Abstract
To evaluate the efficacy and safety of telbivudine (LdT) and tenofovir (TDF) for preventing hepatitis B virus (HBV) vertical transmission for HBV-positive pregnant women.
Pregnant women (n = 145) from January 2013 to June 2017 were enrolled when they met inclusion criteria, which included HBV DNA ≥1.0 × 107 copies/mL and increased alanine aminotransferase (ALT) levels. Groups A (n = 58) and B (n = 51) were treated with LdT and TDF, respectively. Group C (n = 36) received no antiviral treatment. All infants were vaccinated with hepatitis B immunoglobulin and HBV vaccine. Vertical transmission of HBV was indicated by the presence of hepatitis B surface antigen (HBsAg) in infants 6 months and 12 months after birth.
There is no difference of clinical characteristics of patients among the 3 groups. Serum HBV DNA levels of the 3 groups were similar at baseline (Group A vs. Group B vs. Group C, 7.88 ± 0.65 vs. 7.91 ± 0.75 vs. 7.69 ± 0.53 P = .25). In addition, the after anti-HBV treatment in Groups A and B were significantly decreased. Also, the serum HBV DNA levels in both Groups A and B were lower than that of Group C (P < .01, both). The HBV infection rate in Group A treated with LdT was not different from Group B treated with TDF. The dynamic changes of serum ALT level were similar. ALT levels were similar among the 3 Groups (P = .171), while there is statistically significant difference between A and C, and between B and C before delivery (P < .01). For the infants, there were no significant differences among body weight, height, head circumference, or Apgar score. However, the HBsAg positivity rates of infants in Groups A, B, C at postpartum 24 weeks and 48 weeks was 0%, 0%, and 11.1%, respectively (P < .001).
Administration of LdT or TDF to HBV-infected mothers are effective and safe to block mother-to-infant HBV transmission.
Keywords: drug efficacy, mother-to-infant transmission, telbivudine, tenofovir, hepatitis B virus
1. Introduction
Hepatitis B virus (HBV) infection is a serious worldwide health burden.[1] China is a highly endemic area with hepatitis B surface antigen (HBsAg) positive rate about 10%.[2] HBV mainly spreads through blood, mother–infant, and sexual transmission.[2,3] The vertical transmission of mother and infant is the main route of transmission of HBV in China. At present, the most effective mother–infant blockade is to perform active–passive double immunization with HBV vaccine combined with hepatitis B immunoglobulin (HBIG) within 24 hours after birth.[4] However, there are still 5% to 10% of infants who have immune failure.[4,5] The mother-to-infant transmission of HBV mainly includes intrauterine transmission (prenatal delivery), transmission during childbirth, and postpartum transmission. The cause of HBV vertical transmission block failure has not been fully understood. It is generally believed that the main factors include HBeAg-positive, HBV-DNA high viral load, intrauterine infection, and viral mutation.[5–7] Among them, intrauterine infection is the main reason for the failure of active and passive dual immunization.
Nucleoside analogs inhibit HBV-DNA replication and are currently approved to anti-HBV treatment.[8–10] Among them, tenofovir (TDF) and telbivudine (LdT), which can rapidly reduce serum HBV-DNA levels, were used to block vertical transmission of HBV. In previous study, LdT did not determine mutations, carcinogenesis, and associated embryonic or fetal toxicity in animal experiment.[11,12] Therefore, LdT is approved by FDA for blocking vertical transmission of HBV. However, the drug resistance barrier of LdT is low.[13] TDF is another effective anti-HBV drug due to its potency and high resistance barrier.[14,15] In HIV monoinfected and HIV/HBV c-infected mothers, it was reported that TDF had a favorable efficacy and safe profile to block vertical transmission.[16,17] However, to our knowledge, there are limited data available in the literature to compare the effectiveness of TDF and LdT therapy during pregnancy in highly viremic mothers with chronic hepatitis B and its impact on the perinatal transmission of HBV.
To identify effective approaches for preventing mother-to-infant transmission of HBV, the present study evaluated the efficacy and safety of LdT and TDF for treating HBV-positive pregnant women.
2. Methods
2.1. Patients
The Institutional Review Board had approved the study. Pregnant women (n = 145) from January 2013 to June 2017 who met the following inclusion criteria were enrolled: 20 to 35 years old; 20 to 28 weeks gestation; HBsAg and HBeAg-positive, and HBV DNA ≥1.0 × 107 copies/mL; elevated alanine aminotransferase (ALT) ≥2 upper limit of normal.
ALT levels were examined using the Olympus AU5400 biochemical analyzer (Tokyo, Jpan). Levels of HBV serological markers were determined using a commercially available radioimmunoassay (ARCHITECT i2000SR, Abbott Laboratories, Lake Bluff, IL) and serum HBV DNA viral load was detected using Daan real-time PCR test (Daan Gene Co, Ltd of Sun Yat-sen University, Guangdong, China, with linear range of 102–108 IU/mL).
Criteria for exclusion were as followed: patients have received interferon or other nucleos(t)ide analogues treatment previously, coinfected with hepatitis C virus, hepatitis D virus, hepatitis E virus, and HIV and received treatment of immunosuppressive or cytotoxic drugs, or corticosteroids during pregnancy.
2.2. Grouping and management
The patients were allocated to 3 groups according to their antiviral agents. Patients who received LdT were assigned to Group A. Patients who received TDF were assigned to Group B. patients in Group C received no antiviral therapy. Maternal blood samples were collected before treatment, before delivery, and at 12 weeks postpartum to detect HBV DNA and ALT levels.
All infants born to the enrolled women were vaccinated with 200 IU of HBIG within 6 hours. Then they were vaccinated with 20 μg of hepatitis B vaccine within 12 hours after birth, and at postpartum 4 and 24 weeks. The following data were collected at birth: height, weight, head circumference, 1-minute Apgar score, and birth defects (the presence of any deformities). In addition, adverse pregnancy events (e.g., eclampsia, premature rupture of membranes, and premature delivery), postpartum hemorrhage, and cesarean section were recorded. Serum HBsAg in infants were tested at 6 and 12 month postpartum.
2.3. Statistical analyses
The data were analyzed using software SPSS 17.0 (Chicago). The chi-squared test was applied to compare categorical variables among groups. Continuous variables are presented as mean ± standard deviation. Differences among groups were compared with ANOVA test. P < .05 was considered statistically significant.
3. Results
3.1. Pregnancy characteristics of patients enrolled
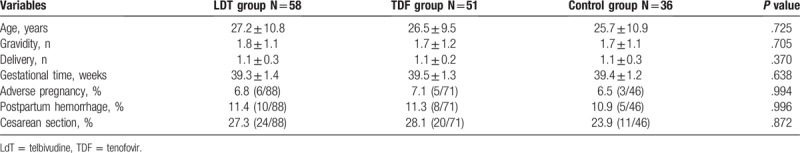
In this study, 145 pregnant women were enrolled and 58 of them treated with LDT while 51 treated with TDF during pregnancy. All infants enrolled have neonatal standard immunoprevention. Clinical characteristics of patients in Groups A, B, and C were similar with regard to age, gravidity, delivery, and gestational age at delivery, as shown in Table 1.
Table 1.
Pregnancy characteristics and safety rates of the 3 groups.
3.2. Serum HBV DNA levels before and after treatment
The dynamic changes of serum HBV DNA level were shown in Figure 1. Serum HBV DNA levels of the 3 groups were similar (Group A vs. Group B vs. Group C, 7.88 ± 0.65 vs. 7.91 ± 0.75 vs. 7.69 ± 0.53, P = .25). The HBV DNA viral load in Groups A and B were significantly decreased after treatment in Group A and B. The serum HBV DNA levels in both Groups A and B were lower than that of Group C (P < .01, both). There were no significant differences in HBV DNA levels between Groups A and B before delivery or at 12 weeks postpartum.
Figure 1.
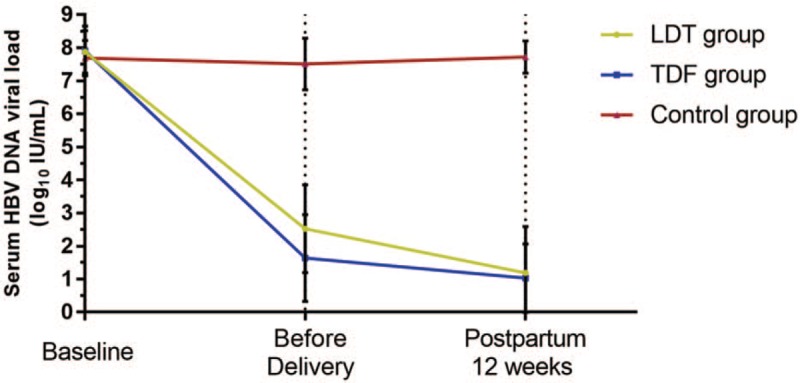
The dynamic changes of serum HBV DNA level in groups. Serum HBV DNA levels of the 3 groups were similar at baseline (Group A vs. Group B vs. Group C, 7.88 ± 0.65 vs. 7.91 ± 0.75 vs. 7.69 ± 0.53 log10 copies/mL, P = .25). The HBV DNA viral load in Groups A and B were significantly decreased after treatment (2.52 ± 1.33 and 1.64 ± 1.31 log10 copies/mL in Group A and B). The serum HBV DNA levels in both Groups A and B were lower than that of Group C (P < .01, both), while no significant differences observed in HBV DNA levels between Groups A and B before delivery and at 12 weeks postpartum. HBV = hepatitis B virus.
3.3. ALT levels before and after treatment
The dynamic changes of serum ALT level were shown in Figure 2. There were no significant differences in ALT level among Groups A, B, and C (P = .171). The differences between A and C, and between B and C were statistically significant before delivery (P < .01), as well as at week 12 postpartum (P < .01). There were no significant differences in ALT level between Groups A and B before delivery or at postpartum 12 weeks.
Figure 2.
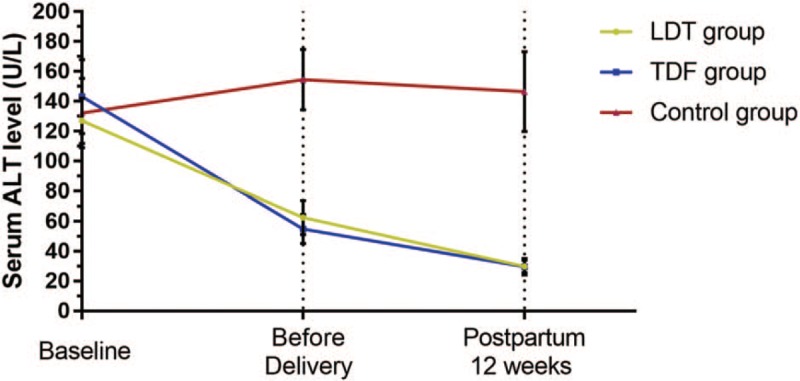
The dynamic changes of serum ALT level were shown. There were no significant differences in ALT level among Groups A, B, and C (127.3 ± 72.2, 143.3 ± 104.6, and 132.3 ± 78.3 in Group A, B, and C, P = .171). The differences between A and C, and between B and C were statistically significant before delivery (62.5 ± 53.7, 54.8 ± 41.2, and 154.6 ± 67.8 U/L in Group A, B, and C, P < .01), as well as at week 12 postpartum (29.9 ± 19.8, 29.8 ± 23.8, and 146.6 ± 89.8 U/L, P < .01). There were no significant differences in ALT level between Groups A and B before delivery or at postpartum 12 weeks. ALT = alanine aminotransferase.
3.4. Safety of LdT and TDF during pregnancy
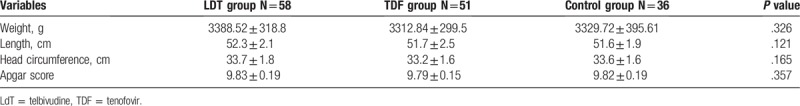
The clinical characteristics of infants were shown in Table 2. There were no significant differences in body weight, height, head circumference, or Apgar score among the infants among the 3 groups. Furthermore, there were no significant differences in the incidence of adverse pregnancy events (including eclampsia, premature rupture of membranes, premature birth, postpartum hemorrhage, or cesarean section) (Table 1). In addition, there were no virologic resistance found assessed by evaluating genotypic changes using HBV polymerase/reverse transcriptase assay.
Table 2.
General conditions of infants in the 3 groups.
3.5. Infant HBV infection rate at week 28 postpartum
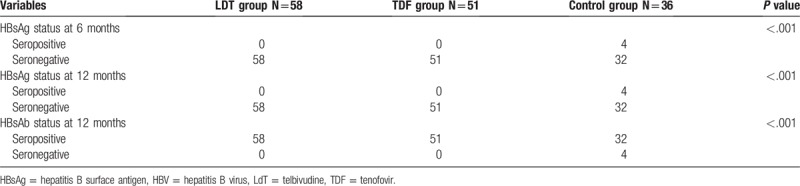
The HBsAg positivity rates of infants in Groups A, B, C at postpartum 6 months and 12 months were 0%, 0%, and 11.1%, respectively (P < .001, Table 3). The differences in HBV infection rate between Groups A and C (P < .01) and between Groups B and C (P < .01) were statistically significant. However, there is no difference observed in HBV infection rate in Group A and Group B.
Table 3.
HBV infection of infants in the 3 groups.
4. Discussion
Our findings showed no vertical transmission in patients receiving LdT or TDF treatment while a vertical transmission rate of 11.1% occurred in patients receiving no anti-HBV treatment, which confirmed the effectiveness of anti-HBV treatment during pregnancy to block vertical transmission. In this retrospective study, the 109 patients in Group A and B receiving anti-HBV drugs during pregnancy did not have complications observed.
Recently, evidence-based medicine has been used to confirm the safety of anti-HBV drugs during pregnancy.[18,19] The experience of human beings using anti-HBV treatment during pregnancy is mainly from lamivudine.[4,20] There are increasing safety data of LdT to prevent vertical transmission in clinical practices.[11,21–23] Many studies reported that TDFs are effective to prevent vertical transmission in HIV-infected mother.[16,17] Among 5 FDA approved oral anti-HBV agents, TDFs are the most effective agents due to their resistance profile and potency.[24,25] However, limited data were presented to compare the effectiveness of LdT and TDF during pregnancy. TDF and LdT are classified as category B for use in pregnancy. According the results of our study, both LdT and TDF are effective and safe used in pregnant mother to block HBV vertical transmission.
This study has some limitations. This is a retrospective study, so data and conclusions may be biased. Prospective controlled studies of pregnancy are still needed. In view of the serious medical and social problems of pregnancy and anti-HBV treatment, how to maximize the risk avoidance and maximize the benefits, we need to accumulate more evidence-based data.
Author contributions
Conceptualization: Hua li, Jianyong Zeng.
Data curation: Hua Li, Caixia Zheng.
Formal analysis: Hua Li, Jianyong Zeng.
Investigation: Hua Li.
Methodology: Hua Li.
Project administration: Hua Li, Caixia Zheng.
Software: Jianyong Zeng.
Supervision: Hua Li, Caixia Zheng.
Validation: Hua Li, Caixia Zheng.
Writing – original draft: Jianyong Zeng, Caixia Zheng.
Writing – review & editing: Jianyong Zeng, Caixia Zheng.
Footnotes
Abbreviations: ALT = alanine aminotransferase, CHB = chronic hepatitis B, HBIG = hepatitis B immunoglobulin, HBsAg = hepatitis B surface antigen, HBV = hepatitis B virus, HCC = hepatocellular carcinoma, HIV = immunodeficiency virus, LdT = telbivudine, NUC = nucleos(t)ide analogues, TDF = tenofovir.
Ethics approval and consent to participate: The study was reviewed and approved by the Medical Ethics Committee of First Affiliated Hospital of Xiamen University. Informed consent was obtained before antiviral treatment.
The authors have no conflicts of interest to disclose.
References
- [1].European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu; European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol 2017;67:370–98. [DOI] [PubMed] [Google Scholar]
- [2].Sarin SK, Kumar M, Lau GK, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int 2016;10:1–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Cai SH, Lu SX, Liu LL, et al. Increased expression of hepatocyte nuclear factor 4 alpha transcribed by promoter 2 indicates a poor prognosis in hepatocellular carcinoma. Therap Adv Gastroenterol 2017;10:761–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Fan R, Yin X, Liu Z, et al. A hepatitis B-free generation in China: from dream to reality. Lancet Infect Dis 2016;16:1103–5. [DOI] [PubMed] [Google Scholar]
- [5].Wang Z, Zhang J, Yang H, et al. Quantitative analysis of HBV DNA level and HBeAg titer in hepatitis B surface antigen positive mothers and their babies: HBeAg passage through the placenta and the rate of decay in babies. J Med Virol 2003;71:360–6. [DOI] [PubMed] [Google Scholar]
- [6].Lee SD, Lo KJ, Tsai YT, et al. Maternal hepatitis B virus DNA in mother-infant transmission. Lancet 1989;1:719. [DOI] [PubMed] [Google Scholar]
- [7].Yin WJ, Shen LP, Wang FZ, et al. Hepatitis B immunoprophylactic failure and characteristics of the hepatitis B virus gene in mother-infant Pairs in parts of China. Biomed Environ Sci 2016;29:790–801. [DOI] [PubMed] [Google Scholar]
- [8].Cai SH, Lv FF, Zhang YH, et al. Dynamic comparison between Daan real-time PCR and Cobas TaqMan for quantification of HBV DNA levels in patients with CHB. BMC Infect Dis 2014;14:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Xiao YB, Cai SH, Liu LL, et al. Decreased expression of peroxisome proliferator-activated receptor alpha indicates unfavorable outcomes in hepatocellular carcinoma. Cancer Manag Res 2018;10:1781–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Xue X, Cai S. Comment on “assessment of liver stiffness in pediatric Fontan patients using transient elastography”. Can J Gastroenterol Hepatol 2016;2016:9343960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hu Y, Xu C, Xu B, et al. Safety and efficacy of telbivudine in late pregnancy to prevent mother-to-child transmission of hepatitis B virus: a multicenter prospective cohort study. J Viral Hepat 2018;25:429–37. [DOI] [PubMed] [Google Scholar]
- [12].Song J, Yang F, Wang S, et al. Efficacy and safety of antiviral treatment on blocking the mother-to-child transmission of hepatitis B virus: a meta-analysis. J Viral Hepat 2019;26:397–406. [DOI] [PubMed] [Google Scholar]
- [13].Cai S, Cao J, Yu T, et al. Effectiveness of entecavir or telbivudine therapy in patients with chronic hepatitis B virus infection pre-treated with interferon compared with de novo therapy with entecavir and telbivudine. Medicine (Baltimore) 2017;96:e7021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Cai S, Li Z, Yu T, et al. Serum hepatitis B core antibody levels predict HBeAg seroconversion in chronic hepatitis B patients with high viral load treated with nucleos(t)ide analogs. Infect Drug Resist 2018;11:469–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Terrault NA, Bzowej NH, Chang KM, et al. AASLD guidelines for treatment of chronic hepatitis B. Hepatology 2016;63:261–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Nachega JB, Uthman OA, Mofenson LM, et al. Safety of tenofovir disoproxil fumarate-based antiretroviral therapy regimens in pregnancy for HIV-infected women and their infants: a systematic review and meta-analysis. J Acquir Immune Defic Syndr 2017;76:1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Siemieniuk RA, Foroutan F, Mirza R, et al. Antiretroviral therapy for pregnant women living with HIV or hepatitis B: a systematic review and meta-analysis. BMJ Open 2017;7:e19022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Park JS, Pan CQ. Viral factors for HBV mother-to-child transmission. Hepatol Int 2017;11:476–80. [DOI] [PubMed] [Google Scholar]
- [19].Sun W, Zhao S, Ma L, et al. Telbivudine treatment started in early and middle pregnancy completely blocks HBV vertical transmission. BMC Gastroenterol 2017;17:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Cai S, Yu T, Jiang Y, et al. Comparison of entecavir monotherapy and de novo lamivudine and adefovir combination therapy in HBeAg-positive chronic hepatitis B with high viral load: 48-week result. Clin Exp Med 2016;16:429–36. [DOI] [PubMed] [Google Scholar]
- [21].Cai S, Ou Z, Liu D, et al. Risk factors associated with liver steatosis and fibrosis in chronic hepatitis B patient with component of metabolic syndrome. United European Gastroenterol J 2018;6:558–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Xue X, Cai S, Ou H, et al. Health-related quality of life in patients with chronic hepatitis B during antiviral treatment and off-treatment. Patient Prefer Adherence 2017;11:85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Wu X, Cai S, Li Z, et al. Potential effects of telbivudine and entecavir on renal function: a systematic review and meta-analysis. Virol J 2016;13:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ou H, Cai S, Liu Y, et al. A noninvasive diagnostic model to assess nonalcoholic hepatic steatosis in patients with chronic hepatitis B. Therap Adv Gastroenterol 2017;10:207–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Zeng J, Cai S, Liu J, et al. Dynamic changes in liver stiffness measured by transient elastography predict clinical outcomes among patients with chronic hepatitis B. J Ultrasound Med 2017;36:261–8. [DOI] [PubMed] [Google Scholar]


