Abstract
Rationale:
Clostridium difficile-associated diarrhea (CDAD) remains a persistent challenge, with substantially increased incidence and severity. The rising burden of CDAD requires urgent identification of preventable risk factors.
Patients concerns:
A 77-year-old man with the symptoms of abdominal pain and watery diarrhea was readmitted to the hospital, who received cephalosporins and proton pump inhibitors (PPIs) during the initial hospitalization for 12 days until discharge. Antibiotic-associated diarrhea was seriously suspected. And the stool sample was immediately sent for inspection for C difficile. He had a history of chronic bronchitis, coronary heart disease, and osteonecrosis.
Diagnosis:
CDAD, renal insufficiency
Interventions:
Oral vancomycin was administered for 14 days.
Outcomes:
On the third day after readmission, the stool sample turned out to be positive for both C difficile toxin and its antigen. After 10-day treatment with vancomycin, diarrhea symptoms disappeared and his stools became normal.
Lessons:
In elderly patients with multiple comorbidities, PPIs must be administered cautiously to minimize the risk for adverse effects including CDAD. It is important to identify the preventable risk factors of CDAD for clinicians and pharmacists. Oral vancomycin therapy seems to be effective in CDAD.
Keywords: antibiotic, cephalosporins, clostridium difficile-associated diarrhea, proton pump inhibitors
1. Introduction
Clostridium difficile (C difficile) is a ubiquitous gram-positive and spore-forming bacillus that may cause gastrointestinal illness, ranging in severity from mild diarrhea to fulminant colitis and even death.[1] It has emerged as the leading cause of approximately 25% of all cases of antibiotic-associated diarrhea worldwide.[2] There has been a dramatic change in the epidemiology of C difficile-associated diarrhea (CDAD) in recent years, with an alarmingly increase in incidence and severity. A recent meta-analysis demonstrated that in Asia the proportion of CDAD in patients with nosocomial diarrhea was 14.8% and the related mortality was found to be 8.9%.[3] As the severity of the disease increases, symptoms include fever, leucocytosis, nausea, dehydration associated with profuse diarrhea, abdominal pain, and distension.[4] Certain risk factors are associated with increased likelihood of CDAD, such as hospitalization, elder age, antibiotic usage, underlying medical conditions, gastrointestinal surgery, nasogastric tubes, and so on.[5] Thus, the identification of preventable risk factors of CDAD has gained urgency and importance. This case revealed the risk factors of CDAD and indicated that the physicians should be aware of the possibility of CDAD when the patient has known risk factors.
Proton pump inhibitors (PPIs) which could reduce the production of gastric acid by blocking the proton pump, are widely prescribed drugs and could be used to treat gastrointestinal diseases, such as dyspepsia peptic ulcer disease, gastroesophageal reflux disease, functional dyspepsia, stress gastritis, and so on. Owing to their high efficacy and relative safety, an excess of drug prescriptions has been widespread in Chinese hospitals.[6] However, recent studies have shown that PPIs are associated with an increased risk of some adverse reactions including osteoporotic-related fractures, C difficile infection, community-acquired pneumonia, Vitamin B12 deficiency, and so on.[7] This case emphasizes the necessity to improve the appropriate and rational use of PPIs for pharmacists.
Here, we report a patient with several comorbidities, who developed CDAD after treatment with cephalosporins and PPIs in the hospital.
2. Case report
A 77-year-old man was admitted to the hospital because of the repeated and paroxysmal pain in upper abdomen along with watery diarrhea for 7 days. He had 3 to 4 loose stools daily along with nausea and acid reflux. On physical examination, his abdomen was distent but tender. The body temperature was 37°C. Laboratory investigation revealed a leucocytosis of 28.48 × 109/L with neutrophil ratio of 92.90% and C-reaction protein of 242.63 mg/L. His serum creatinine was 224 μmol/L, leading to the diagnosis of renal insufficiency.
He had a history of chronic bronchitis for 15 years with relatively higher incidence in winter and spring, without administration of any medication for it. And he had suffered coronary heart disease for more than 10 years, treated with oral betalocton, furosemide, and spironolactone. Furthermore, oral diclofenac was administered for 2 and a half years to release the pain caused by necrosis of the right hip and left knee joint.
The patient was diagnosed as acute gastrointestinal infection and was empirically treated with intravenous cefepime at a dose of 1 g twice per day for 5 days followed by oral cefdinir capsules 0.1 g twice daily until discharge. For the treatment of abdominal discomfort, he received intravenous drop infusion of pantoprazole for 5 days, followed by oral omeprazole until discharge. He was also prescribed oral clostridium butyricum tablets to improve intestinal flora. His symptoms resolved after treatment for 12 days. After discharge, he continued antibacterial treatment with cefdinir capsule for 5 days at home. Moreover, his laboratory test results show a serum albumin level of 2.3 g/dL and hemoglobin level of 80 g/L before discharge.
Six days after discharge, his symptoms recurred, again experiencing abdominal pain and watery diarrhea with the daily passage of 7 to 8 stools. After a duration of symptoms for 6 days, he was readmitted to the hospital. The patient had a soft abdomen, and his body temperature was 37.1°C. Antibiotic-associated diarrhea was seriously suspected. The treatment included empirically anti-infection with oral metronidazole 0.4 g twice per day, parenteral nutrition support, remission of symptoms with montmorillonite powder, regulation of intestinal bacteria flora, and intravenous pantoprazole 160 mg daily to relieve the discomfort. Other than that, the stool sample was immediately sent for inspection for C difficile. On the third day after admission, stool sample was turned out to be positive for both C difficile toxin and its antigen. Leucocytes in stool were 40 to 50/HP and the occult blood test was positive. By a comprehensive consideration for the present clinical manifestations and laboratory test results, metronidazole was withdrawn after treatment for 3 days and 125 mg vancomycin was orally administered every 6 hours as a standard 14-day course. After 10-day treatment of vancomycin, he improved and his stools became normal. At 14th day, multiple ulcers in different parts of the colon were found under colonoscopy, as in Figure 1, which may further indicate the infection of C difficile. The patient was administered intravenous pantoprazole until discharge and continued oral treatment with rabeprazole for 14 days at home.
Figure 1.
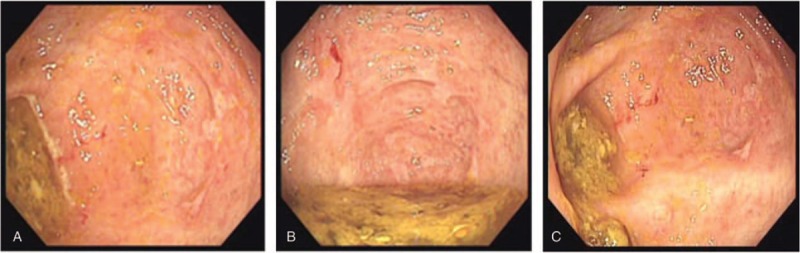
Multiple ulcers in different parts of the colon under colonoscopy.
Since the second discharge until now, diarrhea and abdominal pain symptoms of this patient have been resolved and have not recurred.
3. Discussion
Prior antibiotic therapy, in particular, broad-spectrum antibiotics with activity against enteric bacteria, is considered the single most important risk factor in the development of CDAD.[4] A study on C difficile infection (CDI) after exposure to antibiotics revealed that the second and third generation cephalosporins, as well as carbapenems, were the strongest risk factors for developing CDI.[8] The patient was prescribed the cephalosporins even after the first discharge. The incidence of CDI is projected to increase, partly caused by greater spread of hypervirulent strains resistant to commonly used antibiotics.[9]C difficile could colonize the human intestinal tract after the gut flora altered by antibiotic therapy. Therefore, prudent use of antimicrobials is the first and foremost step in reducing the risk of CDAD.
More recently, PPIs have been implicated as a novel potential contributor to CDI.[10] A recent meta-analysis of 50 studies involving 342,532 individuals showed a significant association between PPIs therapy and increased risk of CDI as compared with nonusers.[11] This result was supported by another systematic review and meta-analysis including 56 studies (40 case–control and 16 cohort) which found the risk of CDI almost 2-times higher in PPIs users than in nonusers.[12] Nevertheless, almost all the published literatures were observational up to now, the causality and the precise mechanism regarding this positive association remain unclear and need further investigations. The patient had taken intravenous infusions and oral enteric-coated tablets of PPIs during 2 times of hospitalizations. His gastric acid as a barrier to ingested bacteria and bacterial overgrowth was profoundly inhibited after antibiotic and PPIs therapies, which at least in part led to the proliferation of spores and enhance their ability to convert to a vegetative form of C difficile.[13] It is recommended that appropriate treatment duration of PPIs should be prescribed based on well-established indications.[14] Unfortunately, the information regarding the impact of PPIs on the CDI had not been provided in time by the pharmacist during his second hospitalization in this case.
In addition to the broad-spectrum antimicrobial therapy and PPIs, the patient presented other risk factors leading to the development of CDAD, such as advanced age, previous hospitalization, underlying medical conditions, renal insufficiency, hypoalbuminemia, the presence of comorbidities.[12] CDAD is frequently acquired within hospitals where the bacterial load is likely high, so this patient was more prone to exposed to the pathogen C difficile and presented the clinical symptoms of CDAD several days following the initial discharge. Besides, elder age and existing comorbidities of the patient may result in the lack of effective humoral immunity to antitoxin as well as the lower concentrations of circulating and fecal antibodies against C difficile toxins A and B.[5] In addition, he was prescribed oral diclofenac for 2 and a half years which may give rise to the injuries of upper gastrointestinal tract. These host factors of the patient may explain the risk profile for CDAD.
Severe CDI was empirically defined to consist of any 2 of the following variables: age older than 60 years, temperature higher than 101°F (38.4°C), serum albumin less than 2.5 g/dL, and peripheral white blood cell count greater than 15,000/μL.[15] This patient was classified as severe CDAD according to this standard, thus was prescribed oral vancomycin which is recommended as the first-line treatment for severe CDI for more than 2 decades.[16] Furthermore, it is important to take strict hygienic measures for all patients with CDAD to prevent spread of the pathogen.
The case presented here concerned the impact of antibiotic and PPIs therapy on CDAD, indicating the importance of appropriate prescribing of acid-suppression therapy and high-risk antibiotics. Making clinical pathway specified on PPIs use may be a practical way to promote the rational use of PPIs based on evidence-based medicine.[14]
Author contributions
Funding acquisition: Xiaoqun Lv.
Investigation: Yujuan Liu, Weifang Ren.
Resources: Miao Jiang, Zhonghong Fang.
Writing - original draft: Xiaoqun Lv.
Writing - review and editing: Jun Zhang, Zhonghong Fang.
Footnotes
Abbreviations: CDAD = Clostridium difficile-associated diarrhea, CDI = Clostridium difficile infection, PPIs = proton pump inhibitors.
Written informed consent was obtained from the patient to participate to this case report and any accompanying images.
This work was supported by grant from “Qi Hang” Project of Jinshan Hospital, Fudan University (2018-JSYYQH-05).
The authors report no conflicts of interest.
References
- [1].Leffler DA, Lamont JT. Clostridium difficile infection. N Engl J Med 2015;372:1539–48. [DOI] [PubMed] [Google Scholar]
- [2].Sun YX, Zhao YT, Teng LL, et al. Clostridium difficile infection associated with antituberculous agents in a patient with tuberculous pericarditis. Intern Med 2013;52:1495–7. [DOI] [PubMed] [Google Scholar]
- [3].Borren NZ, Ghadermarzi S, Hutfless S, et al. The emergence of Clostridium difficile infection in Asia: a systematic review and meta-analysis of incidence and impact. PLoS One 2017;12:e0176797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Masciullo V, Mainenti S, Lorusso D, et al. Lethal Clostridium difficile colitis associated with paclitaxel and carboplatin chemotherapy in ovarian carcinoma: case report and review of the literature. Obstet Gynecol Int 2010;749789–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bauer MP, Goorhuis A, Koster T, et al. Community-onset Clostridium difficile-associated diarrhoea not associated with antibiotic usage – two case reports with review of the changing epidemiology of Clostridium. Neth J Med 2008;66:207–11. [PubMed] [Google Scholar]
- [6].Xin C, Dong Z, Lin M, et al. The impact of pharmaceutical interventions on the rational use of proton pump inhibitors in a Chinese hospital. Patient Prefer Adherence 2017;12:21–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Maes ML, Fixen DR, Linnebur SA. Adverse effects of proton-pump inhibitor use in older adults: a review of the evidence. Ther Adv Drug Saf 2017;8:273–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hensgens MP, Goorhuis A, Dekkers OM, et al. Time interval of increased risk for Clostridium difficile infection after exposure to antibiotics. J Antimicrob Chemother 2012;67:742–8. [DOI] [PubMed] [Google Scholar]
- [9].Loo VG, Poirier L, Miller MA, et al. A predominantly clonal multi-institutional outbreak of Clostridium difficile-associated diarrhea with high morbidity and mortality. N Engl J Med 2005;353:2442–9. [DOI] [PubMed] [Google Scholar]
- [10].Croft L, Ladd J, Doll M, et al. Inappropriate antibiotic use and gastric acid suppression preceding Clostridium difficile infection. Infect Control Hosp Epidemiol 2016;37:494–5. [DOI] [PubMed] [Google Scholar]
- [11].Cao F, Chen CX, Wang M, et al. Updated meta-analysis of controlled observational studies: proton-pump inhibitors and risk of Clostridium difficile infection. J Hosp Infect 2018;98:4–13. [DOI] [PubMed] [Google Scholar]
- [12].Trifan A, Stanciu C, Girleanu I, et al. Proton pump inhibitors therapy and risk of Clostridium difficile infection: systematic review and meta-analysis. World J Gastroenterol 2017;23:6500–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Amir I, Konikoff FM, Oppenheim M, et al. Gastric microbiota is altered in oesophagitis and Barrett's oesophagus and further modified by proton pump inhibitors. Environ Microbiol 2014;16:2905–14. [DOI] [PubMed] [Google Scholar]
- [14].Li B, Ma H, Wang Z, et al. When omeprazole met with asymptomatic Clostridium difficile colonization in a postoperative colon cancer patient: a case report. Medicine (Baltimore) 2017;96:e9089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Zar FA, Bakkanagari SR, Moorthi KM, et al. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile–associated diarrhea, stratified by disease severity. Clin Infect Dis 2007;45:302–7. [DOI] [PubMed] [Google Scholar]
- [16].Kelly CP. A 76-year-old man with recurrent Clostridium difficile-associated diarrhea: review of C. difficile infection. JAMA 2009;301:954–62. [DOI] [PubMed] [Google Scholar]


