Abstract
Purpose: Worldwide, the burden of adverse health conditions is substantial among travestis and transgender women (trans women). Transcendendo, the first trans-specific cohort in a low- or middle-income country, is an open cohort established in August 2015 to longitudinally evaluate the health aspects of trans women aged ≥18 years in Rio de Janeiro, Brazil.
Methods: Study visits occur on an annual basis. Data on sociodemographics, behavioral, gender transition, affirmation procedures, hormone use, discrimination, violence, clinical and mental health, HIV prevention, and care (for those HIV-infected) are collected. Physical examination, anthropometric measurements, and laboratory tests are performed.
Results: As of July 2017, 322 trans women were enrolled in the cohort with a median age of 31.5 years (interquartile range 25.7–39.5), of whom 174 (54%) were HIV-infected. The Transcendendo baseline information reinforces the scenario of marginalization and deprivation surrounding trans women. Most participants had low income (62.0% were living with below US$ 10.00/day), showed a very high engagement in sex work (78.6%), and reported increased occurrence of sexual (46.3%) and physical (54.0%) violence. Pre-exposure peophylaxis (PReP) was used by 18.8% of the HIV-uninfected trans women, only through research participation. Positive screening for depression (57.8%) and problematic use of tobacco (56.6%), cannabis (28.9%), cocaine (23.8%), and alcohol (21.5%) were high. Almost all participants (94.8%) reported hormone use at some point, mostly without medical supervision (78.7%).
Conclusion: Our results describe a context of exclusion experienced by trans women, exposing vulnerabilities of this population in a middle-income country, with poor access to trans-specific care, HIV prevention and care, and mental health care. Addressing transgender experiences and needs can help the development of strategies to diminish stigma, improve health care environment, guide future research on trans morbidities, substance use, and trans-specific interventions to support health-related recommendations. Ultimately, it contributes to close the gaps concerning transgender health and reinforces that trans care cannot be disentangled from the social environment that surrounds trans women.
Keywords: cohort, HIV, transgender health, transgender person, transgender woman
Introduction
Worldwide, the burden of adverse health conditions is substantial among transgender women and travestis (trans women)—people who were assigned as male at birth but identify themselves as females.1–4 Notwithstanding, they are an underserved group, with several gaps in knowledge regarding their health.2 Although there is no definitive definition, the term travesti has historical and political significance in Brazil and is mostly used to refer to people of the feminine spectrum that, in general, do not wish to undergo reassignment.5
Estimates showed that the transgender population size is small, ranging from 0.5% to 0.9% of the overall population3 but, nevertheless, has a disproportionate HIV risk.1 A systematic review, using data from 2000 to 2011, found that HIV prevalence reached 18% in this population, with a 50-fold increased odds of infection compared with other adults in the reproductive age.6 Some of the highest HIV prevalence rates occur in Latin America, where trans women live in the context of profound social exclusion and is a population most vulnerable to HIV.7 This vulnerability is a result of the complex interactions of risks at the individual level (condomless anal sex, substance use, sex work), interpersonal risks (high-risk partner pool), and structural factors (social exclusion, violence, discrimination, unemployment).8,9 Recent data showed a high HIV prevalence in this population in 12 Brazilian cities.10 One study recently reported 31.2% HIV prevalence among trans women in Rio de Janeiro, Brazil.11
Although trans women are a key population for HIV infection, there are very few tailored prevention and treatment programmes and interventions specifically designed for this population. Historically, trans women were inappropriately grouped with men who have sex with men, which not only hindered the attainment of trans-specific data but also limited their visibility in research and surveillance studies.2,12 Surveillance systems do not usually identify transgender respondents, and scientific data are still scarce, with a dearth of longitudinal data.2 To fill this gap, we established the Oswaldo Cruz Foundation (Fiocruz) Transgender Health Clinical Cohort (Transcendendo) with the primary aim of studying the health outcomes in trans women living in Rio de Janeiro, Brazil. We here describe cohort procedures in addition to trans women baseline characteristics.
Methods
Ethic statement
The study was reviewed and approved by the Evandro Chagas National Institute of Infectious Diseases ethics review board at Fiocruz. All information was de-identified before analysis. All participants signed an informed consent form before study procedures. The files have highly restricted access by any personnel.
Study population
Transcendendo is a prospective, open, clinic-based cohort, established in August 2015, to longitudinally evaluate health outcomes among trans women. Inclusion criteria are: ≥18 years of age and self-reported gender identity different from male sex assigned at birth. We present the baseline data of participants enrolled from August 2015 to July 2017.
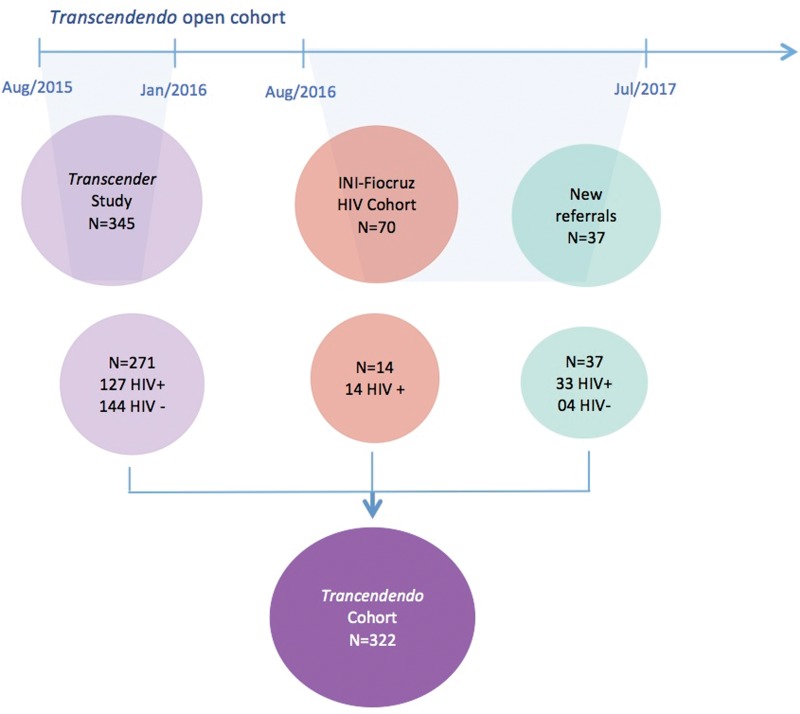
Study participants were derived from three sources, as shown in Figure 1: (1) referral from Transcender, a respondent-driven sampling (RDS) study conducted at our clinic in Fiocruz,11 (2) trans women who reached the site seeking participation in other studies or HIV prevention or care, and (3) from the Fiocruz HIV Clinical Cohort, described elsewhere.13
FIG. 1.
Transcendendo participant flowchart, Rio de Janeiro, Brazil, 2015–2017.
Trans women are a vulnerable population hardly accessible by classic sampling frame techniques for multiple reasons.14 A useful nonprobabilistic methodology for these populations is RDS, which is a chain referral method of recruitment. We employed RDS to recruit 345 trans women to Transcender from August 2015 to January 2016, 271 (78.5%) of whom were enrolled in Transcendendo. Every participant from Transcender had an initial visit scheduled for Transcendendo, but 74 (21.5%) did not return to the appointment (21 were not reachable with the contact information provided, 3 were no longer living in Rio de Janeiro, 14 declined to participate, and 36 were rescheduled for a second appointment but never returned).
After Transcender's closure, inclusion into our cohort was modified to any trans woman who spontaneously sought medical HIV care or prevention at our clinic. During the period from February 2016 to July 2017, 37 trans women reached our services and were enrolled in the cohort. Additionally, during this period, we reviewed the electronic records of patients from the Fiocruz HIV Clinical Cohort to identify potential candidates, which we defined as participants who were assigned as male at birth and had any mention of the term “trans” or social names in their files. This review process identified 70 trans women: as of July 2016, 12 are deceased, 15 were lost to follow-up, and 34 were potential participants. We tried to invite these 34 participants to join the cohort; 14 got enrolled as of July 2017. For the remaining 20 participants, although we tried to contact them, we hardly had any success.
To improve trans women engagement and retention, our setting presented itself as a welcoming, gender-affirming environment by using preferred names and pronouns; respecting gender identities, diversity, and expressions; providing safe, gender-neutral toilets; publicizing trans-affirming posters; offering continuous staff training on gender issues; and enabling friendly interactions between trans women and site staff. Finally, we established partnerships to facilitate social demands, such as adjustment of names in the documents, legal assistance, and providing access to potential interventions that could reduce stigma and socioeconomic disparities.
Study procedures
Measures
Study visits occur on an annual basis (Table 1). Each of these visits comprises face-to-face interviews using structured questionnaires, performed by trained professionals (∼90 min), to collect detailed data on sociodemographics, sexual behavior, gender affirmation procedures, hormone and antiretroviral use (for HIV prevention or treatment), discrimination and violence, alcohol, tobacco, and drug use, physical and mental health, history of sexually transmitted infections (STIs), HIV testing history, HIV prevention, and HIV care information (for those HIV-infected).
Table 1.
Study Procedures: Transcendendo Cohort
| Study procedures | Entry evaluation | Postentry annual evaluation |
|---|---|---|
| Consenting | X | |
| Face-to-face interviews | ||
| Sociodemographic information | X | X |
| Gender transition | X | |
| Gender affirmation procedures | X | X |
| Sexual behaviora | X | X |
| Discriminationb and violencec | X | X |
| History of sexually transmitted infections | X | X |
| HIV testing history | X | X |
| HIV prevention knowledge and risk perception | X | X |
| Substance use | X | X |
| ASSIST questionnaire | X | |
| Depression screening CES-10 | X | |
| Clinical evaluation | ||
| Physical, mental, and medical evaluation | X | X |
| Hormone use | X | X |
| HIV care informationd | X | X |
| Antiretroviral use (prevention or treatment) | X | X |
| Medication history | X | X |
| Anthropometric measurements | X | X |
| Laboratory testing | ||
| Metabolic panel | X | X |
| Serum hormone levels | X | |
| Hematology and chemistry tests | X | X |
| STI testing | X | X |
| Counseling and HIV testinge | X | X |
| HIV drug resistance tests | X | |
| CD4+/CD8+ count and plasma HIV RNAd | X | X |
| Stored serum and plasma for future assessments | X | X |
Sexual behavior was assessed through questions regarding age of sexual debut, sexual orientation, sex work, practices of receptive and insertive anal sex, vaginal sex, use of condoms, and number of partners.
Discrimination was assessed by the participant's perception of being discriminated against in the following settings: at home, school, work, health care, and on the street.
Violence was assessed with questions on sexual and physical violence ever in life and in the past year.
For HIV-infected participants.
For HIV-uninfected participants.
Procedures during visits also included clinical evaluation, as well as morbidity and medication history. We used self-reported information (prior medical diagnosis or use of specific treatment) or current results/measurements to define hypertension, diabetes, and dyslipidemia, as follows: (1) hypertension: average diastolic blood pressure ≥90 mmHg or average systolic blood pressure ≥140 mmHg,15 (2) diabetes: plasma nonfasting glucose ≥200 mg/dL or glycosylated hemoglobin ≥6.5%,16 (3) dyslipidemia: non-HDL-cholesterol ≥160 mg/dL.17 We considered overweight and obesity as body mass index between 25 and 29.9 kg/m2 and ≥30 kg/m2, respectively.
At baseline visit, we performed some additional procedures. We assessed information on gender perception, gender transition, and engagement in sex work. Additionally, participants responded to two validated instruments to assess depression and substance use.18 We screened depression using the 10-item Center for Epidemiologic Studies Depression Scale (CES-D)19–21 and defined it with a score of ≥10. Problematic substance use was defined when the Alcohol, Smoking, and Substance Involvement Screening Test (ASSIST) scored moderate or high risk of experiencing health problems and demanding intervention.22
For HIV-infected trans women, clinical information included date of first HIV-positive test, all antiretroviral therapy (ART) used, as well as other concomitant medications, history of non-AIDS-related comorbidities, and AIDS-defining illnesses, classified according to the CDC.23
Laboratory procedures
Procedures during visits also comprised laboratory testing, as described in Table 1, including STI testing (syphilis, rectal Chlamydia trachomatis and Neisseria gonorrhea, hepatitis B and C), and sample storage at all visits for future assessments. For HIV-infected trans women, we measured CD4+ and CD8+ cells and plasma viral load (VL) during every visit and performed HIV drug resistance test at baseline. For HIV-uninfected trans women who reported condomless anal intercourse in the past 30 days and have a negative HIV rapid test, we also offered pooled HIV RNA testing to identify acute HIV infection.
Additional data collection
We also systematically collected data on hospital admissions (causes and length of stay) and causes of death. For all deaths that occurred, we used a uniform coding system, adapted from the CoDe (“Coding of Death in HIV”) method,24 to revise near-death patient information either for the HIV-infected or uninfected participants. The method includes detailed data collection on the causes of death and contributing factors and a centralized review process of the collected data. Information regarding vital status was regularly checked using the patients' medical charts, via active contact with individuals and family members, and by linkage with the Rio de Janeiro state mortality database, using a previously validated algorithm.25
Study definitions
Sociodemographic characteristics
We categorized self-reported gender identity as “woman,” “transgender woman,” “travesti,” and “other definitions.” “Travesti” is a term used in Brazil that mostly refers to individuals assigned as male at birth and have feminine gender expression, but do not identify as women, and generally do not wish to undergo feminizing vaginoplasty.5 Educational level included four categories, based on the number of years of formal education. Per-person daily income followed the classification of poverty.26 Participants born outside Rio de Janeiro State were classified as internal migrants.
STI diagnosis
We considered active syphilis as a positive treponemal test (rapid immunochromatographic syphilis point-of-care or microhemagglutination assay for Treponema pallidum) plus a positive nontreponemal test Venereal Disease Research Laboratory (VDRL) with titers of at least 1/8 at baseline. Active hepatitis B was defined as a positive HB antigen and a negative anti-HB antigen. HCV prevalence was defined as positive anti-HCV.
Trans-specific characteristics
Feminizing procedures included gender-affirming surgeries (breast augmentation, facial feminization, tracheal shave) and sex reassignment surgeries (SRS) (penectomy, orchiectomy, vaginoplasty). We classified the use of feminizing hormonal therapy (FHT) as “never,” “current,” or “former,” according to the report within the last 30 days. As trans women might be using more than one regimen; we inquired on every hormonal medication and classified them as “ethinylestradiol,” “intramuscular estradiol plus progestogen,” “estradiol plus antiandrogen,” or “other.” We considered “estradiol plus antiandrogen” as an adequate FHT, according to current guidelines27–30 and the use of ethinylestradiol or intramuscular progestagen as inadequate, as these substances are not recommended by international guidelines.27–30 We considered serum testosterone and estradiol levels, respectively, of <55 ng/dL and between 40 and 199 pg/mL as the target levels for FHT.31
Statistical analysis
Descriptive characteristics of cohort participants are given as counts, percentages, medians, and interquartile ranges (IQR). We compared baseline characteristics of participants by HIV status using chi-squared test for categorical variables and Kruskal–Wallis test for continuous variables. In addition, we compared clinical and laboratory data for HIV-infected trans women by age strata.
Results
As described in Table 2, 322 trans women were enrolled from August 2015 to July 2017, of whom 174 (54.0%) were HIV-infected. Median age was 31.5 years (IQR 25.7–39.5). The majority of them self-declared as nonwhite. Overall, participants reported low schooling and income, with 62.0% of them living with below US$ 10.00 per day. Sexual debut occurred before the age of 10 years for 70.2% of the participants. We observed a high prevalence of physical (n=174; 54.0%) and sexual (n=149; 46.3%) violence. Out of the 149 participants who reported a history of sexual violence, 95 (63.8%) informed that it occurred the first time before the age of 18 years, and for 58 (38.9%), it occurred before the age of 14 years. The frequency of past engagement in sex work was high (78.6%). Overall, 186 (57.6%) of trans women had a positive treponemal test, and 27.0% had active syphilis at baseline. The prevalence of active rectal C. trachomatis and N. gonorrhea was 12% and 6%, respectively, and few had active hepatitis B and C. From the 148 HIV-uninfected participants, only 18 (12.2%) were on HIV pre-exposure prophylaxis (PrEP), all of them via participation in a PrEP demonstration study. Hypertension (17.9%) was one of the most common chronic nontransmissible comorbidity. We identified depression in 58% of the participants; depression was significantly higher among the HIV-infected (p=0.05).
Table 2.
Baseline Sociodemographic and Clinical Characteristics of Transgender Women by HIV Serostatus—Transcendendo Cohort, Rio de Janeiro, Brazil, 2015–2017
| Demographics | Total N=322 (%) | HIV-uninfected N=148 (%) | HIV-infected N=174 (%) | p-value |
|---|---|---|---|---|
| Agea | ||||
| 18–24 | 68 (21.1) | 38 (25.7) | 30 (17.2) | 0.128 |
| 25–35 | 132 (41.0) | 63 (42.6) | 69 (39.7) | |
| 36–45 | 73 (22.7) | 29 (19.6) | 44 (25.3) | |
| >45 | 49 (15.2) | 18 (12.2) | 31 (17.8) | |
| Gender identity | <0.001 | |||
| Woman | 74 (23.0) | 49 (33.1) | 25 (14.4) | |
| Transgender woman | 112 (34.8) | 53 (35.8) | 59 (33.9) | |
| Travesti | 128 (39.8) | 43 (29.1) | 85 (48.9) | |
| Other definitions | 8 (2.5) | 3 (2.0) | 5 (2.9) | |
| Self-declared race/color | 0.111 | |||
| White | 80 (24.8) | 39 (26.4) | 41 (23.6) | |
| Black | 73 (22.7) | 26 (17.6) | 47 (27.0) | |
| Mixed | 161 (50.0) | 81 (54.7) | 80 (46.0) | |
| Other | 8 (2.5) | 2 (1.4) | 6 (3.4) | |
| Years of educationa | 0.023 | |||
| <4 | 19 (5.9) | 5 (3.4) | 14 (8.0) | |
| 4–8 | 100 (31.1) | 39 (26.4) | 61 (35.1) | |
| 9–12 | 177 (55.0) | 87 (58.8) | 90 (51.7) | |
| >12 | 26 (8.1) | 17 (11.5) | 9 (5.2) | |
| Income (in US$/day)a,b | <0.001 | |||
| >10.00 | 55 (17.1) | 34 (23.0) | 21 (12.1) | |
| 1.91–10.00 | 161 (50.0) | 85 (56.1) | 78 (44.8) | |
| ≤1.90 | 39 (12.1) | 19 (12.8) | 20 (11.5) | |
| Missing | 67 (20.8) | 12 (8.1) | 55 (31.6) | |
| Internal migrantsc | 87 (27.0) | 45 (30.4) | 42 (24.1) | 0.256 |
| Age at sexual debuta | 0.757 | |||
| <10 | 226 (70.2) | 100 (67.6) | 126 (72.4) | |
| 10–13 | 23 (7.1) | 12 (8.1) | 11 (6.3) | |
| 14–17 | 7 (2.2) | 4 (2.7) | 3 (1.7) | |
| ≥18 | 66 (20.5) | 32 (21.6) | 34 (19.5) | |
| Ever suffered physical violence | 174 (54.0) | 74 (50.0) | 100 (57.5) | 0.219 |
| Ever suffered sexual violence (rape) | 149 (46.3) | 66 (44.6) | 83 (47.7) | 0.656 |
| Engagement in sex work | 0.002 | |||
| Never | 69 (21.4) | 44 (29.7) | 25 (14.4) | |
| Current | 150 (46.6) | 58 (39.2) | 92 (52.9) | |
| Past | 103 (32.0) | 46 (31.1) | 57 (32.8) | |
| Current syphilis | ||||
| No | 184 (57.1) | 106 (71.6) | 78 (44.8) | < 0.001 |
| Yes | 88 (27.3) | 35 (23.6) | 53 (30.5) | |
| Missing | 50 (15.5) | 7 (4.7) | 43 (24.7) | |
| Rectal chlamydia | 0.004 | |||
| No | 241 (74.8) | 121 (81.8) | 120 (69.0) | |
| Yes | 40 (12.4) | 18 (12.2) | 22 (12.6) | |
| Missing | 41 (12.7) | 9 (6.1) | 32 (18.4) | |
| Rectal gonorrhea | 0.002 | |||
| No | 263 (81.7) | 132 (89.2) | 131 (75.3) | |
| Yes | 19 (5.9) | 8 (5.4) | 11 (6.3) | |
| Missing | 40 (12.4) | 8 (5.4) | 32 (18.4) | |
| Hepatitis B | < 0.001 | |||
| No | 268 (83.2) | 140 (94.6) | 128 (73.6) | |
| Yes | 9 (2.8) | 1 (0.7) | 8 (4.6) | |
| Missing | 45 (14.0) | 7 (4.7) | 38 (21.8) | |
| Hepatitis C | 0.157 | |||
| No | 307 (95.3) | 145 (98.0) | 162 (93.1) | |
| Yes | 11 (3.4) | 2 (1.4) | 9 (5.2) | |
| Missing | 4 (1.2) | 1 (0.7) | 3 (1.7) | |
| Diabetes mellitus | 7 (2.2) | 2 (1.4) | 5 (2.9) | 0.459 |
| Dyslipidemia | 24 (7.5) | 8 (5.4) | 16 (9.2) | 0.281 |
| Systemic arterial hypertension | 54 (17.9) | 26 (18.3) | 28 (17.6) | 0.994 |
| Obesity/overweight | ||||
| No | 170 (52.8) | 73 (49.3) | 97 (55.7) | |
| Yes | 138 (42.9) | 73 (49.3) | 65 (37.4) | 0.011 |
| Missing | 14 (4.3) | 2 (1.4) | 12 (6.9) | |
| Depressiond | 186 (57.8) | 77 (52.0) | 109 (62.6) | 0.054 |
| Problematic use of substances (ASSIST scored)e | ||||
| Alcohol | 55 (21.5) | 18 (16.4) | 37 (25.3) | 0.115 |
| Tobacco | 145 (56.6) | 54 (49.1) | 91 (62.3) | 0.047 |
| Cannabis | 74 (28.9) | 22 (20.0) | 52 (35.6) | 0.010 |
| Cocaine | 61 (23.8) | 15 (13.6) | 46 (31.5) | 0.002 |
| Amphetamine stimulants | 7 (2.7) | 2 (1.8) | 5 (3.4) | 0.702 |
Continuous variables were reclassified as categorical.
US$1.00=R$3.85.
Participants born outside Rio de Janeiro state were classified as internal migrants.
Screening by CES-D10.
ASSIST score of 11+ for alcohol and 4+ for other substances.
ASSIST, Alcohol, Smoking and Substance Involvement Screening Test.
Compared to those HIV-uninfected, HIV-infected trans women had lower schooling and income, reported more engagement in sex work, and had higher rates of STIs. HIV-uninfected and HIV-infected trans women significantly differed in gender identity, as HIV-uninfected trans women more frequently self-reported as women (33.1% vs. 14.4%) and less frequently as travestis (29.1% vs. 48.8%) and had higher obesity/overweight prevalence (Table 2).
A high number of trans women reported problematic use of tobacco (56.6%), cannabis (28.9%), cocaine (23.8%), and alcohol (21.5%) (Table 2). Compared to those HIV-uninfected, higher proportions of HIV-infected trans women had problematic use of cannabis, cocaine, and tobacco.
The majority of trans women perceived themselves as transgender during childhood (63.7% aged ≤10), and the median age of gender transition was 16 years (IQR 14–18) (Table 3). Any feminizing procedure was reported by 41.0%, with only 5.9% reporting SRS. SRS was more frequent among HIV-uninfected than in HIV-infected trans women. About a third had gender-affirming surgery, and 49.1% reported prior industrial silicone filler injection. Most trans women have used hormones at some point, and around half (49.1%) were on current FHT, mostly nonprescribed by health professionals (78.7%). Fewer HIV-infected trans women were currently using hormones (both prescribed and nonprescribed) compared with HIV-uninfected trans women (40.2% vs. 59.5%; p<0.001). Among trans women currently using hormones (N=158), most of them were using inadequate FHT (57.6% were using intramuscular estradiol plus progestagen, 37.3% reported current use of ethinylestradiol). Only 13.9% reported the use of an adequate regimen (estradiol plus antiandrogen) and 9.5% were under current medical-guided hormonal use. Among trans women currently on hormones, 56.3% and 29.7% achieved the target testosterone and estradiol levels, respectively; 16.5% had estradiol levels higher than recommended.
Table 3.
Transitioning Characteristics of Transgender Women According to HIV Serostatus—Transcendendo Cohort, Rio de Janeiro, Brazil, 2015–2017
| Transitioning characteristics | Total N=322 | HIV-uninfected N=148 | HIV-infected N=174 | p |
|---|---|---|---|---|
| Age of gender perceptiona | ||||
| ≤7 | 112 (34.8) | 56 (37.8) | 56 (32.2) | 0.254 |
| 8–10 | 93 (28.9) | 39 (26.4) | 54 (31.0) | |
| 11–13 | 66 (20.5) | 29 (19.6) | 37 (21.3) | |
| ≥14 | 47 (14.6) | 24 (16.2) | 23 (13.2) | |
| Missing | 4 (1.2) | 0 (0.0) | 4 (2.3) | |
| Age of gender transitiona | ||||
| <14 | 59 (18.3) | 24 (16.2) | 35 (20.1) | 0.151 |
| 14–17 | 148 (46.0) | 63 (42.6) | 85 (48.9) | |
| ≥18 | 114 (35.4) | 61 (41.2) | 53 (30.5) | |
| Missing | 1 (0.3) | 0 (0.0) | 1 (0.6) | |
| Ever performed gender affirmation proceduresb | 132 (41.0) | 59 (39.9) | 73 (42.0) | 0.790 |
| Ever had sex reassignment surgeryb | 19 (5.9) | 15 (10.1) | 4 (2.3) | 0.006 |
| Ever injected fillers | 158 (49.1) | 57 (38.5) | 101 (58.0) | <0.001 |
| Hormone use | ||||
| Current | 158 (49.1) | 88 (59.5) | 70 (40.2) | 0.002 |
| Past | 147 (45.7) | 52 (35.1) | 95 (54.6) | |
| Never | 16 (5.0) | 8 (5.4) | 8 (4.6) | |
| Missing | 1 (0.3) | 0 (0.0) | 1 (0.6) | |
| Medically guided hormone use | 0.014 | |||
| Never | 240 (78.7) | 100 (71.4) | 140 (84.8) | |
| Current | 29 (9.5) | 21 (15.0) | 8 (4.8) | |
| Past | 19 (6.2) | 10 (7.1) | 9 (5.5) | |
| Missing | 17 (5.6) | 9 (6.4) | 8 (4.8) | |
| Current use of ethinylestradiolc | 59 (37.3) | 35 (39.8) | 24 (34.3) | 0.587 |
| Current use of IM estradiol plus progestagenc | 91 (57.6) | 45 (51.1) | 46 (65.7) | 0.093 |
| Current use of estradiol plus antiandrogenc | 22 (13.9) | 16 (18.2) | 6 (8.6) | 0.133 |
| Total testosteronea,c | ||||
| At target level | 89 (56.3) | 53 (60.2) | 36 (51.4) | 0.221 |
| Above target level | 64 (40.5) | 34 (38.6) | 30 (42.9) | |
| Missing | 5 (3.2) | 1 (1.1) | 4 (5.7) | |
| Estradiola,c | ||||
| Below target level | 80 (50.6) | 47 (53.4) | 33 (47.1) | 0.433 |
| At target level | 47 (29.7) | 26 (29.5) | 21 (30.0) | |
| Above target level | 26 (16.5) | 14 (15.9) | 12 (17.1) | |
| Missing | 5 (3.2) | 1 (1.1) | 4 (5.7) | |
Continuous variables were reclassified as categorical.
Breast augmentation, facial feminization, tracheal shave, or sex reassignment surgery (vaginoplasty, penectomy, and oorchiectomy).
Evaluated to trans women currently using hormones (n=158).
IM, intramuscular.
Among HIV-infected trans women, median time since HIV diagnosis was 1.8 years (IQR 0–7.3); the median CD4+ count was 582 cells/mm3 (IQR 372–800); and 60% of them were using ART. Out of the 70 HIV-infected trans women who were not on ART, 38.6% (27) had been diagnosed with HIV on the same day of Transcendendo enrollment, 57.1% (40) had already been diagnosed with HIV but had never used ART, and 4.3% (3) had been on ART before but were not currently using it. Among those currently using ART, 67.3% had undetectable VL (Table 4). ART use was less frequent among younger HIV-infected trans women, but among those who used ART, they were more likely to have undetectable VL: 80.0% among those aged 18–24 versus 57.1% among those aged ≥46. Forty (23.0%) HIV-infected trans women reported a previous AIDS-defining illness; 16.7% reported previous tuberculosis.
Table 4.
Clinical Characteristics of HIV-Infected Transgender Women, According to Age—Transcendendo Cohort, Rio de Janeiro, Brazil, 2015–2017
| Clinical characteristics | Total N=174 (%) | 18–24 N=30 (%) | 25–35 N=69 (%) | 36–45 N=44 (%) | 46+N=31 (%) | p |
|---|---|---|---|---|---|---|
| Mode of HIV acquisition | ||||||
| Sex with men | 161 (92.5) | 27 (90.0) | 66 (95.7) | 43 (97.7) | 25 (80.6) | 0.042 |
| IDU | 2 (1.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (6.5) | |
| Other/unknown | 11 (6.5) | 3 (10.0) | 3 (4.3) | 1 (2.3) | 4 (12.9) | |
| Current CD4 count (cells/mm3)a | ||||||
| <250 | 21 (12.1) | 2 (6.7) | 11 (15.9) | 4 (9.1) | 4 (12.9) | 0.884 |
| 251–500 | 36 (20.7) | 6 (20.0) | 15 (21.7) | 9 (20.5) | 6 (19.4) | |
| >500 | 84 (48.3) | 16 (53.3) | 29 (42.0) | 25 (56.8) | 14 (45.2) | |
| Missing | 33 (19.0) | 6 (20.0) | 14 (20.3) | 6 (13.6) | 7 (22.6) | |
| Currently on ART | ||||||
| No | 70 (40.2) | 25 (83.3) | 36 (52.2) | 6 (13.6) | 3 (9.7) | <0.001 |
| Yes | 104 (59.8) | 5 (16.7) | 33 (47.8) | 38 (86.4) | 28 (90.3) | |
| Current HIV RNA viral load (copies/mL) (N=103)b | ||||||
| <40 | 70 (67.3) | 4 (80.0) | 23 (69.7) | 27 (71.1) | 16 (57.1) | 0.775 |
| ≥40 | 22 (21.2) | 1 (20.0) | 5 (15.2) | 8 (21.1) | 8 (28.6) | |
| Missing | 12 (11.5) | 0 (0.0) | 5 (15.2) | 3 (7.9) | 4 (14.3) | |
| Had previous AIDS-defining illness | 40 (23.0) | 2 (6.7) | 8 (11.6) | 17 (38.6) | 13 (41.9) | <0.001 |
| Had previous TBc | 29 (16.7) | 2 (6.7) | 11 (15.9) | 11 (25.0) | 10 (32.3) | 0.051 |
Continuous variables were reclassified as categorical.
Calculated only for those on ART.
After HIV diagnosis.
ART, antiretroviral therapy; IDU, injection drug use.
Discussion
Our study presents baseline characteristics of the Transcendendo cohort. Our findings reinforce trans women as a disadvantaged and disenfranchised group, with multiple needs that go beyond health itself. Trans women bear a disproportionate burden of adverse outcomes that are sparingly evaluated, mostly by cross-sectional studies.2 Although transgender populations face adverse experiences worldwide, the context of marginalization and social exclusion may be even worse in a low- or middle-income country (LMIC), with poor access to hormone use supervision, gender-related interventions, HIV prevention and care, and support for problematic substance use. Transcendendo is the first Latin-American cohort to evaluate the social, demographic, and clinical aspects of HIV-infected and HIV-uninfected trans women.
Sociodemographic context of exclusion, discrimination, and violence
Our results reveal that the participants of Transcendendo have low education level and income, experience high physical and sexual violence rates, and frequently engage in sex work. These unfavorable conditions are in accordance with other authors.32–35 The social context that trans women face is a combination of diverse related factors such as exclusion, stigma, violence, lack of justice, and poor access to education and employment, which usually exacerbate one another and increase vulnerability to HIV and other diseases.36 Although Brazilian laws protect transgender rights, transphobia is ingrained diffusely, and Brazil is the leading country of homicides of transgender people, accounting for almost 40% of all reported murders of transgender people worldwide.37
Mental and physical health
We found that depression was very common in our cohort (58%), comparable to those described using similar screening scales (prevalence between 44% and 49%)14,38,39 and more prevalent than observed in the cisgender Brazilian population (13.2–20.2%).40,41 This finding is in accordance with data from Canada and the United States, which observed a higher prevalence of depression among trans women compared to cisgender population.14,42 The factors associated with gender minority stress, such as victimization, rejection, discrimination, and nonaffirmation of gender identity, seem to be associated with depression and other mental health conditions.43,44 We found a lower prevalence of hypertension compared to the data from the general population of Brazil,45 which may be due to the lower age of our sample. Additionally, diabetes and dyslipidemia were less frequent in our population, compared with other general population studies in Brazil46,47 and with the Canadian trans women cohort.14 These contrasts may again be related to the demographic characteristics of the populations, such as age and gender, as well as to the differences in definitions adopted by each study.
Trans-specific interventions
Most trans women used hormones at some point, almost always nonprescribed by health professionals, at higher levels than described in the United States.48–51 We also identified alarming rates of industrial silicon filler injection and lower rates of SRS than described in other countries.52,53 These differences might reflect worse access to general and transgender-specific health care. Gender-affirming hormone therapy is the main intervention sought by transgender people, since it allows the development of characteristics compatible with their gender identity.28 FHT is considered safe under medical supervision,54 but nonprescribed hormones are associated with the uptake of inadequate compounds, improper dosing, and lack of monitoring.48 In our cohort, most trans women did not achieve the recommended levels of hormones; more than a third was using ethinylestradiol (37.3%) and some had higher estradiol levels than recommended. Ethinylestradiol is not recommended to feminization due to its high thrombogenic risk.54 The combination of intramuscular estradiol plus progestagen, commonly used in our setting, is understudied, but might be associated with cardiovascular disease and breast cancer in postmenopausal women.55 In summary, the high rates of inadequate use of hormones in our sample is alarming and points to the unmet needs of this underprivileged group.
HIV infection
Compared with those HIV-uninfected, HIV-infected trans women had lower educational levels, were involved in more sex work, and reported problematic use of some substances. Forty percent were not on ART, especially the younger population with two-thirds of those on ART having an undetectable VL. These results highlight the need for better retention in care, which will ultimately impact HIV transmission. HIV epidemic among trans women is considered a syndemic condition, which generally develops in the context of social disadvantage and inequality.4 It has been suggested that syndemics may also explain the difficulties in the treatment of HIV infection apart from other coexisting social, behavioral, and medical conditions that limit successful and sustained engagement in health care.12
Strengths and limitations
The main strengths of this study are the unique evaluation of an extremely marginalized and vulnerable population, the use of a standardized data collection platform, and the systematic medical and laboratory assessments. Very few studies have evaluated trans women longitudinally, none in LMICs. In addition, trans women are a hard-to-reach and hard-to-retain population for health care.56–58 So, to improve retention and outcome assessment, we established a welcoming, gender-affirming setting, which facilitated the engagement and retention of trans women. Also, we established a linkage with the state-level mortality database to improve lost to follow-up evaluation in our population, since it can help identify deaths not reported to our study team. Our study also has some limitations. Foremost, Transcendendo is an open cohort restricted to a convenience sample from the Rio de Janeiro population, and therefore the findings cannot be generalizable to the whole trans women population. Also, as this is a clinic-based cohort, procedures are linked to medical care, which may lead to some missing data. However, to minimize these occurrences, our study procedures are carried out annually.
Conclusions
Transcendendo is a unique opportunity to longitudinally assess transgender health outcomes among young trans women from a LMIC, including issues regarding hormone and antiretroviral use, incident comorbidities, and complications such as metabolic and cardiovascular events. Baseline results contribute to bridging the gaps concerning transgender people's health and reinforce that transgender care cannot be disentangled from the social environment that surrounds trans women. HIV infection is a major concern among trans women, but transgender members claim that their marginalization needs to be addressed as the highest priority to succeed in a response to HIV.36 Transcendendo results may guide future research and trans-specific interventions by addressing transgender vulnerabilities and needs and supporting the development of strategies to diminish stigma, improve health care environment, guide future research on trans morbidities, substance use, feminizing hormone therapy, and trans-specific interventions to support health-related recommendations.
Acknowledgments
We acknowledge all of the Transcendendo team and participants. The study was reviewed and approved by the Evandro Chagas National Institute of Infectious Diseases ethics review board at Fiocruz. Funding: This work was supported by Fiocruz PMA (Programa de Polìticas Públicas e Modelos de Atenção à Saúde) and the Brazilian Ministry of Health.
Abbreviations Used
- ART
antiretroviral therapy
- ASSIST
Alcohol, Smoking and Substance Involvement Screening Test
- FHT
feminizing hormonal therapy
- IDU
injection drug use
- IM
intramuscular
- IQR
interquartile range
- LMIC
low- or middle-income country
- RDS
respondent-driven sampling
- SRS
sex reassignment surgeries
- STI
sexually transmitted infection
Author Disclosure Statement
No competing financial interests exist.
Cite this article as: Ferreira ACG, Coelho LE, Jalil EM, Luz PM, Friedman RK, Guimarães MRC, Moreira RC, Eksterman LF, Cardoso SW, Castro CV, Derrico M, Moreira RI, Fernandes B, Monteiro L, Kamel L, Pacheco AG, Veloso VG, Grinsztejn B (2019) Transcendendo: A cohort study of HIV-infected and uninfected transgender women in Rio de Janeiro, Brazil, Transgender Health 4:1, 107–117, DOI: 10.1089/trgh.2018.0063.
References
- 1. Poteat T, Reisner SL, Radix A. HIV epidemics among transgender women. Curr Opin HIV AIDS. 2014;9:168–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Reisner SL, Poteat T, Keatley J, et al. Global health burden and needs of transgender populations: a review. Lancet Lond Engl. 2016;388:412–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Poteat T, Scheim A, Xavier J, et al. Global epidemiology of HIV infection and related syndemics affecting transgender people. J Acquir Immune Defic Syndr. 2016;72 Suppl 3:S210–S219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Parsons JT, Millar BM, Moody RL, et al. Syndemic conditions and HIV transmission risk behavior among HIV-negative gay and bisexual men in a U.S. national sample. Health Psychol. 2017;36:695–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kulick D. Travesti: Prostitution, Sex, Gender, and Culture in Brazil [in Portuguese]. Rio de Janeiro: Editora Fiocruz, 2008 [Google Scholar]
- 6. Baral SD, Poteat T, Strömdahl S, et al. Worldwide burden of HIV in transgender women: a systematic review and meta-analysis. Lancet Infect Dis. 2013;13:214–222 [DOI] [PubMed] [Google Scholar]
- 7. Silva-Santisteban A, Eng S, de la Iglesia G, et al. HIV prevention among transgender women in Latin America: implementation, gaps and challenges. J Int AIDS Soc. 2016;19:2079–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Poteat T, Wirtz AL, Radix A, et al. HIV risk and preventive interventions in transgender women sex workers. Lancet. 2015;385:274–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Silva-Santisteban A, Raymond HF, Salazar X, et al. Understanding the HIV/AIDS epidemic in transgender women of Lima, Peru: results from a sero-epidemiologic study using respondent driven sampling. AIDS Behav. 2012;16:872–881 [DOI] [PubMed] [Google Scholar]
- 10. Bastos FI, Bastos LS, Coutinho C, et al. HIV, HCV, HBV, and syphilis among transgender women from Brazil: assessing different methods to adjust infection rates of a hard-to-reach, sparse population. Med Baltim. 2018;97:S16–S24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Grinsztejn B, Jalil EM, Monteiro L, et al. Unveiling of HIV dynamics among transgender women: a respondent-driven sampling study in Rio de Janeiro, Brazil. Lancet HIV. 2017;4:e169–e176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Poteat T, German D, Flynn C. The conflation of gender and sex: gaps and opportunities in HIV data among transgender women and MSM. Glob Public Health. 2016;11:835–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Grinsztejn B, Veloso VG, Friedman RK, et al. Early mortality and cause of deaths in patients using HAART in Brazil and the United States. AIDS Lond Engl. 2009;23:2107–2114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Quinn VP, Nash R, Hunkeler E, et al. Cohort profile: study of Transition, Outcomes and Gender (STRONG) to assess health status of transgender people. BMJ Open. 2017;7:e01812–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018;39:3021–3104 [DOI] [PubMed] [Google Scholar]
- 16. American Diabetes Association (ADA). Introduction: standards of medical care in diabetes—2018. Diabetes Care. 2018;41:S1–S2 [DOI] [PubMed] [Google Scholar]
- 17. Jellinger PS, Handelsman Y, Rosenblit PD, et al. American Association of Clinical Endocrinologists and American College of Endocrinology Guidelines for management of dyslipidemia and prevention of cardiovascular disease. Endocr Pract. 2017;23:1–87 [DOI] [PubMed] [Google Scholar]
- 18. Garvey WT, Mechanick JI, Brett EM, et al. American Association of Clinical Endocrinologists and American College of Endocrinology Comprehnesive Clinical Practice Guidelines for Medical Care of Patients with Obesity. Endocr Pract Off J Am Coll Endocrinol Am Assoc Clin Endocrinol. 2016;22:842–884 [DOI] [PubMed] [Google Scholar]
- 19. Andresen EM, Malmgren JA, Carter WB, et al. Screening for depression in well older adults: evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale). Am J Prev Med. 1994;10:77–84 [PubMed] [Google Scholar]
- 20. Zhang W, O'Brien N, Forrest JI, et al. Validating a shortened depression scale (10 item CES-D) among HIV-positive people in British Columbia, Canada. PLoS One. 2012;7:e4079–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. González P, Nuñez A, Merz E, et al. Measurement properties of the Center for Epidemiologic Studies Depression Scale (CES-D 10): findings from HCHS/SOL. Psychol Assess. 2017;29:372–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. World Health Organization (WHO). WHO | The ASSIST Screening Test Version 3.0. WHO. 2005. www.who.int/substance_abuse/activities/assist_test/en (accessed September30, 2018)
- 23. Centers for Disease Control and Prevention. AIDS-Defining Conditions. 2008;57:9 [Google Scholar]
- 24. Kowalska JD, Friis-Møller N, Kirk O, et al. The coding causes of death in HIV (CoDe) project: initial results and evaluation of methodology. Epidemiology. 2011;22:516–523 [DOI] [PubMed] [Google Scholar]
- 25. Pacheco AG, Saraceni V, Tuboi SH, et al. Validation of a hierarchical deterministic record-linkage algorithm using data from 2 different cohorts of human immunodeficiency virus-infected persons and mortality databases in Brazil. Am J Epidemiol. 2008;168:1326–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Beltekian D, Ortiz-Ospina E. Extreme poverty is falling: How is poverty changing for higher poverty lines? March 5, 2018. https://ourworldindata.org/poverty-at-higher-poverty-lines (accessed March28, 2019)
- 27. Hembree WC, Cohen-Kettenis P, Delemarre-van de Waal HA, et al. Endocrine treatment of transsexual persons: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2009;94:3132–3154 [DOI] [PubMed] [Google Scholar]
- 28. Gardner IH, Safer JD. Progress on the road to better medical care for transgender patients. Curr Opin Endocrinol Diabetes Obes. 2013;20:553–558 [DOI] [PubMed] [Google Scholar]
- 29. Mahan RJ, Bailey TA, Bibb TJ, et al. Drug therapy for gender transitions and health screenings in transgender older adults. J Am Geriatr Soc. 2016;64:2554–2559 [DOI] [PubMed] [Google Scholar]
- 30. University of California, San Francisco. Guidelines for the primary and gender-affirming care of transgender and gender nonbinary people. http://transhealth.ucsf.edu/protocols (accessed October4, 2018)
- 31. Bishop BM. Pharmacotherapy considerations in the management of transgender patients: a brief review. Pharmacotherapy. 2015;35:1130–1139 [DOI] [PubMed] [Google Scholar]
- 32. Grant J, Mottet L, Tanis J, et al. Injustice at every turn: a report of the National Transgender Discrimination Survey. National Center for Trangender Equality, National Gay and Lesbion Task Force. 2011. Available at: www.ncgs.org/research/database/injustice-at-every-turn-a-report-of-the-national-trangender-discrimination-survey Accessed November 29, 2018 [Google Scholar]
- 33. Movement Advancement Project. A broken bargain for transgender workers. www.lgbtmap.org/policy-and-issue-analysis/transgender-workers (accessed November29, 2018)
- 34. UNESCO. Education sector responses to homophobic bullying. Good policy and practice in HIV and health education. Booklet 8. 2012. Available at: https://unesdoc.unesco.org/ark:/48223/pf0000216493 Accessed November 29, 2018 [Google Scholar]
- 35. Hiller R, Moreno A, Mallimaci A, et al. Cumbia, copeteo y lágrimas: informe nacional sobre la situación de las travestis transexuales y transgéneros. Buenos Aires: Asociación de Lucha por la Identidad Travesti-Transexual, A.L.I.T.T., 2007 [Google Scholar]
- 36. Divan V, Cortez C, Smelyanskaya M, et al. Transgender social inclusion and equality: a pivotal path to development. J Int AIDS Soc. 2016;19:2080–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Balzer C, LaGata C, Berredo L. TMM annual report 2016—an introduction to the Trans Murder Monitoring project. In: TvT Publication Series. 2016. www.tgeu.org (accessed November29, 2018)
- 38. Nuttbrock L, Bockting W, Rosenblum A, et al. Gender abuse, depressive symptoms, and HIV and other sexually transmitted infections among male-to-female transgender persons: a three-year prospective study. Am J Public Health. 2013;103:300–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nemoto T, Bödeker B, Iwamoto M, et al. Practices of receptive and insertive anal sex among transgender women in relation to partner types, sociocultural factors, and background variables. AIDS Care. 2014;26:434–440 [DOI] [PubMed] [Google Scholar]
- 40. Fleck MP, Lima AF, Louzada S, et al. [Association of depressive symptoms and social functioning in primary care service, Brazil]. Rev Saude Publica. 2002;36:431–438 [DOI] [PubMed] [Google Scholar]
- 41. Munhoz TN, Nunes BP, Wehrmeister FC, et al. A nationwide population-based study of depression in Brazil. J Affect Disord. 2016;192:226–233 [DOI] [PubMed] [Google Scholar]
- 42. Steele LS, Daley A, Curling D, et al. LGBT identity, untreated depression, and unmet need for mental health services by sexual minority women and trans-identified people. J Womens Health Larchmt. 2017;26:116–127 [DOI] [PubMed] [Google Scholar]
- 43. Jäggi T, Jellestad L, Corbisiero S, et al. Gender minority stress and depressive symptoms in transitioned Swiss transpersons. BioMed Res Int. 2018;2018:863926–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tebbe EA, Moradi B. Suicide risk in trans populations: an application of minority stress theory. J Couns Psychol. 2016;63:520–533 [DOI] [PubMed] [Google Scholar]
- 45. Picon RV, Fuchs FD, Moreira LB, et al. Trends in prevalence of hypertension in Brazil: a systematic review with meta-analysis. PLoS One. 2012;7:e4825–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Telo GH, Cureau FV, de Souza MS, et al. Prevalence of diabetes in Brazil over time: a systematic review with meta-analysis. Diabetol Metab Syndr. 2016;8:6–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lotufo PA, Santos RD, Figueiredo RM, et al. Prevalence, awareness, treatment, and control of high low-density lipoprotein cholesterol in Brazil: baseline of the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil). J Clin Lipidol. 2016;10:568–576 [DOI] [PubMed] [Google Scholar]
- 48. Rotondi NK, Bauer GR, Scanlon K, et al. Nonprescribed hormone use and self-performed surgeries: ‘do-it-yourself’ transitions in transgender communities in Ontario, Canada. Am J Public Health. 2013;103:1830–1836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wilson EC, Arayasirikul S, Johnson K. Access to HIV Care and Support Services for African American transwomen living with HIV. Int J Transgenderism. 2013;14:182–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Clark K, Fletcher JB, Holloway IW, et al. Structural inequities and social networks impact hormone use and misuse among transgender women in Los Angeles County. Arch Sex Behav. 2018;47:953–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. de Haan G, Santos G-M, Arayasirikul S, et al. Non-prescribed hormone use and barriers to care for transgender women in San Francisco. LGBT Health. 2015;2:313–323 [DOI] [PubMed] [Google Scholar]
- 52. Rapues J, Wilson EC, Packer T, et al. Correlates of HIV infection among transfemales, San Francisco, 2010: results from a respondent-driven sampling study. Am J Public Health. 2013;103:1485–1492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bellhouse C, Walker S, Fairley CK, et al. Patterns of sexual behaviour and sexual healthcare needs among transgender individuals in Melbourne, Australia, 2011–2014. Sex Transm Infect. 2018;94:212–215 [DOI] [PubMed] [Google Scholar]
- 54. World Professional Association for Transgender Health (WPATH). Standards of care for the health of transsexual, transgender, and gender nonconforming people. 2009. https://www.wpath.org/publications/soc (accessed September8, 2018)
- 55. Gooren LJ, T'Sjoen G. Endocrine treatment of aging transgender people. Rev Endocr Metab Disord. 19:253–262 [DOI] [PubMed] [Google Scholar]
- 56. Melendez RM, Exner TA, Ehrhardt AA, et al. Health and health care among male-to-female transgender persons who are HIV positive. Am J Public Health. 2006;96:1034–1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sevelius JM, Carrico A, Johnson MO. Antiretroviral therapy adherence among transgender women living with HIV. J Assoc Nurses AIDS Care. 2010;21:256–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sevelius JM, Patouhas E, Keatley JG, et al. Barriers and facilitators to engagement and retention in care among transgender women living with human immunodeficiency virus. Ann Behav Med. 2014;47:5–16 [DOI] [PMC free article] [PubMed] [Google Scholar]