were introduced into host embryos by blastocyst injection to generate chimaeric germ line transmitting mice. Both Spry4H2B-Venus heterozygous and homozygous adult male and female mice were viable and fertile (Table 1,2) and, at all stages analyzed, embryos were indistinguishable from their wild-type littermates (Fig. S2B,D,E). However, as reported for Spry4−/− mice [43], a fraction (29%) of Spry4H2B-Venus/H2B-Venus mice exhibited polysyndactyly (Fig. S3A) suggesting that the introduction of the H2B-Venus construct disrupted Spry4 expression. Quantitative RT-PCR in wild-type, heterozygous and homozygous pre-implantation embryos revealed a significant reduction in Spry4 mRNA expression in Spry4H2B-Venus/H2B-Venus embryos, further indicating that the reporter generates a loss of function allele for Spry4 (Fig. S3B). As Spry4 is a negative regulator of FGF/ERK activity, we investigated whether the perturbation at the Spry4 locus affected signaling activity. Surprisingly, we observed a slight reduction in ppERK levels within Spry4H2B-Venus/+ ESCs relative to the wild-type parental KH2 ESC line (Figure S3C,D), which may be attributed to the complex feedback interactions regulating FGF/ERK signaling [67].
Table 1.
Survival of homozygous mice from Spry4H2B-Venus/+ x Spry4H2B-Venus/+ crosses.
| Homozygous (−/−) | Total mice | |
|---|---|---|
| Number | 47 | 202 |
| Percentage | 23% | |
Table 2.
Survival of adult mice from Spry4H2B-Venus/H2B-Venus x Spry4H2B-Venus/+ crosses. The number of female (F) and males (M) are shown in brackets.
| Heterozygous (+/−) | Homozygous (−/−) | |
|---|---|---|
| Number | 14 (7F, 7M) | 15 (5F, 10M) |
| Percentage | 48 | 52 |
To assess the early response of the reporter to FGF signaling, we analyzed the expression of Spry4H2B-Venus throughout pre-implantation development. The majority of data presented in the figures were collected from Spry4H2B-Venus/+ embryos, unless otherwise stated in the figures. In fixed samples, Venus was first detected from the 64–90 cell stage, specifically in ICM cells, and increased as blastocysts developed (Fig. 3A, S2A). When immunostained with an anti-GFP antibody, Venus was detectable at earlier stages, from the 32–64 cell stage (Fig. 3B, S2A). Spry4H2B-Venus/H2B-Venus embryos showed higher levels of Venus than heterozygous Spry4H2B-Venus/+ embryos, although this difference was less evident when detected with anti-GFP (Fig. 3C,D), probably due to non-linear signal amplification by the secondary antibody. Venus levels within the TE were comparable to those of non-transgenic (wild-type) littermates at all stages analyzed (Fig. 3C,D, S2A) hence we conclude that Spry4 is not expressed at detectable levels within these cells. Both Spry4H2B-Venus/+ and Spry4H2B-Venus/H2B-Venus embryos exhibited normal blastocyst development as assessed by morphology, total cell number and lineage composition (Fig. 3A,B, S2B,D,E), comparable to that of wild-type littermates and previous studies [56].
Venus expression within the ICM was heterogeneous (Fig. 3A,B) hence we asked whether different Venus levels were associated with different ICM lineages. Cell identities within the ICM were assigned based on relative NANOG and GATA6 expression as previously described [52, 56]. Cells were classified as Epi (NANOG positive (+), PrE (GATA6+), double positive (DP, NANOG+, GATA6+) or double negative (DN, NANOG negative (−), GATA6−) (Fig. S2C,D). We observed no difference in Venus or anti-GFP::AlexaFluor®488 (AF®488) levels between ICM cell types at any stage, in either Spry4H2B-Venus/+ (Fig. 3E) or Spry4H2B-Venus/H2B-Venus (Fig. S2F) embryos. These data suggest that the FGF/ERK pathway is active in all ICM lineages, consistent with the reported expression patterns for Fgfr1, Fgfr2 and Spry4 in the ICM [18, 19, 64] and the phenotype observed in both the Epi and PrE in the absence of FGF signaling [14, 18, 19].
3.3. Spry4H2B-Venus is a faithful readout of FGF/ERK activity in vivo
Manipulation of the FGF pathway during pre-implantation development has a profound effect on cell fate choice within the ICM [12, 13, 15, 16]. Ex vivo culture of pre-implantation embryos in the presence of FGF4 or inhibitors of MEK or FGFRs directs ICM cells towards a PrE or Epi fate respectively [15, 16, 56]. Genetic perturbation of pathway components similarly alters the balance of ICM lineages [12, 14, 17–19]. To establish whether Spry4H2B-Venus expression is a faithful readout of FGF/ERK activity in vivo, we analyzed reporter expression in Fgf4, Fgfr1 and Fgfr2 null embryos.
In the absence of Fgf4, PrE cells are not specified and the entire ICM adopts an Epi-like fate by the late blastocyst stage (>64-cells) [14, 17]. Consistent with a reduction in FGF signaling activity, Spry4H2B-Venus expression was decreased within the ICM of Fgf4−/− versus Fgf4+/+ Spry4H2B-Venus embryos (Fig. 4AF, S4A–C). In Fgf4−/− ICMs, Venus expression was reduced to a level comparable to the background signal observed in TE cells (Fig. S4A–C), suggesting that FGF/ERK activity was lost within all cells of Fgf4−/− embryos. Of note, a small number of ICM cells were assigned as DP and PrE cells in 64–90 and 90–120 cell embryos owing to the unbiased and automated methods used for lineage assignment based on relative NANOG and GATA6 expression in cells (see Fig. S2C,D, S4D,E and Materials and methods). This was also the case for DP cells assigned in Fgfr1−/− embryos at the 90–120 cell stage (see below).
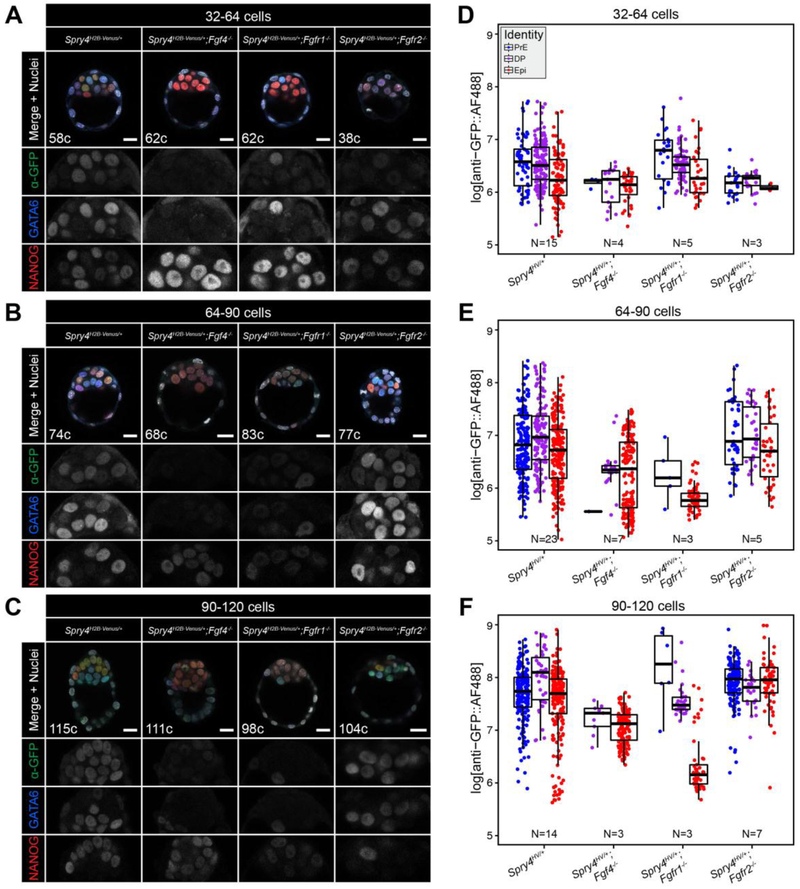
Figure 4. Spry4H2B-Venus reporter expression is altered in the developing ICM by genetic modulation of the FGF pathway components.
A-C. Representative images showing 5 μm maximum intensity projections of confocal optical sections of wild-type, Fgf4−/−, Fgfr1−/− and Fgfr2−/− embryos fixed and immunostained at sequential stages of pre-implantation development (A, 32–64 cell stage; B, 64–90 cell stage; C, 90–120 cell stage). Venus signal from the Spry4 locus was detected by immunostaining using an anti-GFP antibody and an AlexaFluor® 488 coupled secondary antibody. All embryos were stained for NANOG and GATA6 to identify the Epi, PrE and double positive (DP) populations. Color-coding is indicated. Total cell number (c) for each individual embryo shown is indicated. ICM magnifications for each channel are shown in grayscale. D-F. Boxplots showing relative Venus levels in indicated ICM lineages of wild-type, Fgf4−/−, Fgfr1−/− and Fgfr2−/− embryos (x-axis) at sequential stages of pre-implantation development. (D, 32–64 cell stage; E, 64–90 cell stage; F, 90–120 cell stage). Scattered points represent measurements in individual nuclei, color-coded for identity as indicated. In all boxplots, top and bottom edges of boxes represent third and first quartiles, respectively (interquartile range, IQR). Middle lines mark the median. Whiskers extend to 1.5 * IQR. N indicates number of embryos analyzed for each group. Scale bars, 20 μm.
Loss of Fgfr1, a pan-ICM receptor, results in an ICM comprised predominantly of Epi cells, but rare PrE cells are also specified [18, 19]. Here we observed that the few PrE cells present exhibited comparable Venus levels to those observed in Fgfr1+/+ embryos (Fig. 4A–F, S4A–C) indicating that, in the absence of FGFR1, the PrE-specific receptor FGFR2 [64, 65] was sufficient to trigger normal FGF/ERK activity in these cells. Consistent with the fact that Epi cells only express Fgfr1 and not Fgfr2 [18, 19, 64], at the 64–90 and 90–120 cell stages the expression of Spry4H2B-Venus was substantially reduced within Epi cells of Fgfr1−/− versus Fgfr1+/+;Spry4H2B-Venus/+ embryos (Fig. 4A–F).
In contrast to Fgfr1 mutant embryos, loss of the PrE-specific receptor Fgfr2 does not affect PrE or Epi fate specification [18, 19]. In the absence of FGFR2, FGFR1 is able to transduce the FGF4 signal to specify the two ICM lineages. Consistent with this, we observed no effect on cell fate specification or Spry4H2B-Venus expression levels within ICM cells of Fgfr2−/−;Spry4H2B-Venus/+ embryos (Fig. 4A–F, S4A–C).
While Venus levels were reduced in Fgf4−/− ICM and Fgfr1−/− Epi cells relative to non-mutant ICM cells, we still observed an increase in Spry4H2B-Venus reporter expression over developmental time (Fig. 4D–F, S4A–C). This may be due to an FGF-independent increase in basal Spry4 transcription within ICM cells as they mature.
To assess the response of the reporter to FGF/ERK signaling modulation, we cultured E2.5 Spry4H2B-Venus/+ embryos for 48 hours in the presence of saturating doses of FGF4 (1 μg/ml) or inhibitors of the FGF/ERK axis (MEKi, or the FGFR inhibitor AZD4547 - FGFRi) (Fig. 5A). In these conditions, embryos progress developmentally but all ICM cells adopt the same identity (Fig. S5A–C). As previously shown [16], all ICM cells of embryos cultured in the presence of FGF4 expressed GATA6 and not NANOG at the end of the culture period, indicating a PrE identity (Fig. 5B, S5B,C). Conversely, in embryos cultured with either MEKi or FGFRi, all ICM cells expressed NANOG and not GATA6, indicating an Epi identity (Fig. 5B, S5B,C) [15]. These effects were irrespective of the embryo genotype (wild-type or Spry4H2B-Venus/+) (Fig. S5A–C). Consistent with FGF4 stimulating an increase in FGF/ERK activity, Spry4H2B-Venus/+ embryos exposed to FGF4 showed elevated levels of Venus in the ICM compared to untreated littermates (Fig. 5B,C). Conversely, when Spry4H2B-Venus/+ embryos were cultured in the presence of MEKi or FGFRi, Venus expression was completely abrogated (Fig. 5B,C).
We next took advantage of the temporal control that cytokine and small molecule treatments afford to visualize changes in Venus expression in response to FGFR inhibitor and MEK inhibitor treatments in embryos. We have previously shown that stimulation or inhibition of the FGF/ERK axis at blastocyst stages does not force all ICM cells to adopt the same identity [56]. While inhibitor treatment from the 8-cell stage results in all ICM cells adopting an Epi fate (Fig. 5A,B, S5B,C) [15], treatment of blastocysts results in embryos comprising a mix of both Epi and PrE [15, 56]. Hence, by treating blastocysts rather than morulae with FGFRi or MEKi, we can decouple Spry4 expression from cell fate choice.
Embryos treated in this way from the ~64-cell stage (Fig. 5A) had comparable total cell numbers to control embryos and developed ICMs with both Epi and PrE cells (Fig. 5D, S5D–F), but with reduced levels of Spry4H2B-Venus compared to controls (Fig. 5D,E). However, the Venus signal within these embryos was above background levels (Fig. 5D,E, S5G), suggesting either perdurance of the fluorescent protein and/or the activity of other pathways at these stages that may also stimulate Spry4 expression. To assess the dynamics of Spry4H2B-Venus expression in treated embryos, we performed time-lapse imaging (Fig. 5F) and quantified the fluorescent signal. When the Venus signal was normalized against the corresponding timepoint of control embryos for each experiment, a decrease was evident in embryos treated with either FGFRi or MEKi (Fig. 5F,G). This reduction was especially apparent from 8 hours into the culture (Fig. 5G, S5G), which is in agreement with the half-life for the H2B-Venus fusion protein observed in ESC cultures (Fig. S1B, C). These data demonstrate that the Spry4H2B-Venus reporter may be utilized to monitor FGF/ERK pathway dynamics in vivo as well as in vitro and in fixed samples.
3.4. The Spry4H2B-Venus reporter reveals post-implantation expression within the VE
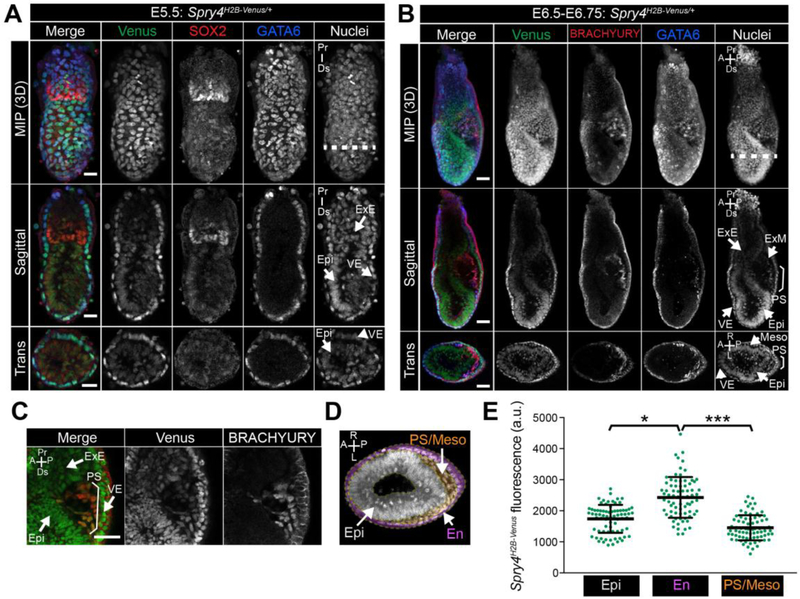
At post-implantation stages of development, FGF is produced by the Epi and stimulates ERK-mediated proliferation of the adjacent diploid TE cells within the extraembryonic ectoderm (ExE) [21, 26, 37]. Prior to the onset of gastrulation, at E5.5, Spry4H2B-Venus was expressed within the VE and Venus signal was also observed at lower levels within the Epi and ExE (Fig. 6A).
Figure 6. Expression of Spry4H2B-Venus/+ at early post-implantation stages.
A-B. Confocal images of representative E5.5 (A, scale bars, 25 μm) and E6.5-E6.75 (B, scale bars, 50 μm) Spry4H2B-Venus/+ embryos after wholemount immunofluorescence staining. Upper to lower panels show: maximum intensity projections (MIP), optical sagittal sections and optical transverse sections. Nuclei are stained with Hoechst. Dashed white line indicates plane of transverse optical section. Bracket demarcates the primitive streak. C. High magnification confocal image of the primitive streak region of an E6.5-E6.75 Spry4H2B-Venus/+ embryo. Epi, epiblast; VE, visceral endoderm; ExE, extraembryonic ectoderm; ExM, extraembryonic mesoderm; PS, primitive streak; Meso, mesoderm; Pr, proximal, Ds, distal; A, anterior; P, posterior; R, right; L, left. Non-nuclear fluorescence is observed in VE cells stained with the anti-BRACHYURY antibody and likely represents non-specific binding. D. Transverse confocal optical section of an E6.5-E6.75 embryo marked with the regions defined for quantification in E. Regions were defined based on positional information and include epiblast (Epi, gray) – inner epithelial cells, endoderm (En, pink) - outer epithelial cell layer, primitive streak and mesoderm (PS/Meso, orange) - cells leaving the epithelial layer at the posterior of the Epi and migrating between the Epi and outer endoderm layers. E. Quantification of mean fluorescence levels of Venus in transverse optical sections of E6.5-E6.75 Spry4H2B-Venus/+ embryos, carried out as described in Materials and methods. Fluorescence, measured in arbitrary units (a.u.) was measured in 15 randomly selected cells per region (defined in panel D) per embryo (n = 5 embryos, 225 cells total). Each point marks a single cell. * p = <0.05, *** p = <0.0005, unpaired t-test.
In the mouse, gastrulation is initiated at approximately E6.25. Cells within the posterior Epi undergo an epithelial to mesenchymal transition (EMT) at the primitive streak (PS) and subsequently migrate bilaterally around the embryo between the Epi and VE cell layers [68]. FGF signaling regulates EMT and cell migration during gastrulation [28, 29, 33, 34, 69]. We therefore asked whether Spry4H2B-Venus expression would report areas of FGF/ERK signaling during gastrulation. At E6.5-E7.0, Venus was most highly expressed within cells of the endoderm (marked by FOXA2, SOX17 and GATA6) while lower levels of Venus were observed within cells of the ExE, Epi, PS (marked by BRACHYURY) and nascent mesoderm (marked by BRACHYURY and GATA6) (Fig. 6B,C, S6A,B). To quantify the relative levels of Venus in different cell types of the early gastrulating embryo, we defined three regions based on positional information, the Epi – inner epithelial layer, the endoderm (En) – outer epithelial layer comprised of both VE and definitive endoderm cells, the PS and mesoderm (PS/Meso) – cells that had left the Epi epithelial layer (Fig. 6D). Venus levels were significantly higher within endoderm cells compared to the Epi and PS/Meso populations (Fig. 6E). While at E6.5 Spry4 mRNA is present at elevated levels within cells of the PS relative to cells of the Epi (Fig. S7A), we did not observe an elevation of Venus within the PS at this stage (Fig. 6B–E, S6A,B). This likely represents a time lag of reporter expression and signal relative to the endogenous mRNA expression, as observed in ESCs.
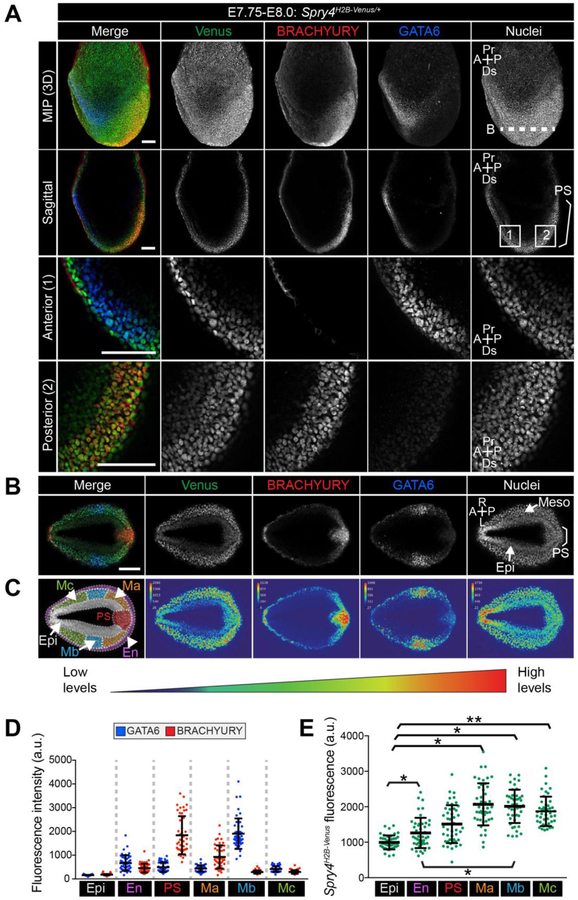
At E7.75-E8.0, Spry4 is expressed within cells exiting the PS (Fig. S7A,B) [39]. At this stage, Venus expression was observed within the Epi and VE, and was elevated within cells exiting the PS (Fig. 7A,B). Due to the more complex array of cell populations present at this later gastrulation stage, we utilized both positional information and markers, BRACHYURY and GATA6, to define cell populations (Fig. 7C,D and Materials and methods);); Epi – inner embryonic cells expressing neither BRACHYURY nor GATA6, endoderm (En) – cells circumventing the outside of the embryo, primitive streak (PS) - cells at the posterior of the embryo leaving the Epi and expressing high levels of BRACHYURY, Mesoderm a (Ma) - cells close to the PS expressing intermediate levels of BRACHYURY and GATA6. Mesoderm b (Mb) - cells further from the PS expressing high levels of GATA6 and low levels of BRACHYURY, likely cardiac mesoderm precursors [46], Mesoderm c (Mc) – mesenchymal cells positioned most anteriorly. Spry4H2B-Venus was expressed at lowest levels within Epi cells, at higher levels within the endoderm, comprised of both VE and definitive endoderm cells, and at approximately 2-fold higher levels within all of the mesoderm subtypes analyzed (Fig. 7E).
Figure 7. Expression of Spry4H2B-Venus/+ at E7.75-E8.0.
A. Confocal images of a representative E7.75-E8.0 Spry4H2B-Venus/+ embryo after wholemount immunofluorescence staining for BRACHYURY and GATA6. Upper to lower panels show: maximum intensity projections (MIP), optical sagittal sections and high magnification images of anterior and posterior regions. Nuclei are stained with Hoechst. Dashed white line (B) indicates plane of transverse optical section shown in panel B. White boxes, 1 and 2, indicate regions shown in lower panel high magnification anterior and posterior images. B. Image showing a confocal transverse optical section through the E7.75-E8.0 Spry4H2B-Venus/+ embryo in panel A. Epi, epiblast; VE, visceral endoderm; PS, primitive streak; M, mesoderm; A, anterior; P, posterior; R, right; L, left; Pr, proximal; Ds, distal. Bracket demarcates the primitive streak. Scale bars, 100 μm. C. Left-most panel shows the transverse confocal optical section of embryo from panel B marked with the regions defined for quantification in D and E. Regions include epiblast (Epi, gray), endoderm (En, pink) - identified as the outermost cell layer, primitive streak (PS, red) - identified as cells leaving the epithelial layer at the posterior of the epiblast, mesoderm A (Ma, orange) defined as GATA6-low cells closest to the PS, mesoderm B (Mb, blue) defined as GATA6-high cells further from the PS, mesoderm C (Mc, green), cells that expressed neither GATA6 nor BRACHYURY that had migrated most anteriorly. Other panels display a look up table (LUT) visualization of fluorescence intensity of each fluorophore in panel B using a range indicator to show high levels of expression in red and low levels in blue. D. Quantification of mean fluorescence levels of BRACHYURY and GATA6 immunostaining in transverse optical sections of E7.75-E8.0 Spry4H2B-Venus/+ embryos. Fluorescence, measured in arbitrary units (a.u.) was measured in 15 randomly selected cells per region (defined in panel C) per embryo (n = 3 embryos, 225 cells total). Each point denotes a single cell. Quantification was carried out as described in the Materials and methods. BRACHYURY was observed at the membrane of endoderm cells and likely represented non-specific antibody binding. As only nuclei were selected, this non-specific signal was excluded from the quantification. E. Quantification of mean fluorescence levels of Venus in transverse optical sections of E7.75-E8.0 Spry4H2B-Venus/+ embryos as described in D. * p = <0.05, ** p = <0.01, unpaired t-test.
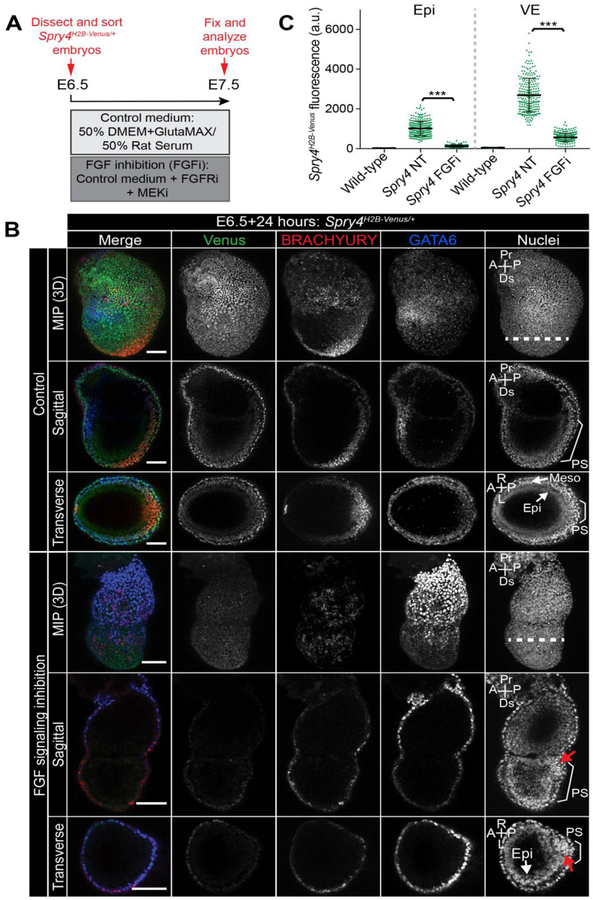
As Spry4 expression in the Epi and VE has not been previously described [39], and we could not detect localization within the VE by mRNA in situ hybridization for Spry4 (Fig. S7A,B), hence the Venus signal could either be attributed to perdurance of the Venus fluorescent protein from earlier pre-implantation stages or indicate a higher sensitivity of the fluorescent reporter compared to RNA in situ or ppERK immunoreactivity [37, 39]. To distinguish between these possibilities, E6.5 Spry4H2B-Venus/+ embryos were isolated and cultured ex vivo for 24 hours in the presence of FGFRi and MEKi, which in combination we refer to here as FGFi (Fig. 8A). It should be noted that this inhibitor combination also inhibits ERK activity downstream of non-FGF ligands, such as PGDF and EGF, which could also regulate Spry4 expression in this context.
Figure 8. Response of Spry4H2B-Venus reporter to FGF signaling inhibition during early post-implantation development.
A. Schematic diagram of experimental design. E6.5 Spry4H2B-Venus/+ embryos were dissected and cultured ex vivo for 24 hours either in control medium containing 50% DMEM with GlutaMAX and 50% rat serum or in control medium with 2 μM of the FGF receptor inhibitor (FGFRi) AZD4547 in combination with 1 μM of the MEK inhibitor (MEKi) PD0325901. B. Confocal images of E6.5 Spry4H2B-Venus/+ embryos after 24 hours of ex vivo culture in described conditions. Upper to lower panels show: maximum intensity projections (MIP), optical sagittal sections and optical transverse sections. Nuclei are stained with Hoechst. Epi, epiblast; PS, primitive streak; M, mesoderm; Pr, proximal; Ds, distal; A, anterior; P, posterior; R, right; L, left. Dashed white line indicates plane of transverse optical section. Bracket demarcates the primitive streak. Scale bars, 100 μm. C. Quantification of mean Venus fluorescence in embryos after ex vivo culture. Venus fluorescence, measured in arbitrary units (a.u.) was measured in 100 randomly selected cells/embryo (50 visceral endoderm (VE) and 50 epiblast (Epi) cells) in wild-type embryos (n = 3 embryos, 300 cells), Spry4H2B-Venus/+ non-treated (NT) embryos (n = 5 embryos, 500 cells) or Spry4H2B-Venus/+ embryos cultured with FGF signaling inhibitors (FGFi) (n = 6 embryos, 600 cells). Each point marks a single cell. Quantification was carried out as described in the Materials and methods. *** p = <0.001, unpaired t-test.
Consistent with the known role of FGF signaling at gastrulation [28, 29, 33, 34, 36], and confirming that the inhibitor treatment was effective, culturing embryos in FGFi prevented normal PS progression, reduced BRACHYURY expression and consequently blocked dispersal of the VE [70] (Fig. 8B). While some cells had left the Epi layer, perhaps prior to FGFi treatment, the majority of cells did not move efficiently away from the PS and instead accumulated at the posterior of the embryo (Fig. 8B, red arrows), consistent with cell migration defects observed within the PS of Fgf8 and Fgfr1 mutant mice [28, 29, 33, 34, 36]. Upon FGFi treatment, we observed an almost 5-fold reduction in Venus levels within the VE andã7.5-fold reduction within the Epi (Fig. 8B,C). This revealed that the Venus signal was not solely due to reporter perdurance and that the majority of the signal observed within the VE was stimulated by signaling downstream of the FGFR and/or ERK.
3.5. Spry4H2B-Venus expression at somite stages in FGF/ERK signaling centers
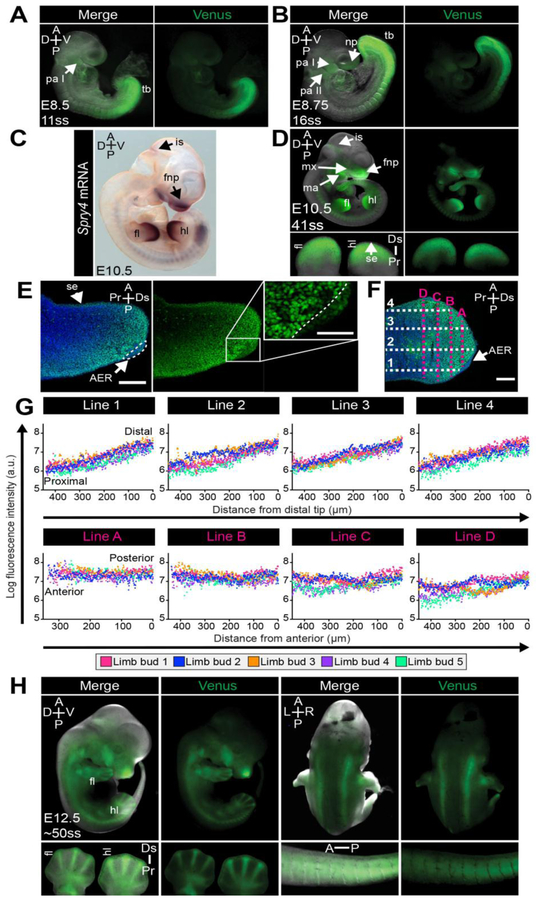
Sprouty gene expression during later somite stages of development has been characterized by RNA in situ hybridization (Fig. S7A–D) [39, 71]. At E8.5-E10.5, Venus expression in Spry4H2B-Venus reporter line recapitulated the known, highly localized expression pattern of Spry4 at these stages within the tailbud presomitic mesoderm (PSM), the newly formed somites [72] and, at lower levels, in the pharyngeal arches (Fig. 9A–D, Fig. S7AC,D), confirming its utility as a faithful reporter. We did not observe waves of reporter expression within the PSM (data not shown) that might be expected from the oscillatory expression of Spry4 within this tissue [72]. This is likely due to the relatively long half-life of the reporter protein compared to the period of the oscillations. As previously reported [39], at E10.5 (Fig. 9C), Venus was observed in the frontonasal processes, maxillary and mandibular arches, as well as the isthmic constriction (Fig. 9D), an FGF-dependent organizer at the midbrain-hindbrain junction [73–75]. At E12.5 we observed continued expression of Spry4H2B-Venus within the tail, craniofacial structures, somites and isthmus (Fig. 9E). This localization was also consistent with known patterns of FGF/ERK activity at these developmental stages [37].
Figure 9. Expression of Spry4H2B-Venus/+ at somite stages.
A,B,D,H. Representative wholemount images of somite stage Spry4H2B-Venus/+ embryos, showing either Venus fluorescence alone or merged with the brightfield image. A. Lateral view of E8.5 embryo. B. Lateral view of E8.75 embryo. D. Lateral view of E10.5 embryo. Limb buds were removed and imaged at higher magnification (lower panels). H. Lateral and dorsal view of E12.5 embryo. Limbs and tail were removed and imaged at higher magnification (lower panels). Ss, somite stage; tb, tail bud; pa I/II, pharyngeal arch one and two; fl, forelimb; hl, hindlimb; fnp, frontonasal process; mx, maxillary arch; ma, mandibular arch; np, nasal placode; is, isthmic constriction; aer, apical ectodermal ridge; se, surface ectoderm; A, anterior; P, posterior; D, dorsal; V, ventral; Pr, proximal; Ds, distal; L, left; R, right. C. Wholemount in situ hybridization labeling of Spry4 mRNA in an embryonic day (E) 10.5 embryo. E. Confocal image of a cryosectioned forelimb bud from an E10.5 embryo showing Spry4H2B-Venus alone and as a merge with the nuclei stain (Hoechst). Inset shows high magnification region of AER. AER, apical ectodermal ridge. Scale bars, 50 μm. F. Confocal image of an E10.5 cryosectioned limb bud depicting the arrangement of example measurement lines used for the quantification in panel G. Four lines were made along the proximal to distal axis of the limb bud (1–4) and four across the anterior-posterior axis (A-D). Spry4H2B-Venus fluorescence intensity was measured (in arbitrary units – a.u.) along these lines using ImageJ software as described in the Materials and methods. Scale bars, 100 μm. G. Quantification of Spry4H2B-Venus signal gradient in E10.5 forelimb limb buds (n = 5 limb buds, 5 cryosections per limb bud). Each dot represents the average fluorescence intensity per limb bud at a particular point along the line.
FGF/ERK signaling plays a central role in limb formation [76–78]. FGF produced by the apical ectodermal ridge (AER) [76–78], a thickening of the epithelium at the distal tip of the limb bud, induces the expression of negative FGF/ERK regulators, including Sprouty genes, in the underlying limb bud mesenchyme (Fig. S7D) [39, 76]. Accordingly, we observed Venus within the limb buds (E10.5) (Fig. 9D,E) [71, 77–79]. High Venus levels were observed within the mesenchyme and surface ectoderm and at lower levels within the AER (Fig. 9E). Downstream targets of the FGF/ERK pathway, including ppERK [37, 80], Spry1 and Spry4 [39, 78], Etv4/5 [81] and Dusp6 [82], are present in a proximal-distal gradient in the limb bud mesenchyme. Consistently, we also observed a Venus proximal-distal gradient in the limb bud mesenchyme of Spry4H2B-Venus/+ embryos (Fig. 9E). We quantified this gradient by measuring Venus fluorescence intensity from the distal tip to the proximal base of E10.5 forelimb buds (Fig. 9F, Lines 1–4), as well as across the anterior-posterior limb bud axis (Fig. 9F, Lines A-D). While a Venus gradient was observed along the proximal-distal axis of all limb buds analyzed (Fig. 9G, upper panels), we did not observe an anterior-posterior gradient (Fig. 9G, lower panels).
At E12.5, we noted Venus fluorescence within the developing digits (Fig. 9H). As expression of Spry4 within the digits has not been previously reported, and was not evident by in situ hybridization (Fig. S8A,B), we examined this further by confocal imaging of wholemount and cryosectioned forepaws of E12.5 Spry4H2B-Venus/+ embryos. The nuclear-localized Venus signal at the distal edge of forepaws recapitulated labeling of Spry4 mRNA by in situ hybridization (Fig. S8A–D). However, the fluorescence observed within the more proximal digit and interdigit regions was non-nuclear and exhibited autofluorescence in the red channel (Fig. S8D), hence represented non-specific signal which we attribute to the abundance of apoptotic cells within this region [83].
To determine whether the reporter could be utilized at these later developmental stages for high-resolution analysis of Venus within individual cells, we imaged cryosections of E10.5 and E13.5 embryos. We observed localized Spry4H2B-Venus expression within cells at known sites of FGF/ERK activity and Spry4 expression including the midbrain [79], olfactory mesenchyme [79], distal mesenchyme of the lungs [71, 79], kidneys [79], duodenum [84, 85], liver [86] and dorsal root ganglion [79, 87] (Fig. S9). Venus positive cells were often observed within the mesenchyme immediately underlying an epithelial layer (Fig. S9). At E13.5, Venus was present within mesenchymal cells between the primary lens fiber and neuroretinal layers of the optic cup (Fig. S9, yellow arrowheads), a population also marked by Spry2 expression [88]. Additionally, Venus was observed within Rathke’s pouch and in the mesenchyme of the genital tubercle (Fig. S9), tissues previously shown to express Spry1 and Spry2 but not significant levels of Spry4 [79, 89]. Therefore, the Spry4H2B-Venus mouse line is a useful tool to identify individual cells or cell types within tissues that express Spry4, and may be used to identify novel sites of low-level expression that have not previously been characterized.
3.6. Spry4H2B-Venus exhibits highly localized expression in adult organs
FGF signaling is important not only during development but also for maintaining homeostasis throughout the lifetime of the adult. Disruption of the FGF pathway results in defects in almost all organ systems analyzed, including lung, heart, stomach, pancreas, kidney, muscle, bone, brain and intestine [2]. Spry2/4 double mutants also exhibit severe brain, craniofacial and lung defects [43]. To characterize the expression of the Spry4H2B-Venus reporter in adult mice, organs were imaged whole-mount followed by cryosectioning to generate higher resolution data. In keeping with the widespread role of FGF signaling, and signaling stimulated by other growth factors such as EGF, PDGF and WNT that can also regulate Sprouty gene expression [40–43], the Venus reporter was observed in many organs. Previous reports demonstrated Spry4 expression in whole tissue lysates of lungs, heart and kidney [39]. Here, we confirmed this expression as well as expression in the uterus, oviducts, liver, stomach and spleen (Fig. 10).
Figure 10. Expression of Spry4H2B-Venus/+ in adult organs.
Adult organs were dissected from approximately 3 month old mice. Organs were imaged in wholemount (upper panels of each organ) followed by cryosectioning (lower panels). Cryosections were imaged by confocal microscopy and single optical sections are shown. Nuclei are stained with Hoechst. ems, endometrial stroma; le, luminal epithelium; sm, smooth muscle; cm, corpus mucosa; mes, mesothelium; wp, white pulp; rp, red pulp. Scale bars, 100 μm
FGF/ERK signaling plays a role in kidney morphogenesis during development [90], and FGFs and FGFRs are also expressed in the adult kidney [91]. We observed localized Venus within the kidney glomeruli (Fig. 10) where FGF receptors and ligands are expressed in rat [91, 92] but, to our knowledge, have not been characterized in mouse. Additionally, Venus was present in theca cells of the ovary (Fig. 10), a cell type exhibiting ERK activity in bovine samples [93]. FGF signaling also plays a key role in mediating crosstalk between the uterus epithelium and underlying mesenchyme for embryo implantation [94–96]. We observed a highly localized Venus signal in the mesenchyme immediately underlying the uterine epithelium (Fig. 10) indicating that this reporter will be a useful tool to study paracrine interactions between different cell types in the uterus both under steady state conditions as well as during pregnancy.
In addition to playing a critical role during early brain development, FGF signaling is important within the adult brain [97, 98]. To determine whether localized expression of the Spry4H2B-Venus reporter could be observed within the adult mouse brain, we isolated and cryosectioned brains of 2 month old mice and imaged sections by confocal microscopy. Venus-positive cells were present throughout the cerebral cortex as well as in cells within the Purkinje cell layer (PCL) of the cerebellum, likely marking Bergmann glia [99], in cells underlying the epithelium of the choroid plexus, also a known site of expression of Spry1/2 and members of the Sprouty-related Spred gene family [100, 101], and in cells within both the glomerular and periglomerular layers of the olfactory bulb (Fig. S10).
Therefore, the Spry4H2B-Venus reporter may be used to generate high-resolution spatial information on the localization of Spry4 and give new insights into the roles of FGF/ERK activity and regulation in the adult mouse.
4. Discussion
A reporter of FGF signaling activity has long been sought to facilitate the quantitative real-time analysis of pathway activity at single-cell resolution. Here we report the generation of a Spry4H2B-Venus reporter allele in ESCs and mice that recapitulates known patterns of FGF/ERK signaling during development. The emerging roles of Sprouty genes in cancer and ESC biology [102–104] also make this a valuable tool to study the role of signal regulation by Spry4 in these processes.
In vitro, the Spry4H2B-Venus signal was elevated in response to FGF ligands and was abrogated by small molecule inhibitors of MEK or in the absence of FGF stimulation (Fig. 2), validating the reporter as a read out FGF/ERK signaling activity. The rapid maturation time of the Venus fluorescent protein [105] renders this a useful tool to study the onset of signaling. The half-life of Venus in ESCs was ~9 hours (Fig. S1B,C) hence, in its current form, the reporter might be utilized as a short-term trace of signaling history. To visualize more rapid signaling dynamics, additional modifications may be introduced into the Venus construct, for example a destabilizing PEST sequence [106, 107], or alternatively sensors directly reporting ERK activity could be utilized [108].
The Spry4H2B-Venus reporter exhibited heterogeneity of expression within ESC cultures (Fig. 1D,E). This indicated that individual cells may exhibit distinct temporal responses, have different sensitivities to FGF ligands or can activate distinct downstream targets. Alternatively, this could represent a spatially heterogeneous distribution of FGF ligands across the culture. On a population level, Venus expression increased and expression of the pluripotency-associated marker NANOG decreased in response to increasing doses of FGF, consistent with FGF/ERK inducing ESC differentiation [109]. However, we did not observe a negative correlation between Venus and NANOG levels within individual cells (Fig. S1E–G), hence the majority of the Spry4H2B-Venus signal observed within ESCs was associated with FGF/ERK activity below the threshold needed to trigger differentiation.
FGF signaling plays a multitude of roles in development and disease. Although genetic mutations and inhibitor experiments have yielded valuable information regarding the functions of FGF signaling, many open questions remain such as when this signaling pathway is first active in development; which cells within a tissue or organism respond to FGF; if the response is synchronous across a population of cells; how signaling changes over time. A relatively simple system to address many of these questions is the pre-implantation mouse embryo. While there is evidence for a role of FGF signaling in the TE at pre-implantation stages [20, 23, 110], its function is unclear [111] and we did not observe Spry4H2B-Venus fluorescence within the TE. Conversely, the role of FGF signaling in the ICM of the blastocyst, to regulate PrE specification and Epi maturation, has been well characterized [12–17, 64, 65, 112]. Here we observed heterogeneous Spry4H2B-Venus expression within the ICM (Fig. 3A–D), though there was no association of Venus fluorescence with a particular cell state (GATA6 and NANOG double positive – DP cells; GATA6+ PrE; NANOG+ Epi; GATA6 and NANOG double negative – DN cells). This suggests that all cells transduce the FGF4 signal, but that individual ICM cells of all lineages experience varying levels of FGF/ERK activity, and hence the Spry4H2B-Venus reporter represents a useful tool to probe the effect of signaling dynamics on early lineage specification.
We additionally observed previously unreported sites of Spry4 expression, such as within cells of the VE of the early post-implantation embryo. Throughout early post-implantation development (E5.5–7.5) we observed Spry4H2B-Venus expression within the Epi and VE of the embryo (Fig. 6–8). Although in situ hybridization labeling of Spry4 mRNA does not display a clear signal in either of these cell types (Fig. S7A,B) [39], the Epi and VE Venus signal was responsive to inhibition of signaling through FGFR and/or ERK and therefore does not represent reporter perdurance. FGF pathway components and targets are expressed within the Epi and VE, including Fgf4 [113–115], Fgf5 [116, 117], Fgf8 [118], Ets2 [119], Eras [120] and Etv5 [121]. Furthermore, although FGF/ERK activity has not been described within the VE, abnormal development of the VE and its derivatives has been noted in mouse embryos and in vitro cell lines with mutations in the FGF pathway receptor Fgfr1 [29, 34, 122]. Hence, FGF/ERK signaling may play a role in the VE that has yet to be fully elucidated. Spry4 expression within the VE may also be stimulated by non-FGF ligands, such as epidermal growth factor (EGF), platelet-derived growth factor (PDGF) or WNT, which can also regulate the expression of Sprouty family members [40–43, 123]. Nevertheless, as the Spry4H2B-Venus construct contains a NEO selection cassette, which could affect expression [124, 125], further transcriptional validation of novel Spry4 domains identified by this reporter may be required or excision of the selection cassette using a Dre recombinase mouse line should be performed [50].
FGF/ERK signaling has been implicated in epithelial-mesenchymal interactions in a wide range of tissues [126–131]. During embryonic organogenesis and in adult organs, we often observed Spry4H2B-Venus expression localized to mesenchyme immediately underlying an adjacent epithelial layer, for example in the limb bud, cephalic, olfactory, genital tubercle and uterine mesenchyme (Fig. 10, Fig. S9). This is consistent with the hypothesis that the epithelial or mesenchymal – restricted expression of distinct Sprouty family members within adjacent cells can mediate the cell type specific regulation of RTK signaling [127]. Hence, the reporter may be useful to reveal novel interactions between different adjacent cell types in vivo. FGF/ERK signaling also plays a central role in limb bud formation and FGF ligands and receptors are expressed both within the limb bud epithelium and mesenchyme [2]. At E12.5 we observed nuclear-localized Venus at high levels within the mesenchyme and at low levels within the epithelium, including the AER (Fig. 9E). The difference in Venus levels between the epithelium and mesenchyme of the limb bud may indicate a differential regulation of FGF/ERK signaling within these adjacent cell types, as described in other contexts [127].
In summary, this mouse line reports Spry4 expression throughout embryonic development and in adult organs, potentially uncovering new aspects of FGF signaling regulation. Furthermore, in combination with an Fgf reporter, this could be used to map autocrine and paracrine FGF signaling activities in tissues and organisms.
Supplementary Material
Figure S1. Spry4H2B-Venus is heterogeneously expressed in mouse ESC cultures. A. Estimation of Venus half-life in ESCs. Stills from time-lapse imaging of Venus fluorescence in Spry4H2B-Venus reporter cells cultured in serum/LIF conditions (upper row) or serum/LIF supplemented with 1 μM PD0325901 at the start of recording (lower row). White circle indicates a nuclear region of interest (ROI) tracked for determining protein half-life. B. Quantification of Venus fluorescence intensity in nuclear ROI for representative cells grown in serum/LIF conditions (left) or serum/LIF supplemented with 1 μM PD0325901 at the beginning of the recording (right). Black solid line in right panel indicates exponential fit to the data. C. Mean +/− SD of Venus half-life values from exponential fits as in (B). N = 11 cells randomly chosen from 3 distinct colonies. D. Sequence of Fgf4 wild-type locus and the locus mutated in Spry4H2B-Venus/+ ESCs using the CRISPR/Cas9 system and a single stranded oligonucleotide repair template. Bold font indicates mismatches between repair template and wild-type sequence. E. Venus expression and immunostaining for NANOG in Spry4H2B-Venus reporter cells grown in serum/LIF conditions supplemented with indicated concentrations of inhibitors for 24 hours. F. Quantification of fluorescence in single cells cultured as in (E). When plotting all treatment conditions together, the average fluorescence levels (solid circles) of NANOG and Venus are inversely related. G. Plotting NANOG and inverse Venus fluorescence levels for individual conditions separately reveals poor correlation within each treatment group.
Figure S9. Cryosections of E10.5 and E13.5 Spry4H2B-Venus/+ embryos. Cryosections were imaged by confocal microscopy. Nuclei are stained with Hoechst. Yellow arrowheads in the optic cup cryosection indicate Spry4H2B-Venus‒expressing cells between the neuroretina and primary lens fiber layers. oe, olfactory epithelium; om, olfactory mesenchyme; nr, neuroretina; 1° LF, primary lens fibers; nt, neural tube; drg, dorsal root ganglion. Scale bars, 100 μm.
Figure S10. Expression of Spry4H2B-Venus/+ in the adult brain. Panels show confocal images of cryosectioned brain from an approximately 2 month old mouse. Nuclei are stained with Hoechst. IGL, inner granule layer; PCL, Purkinje cell layer. Scale bars, 100 μm
Figure S2. Spry4H2B-Venus/+ embryos show comparable growth and lineage composition to wild-type littermates. A. Boxplot showing Venus levels in ICM (purple) and TE (green) cells, at sequential developmental stages, as detected either directly (top) or by immunostaining using an anti-GFP antibody and an AlexaFluor® 488 (AF®488) coupled secondary antibody (bottom). Gray dots represent values for both ICM and TE cells of wild-type littermates for each stage (autofluorescence (top) or non-specific primary antibody binding (bottom)). Black dots correspond to values of wild-type embryos stained only with AF®488. B. Boxplots comparing the size of individual littermates of each genotype across developmental stages. The total cell count of each embryo was divided by the average cell count of all Spry4H2B-Venus/+ in its litter. Spry4H2B-Venus/+ were used because they were present in all litters. A ratio around 1 indicates no difference in size between different genotypes in each litter. Each dot represents the ratio for a single embryo. Color-coding is indicated. C. Scatterplot showing distribution of ICM cells of embryos of all three genotypes based on their GATA6 and NANOG levels (in log scale, after correction for imaging artifacts, see Materials and methods). Clusters formed were used to assign lineage identity (Epi, PrE, DP or DN, see Materials and methods). Labels indicate cluster centers. Color-coding indicates total cell count of the corresponding embryo (developmental stage). D. Scatterplots showing distribution of individual ICM cells, based on GATA6 and NANOG levels (as in C), for each developmental stage and genotype, color-coded for their lineage identity as determined in (C). Cluster progression is comparable between all three genotypes. Cells progress from a single cluster (left-most panel), mostly dominated by DP progenitors to two distinct clusters (right-most panel), epiblast (Epi - NANOG+) and primitive endoderm (PrE - GATA6+) cells, as described [52, 55, 56]. E. Lineage composition of wild-type, Spry4H2B-Venus/+ and Spry4H2B-Venus/H2B-Venus embryos shown as % of the total, for each of the stages analyzed. F. Venus levels in each ICM cell type of Spry4H2B-Venus/H2B-Venus embryos, as detected directly (top) or by immunostaining using anti-GFP and an AlexaFluor® 488 coupled secondary antibody (bottom), at sequential developmental stages. Boxes are color-coded as indicated. Gray dots indicate levels in ICM cells of wild-type littermates for each developmental stage, representing the autofluorescence (top) or non-specific primary antibody binding (bottom). TE, trophectoderm; PrE, primitive endoderm (GATA6+); DP, double positive (NANOG+, GATA6+); Epi, epiblast (NANOG+); DN, double negative (NANOG−, GATA6−). In all boxplots, top and bottom edges of boxes represent third and first quartiles, respectively (interquartile range, IQR). Middle lines mark the median. Whiskers extend to 1.5 * IQR. Open circles represent outliers (values beyond 1.5 * IQR).
Figure S3. H2B-Venus targeting of Spry4 locus leads to a slight reduction in FGF/ERK activity. A. Images of forelimb mouse paws from wild-type and Spry4H2B-Venus/H2B-Venus adult mice. A fraction of Spry4H2B-Venus/H2B-Venus exhibited polysyndactyly, a phenotype observed in Spry4−/− mice [43], characterized by fusion and duplication of digits on the forelimbs. Digits are numbered I-V in an anterior to posterior direction. Affected digits are highlighted with asterisks. This phenotype was partially penetrant with 7/21 Spry4H2B-Venus/H2B-Venus mice (males and females) exhibiting this phenotype in one or both forelimb paws. This suggested that the reporter results in a reduction of Spry4 expression. B. Histograms showing relative mRNA expression levels of endogenous Spry4 assessed by qRT-PCR in blastocysts of the following genotypes: wild-type (wt, Spry4+/+), heterozygous (het, Spry4H2B-Venus/+), homozygous (homo, Spry4H2B-Venus/H2B-Venus). Data were normalized to mean expression values of Actb and Gapdh. Histogram height represents mean value of biological replicates (n = 3 for each group). Error bars represent SEM. C. Immunoblotting for phosphorylated (ppERK), total ERK (ERK) and a TUBULIN loading control in the parental and Spry4H2B-Venus/+ reporter ESC line. D. Quantification of results from (A) showing the mean ° 95% confidence interval of 3 independent replicates. Data shows ppERK normalized to total ERK signal and normalized to 1. The reporter cell line shows slightly decreased ppERK levels, * p = ≤ 0.05.
Figure S4. Spry4H2B-Venus reporter expression is altered in the developing ICM by genetic modulation of the FGF pathway components. A-C. Boxplots showing relative Venus levels in indicated embryo lineages of wild-type, Fgf4−/−, Fgfr1−/− and Fgfr2−/− embryos (x-axis) at sequential stages of pre-implantation development. Scattered points represent measurements in individual nuclei, color-coded for identity as indicated. (A, 32–64 cell stage; B, 64–90 cell stage; C, 90–120 cell stage). N indicates number of embryos analyzed for each group. D. Scatterplots showing distribution of individual ICM cells, based on GATA6 and NANOG levels, for each developmental stage and genotype, color-coded for their lineage identity as determined in Fig. S2C. E. Relative ICM composition of embryos shown in Fig. 4A–C represented as percentage of the ICM. In all boxplots, top and bottom edges of boxes represent third and first quartiles, respectively (interquartile range, IQR). Middle lines mark the median. Whiskers extend to 1.5 * IQR.
Figure S5. Growth and lineage composition of embryos grown ex vivo with FGF/RTK pathway inhibitors. A. Total cell count of embryos grown from the 8-cell stage, as indicated in Fig. 5A, in the conditions indicated. Genotype is color-coded as indicated. B. Relative levels of GATA6 and NANOG in ICM cells of wild-type (wt, Spry4+/+) and heterozygous (Spry4H2B-Venus/+) embryos for each treatment shown in A. Cell identity is color-coded as indicated. For details on cell identity assignment, see Materials and methods. C. Relative ICM composition of embryos shown in A,B represented as percentage of the ICM. D., Total cell count of Spry4H2B-Venus/+ embryos grown from the mid-blastocyst stage as indicated in Fig. 5A, in the conditions indicated. E. Relative levels of GATA6 and NANOG in ICM cells of embryos shown in D. Cell identity is color-coded as indicated. F. Relative ICM composition of embryos shown in D,E represented as percentage of the ICM. FGFRi or MEKi treatment from the mid-blastocyst stage do not result in total conversion to an Epi state, as described in [56]. G. Venus levels (as detected by immunostaining with an anti-GFP antibody and an AlexaFluor® 488 coupled secondary antibody) in each of the ICM lineages in embryos shown in D-F and Fig. 5D–G. Cell identity is color-coded as indicated. Scattered dots represent TE cells that do not express Spry4H2B-Venus and thus indicate the baseline Venus signal, acting as an internal negative control. In all boxplots, top and bottom edges of boxes represent third and first quartiles, respectively (interquartile range, IQR). Middle lines mark the median. Whiskers extend to 1.5 * IQR. Open circles represent outliers (values beyond 1.5 * IQR).
Figure S6. Expression of Spry4H2B-Venus at E6.75 and E7.0. A,B. Confocal images of a representative E6.75-E7.0 Spry4H2B-Venus/+ embryos after wholemount immunofluorescence staining for SOX17, FOXA2 and GATA6. Upper to lower panels show: maximum intensity projections (MIP), optical sagittal sections and optical transverse sections. Nuclei are stained with Hoechst. Dashed white line indicates plane of transverse optical section. Dashed red line approximately demarcates the primitive streak. Epi, epiblast; VE, visceral endoderm; PS, primitive streak; Pr, proximal; Ds, distal; A, anterior, P, posterior. Dashed line indicates plane of transverse optical section. Scale bars, 50 μm.
Figure S7. In situ hybridization labeling of Spry4 mRNA during embryonic development. A. Wholemount in situ hybridization labeling of Spry4 mRNA in embryonic day (E) 7.5–10.5 embryos. Dashed lines in E8.0 embryo mark the approximate plane of transverse sections in panel B. B-D. Transverse cryosections of comparable wholemount embryos to those depicted in panel A. Brackets demarcate the primitive streak (PS). Is, isthmus; pa, pharyngeal arch; np, nasal placode; tb, tailbud; fnp, frontonasal process; fl, forelimb; hl, hindlimb; AER, apical ectodermal ridge; A, anterior; P, posterior; Pr, proximal; Ds, distal; D, dorsal; V, ventral; L, left; R, right.
Figure S8. Venus signal in E12.5 Spry4H2B-Venus/+ paws replicates the domain of Spry4 expression. A. Whole E12.5 forepaw labeled by wholemount in situ hybridization for Spry4 mRNA. Dashed line indicates approximate plane of transverse section shown in B. B. Sagittal (left panel) and transverse (right panel) cryosections of E12.5 forepaws labeled by wholemount in situ hybridization. C. Confocal imaging of whole E12.5 Spry4H2B-Venus/+ forepaw. Dashed line indicates approximate plane of transverse section shown in D. D. Confocal images of cryosectioned E12.5 Spry4H2B-Venus/+ forepaws. The sample was also imaged using red fluorescence to indicate regions of autofluorescence. Asterisks mark regions exhibiting both red and green fluorescence, hence corresponding to tissue autofluorescence. Middle panel shows high magnification image of region marked by box in left panel. Right panel indicates a transverse cryosection through a more proximal region of the forepaw. Pr, proximal; Ds, distal. Scale bars, 100 μm.
Highlights.
Spry4 transcription is a rapid and robust response to FGF/ERK signaling in ESCs.
Nuclear-localized reporter for Spry4 (Spry4H2B-Venus) transcription, an FGF/ERK target
Venus localized to known sites of FGF/ERK activity in vivo.
Venus exhibited highly localized cell type-specific expression in adult organs.
Reporter expression responds to genetic and chemical perturbation of FGF/ERK.
Acknowledgements
We thank the Wellcome Trust-Medical Research Council Centre for Stem Cell Research Transgenics Facility, Cambridge, UK for generating germline transmitting chimeras; members of the Hadjantonakis lab for critical discussions of the reporter and comments on the manuscript; Lenka Filipkova, Sung Ly, Michelle Protzek and Daniel Stephen for technical assistance and Sho Fujisawa of from MSKCC’s Molecular Cytology Core Facility for assistance with quantification of time-lapse data. This work was supported by grants from the NIH (R01HD094868, R01DK084391 and P30CA00874 - AKH), the Max-Planck Society (CS and DR). The work of AMA, JN and CS is supported by a BBSRC project grant (BB/M023370/1). SMM is supported by a Wellcome Trust Sir Henry Wellcome postdoctoral fellowship. NS was supported by a Starr Foundation Tri-Institutional Stem Cell Initiative postdoctoral fellowship for part of this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests
The authors have no competing interests to declare.
References
- 1.Brewer JR, Mazot P, and Soriano P, Genetic insights into the mechanisms of Fgf signaling. Genes Dev, 2016. 30(7): p. 751–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ornitz DM and Itoh N, The Fibroblast Growth Factor signaling pathway. Wiley Interdiscip Rev Dev Biol, 2015. 4(3): p. 215–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Itoh N and Ornitz DM, Fibroblast growth factors: from molecular evolution to roles in development, metabolism and disease. J Biochem, 2011. 149(2): p. 121–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oulion S, Bertrand S, and Escriva H, Evolution of the FGF Gene Family. Int J Evol Biol, 2012. 2012: p. 298147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dorey K and Amaya E, FGF signalling: diverse roles during early vertebrate embryogenesis. Development, 2010. 137(22): p. 3731–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nies VJ, et al. , Fibroblast Growth Factor Signaling in Metabolic Regulation. Front Endocrinol (Lausanne), 2015. 6: p. 193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kelleher FC, et al. , Fibroblast growth factor receptors, developmental corruption and malignant disease. Carcinogenesis, 2013. 34(10): p. 2198–205. [DOI] [PubMed] [Google Scholar]
- 8.Turner N and Grose R, Fibroblast growth factor signalling: from development to cancer. Nat Rev Cancer, 2010. 10(2): p. 116–29. [DOI] [PubMed] [Google Scholar]
- 9.Su N, Jin M, and Chen L, Role of FGF/FGFR signaling in skeletal development and homeostasis: learning from mouse models. Bone Res, 2014. 2: p. 14003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsai SM, Liu DW, and Wang WP, Fibroblast growth factor (Fgf) signaling pathway regulates liver homeostasis in zebrafish. Transgenic Res, 2013. 22(2): p. 301–14. [DOI] [PubMed] [Google Scholar]
- 11.Turner CA, et al. , Dysregulated fibroblast growth factor (FGF) signaling in neurological and psychiatric disorders. Seminars in Cell & Developmental Biology, 2016. 53: p. 136–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chazaud C, et al. , Early lineage segregation between epiblast and primitive endoderm in mouse blastocysts through the Grb2-MAPK pathway. Dev Cell, 2006. 10(5): p. 615–24. [DOI] [PubMed] [Google Scholar]
- 13.Frankenberg S, et al. , Primitive endoderm differentiates via a three-step mechanism involving Nanog and RTK signaling. Dev Cell, 2011. 21(6): p. 1005–13. [DOI] [PubMed] [Google Scholar]
- 14.Kang M, et al. , FGF4 is required for lineage restriction and salt-and-pepper distribution of primitive endoderm factors but not their initial expression in the mouse. Development, 2013. 140(2): p. 267–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nichols J, et al. , Suppression of Erk signalling promotes ground state pluripotency in the mouse embryo. Development, 2009. 136(19): p. 3215–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamanaka Y, Lanner F, and Rossant J, FGF signal-dependent segregation of primitive endoderm and epiblast in the mouse blastocyst. Development, 2010. 137(5): p. 715–24. [DOI] [PubMed] [Google Scholar]
- 17.Krawchuk D, et al. , FGF4 is a limiting factor controlling the proportions of primitive endoderm and epiblast in the ICM of the mouse blastocyst. Dev Biol, 2013. 384(1): p. 65–71. [DOI] [PubMed] [Google Scholar]
- 18.Kang MJ, Garg V, and Hadjantonakis AK, Lineage Establishment and Progression within the Inner Cell Mass of the Mouse Blastocyst Requires FGFR1 and FGFR2. Developmental Cell, 2017. 41(5): p. 496–+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Molotkov A, et al. , Distinct Requirements for FGFR1 and FGFR2 in Primitive Endoderm Development and Exit from Pluripotency. Dev Cell, 2017. 41(5): p. 511–526 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu CW, et al. , Ras-MAPK signaling promotes trophectoderm formation from embryonic stem cells and mouse embryos. Nat Genet, 2008. 40(7): p. 921–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Orr-Urtreger A, et al. , Developmental localization of the splicing alternatives of fibroblast growth factor receptor-2 (FGFR2). Dev Biol, 1993. 158(2): p. 475–86. [DOI] [PubMed] [Google Scholar]
- 22.Rossant J and Cross JC, Placental development: lessons from mouse mutants. Nat Rev Genet, 2001. 2(7): p. 538–48. [DOI] [PubMed] [Google Scholar]
- 23.Nichols J, et al. , Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell, 1998. 95(3): p. 379–91. [DOI] [PubMed] [Google Scholar]
- 24.Brons IG, et al. , Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature, 2007. 448(7150): p. 191–5. [DOI] [PubMed] [Google Scholar]
- 25.Tesar PJ, et al. , New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature, 2007. 448(7150): p. 196–9. [DOI] [PubMed] [Google Scholar]
- 26.Tanaka S, et al. , Promotion of trophoblast stem cell proliferation by FGF4. Science, 1998. 282(5396): p. 2072–5. [DOI] [PubMed] [Google Scholar]
- 27.Bertrand V, et al. , Neural tissue in ascidian embryos is induced by FGF9/16/20, acting via a combination of maternal GATA and Ets transcription factors. Cell, 2003. 115(5): p. 615–27. [DOI] [PubMed] [Google Scholar]
- 28.Ciruna B and Rossant J, FGF signaling regulates mesoderm cell fate specification and morphogenetic movement at the primitive streak. Dev Cell, 2001. 1(1): p. 37–49. [DOI] [PubMed] [Google Scholar]
- 29.Deng CX, et al. , Murine FGFR-1 is required for early postimplantation growth and axial organization. Genes Dev, 1994. 8(24): p. 3045–57. [DOI] [PubMed] [Google Scholar]
- 30.Isaacs HV, Pownall ME, and Slack JM, eFGF regulates Xbra expression during Xenopus gastrulation. EMBO J, 1994. 13(19): p. 4469–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Popovici C, et al. , An evolutionary history of the FGF superfamily. Bioessays, 2005. 27(8): p. 849–57. [DOI] [PubMed] [Google Scholar]
- 32.Stathopoulos A, et al. , pyramus and thisbe: FGF genes that pattern the mesoderm of Drosophila embryos. Genes Dev, 2004. 18(6): p. 687–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun X, et al. , Targeted disruption of Fgf8 causes failure of cell migration in the gastrulating mouse embryo. Genes Dev, 1999. 13(14): p. 1834–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamaguchi TP, et al. , fgfr-1 is required for embryonic growth and mesodermal patterning during mouse gastrulation. Genes Dev, 1994. 8(24): p. 3032–44. [DOI] [PubMed] [Google Scholar]
- 35.Matus DQ, Thomsen GH, and Martindale MQ, FGF signaling in gastrulation and neural development in Nematostella vectensis, an anthozoan cnidarian. Dev Genes Evol, 2007. 217(2): p. 137–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ciruna BG, et al. , Chimeric analysis of fibroblast growth factor receptor-1 (Fgfr1) function: a role for FGFR1 in morphogenetic movement through the primitive streak. Development, 1997. 124(14): p. 2829–41. [DOI] [PubMed] [Google Scholar]
- 37.Corson LB, et al. , Spatial and temporal patterns of ERK signaling during mouse embryogenesis. Development, 2003. 130(19): p. 4527–37. [DOI] [PubMed] [Google Scholar]
- 38.Nowotschin S, et al. , A bright single-cell resolution live imaging reporter of Notch signaling in the mouse. BMC Dev Biol, 2013. 13: p. 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Minowada G, et al. , Vertebrate Sprouty genes are induced by FGF signaling and can cause chondrodysplasia when overexpressed. Development, 1999. 126(20): p. 4465–75. [DOI] [PubMed] [Google Scholar]
- 40.Gross I, et al. , Mammalian Sprouty proteins inhibit cell growth and differentiation by preventing Ras activation. Journal of Biological Chemistry, 2001. 276(49): p. 46460–46468. [DOI] [PubMed] [Google Scholar]
- 41.Katoh Y and Katoh M, FGF signaling inhibitor, SPRY4, is evolutionarily conserved target of WNT signaling pathway in progenitor cells. Int J Mol Med, 2006. 17(3): p. 529–32. [PubMed] [Google Scholar]
- 42.Reich A, Sapir A, and Shilo BZ, Sprouty is a general inhibitor of receptor tyrosine kinase signaling. Development, 1999. 126(18): p. 4139–4147. [DOI] [PubMed] [Google Scholar]
- 43.Taniguchi K, et al. , Sprouty2 and Sprouty4 are essential for embryonic morphogenesis and regulation of FGF signaling. Biochem Biophys Res Commun, 2007. 352(4): p. 896–902. [DOI] [PubMed] [Google Scholar]
- 44.Turner DA, et al. , An interplay between extracellular signalling and the dynamics of the exit from pluripotency drives cell fate decisions in mouse ES cells. Biol Open, 2014. 3(7): p. 614–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Skarnes WC, et al. , A conditional knockout resource for the genome-wide study of mouse gene function. Nature, 2011. 474(7351): p. 337–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Freyer L, et al. , A loss-of-function and H2B-Venus transcriptional reporter allele for Gata6 in mice. BMC Dev Biol, 2015. 15: p. 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schroter C, et al. , FGF/MAPK signaling sets the switching threshold of a bistable circuit controlling cell fate decisions in embryonic stem cells. Development, 2015. 142(24): p. 4205–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Beard C, et al. , Efficient method to generate single-copy transgenic mice by site-specific integration in embryonic stem cells. Genesis, 2006. 44(1): p. 23–8. [DOI] [PubMed] [Google Scholar]
- 49.Ying QL, et al. , The ground state of embryonic stem cell self-renewal. Nature, 2008. 453(7194): p. 519–U5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Anastassiadis K, et al. , Dre recombinase, like Cre, is a highly efficient site-specific recombinase in E-coli, mammalian cells and mice. Disease Models & Mechanisms, 2009. 2(9–10): p. 508–515. [DOI] [PubMed] [Google Scholar]
- 51.Behringer R, Gertsenstein M, Nagy K, Nagy A, Manipulating the Mouse Embryo: A Laboratory Manual. Vol. 4 2014, Cold Spring Harbor, New York, USA: Cold Spring Harbor Laboratory Press. [Google Scholar]
- 52.Saiz N, et al. , Quantitative Analysis of Protein Expression to Study Lineage Specification in Mouse Preimplantation Embryos. J Vis Exp, 2016(108): p. 53654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Conlon RA and Rossant J, Exogenous retinoic acid rapidly induces anterior ectopic expression of murine Hox-2 genes in vivo. Development, 1992. 116(2): p. 357–68. [DOI] [PubMed] [Google Scholar]
- 54.Artus J, et al. , Impaired mitotic progression and preimplantation lethality in mice lacking OMCG1, a new evolutionarily conserved nuclear protein. Mol Cell Biol, 2005. 25(14): p. 6289–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lou X, et al. , A rapid and efficient 2D/3D nuclear segmentation method for analysis of early mouse embryo and stem cell image data. Stem Cell Reports, 2014. 2(3): p. 382–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Saiz N, et al. , Asynchronous fate decisions by single cells collectively ensure consistent lineage composition in the mouse blastocyst. Nat Commun, 2016. 7: p. 13463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Morgani S.M.S., Nestor; Garg Vidur; Hadjantonakis Anna-Katerina, A Sprouty4 reporter to monitor FGF/ERK signaling activity in ESCs and mice. Figshare, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang SH, et al. , A genome-wide RNAi screen reveals MAP kinase phosphatases as key ERK pathway regulators during embryonic stem cell differentiation. PLoS Genet, 2012. 8(12): p. e1003112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Collins PJ, Hale CM, and Xu H, Edited course of biomedical research: leaping forward with CRISPR. Pharmacol Res, 2017. 125(Pt B): p. 258–265. [DOI] [PubMed] [Google Scholar]
- 60.Chambers I, et al. , Nanog safeguards pluripotency and mediates germline development. Nature, 2007. 450(7173): p. 1230–U8. [DOI] [PubMed] [Google Scholar]
- 61.Singh AM, et al. , A heterogeneous expression pattern for Nanog in embryonic stem cells. Stem Cells, 2007. 25(10): p. 2534–42. [DOI] [PubMed] [Google Scholar]
- 62.Santostefano KE, et al. , Fibroblast Growth Factor Receptor 2 Homodimerization Rapidly Reduces Transcription of the Pluripotency Gene Nanog without Dissociation of Activating Transcription Factors. Journal of Biological Chemistry, 2012. 287(36): p. 30507–30517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim SH, et al. , ERK1 phosphorylates Nanog to regulate protein stability and stem cell self-renewal. Stem Cell Research, 2014. 13(1): p. 1–11. [DOI] [PubMed] [Google Scholar]
- 64.Ohnishi Y, et al. , Cell-to-cell expression variability followed by signal reinforcement progressively segregates early mouse lineages. Nat Cell Biol, 2014. 16(1): p. 27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Guo G, et al. , Resolution of cell fate decisions revealed by single-cell gene expression analysis from zygote to blastocyst. Dev Cell, 2010. 18(4): p. 675–85. [DOI] [PubMed] [Google Scholar]
- 66.Arman E, et al. , Targeted disruption of fibroblast growth factor (FGF) receptor 2 suggests a role for FGF signaling in pregastrulation mammalian development. Proceedings of the National Academy of Sciences of the United States of America, 1998. 95(9): p. 5082–5087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lake D, Correa SA, and Muller J, Negative feedback regulation of the ERK1/2 MAPK pathway. Cell Mol Life Sci, 2016. 73(23): p. 4397–4413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Arnold SJ and Robertson EJ, Making a commitment: cell lineage allocation and axis patterning in the early mouse embryo. Nature Reviews Molecular Cell Biology, 2009. 10(2): p. 91–103. [DOI] [PubMed] [Google Scholar]
- 69.Goto H, et al. , FGF and canonical Wnt signaling cooperate to induce paraxial mesoderm from tailbud neuromesodermal progenitors through regulation of a two-step epithelial to mesenchymal transition. Development, 2017. 144(8): p. 1412–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Viotti M, Nowotschin S, and Hadjantonakis AK, SOX17 links gut endoderm morphogenesis and germ layer segregation. Nat Cell Biol, 2014. 16(12): p. 1146–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.de Maximy AA, et al. , Cloning and expression pattern of a mouse homologue of drosophila sprouty in the mouse embryo. Mech Dev, 1999. 81(1–2): p. 213–6. [DOI] [PubMed] [Google Scholar]
- 72.Hayashi S, et al. , Sprouty4, an FGF inhibitor, displays cyclic gene expression under the control of the notch segmentation clock in the mouse PSM. PLoS One, 2009. 4(5): p. e5603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Crossley PH, Martinez S, and Martin GR, Midbrain development induced by FGF8 in the chick embryo. Nature, 1996. 380(6569): p. 66–8. [DOI] [PubMed] [Google Scholar]
- 74.Chi CL, et al. , The isthmic organizer signal FGF8 is required for cell survival in the prospective midbrain and cerebellum. Development, 2003. 130(12): p. 2633–44. [DOI] [PubMed] [Google Scholar]
- 75.Basson MA, et al. , Specific regions within the embryonic midbrain and cerebellum require different levels of FGF signaling during development. Development, 2008. 135(5): p. 889–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kawakami Y, et al. , MKP3 mediates the cellular response to FGF8 signalling in the vertebrate limb. Nat Cell Biol, 2003. 5(6): p. 513–9. [DOI] [PubMed] [Google Scholar]
- 77.Martin GR, The roles of FGFs in the early development of vertebrate limbs. Genes Dev, 1998. 12(11): p. 1571–86. [DOI] [PubMed] [Google Scholar]
- 78.Probst S, et al. , SHH propagates distal limb bud development by enhancing CYP26B1-mediated retinoic acid clearance via AER-FGF signalling. Development, 2011. 138(10): p. 1913–1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang S, et al. , Expression of Sprouty genes 1, 2 and 4 during mouse organogenesis. Mech Dev, 2001. 109(2): p. 367–70. [DOI] [PubMed] [Google Scholar]
- 80.Gros J, et al. , WNT5A/JNK and FGF/MAPK Pathways Regulate the Cellular Events Shaping the Vertebrate Limb Bud. Current Biology, 2010. 20(22): p. 1993–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mao JH, et al. , Fgf-Dependent Etv4/5 Activity Is Required for Posterior Restriction of Sonic hedgehog and Promoting Outgrowth of the Vertebrate Limb. Developmental Cell, 2009. 16(4): p. 600–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li CY, et al. , Dusp6 (Mkp3) is a negative feedback regulator of FGF-stimulated ERK signaling during mouse development. Development, 2007. 134(1): p. 167–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fernandez-Teran MA, Hinchliffe JR, and Ros MA, Birth and death of cells in limb development: A mapping study. Developmental Dynamics, 2006. 235(9): p. 2521–2537. [DOI] [PubMed] [Google Scholar]
- 84.Kanard RC, et al. , Fibroblast growth factor-10 serves a regulatory role in duodenal development. Journal of Pediatric Surgery, 2005. 40(2): p. 313–316. [DOI] [PubMed] [Google Scholar]
- 85.Thys A, et al. , Hyperplasia of Interstitial Cells of Cajal in Sprouty Homolog 4 Deficient Mice. Plos One, 2015. 10(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Itoh N, Nakayama Y, and Konishi M, Roles of FGFs As Paracrine or Endocrine Signals in Liver Development, Health, and Disease. Front Cell Dev Biol, 2016. 4: p. 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Alsina FC, et al. , Sprouty4 Is an Endogenous Negative Modulator of TrkA Signaling and Neuronal Differentiation Induced by NGF. Plos One, 2012. 7(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Boros J, et al. , Sef and Sprouty expression in the developing ocular lens: implications for regulating lens cell proliferation and differentiation. Semin Cell Dev Biol, 2006. 17(6): p. 741–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ching ST, et al. , Coordinated activity of Spry1 and Spry2 is required for normal development of the external genitalia. Developmental Biology, 2014. 386(1): p. 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Walker KA, Sims-Lucas S, and Bates CM, Fibroblast growth factor receptor signaling in kidney and lower urinary tract development. Pediatr Nephrol, 2016. 31(6): p. 885–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cancilla B, Ford-Perriss MD, and Bertram JF, Expression and localization of fibroblast growth factors and fibroblast growth factor receptors in the developing rat kidney. Kidney Int, 1999. 56(6): p. 2025–39. [DOI] [PubMed] [Google Scholar]
- 92.Ford MD, et al. , Expression of fibroblast growth factors and their receptors in rat glomeruli. Kidney Int, 1997. 51(6): p. 1729–38. [DOI] [PubMed] [Google Scholar]
- 93.Tajima K, et al. , Luteinizing hormone-induced extracellular-signal regulated kinase activation differently modulates progesterone and androstenedione production in bovine theca cells. Endocrinology, 2005. 146(7): p. 2903–10. [DOI] [PubMed] [Google Scholar]
- 94.Li QX, et al. , The Antiproliferative Action of Progesterone in Uterine Epithelium Is Mediated by Hand2. Science, 2011. 331(6019): p. 912–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chung D, et al. , Estrogen mediated epithelial proliferation in the uterus is directed by stromal Fgf10 and Bmp8a. Molecular and Cellular Endocrinology, 2015. 400(C): p. 48–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hantak AM, Bagchi IC, and Bagchi MK, Role of uterine stromal-epithelial crosstalk in embryo implantation. International Journal of Developmental Biology, 2014. 58(2–4): p. 139–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Iwata T and Hevner RF, Fibroblast growth factor signaling in development of the cerebral cortex. Dev Growth Differ, 2009. 51(3): p. 299–323. [DOI] [PubMed] [Google Scholar]
- 98.Kang WF and Hebert JM, FGF Signaling Is Necessary for Neurogenesis in Young Mice and Sufficient to Reverse Its Decline in Old Mice. Journal of Neuroscience, 2015. 35(28): p. 10217–10223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yu T, et al. , Sprouty genes prevent excessive FGF signalling in multiple cell types throughout development of the cerebellum. Development, 2011. 138(14): p. 2957–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tuduce IL, Schuh K, and Bundschu K, Spred2 expression during mouse development. Dev Dyn, 2010. 239(11): p. 3072–85. [DOI] [PubMed] [Google Scholar]
- 101.Faedo A, Borello U, and Rubenstein JL, Repression of Fgf signaling by sprouty1–2 regulates cortical patterning in two distinct regions and times. J Neurosci, 2010. 30(11): p. 4015–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Felfly H and Klein OD, Sprouty genes regulate proliferation and survival of human embryonic stem cells. Sci Rep, 2013. 3: p. 2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lee JY, et al. , Novel Function of Sprouty4 as a Regulator of Stemness and Differentiation of Embryonic Stem Cells. Dev Reprod, 2016. 20(2): p. 171–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Masoumi-Moghaddam S, Amini A, and Morris DL, The developing story of Sprouty and cancer. Cancer Metastasis Rev, 2014. 33(2–3): p. 695–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nagai T, et al. , A variant of yellow fluorescent protein with fast and efficient maturation for cell-biological applications. Nat Biotechnol, 2002. 20(1): p. 87–90. [DOI] [PubMed] [Google Scholar]
- 106.Li X, et al. , Generation of destabilized green fluorescent protein as a transcription reporter. J Biol Chem, 1998. 273(52): p. 34970–5. [DOI] [PubMed] [Google Scholar]
- 107.Vilas-Boas F, et al. , A novel reporter of notch signalling indicates regulated and random Notch activation during vertebrate neurogenesis. BMC Biol, 2011. 9: p. 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Komatsu N, et al. , Development of an optimized backbone of FRET biosensors for kinases and GTPases. Mol Biol Cell, 2011. 22(23): p. 4647–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kunath T, et al. , FGF stimulation of the Erk1/2 signalling cascade triggers transition of pluripotent embryonic stem cells from self-renewal to lineage commitment. Development, 2007. 134(16): p. 2895–902. [DOI] [PubMed] [Google Scholar]
- 110.Tanaka S, Derivation and culture of mouse trophoblast stem cells in vitro. Methods Mol Biol, 2006. 329: p. 35–44. [DOI] [PubMed] [Google Scholar]
- 111.Sasaki H, Mechanisms of trophectoderm fate specification in preimplantation mouse development. Development Growth & Differentiation, 2010. 52(3): p. 263–273. [DOI] [PubMed] [Google Scholar]
- 112.Feldman B, et al. , Requirement of FGF-4 for postimplantation mouse development. Science, 1995. 267(5195): p. 246–9. [DOI] [PubMed] [Google Scholar]
- 113.Niswander L and Martin GR, Fgf-4 Expression during Gastrulation, Myogenesis, Limb and Tooth Development in the Mouse. Development, 1992. 114(3): p. 755–768. [DOI] [PubMed] [Google Scholar]
- 114.Sakaki-Yumoto M, et al. , The murine homolog of SALL4, a causative gene in Okihiro syndrome, is essential for embryonic stem cell proliferation, and cooperates with Sall1 in anorectal, heart, brain and kidney development. Development, 2006. 133(15): p. 3005–3013. [DOI] [PubMed] [Google Scholar]
- 115.Wright TJ, et al. , Expression of mouse fibroblast growth factor and fibroblast growth factor receptor genes during early inner ear development. Dev Dyn, 2003. 228(2): p. 267–72. [DOI] [PubMed] [Google Scholar]
- 116.Haub O and Goldfarb M, Expression of the Fibroblast Growth Factor-5 Gene in the Mouse Embryo. Development, 1991. 112(2): p. 397–&. [DOI] [PubMed] [Google Scholar]
- 117.Hebert JM, Boyle M, and Martin GR, Messenger-Rna Localization Studies Suggest That Murine Fgf-5 Plays a Role in Gastrulation. Development, 1991. 112(2): p. 407–415. [DOI] [PubMed] [Google Scholar]
- 118.Crossley PH and Martin GR, The mouse Fgf8 gene encodes a family of polypeptides and is expressed in regions that direct outgrowth and patterning in the developing embryo. Development, 1995. 121(2): p. 439–51. [DOI] [PubMed] [Google Scholar]
- 119.Donnison M, Broadhurst R, and Pfeffer PL, Elf5 and Ets2 maintain the mouse extraembryonic ectoderm in a dosage dependent synergistic manner. Developmental Biology, 2015. 397(1): p. 77–88. [DOI] [PubMed] [Google Scholar]
- 120.Zhao ZA, et al. , The roles of ERAS during cell lineage specification of mouse early embryonic development. Open Biol, 2015. 5(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chotteau-Lelievre A, et al. , Expression patterns of the Ets transcription factors from the PEA3 group during early stages of mouse development. Mech Dev, 2001. 108(1–2): p. 191–5. [DOI] [PubMed] [Google Scholar]
- 122.Esner M, et al. , Targeted disruption of fibroblast growth factor receptor-1 blocks maturation of visceral endoderm and cavitation in mouse embryoid bodies. Int J Dev Biol, 2002. 46(6): p. 817–25. [PubMed] [Google Scholar]
- 123.Winn RA, et al. , Restoration of Wnt-7a expression reverses non-small cell lung cancer cellular transformation through frizzled-9-mediated growth inhibition and promotion of cell differentiation. J Biol Chem, 2005. 280(20): p. 19625–34. [DOI] [PubMed] [Google Scholar]
- 124.Pan YC, et al. , Insertion of a knockout-first cassette in Ampd1 gene leads to neonatal death by disruption of neighboring genes expression. Scientific Reports, 2016. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Scarff KL, et al. , A retained selection cassette increases reporter gene expression without affecting tissue distribution in SPI3 knockout/GFP knock-in mice. Genesis, 2003. 36(3): p. 149–157. [DOI] [PubMed] [Google Scholar]
- 126.Huh SH, Warchol ME, and Ornitz DM, Cochlear progenitor number is controlled through mesenchymal FGF receptor signaling. Elife, 2015. 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Klein OD, et al. , Sprouty genes control diastema tooth development via bidirectional antagonism of epithelial-mesenchymal FGF signaling. Developmental Cell, 2006. 11(2): p. 181–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rice R, et al. , Disruption of Fgf10/Fgfr2b-coordinated epithelial-mesenchymal interactions causes cleft palate. Journal of Clinical Investigation, 2004. 113(12): p. 1692–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhang XQ, et al. , Reciprocal epithelial-mesenchymal FGF signaling is required for cecal development. Development, 2006. 133(1): p. 173–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.El Ramy R, et al. , Fibroblast growth factor (FGF) 2 and FGF9 mediate mesenchymal-epithelial interactions of peritubular and Sertoli cells in the rat testis. Journal of Endocrinology, 2005. 187(1): p. 135–147. [DOI] [PubMed] [Google Scholar]
- 131.Volckaert T and De Langhe SP, Wnt and FGF Mediated Epithelial-Mesenchymal Crosstalk During Lung Development. Developmental Dynamics, 2015. 244(3): p. 342–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Spry4H2B-Venus is heterogeneously expressed in mouse ESC cultures. A. Estimation of Venus half-life in ESCs. Stills from time-lapse imaging of Venus fluorescence in Spry4H2B-Venus reporter cells cultured in serum/LIF conditions (upper row) or serum/LIF supplemented with 1 μM PD0325901 at the start of recording (lower row). White circle indicates a nuclear region of interest (ROI) tracked for determining protein half-life. B. Quantification of Venus fluorescence intensity in nuclear ROI for representative cells grown in serum/LIF conditions (left) or serum/LIF supplemented with 1 μM PD0325901 at the beginning of the recording (right). Black solid line in right panel indicates exponential fit to the data. C. Mean +/− SD of Venus half-life values from exponential fits as in (B). N = 11 cells randomly chosen from 3 distinct colonies. D. Sequence of Fgf4 wild-type locus and the locus mutated in Spry4H2B-Venus/+ ESCs using the CRISPR/Cas9 system and a single stranded oligonucleotide repair template. Bold font indicates mismatches between repair template and wild-type sequence. E. Venus expression and immunostaining for NANOG in Spry4H2B-Venus reporter cells grown in serum/LIF conditions supplemented with indicated concentrations of inhibitors for 24 hours. F. Quantification of fluorescence in single cells cultured as in (E). When plotting all treatment conditions together, the average fluorescence levels (solid circles) of NANOG and Venus are inversely related. G. Plotting NANOG and inverse Venus fluorescence levels for individual conditions separately reveals poor correlation within each treatment group.
Figure S9. Cryosections of E10.5 and E13.5 Spry4H2B-Venus/+ embryos. Cryosections were imaged by confocal microscopy. Nuclei are stained with Hoechst. Yellow arrowheads in the optic cup cryosection indicate Spry4H2B-Venus‒expressing cells between the neuroretina and primary lens fiber layers. oe, olfactory epithelium; om, olfactory mesenchyme; nr, neuroretina; 1° LF, primary lens fibers; nt, neural tube; drg, dorsal root ganglion. Scale bars, 100 μm.
Figure S10. Expression of Spry4H2B-Venus/+ in the adult brain. Panels show confocal images of cryosectioned brain from an approximately 2 month old mouse. Nuclei are stained with Hoechst. IGL, inner granule layer; PCL, Purkinje cell layer. Scale bars, 100 μm
Figure S2. Spry4H2B-Venus/+ embryos show comparable growth and lineage composition to wild-type littermates. A. Boxplot showing Venus levels in ICM (purple) and TE (green) cells, at sequential developmental stages, as detected either directly (top) or by immunostaining using an anti-GFP antibody and an AlexaFluor® 488 (AF®488) coupled secondary antibody (bottom). Gray dots represent values for both ICM and TE cells of wild-type littermates for each stage (autofluorescence (top) or non-specific primary antibody binding (bottom)). Black dots correspond to values of wild-type embryos stained only with AF®488. B. Boxplots comparing the size of individual littermates of each genotype across developmental stages. The total cell count of each embryo was divided by the average cell count of all Spry4H2B-Venus/+ in its litter. Spry4H2B-Venus/+ were used because they were present in all litters. A ratio around 1 indicates no difference in size between different genotypes in each litter. Each dot represents the ratio for a single embryo. Color-coding is indicated. C. Scatterplot showing distribution of ICM cells of embryos of all three genotypes based on their GATA6 and NANOG levels (in log scale, after correction for imaging artifacts, see Materials and methods). Clusters formed were used to assign lineage identity (Epi, PrE, DP or DN, see Materials and methods). Labels indicate cluster centers. Color-coding indicates total cell count of the corresponding embryo (developmental stage). D. Scatterplots showing distribution of individual ICM cells, based on GATA6 and NANOG levels (as in C), for each developmental stage and genotype, color-coded for their lineage identity as determined in (C). Cluster progression is comparable between all three genotypes. Cells progress from a single cluster (left-most panel), mostly dominated by DP progenitors to two distinct clusters (right-most panel), epiblast (Epi - NANOG+) and primitive endoderm (PrE - GATA6+) cells, as described [52, 55, 56]. E. Lineage composition of wild-type, Spry4H2B-Venus/+ and Spry4H2B-Venus/H2B-Venus embryos shown as % of the total, for each of the stages analyzed. F. Venus levels in each ICM cell type of Spry4H2B-Venus/H2B-Venus embryos, as detected directly (top) or by immunostaining using anti-GFP and an AlexaFluor® 488 coupled secondary antibody (bottom), at sequential developmental stages. Boxes are color-coded as indicated. Gray dots indicate levels in ICM cells of wild-type littermates for each developmental stage, representing the autofluorescence (top) or non-specific primary antibody binding (bottom). TE, trophectoderm; PrE, primitive endoderm (GATA6+); DP, double positive (NANOG+, GATA6+); Epi, epiblast (NANOG+); DN, double negative (NANOG−, GATA6−). In all boxplots, top and bottom edges of boxes represent third and first quartiles, respectively (interquartile range, IQR). Middle lines mark the median. Whiskers extend to 1.5 * IQR. Open circles represent outliers (values beyond 1.5 * IQR).
Figure S3. H2B-Venus targeting of Spry4 locus leads to a slight reduction in FGF/ERK activity. A. Images of forelimb mouse paws from wild-type and Spry4H2B-Venus/H2B-Venus adult mice. A fraction of Spry4H2B-Venus/H2B-Venus exhibited polysyndactyly, a phenotype observed in Spry4−/− mice [43], characterized by fusion and duplication of digits on the forelimbs. Digits are numbered I-V in an anterior to posterior direction. Affected digits are highlighted with asterisks. This phenotype was partially penetrant with 7/21 Spry4H2B-Venus/H2B-Venus mice (males and females) exhibiting this phenotype in one or both forelimb paws. This suggested that the reporter results in a reduction of Spry4 expression. B. Histograms showing relative mRNA expression levels of endogenous Spry4 assessed by qRT-PCR in blastocysts of the following genotypes: wild-type (wt, Spry4+/+), heterozygous (het, Spry4H2B-Venus/+), homozygous (homo, Spry4H2B-Venus/H2B-Venus). Data were normalized to mean expression values of Actb and Gapdh. Histogram height represents mean value of biological replicates (n = 3 for each group). Error bars represent SEM. C. Immunoblotting for phosphorylated (ppERK), total ERK (ERK) and a TUBULIN loading control in the parental and Spry4H2B-Venus/+ reporter ESC line. D. Quantification of results from (A) showing the mean ° 95% confidence interval of 3 independent replicates. Data shows ppERK normalized to total ERK signal and normalized to 1. The reporter cell line shows slightly decreased ppERK levels, * p = ≤ 0.05.
Figure S4. Spry4H2B-Venus reporter expression is altered in the developing ICM by genetic modulation of the FGF pathway components. A-C. Boxplots showing relative Venus levels in indicated embryo lineages of wild-type, Fgf4−/−, Fgfr1−/− and Fgfr2−/− embryos (x-axis) at sequential stages of pre-implantation development. Scattered points represent measurements in individual nuclei, color-coded for identity as indicated. (A, 32–64 cell stage; B, 64–90 cell stage; C, 90–120 cell stage). N indicates number of embryos analyzed for each group. D. Scatterplots showing distribution of individual ICM cells, based on GATA6 and NANOG levels, for each developmental stage and genotype, color-coded for their lineage identity as determined in Fig. S2C. E. Relative ICM composition of embryos shown in Fig. 4A–C represented as percentage of the ICM. In all boxplots, top and bottom edges of boxes represent third and first quartiles, respectively (interquartile range, IQR). Middle lines mark the median. Whiskers extend to 1.5 * IQR.
Figure S5. Growth and lineage composition of embryos grown ex vivo with FGF/RTK pathway inhibitors. A. Total cell count of embryos grown from the 8-cell stage, as indicated in Fig. 5A, in the conditions indicated. Genotype is color-coded as indicated. B. Relative levels of GATA6 and NANOG in ICM cells of wild-type (wt, Spry4+/+) and heterozygous (Spry4H2B-Venus/+) embryos for each treatment shown in A. Cell identity is color-coded as indicated. For details on cell identity assignment, see Materials and methods. C. Relative ICM composition of embryos shown in A,B represented as percentage of the ICM. D., Total cell count of Spry4H2B-Venus/+ embryos grown from the mid-blastocyst stage as indicated in Fig. 5A, in the conditions indicated. E. Relative levels of GATA6 and NANOG in ICM cells of embryos shown in D. Cell identity is color-coded as indicated. F. Relative ICM composition of embryos shown in D,E represented as percentage of the ICM. FGFRi or MEKi treatment from the mid-blastocyst stage do not result in total conversion to an Epi state, as described in [56]. G. Venus levels (as detected by immunostaining with an anti-GFP antibody and an AlexaFluor® 488 coupled secondary antibody) in each of the ICM lineages in embryos shown in D-F and Fig. 5D–G. Cell identity is color-coded as indicated. Scattered dots represent TE cells that do not express Spry4H2B-Venus and thus indicate the baseline Venus signal, acting as an internal negative control. In all boxplots, top and bottom edges of boxes represent third and first quartiles, respectively (interquartile range, IQR). Middle lines mark the median. Whiskers extend to 1.5 * IQR. Open circles represent outliers (values beyond 1.5 * IQR).
Figure S6. Expression of Spry4H2B-Venus at E6.75 and E7.0. A,B. Confocal images of a representative E6.75-E7.0 Spry4H2B-Venus/+ embryos after wholemount immunofluorescence staining for SOX17, FOXA2 and GATA6. Upper to lower panels show: maximum intensity projections (MIP), optical sagittal sections and optical transverse sections. Nuclei are stained with Hoechst. Dashed white line indicates plane of transverse optical section. Dashed red line approximately demarcates the primitive streak. Epi, epiblast; VE, visceral endoderm; PS, primitive streak; Pr, proximal; Ds, distal; A, anterior, P, posterior. Dashed line indicates plane of transverse optical section. Scale bars, 50 μm.
Figure S7. In situ hybridization labeling of Spry4 mRNA during embryonic development. A. Wholemount in situ hybridization labeling of Spry4 mRNA in embryonic day (E) 7.5–10.5 embryos. Dashed lines in E8.0 embryo mark the approximate plane of transverse sections in panel B. B-D. Transverse cryosections of comparable wholemount embryos to those depicted in panel A. Brackets demarcate the primitive streak (PS). Is, isthmus; pa, pharyngeal arch; np, nasal placode; tb, tailbud; fnp, frontonasal process; fl, forelimb; hl, hindlimb; AER, apical ectodermal ridge; A, anterior; P, posterior; Pr, proximal; Ds, distal; D, dorsal; V, ventral; L, left; R, right.
Figure S8. Venus signal in E12.5 Spry4H2B-Venus/+ paws replicates the domain of Spry4 expression. A. Whole E12.5 forepaw labeled by wholemount in situ hybridization for Spry4 mRNA. Dashed line indicates approximate plane of transverse section shown in B. B. Sagittal (left panel) and transverse (right panel) cryosections of E12.5 forepaws labeled by wholemount in situ hybridization. C. Confocal imaging of whole E12.5 Spry4H2B-Venus/+ forepaw. Dashed line indicates approximate plane of transverse section shown in D. D. Confocal images of cryosectioned E12.5 Spry4H2B-Venus/+ forepaws. The sample was also imaged using red fluorescence to indicate regions of autofluorescence. Asterisks mark regions exhibiting both red and green fluorescence, hence corresponding to tissue autofluorescence. Middle panel shows high magnification image of region marked by box in left panel. Right panel indicates a transverse cryosection through a more proximal region of the forepaw. Pr, proximal; Ds, distal. Scale bars, 100 μm.