Abstract
Stereotactic body radiation therapy (SBRT) has emerged to be a preference treatment for locally advanced pancreatic cancer (LAPC) patients. In this study, we aimed to investigate the prognostic roles of 18F-FDG PET/CT metabolic parameters and clinical figures in LAPC patients underwent chemo-SBRT combined therapy.
During January 2013 to January 2017, 23 LAPC patients who underwent 18F-FDG PET/CT within 2 weeks before treatment were recruited and retrospectively analyzed. Maximum standardized uptake values (SUVmax), SUVmean, metabolic tumor volume (MTV), total lesion glycolysis (TLG), chemoradiotherapy (CRT) sequence, and relevant clinical figures were grouped upon the median values, then analyzed by Kaplan–Meier method and Cox proportional hazard models for their prognostic evaluation.
The median overall survival (OS) and progression-free survival (PFS) of all patients were 16.7 months and 11.3 months, respectively. According to the statistic results, the longest diameter of tumor (LDT), MTV, TLG, and CRT sequence were associated with OS (all P <.05). Among which, LDT and MTV were proved to be the independent prognostic factors for OS (hazard ratio [HR]: 3.437, 3.015, both P <.05). Additionally, LDT and CRT sequence were found associated with PFS (both P <.05), and CRT sequence was the independent prognostic factor for PFS in chemo-SBRT treated LAPC patients (HR: 0.130, P <.05).
For LAPC patients received chemotherapy and SBRT combined therapy, MTV and LDT showed independent prognostic values for OS. Meanwhile, CRT sequence was an independent PFS prediction factor.
Keywords: 18F-FDG PET/CT, chemoradiotherapy sequence, locally advanced pancreatic caner, prognostic evaluation, stereotactic body radiation therapy
1. Introduction
Pancreatic Ductal Adenocarcinoma (PDAC) is a highly malignant gastrointestinal cancer, whose global incidence is continually increasing with its 5-year survival rate stays less than 5%.[1] To date, surgical resection is considered as the most effective way to cure PDAC. However, only about 20% of PDAC patients present with surgical indications when diagnosed.[2] Meanwhile, 45% to 50% of patients have developed distant metastases at diagnosis, whose median survival time is only 3 to 6 months.[3] Additionally, there are about 20% to 40% PDAC patients could be defined as locally advanced pancreatic cancer (LAPC) when diagnosed,[4,5] for which no distant metastasis found, but tumor tissue cannot be surgically removed because of surrounding normal structures infiltration.
Single-agent chemotherapy and multi-drug combination chemotherapy have been recommended by the American Society of Clinical Oncology (ASCO) for LAPC patients,[6] and the efficiency has been well documented. Chemotherapy could significantly increase the overall survival (OS) of LAPC patients, also delay the progression of disease.[7] However, the treatment value of conventional radiotherapy in LAPC was argued by many researchers.[8,9] On 1 hand, pancreatic cancer is a cancer of moderate radiosensitivity.[10] On the other hand, pancreas is closely surrounded by stomach, duodenum, small intestine and kidney, which are highly sensitive to radiation. Thus, traditional radiotherapy normally related with poor treatment efficiency and severe gastrointestinal adverse reactions. Recently, the target position of radiotherapy in LAPC treatment has gradually been recognized,[11,12] because of an emerging 3-dimensional conformal radiation technology, stereotactic body radiation therapy (SBRT). With the SBRT delivery system, a high-dose irradiation could be applied to the target tumor area, meanwhile the protection for surrounding organs was severely ensured. Hence, radiation-induced toxicities were greatly reduced, and the life qualities and survival duration were significantly improved.[13] As the SBRT technology has been widely used in clinic, chemoradiotherapy (CRT) has been listed as a recommend therapy by ASCO (6) and become 1 of the routine treatments for LAPC patients.[14]
Through a remarkable achievement has been obtained in LAPC treatment, the median survival of LAPC patients after therapy remains 6 to 14 months.[15] Most patients still have distant metastases developed, while the local lesions were well controlled. Therefore, figuring out effective prognostic factors is crucial for LAPC patients management. The 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography-computed tomography (PET/CT) has been widely used in the diagnosis, staging and therapeutic evaluation of various malignancies.[16,17] Also, the prognostic prediction value in PDAC was reported by several researchers.[18–20] For resected PDAC patients, 18F-FDG PET/CT metabolic parameters maximum standardized uptake values (SUVmax), metabolic tumor volume (MTV) and total lesion glycolysis (TLG) were illustrated to strongly correlate with OS and progression-free survival (PFS). On the basis, the aim of this study was to further evaluate the prognostic values of 18F-FDG PET/CT metabolic parameters and clinical figures in LAPC patients treated with chemo-SBRT combined therapy.
2. Material and methods
2.1. Patients
Medical data of 23 LAPC patients, who were diagnosed and treated in Shanghai Changhai Hospital from January 2013 to January 2017, was collected and retrospectively analyzed.
Inclusion criteria:
-
1.
Initially diagnosed with pancreatic cancer.
-
2.
Histopathologically confirmed as LAPC.
-
3.
Received chemotherapy and SBRT combination treatment.
-
4.
Underwent 18F-FDG PET/CT scan within 2 weeks before treatment.
Exclusion criteria:
-
1.
Previous history of tumor treatment at the pancreatic site.
-
2.
Merged with other tumors.
-
3.
Distal metastases found by 18F-FDG PET/CT.
-
4.
Deaths caused by diseases other than pancreatic cancer during follow-ups.
-
5.
Lost to follow-up.
Patients were followed up by telephone or through clinic. By January 2018, the median follow-up time was 35.5 months (18–55 months). OS and PFS were used as prognostic indicators, where OS refers to the time period between first day receiving treatment to the date of disease-related death or last visit, and PFS is defined as the period started from the day of initial treatment applied to the date of first progression or the death.
2.2. 18F-FDG PET/CT image acquisition and image analysis
All scans were performed on a Biograph trupoint 64-layer 52-ring HD PET/CT scanner (Siemens, Germany), 18F-FDG was provided by Shanghai Atomic Kexing Pharmaceutical Co., Ltd., with radiochemical purity above 95%. Before imaging, patients fasted for 6 hours or above, and the blood glucose was confirmed <11.1 mmol/L. The 18F-FDG was injected 45 to 60 minutes before scanning at a dose of 3.70 to 5.55 MBq/kg. A routine PET scan started from the skull to the mid-femur, 5 to 6 beds, and 2 minutes per bed in 3-D mode. For the whole body CT scan, 170 mA current and 120 kV voltage were used, scanning time was approximately 18.67 to 21.93 seconds, and the slice width was 3 mm. The images were reconstructed and post-processed to obtain CT, PET, PET/CT fusion images and 3D reconstruction images, which were performed using ordered subset expectation maximization (OSEM) and the Multimodality Workstation, respectively.
All images were blind read by 2 experienced nuclear medicine physicians. 40% of SUVmax was set up as the baseline for the assessment of primary pancreatic cancer lesions, which was carried out based on the cubic-shaped volume of interest (VOI) to maximally include the entire lesion, and to avoid the surrounding high-metabolic tissues. SUVmax, SUVmean, MTV (cm3), and TLG (g) were offered by the TrueD system automatically.
2.3. Stereotactic body radiation therapy protocol
SBRT therapy was carried out by CyberKnife, SRS G4 treatment system (Accuray Incorporated, Sunnyvale). The real-time imaging was performed by using the vertebral tracking technology. Gross tumor volume (GTV) was defined as the radiographic evident tumor range in CT images, which was assessed by experienced radiation clinicians, after the fusion of Cyberspace Data Management System (CDMS) and CT images. Planning target volume (PTV) was defined as the GTV with extended margins (2–3 mm) in X, Y, and Z axis. The single radiation dose and repeated times were adjusted individually, normally the single irradiation was performed at a dose of 6 to 8 Gy, 4 to 8 times repeated.
2.4. Chemotherapy devilry
All patients included received chemotherapy. Among them, 13 patients received gemcitabine-based chemotherapy, 9 patients received S-1 based chemotherapy, and 1 patient received gemcitabine and S-1 combined chemotherapy. Meanwhile, 5 of 23 patients were treated with chemotherapy first, and 18 patients were first applied with SBRT therapy.
2.5. Statistical analysis
Statistics were performed using SPSS software (Version 22, IBM, Portsmouth, UK). Normal distributions of all parameters were analyzed by Kolmogorow-Smirnov test. For the correlation of CRT sequence and clinical figures, the figures coincided with normal distributions were analyzed by Chi-square test, and the non-normal distributed figures were applied with Mann–Whitney U test. All survival related variables were grouped upon median value and subsequently analyzed by Kaplan–Meier methods for univariate analysis. Significant factors from Log-rank test were then characterized by Cox proportional hazards models for multivariate analysis. The method for the independent variables screening was conditional parameter estimation likelihood ratio test (Forward: Conditional, the probability for entry stepwise was 0.05, and the probability for removal was 0.10). P <.05 was considered as statistically significant.
3. Results
3.1. Patient characteristics and follow-ups
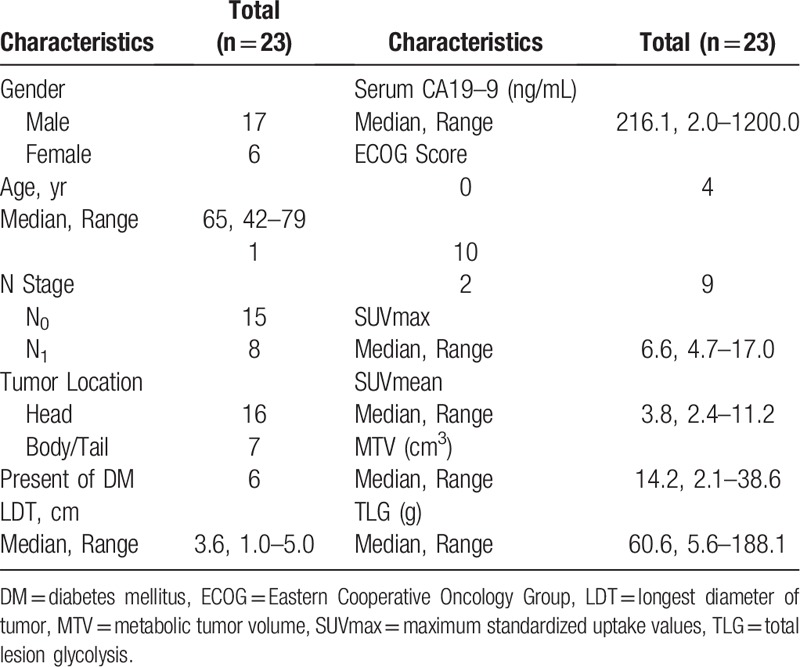
The patient characteristics were summarized in Table 1. In this study, the median follow-up duration was 35.5 months (18.0–55.0 months), and 5 patients (21.7%) were alive by the end of follow-ups. The median OS time was 16.7 months (6.5–33.0 months), and the 1-year and 2-year survival rates were 78.3% and 33.5%, respectively. Meanwhile, the median PFS period was 11.3 months (2.8–28.5 months), and the 1-year PFS rate was 47.8%.
Table 1.
Basic characteristics of patients.
3.2. Correlation between CRT sequence and clinical figures
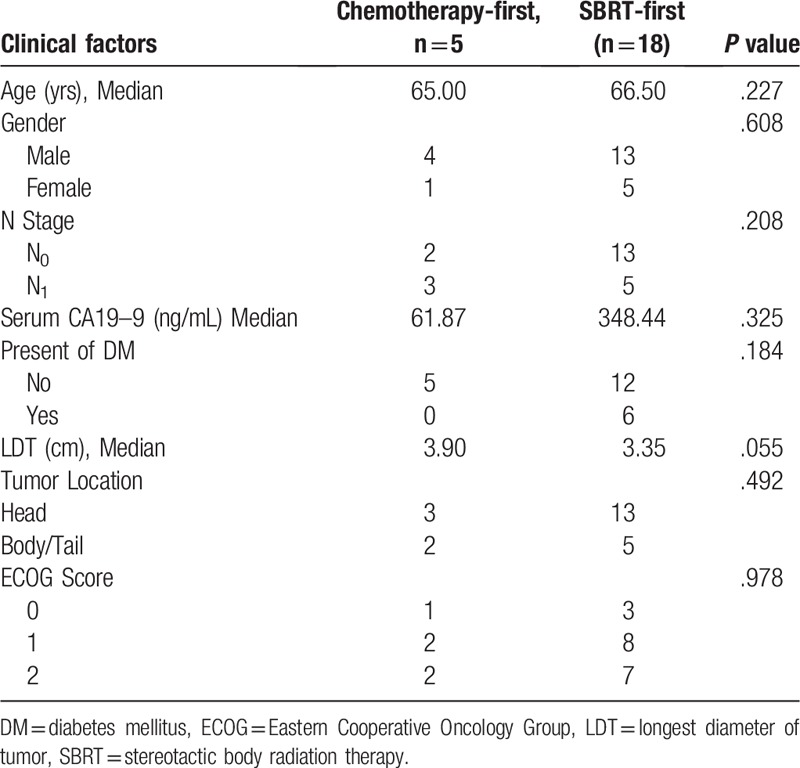
Patients were grouped into 2 based on CRT sequence and showed no statistically significant difference on pre-treatment ECOG score (P = .978). Additionally, no significant differences in group distributions were found upon other clinical factors as well (Table 2), indicating the prognostic results of CRT sequence were not impacted by pre-treatment states of patients.
Table 2.
Distribution of clinical factors upon chemoradiotherapy sequence.
3.3. Survival analysis and multivariate analysis
The survival analysis of all patients was carried out by Log-rank test (Table 3). Among 18F-FDG PET/CT metabolic parameters, MTV and TLG were demonstrated to be significant prognostic indicators for OS (Fig. 1A, B). For clinical factors, the longest diameter of tumor (LDT) and CRT sequence were revealed to have prognostic significance for OS (Fig. 1C, D). The above 4 variables were analyzed by Cox proportional hazards models afterward, MTV and LDT were proved to be the independent prognostic factors for OS (Table 4), the hazard ratio (HR) was 3.015 (95% CI: 1.107–8.212, P = .031) and 3.756 (95% CI: 1.352–10.440, P = .011), respectively.
Table 3.
Univariate analysis of prognostic factors for 23 locally advanced pancreatic cancer patients.
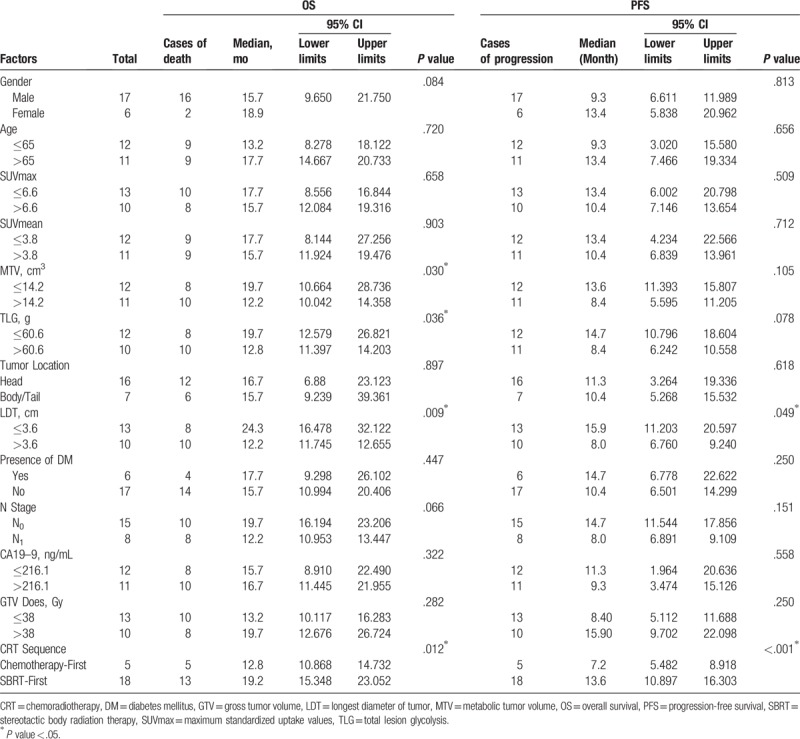
Figure 1.
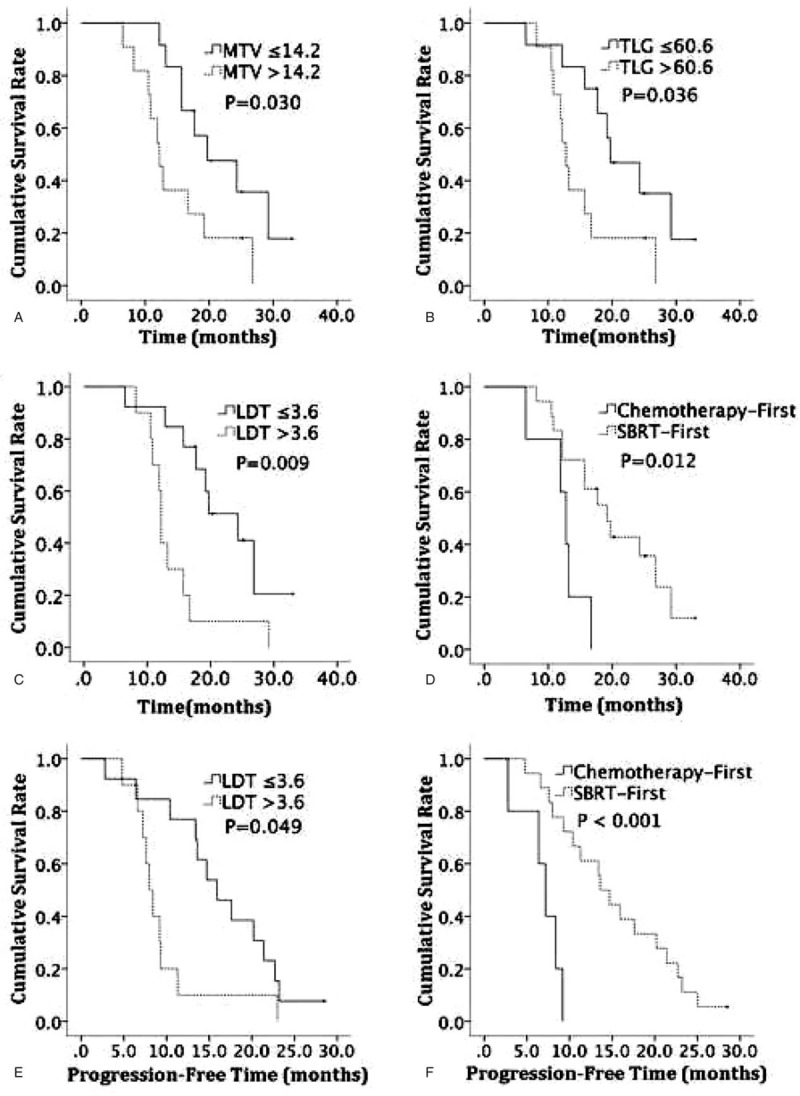
Cumulative OS according to MTV (A), TLG (B), LDT (C) and CRT sequence (D). Cumulative PFS according to LDT (E) and CRT sequence (F). LDT = longest diameter of tumor, MTV = metabolic tumor volume, OS = overall survival, PFS = progression-free survival, TLG = total lesion glycolysis.
Table 4.
Multivariate analysis of prognostic factors for overall survival in locally advanced pancreatic cancer patients.

As for PFS, LDT, and CRT sequence were found to be the significant prognostic variables (Table 3). In the following multivariate analysis, CRT sequence was the only independent prognostic factor for PFS (HR: 0.130, 95% CI: 0.034–0.495, P = .003) (Table 5). Compared to LAPC patients received chemotherapy first, patients in SBRT-first group demonstrated longer PFS durations.
Table 5.
Multivariate analysis of prognostic factors for progression free survival in locally advanced pancreatic cancer patients.

Typical cases of 18F-FDG PET/CT images were shown in Figures 2 and 3.
Figure 2.
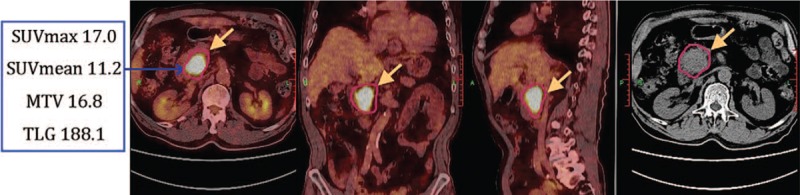
Male, 70 years old, pancreatic head cancer. 18F-FDG PET/CT fusion images showed a massive shadow in pancreatic head, with increased radioactivity uptake. Four metabolic parameters were all above the cut-off value, and the LDT was 3.9 cm. S-1 based chemotherapy was applied first, followed by SBRT treatment. OS of the patient was 16.7 months, and PFS was 9.2 months. LDT = longest diameter of tumor, OS = overall survival, PFS = progression-free survival, SBRT = stereotactic body radiation therapy.
Figure 3.
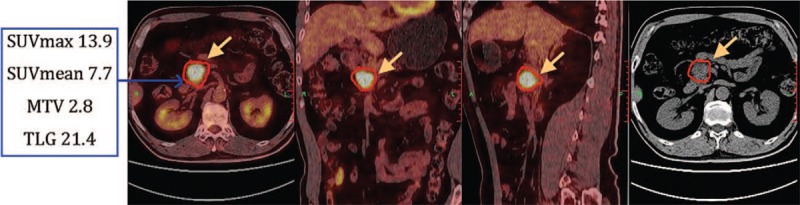
Male, 65 years old, pancreatic head cancer. 18F-FDG PET/CT image showed a lesion located in pancreatic head with increased radioactivity uptake. SUVmax and SUVmean above the cut-off value, MTV and TLG were below the cut-off value, and the LDT was 3.2 cm. The patient was firstly treated with SBRT therapy, followed by the S-1 based chemotherapy. OS of this patient was 33 months, and PFS was 28.5 months. MTV = metabolic tumor volume, OS = overall survival, PFS = progression-free survival, SBRT = stereotactic body radiation therapy, TLG = total lesion glycolysis.
4. Discussion
LAPC was considered as one of the most lethal cancers, presented with extremely poor prognosis. At present, lacking of effective treatment methods makes it essential to determine reliable prognostic indicators for LAPC patients.
However, clinically confirmed prognostic markers so far have been few, and tumor burden was considered to be the most promising one.[21] The absence of parameters which could sensitively and accurately describe the tumor burden has restricted its current application in clinic. Tumor diameter was the most commonly used clinical indicator for measuring tumor burden, and a relatively intuitive one as well. Jeong et al[22] found among 87 PDAC patients, the tumor size of the relapse-free group (1.8 ± 1.0 cm) was significantly lower than the relapse group (2.3 ± 0.8 cm, P = .01), and tumor diameter was confirmed as an independent factor for OS and relapse-free survival (RFS) for PDAC patients. In our study with LAPC patients received chemo-SBRT therapy; the analysis also suggested that LDT was associated with OS and PFS, and an independent prognostic factor for OS. However, as a 1-dimensional parameter, the tumor diameter cannot precisely reflect the tumor burden, not mention the active tumor cell loads in primary lesions, which has largely restricted tumor diameter to become a precision prognosis evaluation factor.
The volume-based 18F-FDG PET/CT parameters could offer information about the metabolism of tumor tissues, as well as the tumor volume, potentially have higher prognostic values than tumor diameter. In the early studies,[23] SUVmax was reported with prognostic value for PDAC patients, but recent studies revealed the shortages of SUVmax in prognosis prediction.[24,25] SUVmax only reflected the metabolic level of a single pixel in tumor tissue, which was unable to include the information of tumor volume and cannot provide a full picture of the overall tumor metabolic situation. In this study, through a research of LAPC patients treated with chemo-SBRT therapy, SUVmax and SUVmean showed no prognostic values (both P >.05).
MTV and TLG could provide details on both tumor volume and metabolism, which have been regarded as reliable measures of tumor burden in recent studies, and their roles in prognostic evaluation have been gradually confirmed. In a retrospective study done by Choi HJ et al,[26] MTV and TLG (SUVmax 2.5 as the delineation threshold) were both independent prognostic predictors for LAPC patients. Another study performed by Hyun et al,[27] which included 137 PDAC patients, found that TLG was statistically significant in univariate analysis but was not an independent prognostic factor. In the research carried out by Wu Peng et al,[28] MTV was revealed to be an independent prognostic factor for pancreatic cancer, which was similar to the conclusion of our study. In our study, 40% of SUVmax was used as the cut-off value, MTV obtained was significantly associated with the OS of LAPC patients received CRT. The OS duration of patients with MTV ≤14.2 cm3 was significantly longer than that of high MTV value group, and MTV was further proved to be an independent OS prognostic predictor. Meanwhile, TLG was well associated with OS, but was not an independent indicator.
The importance of MTV and TLG in pancreatic cancer prognostic predication varied in different clinical trials, which could be caused by inconsistent inclusion criteria. The variation of tumor differentiations, clinical stages, and treatment options would lead to diverse results. Additionally, the variation could also be caused by the measurement of MTV, which was influenced by many factors, especially the tumor boundary delineation. The setting of delineation thresholds could affect the authenticity of how much the actual tumor size was reflected by MTV. Several studies have suggested[29] 40% to 50% of SUVmax as the best thresholds for MTV calculation, MTV obtained within this range could offer a veracious description of actual tumor volume. Therefore, in this study, 40% of SUVmax was used as the cut-off value in tumor boundary delineations. Furthermore, automatic delineation was induced instead of manual operation, to avoid subjective errors and to offer a more objective description of the actual tumor volume.
In our study, more than confirming the prognostic values of 18F-FDG PET/CT parameters for CRT treated LAPC patients, the CRT sequence was also found to play a role in prognostic prediction. In clinic practice, to start CRT treatment with chemotherapy or SBRT therapy was normally determined by physicians’ personal experiences, there were no guidelines nor conclusive agreements about the treatment efficiency of altered CRT sequence.[14,30] Meanwhile, only few correlational studies has been performed to discuss the relationship between CRT sequence and survival results.[31] In our research, all LAPC patients were just treated with CRT, only in 2 different manners, no induction chemotherapy or other interference therapies included. The results illustrated that the median OS and PFS durations of patients in the SBRT-first group were significantly longer than those in the chemotherapy-first group, and CRT sequence was an independent prognostic factor for PFS. The possible reasons were analyzed as follows: to start with, the radiation-induced bystander effect (RIBE) and abscopal effect. The control of both local pancreatic tumor and micro-metastasis in circulatory system could be achieved by signal transduction and the like.[32] Moreover, according to the reports,[33] radiation could induce a significant up-regulation of the major histocompatibility complex (MHC)-I molecules and the tumor-associated antigens (TAAs) on the surface of tumor cells. A research performed by Sharma et al[34] also confirmed the significantly elevated level of a specific TAAs: tumor-testis antigen (CTA) in PDAC patients after radiation exposure. Extensive exposure of tumor cell surface antigens not only facilitated the activation of the immune system in vivo, also improved direct killing of primary tumor cells by secreting perforin and granzyme. Additionally, radio-induced antigen exposure could mediate tumor cell apoptosis via the Fas-FasL pathway and subsequently led to micro-metastases culling.[35] Furthermore, for LAPC patients who received chemotherapy after SBRT treatment, the exposure of MHC-I and TAAs was conducive to exert the effects of chemotherapeutic drugs, and conduced to a better drug efficacy, eventually resulted in a prolonged survival time. Even so, it is still necessary to conduct prospective studies with a larger sample size to optimize the prognostic role of CRT sequence, and to maximize the therapy efficiency for LAPC patients.
There were several limitations in this study: First, as a retrospective study, patients enrolled received various chemotherapy regimens, due to the case number difference in the 3 groups, statistical analysis cannot be performed to assess the prognostic role of various chemotherapy regimens for LAPC patients. Second, although the patient data were collected between January 2013 and January 2017, the total case number included was not quite large. Meanwhile, the number of chemotherapy-first group was relatively small, which might induce biased results to present study. Hence, for chemo-SBRT therapy treated LAPC patients, a prospective, multi-center, large-sample study may be necessary to verify the roles of 18F-FDG PET/CT metabolic parameters and the CRT sequence in prognostic evaluation.
5. Conclusions
In conclusion, for chemo-SBRT therapy treated LAPC patients, 18F-FDG PET/CT metabolic parameter MTV and clinical figure LDT were reliable independent prognostic evaluators for OS. Smaller tumor diameter and lower pretreatment MTV value were correlated with longer OS duration. Meanwhile, CRT sequence was the independent prognostic factor of PFS. Compared to LAPC patients received chemotherapy first, those who first received SBRT therapy tended to have longer PFS.
Author contributions
Conceptualization: Anyu Zhang, Shengnan Ren.
Data curation: Shengnan Ren, Yuan Yuan.
Formal analysis: Anyu Zhang.
Investigation: Xiaofei Zhu, Lingong Jiang, Danni Li.
Project administration: Changjing Zuo.
Supervision: Changjing Zuo.
Writing – Original Draft: Anyu Zhang.
Writing – Review & Editing: Anyu Zhang, Xiao Li, Changjing Zuo.
Footnotes
Abbreviations: 18F-FDG = 18F-fluorodeoxyglucose, ASCO = American Society of Clinical Oncology, CDMS = Cyberspace Data Management System, CI: confidence interval, CRT = chemoradiotherapy, DM = diabetes mellitus, ECOG = Eastern Cooperative Oncology Group, GTV = gross tumour volume, HR = hazard ratio, LAPC = locally advanced pancreatic cancer, LDT = longest diameter of tumor, MTV = metabolic tumor volume, OS = overall survival, OSEM = ordered subset expectation maximization, PDAC = pancreatic ductal adenocarcinoma, PET/CT = positron emission tomography-computed tomography, PFS = progression-free survival, PTV = planning target volume, SBRT = stereotactic body radiation therapy, SUVmax = maximum standardized uptake values, TLG = total lesion glycolysis.
AZ, SR and YY were equally contributed to the article, and were considered as co-first authors.
The authors declare that they have no conflict of interest.
References
- [1].Ferlay J, Soerjomataram I, Ervik M, et al. Cancer Incidence and Mortality Worldwide: IARC Cancer Base No. 11 [Internet]. Lyons, GLOBOCAN 2012 v1.0, International Agency for Research on Cancer, 2013, Available at: http://globocan.iarc.fr/ (accessed 11 January 2013). [Google Scholar]
- [2].Bilimoria KY, Bentrem DJ, Ko CY, et al. Validation of the 6th edition AJCC pancreatic cancer staging system: report from the national cancer database. Cancer 2007;110:738–44. [DOI] [PubMed] [Google Scholar]
- [3].Kauhanen SP, Komar G, Seppänen MP, et al. A prospective diagnostic accuracy study of 18F-fluorodeoxyglucose positron emission tomography/computed tomography, multidetector row computed tomography, and magnetic resonance imaging in primary diagnosis and staging of pancreatic cancer. Ann Surg 2009;250:957–63. [DOI] [PubMed] [Google Scholar]
- [4].Hammel P, Huguet F, van Laethem J-L, et al. Effect of chemoradiotherapy vs chemotherapy on survival in patients with locally advanced pancreatic cancer controlled after 4 months of gemcitabine with or without erlotinib: the LAP07 randomized clinical trial. JAMA 2016;315:1844–53. [DOI] [PubMed] [Google Scholar]
- [5].Real FX. A “catastrophic hypothesis” for pancreas cancer progression. Gastroenterology 2003;124:1958–64. [DOI] [PubMed] [Google Scholar]
- [6].Balaban EP, Mangu PB, Khorana AA, et al. Locally advanced, unresectable pancreatic cancer: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 2016;34:2654–68. [DOI] [PubMed] [Google Scholar]
- [7].Grainne M, Knox JJ. Locally advanced pancreatic cancer: an emerging entity. Curr Probl Cancer 2018;42:12–25. [DOI] [PubMed] [Google Scholar]
- [8].Neoptolemos J, Stocken D, Smith CT, et al. Adjuvant 5-fluorouracil and folinic acid vs observation for pancreatic cancer: composite data from the ESPAC-1 and-3 (v1) trials. Brit J Cancer 2009;100:246–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Chauffert B, Mornex F, Bonnetain F, et al. Phase III trial comparing intensive induction chemoradiotherapy (60 Gy, infusional 5-FU and intermittent cisplatin) followed by maintenance gemcitabine with gemcitabine alone for locally advanced unresectable pancreatic cancer. Definitive results of the 2000–01 FFCD/SFRO study. Ann Oncol 2008;19:1592–9. [DOI] [PubMed] [Google Scholar]
- [10].Jumeau R, Delouya G, Roberge D, et al. Stereotactic body radiotherapy (SBRT) for patients with locally advanced pancreatic cancer: a single center experience. Digest Liver Dis 2018;50:396–400. [Google Scholar]
- [11].Wang BH, Cao WM, Yu J, et al. Gemcitabine-based concurrent chemoradiotherapy versus chemotherapy alone in patients with locally advanced pancreatic cancer. Asian Pac J Cancer Prev 2012;13:2129–32. [DOI] [PubMed] [Google Scholar]
- [12].Landau E, Kalnicki S. The evolving role of radiation in pancreatic cancer. Surg Clin N Am 2018;98:113–25. [DOI] [PubMed] [Google Scholar]
- [13].De Bari B, Porta L, Mazzola R, et al. Hypofractionated radiotherapy in pancreatic cancer: lessons from the past in the era of stereotactic body radiation therapy. Crit Rev Oncol Hemat 2016;103:49–61. [DOI] [PubMed] [Google Scholar]
- [14].Tempero MA, Malafa MP, Al-Hawary M, et al. Pancreatic adenocarcinoma, version 2.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Ne 2017;15:1028–61. [DOI] [PubMed] [Google Scholar]
- [15].Willett CG, Czito BG, Bendell JC, et al. Locally advanced pancreatic cancer. J Clin Oncol 2005;23:4538–44. [DOI] [PubMed] [Google Scholar]
- [16].Roberto M, Falcone R, Mazzuca F, et al. The role of stereotactic body radiation therapy in oligometastatic colorectal cancer: clinical case report of a long-responder patient treated with regorafenib beyond progression. Medicine 2017;96:e9023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Zhu D, Wang Y, Wang L, et al. Prognostic value of the maximum standardized uptake value of pre-treatment primary lesions in small-cell lung cancer on 18F-FDG PET/CT: a meta-analysis. Acta Radiol 2018;59:1082–90. [DOI] [PubMed] [Google Scholar]
- [18].Kang CM, Lee SH, Hwang HK, et al. Preoperative volume-based PET parameter, MTV2. 5, as a potential surrogate marker for tumour biology and recurrence in resected pancreatic cancer. Medicine 2016;95:e2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Pacak K, Ilias I, Chen CC, et al. The role of [18F] fluorodeoxyglucose positron emission tomography and [111In]-diethylenetriaminepentaacetate-D-Phe-pentetreotide scintigraphy in the localization of ectopic adrenocorticotropin-secreting tumours causing Cushing's syndrome. J Clin Endocr Metab 2004;89:2214–21. [DOI] [PubMed] [Google Scholar]
- [20].Jung W, Jang JY, Kang MJ, et al. The clinical usefulness of 18F-fluorodeoxyglucose positron emission tomography–computed tomography (PET–CT) in follow-up of curatively resected pancreatic cancer patients. HPB 2016;18:57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Xu HX, Chen T, Wang WQ, et al. Metabolic tumour burden assessed by 18 F-FDG PET/CT associated with serum CA19–9 predicts pancreatic cancer outcome after resection. Eur J Nucl Med Mol I 2014;41:1093–102. [DOI] [PubMed] [Google Scholar]
- [22].Lee JW, Kang CM, Choi HJ, et al. Prognostic value of metabolic tumour volume and total lesion glycolysis on preoperative 18F-FDG PET/CT in patients with pancreatic cancer. J Nucl Med 2014;55:898–904. [DOI] [PubMed] [Google Scholar]
- [23].Nakata B, Chung YS, Nishimura S, et al. 18F-fluorodeoxyglucose positron emission tomography and the prognosis of patients with pancreatic adenocarcinoma. Cancer 1997;79:695–9. [PubMed] [Google Scholar]
- [24].Beltrame V, Gruppo M, Merigliano S, et al. Preoperative 18-FDG PET predicts survival in resectable pancreatic cancer. Eur J Surg Oncol 2015;41:S9–10. [Google Scholar]
- [25].Moon SY, Joo KR, So YR, et al. Predictive value of maximum standardized uptake value (SUVmax) on 18F-FDG PET/CT in patients with locally advanced or metastatic pancreatic cancer. Clin Nucl Med 2013;38:778–83. [DOI] [PubMed] [Google Scholar]
- [26].Choi HJ, Lee JW, Kang B, et al. Prognostic significance of volume-based FDG PET/CT parameters in patients with locally advanced pancreatic cancer treated with chemoradiation therapy. Yonsei Med J 2014;55:1498–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Hyun SH, Kim HS, Choi SH, et al. Intratumoural heterogeneity of 18F-FDG uptake predicts survival in patients with pancreatic ductal adenocarcinoma. Eur J Nucl Med Mol I 2016;43:1461–1468. [DOI] [PubMed] [Google Scholar]
- [28].Wu P, Yu LJ, Li XY. Prognostic value of metabolic tumour volume on 18F-FDG PET/CT imaging in patients with pancreatic cancer. Chin J Nucl Med Mol I 2016;36:408–12. [Google Scholar]
- [29].Uto F, Shiba E, Onoue S, et al. Phantom study on radiotherapy planning using PET/CT. J Radiat Res 2010;51:157–64. [DOI] [PubMed] [Google Scholar]
- [30].Gillen S, Schuster T, Zum CM, et al. Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. Plos Med 2010;7:e1000267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Huguet F, André T, Hammel P, et al. Impact of chemoradiotherapy after disease control with chemotherapy in locally advanced pancreatic adenocarcinoma in GERCOR phase II and III studies. J Clin Oncol 2007;25:326–31. [DOI] [PubMed] [Google Scholar]
- [32].Najafi M, Fardid R, Hadadi G, et al. The mechanisms of radiation-induced bystander effect. J Biomed Phys Eng 2014;4:163–72. [PMC free article] [PubMed] [Google Scholar]
- [33].Gameiro SR, Jammed ML, Wattenberg MM, et al. Radiation-induced immunogenic modulation of tumour enhances antigen processing and calreticulin exposure, resulting in enhanced T-cell killing. Oncotarget 2014;5:403–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Sharma A, Bode B, Wenger RH, et al. γ-Radiation promotes immunological recognition of cancer cells through increased expression of cancer-testis antigens in vitro and in vivo. Plos One 2011;6:e28217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Kepp O, Senovilla L, Vitale I, et al. Consensus guidelines for the detection of immunogenic cell death. Oncoimmunology 2014;3:e955691. [DOI] [PMC free article] [PubMed] [Google Scholar]


