Abstract
Context
Recent studies have examined level and rate of change of anti-Müllerian hormone (AMH) for predicting time to menopause. Limited prospective, longitudinal data exists evaluating measures of ovarian reserve (MOR) in cancer survivors.
Purpose
Determine the rate of change of MOR in survivors (15 to 39 years) compared with similar-aged controls and compared with late reproductive-aged controls (40 to 50 years).
Design
Prospective cohort.
Setting
Quaternary university hospital.
Participants
Survivors at least 1 year from therapy completion, similar-aged controls, and late reproductive-aged controls.
Interventions
Annual visits with early follicular-phase hormone analysis and ultrasound.
Main Outcome Measure
Changes in AMH and antral follicle count (AFC) were modeled using random effects linear regression.
Results
Cancer survivors (170) and 135 similar-aged controls had annual visits for an average of 38 months; 71 late reproductive-aged controls were followed for an average of 24 months. In models adjusted for body mass index, time since cancer therapy (for survivors), and exogenous hormone use, the geometric mean AMH and AFC levels were lower in the survivors than similar-aged controls at all ages. After age 24.5 AMH and AFC declined in both groups at rates that were similar (P = 0.78 for AMH, P = 0.37 for AFC). Late reproductive-aged controls declined at a much more precipitous rate of 30% per year for AMH and 16% per year for AFC (P < 0.01 compared with survivors).
Conclusions
Although survivors had lower levels of AMH and AFC at the time of enrollment, the rate of change of AMH and AFC is not significantly different than similar-aged controls.
Cancer survivors were followed longitudinally. Although the absolute levels of anti-Müllerian hormone and antral follicle count were lower in survivors than similar-aged controls at all ages, the rate of decline over time did not differ.
Cancer survival has improved for nearly all types of cancer over the past decade (1), allowing cancer patients to look beyond their diagnosis and treatment and to plan for their lives after cancer therapy. Future fertility and the length of the reproductive window are important concerns for many cancer survivors (2, 3). Whereas many studies have retrospectively examined the deleterious effects of cancer therapy, including sterilization, early menopause, and osteoporosis (4–7), there exist limited prospective data examining changes to ovarian reserve in cancer survivors (8–10) and even less data to estimate how these changes impact clinical outcomes, such as incidence of pregnancy or time to menopause (11–13).
Serum hormone levels and some ultrasound measures have been used as a proxy to estimate fertility potential. In particular, anti-Müllerian hormone (AMH) and antral follicle count (AFC) have shown some use as measures of ovarian reserve (MOR) (14–17). However, much of the data currently available about these measures is primarily cross-sectional, and values range widely at any given age (18, 19). A lack of normative data and specifically, a lack of repeated measures over time within individuals, means that it is difficult to interpret a single isolated level of AMH or a single value of AFC and determine how it applies to the reproductive window or fecundity (20, 21). There is some evidence that the examination of within subject rate of change in repeated measures of AMH may help to predict the time to menopause (22–24); however, it is unknown whether that normative data can be applied to cancer survivors.
The primary objective of this study was to collect prospective longitudinal MOR in both reproductive-aged cancer survivors and healthy controls to evaluate the effect of cancer therapy on both the absolute levels and rate of change over time of ovarian reserve measures. Our hypothesis was that both levels and the rate of change of these measures would be different in cancer survivors compared with similar-aged controls and that the levels and rates of change of the survivors may more closely resemble late reproductive-aged controls. We also hypothesized that within the group of cancer survivors, there may be differences in the levels and rate of change of ovarian reserve based on individual and treatment characteristics, and we sought to model those changes.
Materials and Methods
This report is the result of a prospective cohort study at the University of Pennsylvania (Penn) that is part of the Ovarian Reserve After Cancer: Longitudinal Effects (or ORACLE) study. Summary statistics from the enrollment study visit in an initial cohort have been published previously (25). After enrollment, participants were seen for annual study visits that included a questionnaire, physical examination, pelvic ultrasonography, and hormone analysis.
Subjects
Postmenarchal, premenopausal cancer survivors were recruited primarily from the Children’s Hospital of Philadelphia Survivorship Program and the Transition Program at Penn’s Living Well After Cancer Survivorship Program during the years 2006 to 2017. At the time of enrollment, participants were required to be aged 15 to 39 years, be at least 1 year from the end of cancer therapy without evidence of disease, have undergone cancer therapy with chemotherapy, and have a uterus and both ovaries. Participants were excluded if they had a history of a brain or ovarian malignancy, been pregnant or lactating within 3 months, or any other medical history associated with ovarian dysfunction. Participants who reported use of a hormonal contraceptive or hormone therapy were required to discontinue hormone use for 4 weeks before a scheduled study visit and were seen during the subsequent menstrual cycle.
Unexposed controls, similar in age to the cancer survivor cohort (ages 15 to 39) and late reproductive-aged controls (ages 40 to 50) were identified through health practices affiliated with Penn and through advertising. Controls were required to be postmenarchal, with regular menstrual cycles (21 to 35 days), a uterus, and both ovaries. Exclusion criteria were the same as for survivors.
The Institutional Review Board at Penn approved this study, and informed consent was obtained from all participants. Study visits occurred on days 1 to 4 of the menstrual cycle, with the exception of cancer survivors with irregular cycles or no menses for 6 weeks after stopping hormones for whom study visits occurred at any point.
Questionnaires
During a structured interview, detailed information was collected regarding demographics, medical history, menstrual characteristics, pregnancies, infertility history, contraception, medications, and substance use. Use of exogenous hormones was recorded at each visit, including use of hormonal contraceptives, tamoxifen, gonadotropin-releasing hormone agonists, or hormone replacement therapy. Participants were given a menstrual diary and were asked to provide the dates of their two most recent menses. Women were categorized as having regular menstrual cycles if they reported regular menses (21 to 35 days).
Cancer therapy
Diagnosis and treatment exposure data were obtained by abstraction of medical records. Treatment was summarized for chemotherapeutic type, duration, cumulative dose, and radiation dose and location. Cyclophosphamide equivalent doses (CEDs) were calculated (26) to allow for comparisons of therapy toxicity across disparate cancer types.
Physical examination
Height and weight were measured for calculating body mass index (BMI).
Pelvic ultrasonography
Mean ovarian volume (MOV) and AFC were determined by ultrasonography. Transvaginal ultrasonography was preferred, although transabdominal ultrasound was performed in participants uncomfortable with the transvaginal approach. MOV was calculated using the ellipse formula (A × B × C × 0.52). AFC was determined as the number of follicles, 2 to 10 mm in average diameter, for subjects undergoing transvaginal ultrasonography when both ovaries were visualized.
Hormone analysis
Blood samples were obtained at each study visit for determining levels of follicle-stimulating hormone (FSH), luteinizing hormone (LH), estradiol (E2), inhibin B, and AMH. Serum hormones were frozen and initially thawed for measurement in batches at Penn’s Clinical Translational Research Center using FSH and E2 Coat-A-Count kits (Diagnostic Products Corp., Los Angeles, CA) and inhibin B and AMH ELISA kits (Diagnostic Systems, Webster, TX).
With the development of newer assays to measure AMH levels better, closer to the lower limit of detection, additional frozen serum aliquots from the same study visits that had never been thawed were sent to Ansh Laboratories (Webster, TX) for thaw and reanalysis using newer assays. Serum AMH was measured using the picoAMH ELISA (lower limit of detection 0.0012 ng/mL; 1 ng/mL = 7.14 pM; Ansh Laboratories). The AMH ELISA had intra- and interassay coefficients of variation of 3.7% and 4.5%, respectively. All of the AMH, E2, and FSH results in this paper reflect the Ansh Laboratories data, with the exception of 9% of visits for which no frozen serum aliquots remained to send to Ansh Laboratories for reanalysis with the new assays. In those cases, the AMH values from the Diagnostic Systems assay at Penn were used for analysis and were converted to picograms per milliliter using the results of a model built from the 501 samples for which we had both Diagnostic Systems and Ansh Laboratories assay results available (27). The correlation between these two assays was excellent, with a Spearman correlation coefficient of 0.943. A sensitivity analysis was performed at the conclusion of analysis, with these 9% of AMH values censored to ensure that the major study findings remained consistent. The range of the Ansh Laboratories E2 is 25 to 2000 pg/mL, with a lower limit of detection of 9.7 pg/mL and intra- and interassay coefficients of variation of 6.8% and 7.2%, respectively. The Ansh Laboratories FSH assay has a range of 0.16 to 100 mIU/mL, with intra- and interassay coefficients of variation of 7.9% and 7.5%, respectively. The LH and inhibin B measures were run only at Penn. The range of the inhibin B ELISA is 10 to 531 pg/mL, with a sensitivity of 7 pg/mL and interassay and intra-assay coefficients of variation <8% and <6%, respectively. The LH immunoradiometric assay has a range of 1.5 to 300 mIU/mL, sensitivity of 0.15 mIU/mL, and interassay and intra-assay coefficients of variation of <7% and <2%, respectively.
Data analysis
An a priori sample-size calculation assumed equal numbers of participants in each of the cancer survivor and similar-aged control groups, 80% power, and type I alpha error of 5%. The SD for log AMH change from 1 year of follow-up data was 0.83 for survivors and 0.5 for control women. Given these assumptions, a sample size of 135 women per group was necessary to detect a difference of −0.3 of the SD effect size in the annual change in log-transformed AMH per year between the groups. This corresponds to a geometric mean (GM) ratio of 0.74 or greater or adequate power to detect a 26% slower rate of decline in cancer survivors compared with similarly aged control women. Baseline characteristics, log-transformed hormone levels, and ultrasound data at the time of enrollment were summarized using Pearson χ2 analyses for categorical data and t tests or linear-regression models controlling for age, BMI, and exogenous hormone use for continuous data.
To investigate ovarian reserve changes over time (slope), log-transformed hormone levels and ultrasound data were modeled using general linear-regression models (random effects/growth curve) over study visits, accounting for correlations within individuals. Graphical inspection illustrated nonlinear trends over time. Models assuming a quadratic time trend were observed to have a point of maximization for ages in the range 24 to 25 years. A changepoint model (28), with a knot at 24.5 years of age for AMH, best fit the data and is consistent with previous descriptions of AMH distribution over the population (19). The changepoint model and quadratic model fit the data similarly, and the results for testing group differences were similar. Model fit was assessed using Akaiki Information Criteria (29). Given that our primary goal was to describe the decline of AMH over time, we present the changepoint model, where the rate of change in AMH is estimated separately for ages less than vs greater than age 24.5. Significance tests of variance components compared models using both a random slope and random intercept to random intercept-only models and indicated that the simpler random intercept-only (fixed effect for slope) model had a better fit to the data. Statistical tests of interaction between slopes and important risk factors were performed to examine whether these factors modified the rate of change over time. Other covariates, such as BMI, time elapsed since the end of cancer therapy, and exogenous hormone use before the 4-week washout period, were also considered and included as covariates in the final multivariable model. BMI was included in models as a dichotomous variable with median cut point, and time since cancer therapy was included in the model as a variable with quartiles. Tests of collinearity were performed between age of survivors and time since cancer therapy, and they were acceptable. Study data were collected and managed using REDCap electronic data capture tools contained at Penn. Statistical analysis was performed using STATA v15.0 (StataCorp, College Station, TX).
Results
Cancer survivors (170), 135 similarly-aged controls, and 71 late reproductive-aged controls were enrolled in this study. Baseline characteristics at the time of enrollment are presented in Table 1. Cancer survivors and similar-aged controls were comparable in terms of age, race, and BMI. Cancer survivors were less likely to have completed college (although reported a higher income), less likely to report having ever been pregnant, and more likely to have used exogenous hormones within the past year.
Table 1.
Demographics of Participants at Baseline Visit
| Participants With Cancer (n = 170) | Similar-Aged Controls (n = 135) | P a | Late Reproductive-Aged Controls (n = 71) | P b | |
|---|---|---|---|---|---|
| Age, y (mean range) | 24.5 (15–39) | 25.8 (14–39) | 0.04 | 45.1 (39–50) | <0.01 |
| BMI (mean range) | 24.2 (15.2–50.6) | 24.9 (18.0–54.3) | 0.28 | 26.8 (19.3–44.0) | <0.01 |
| Race: White (%/n) | 92 (157/170) | 83 (112/135) | 0.27 | 93 (66/71) | 0.81 |
| Education ≥ college graduate (%/n) | 53 (78/170) | 70 (94/135) | <0.01 | 67 (48/71) | <0.01 |
| Income > 50 k (%/n) | 43 (69/161) | 23 (24/102) | <0.01 | 62 (42/68) | <0.01 |
| Marital status: single (%/n) | 68 (115/170) | 79 (106/135) | 0.13 | 42 (30/71) | <0.01 |
| Previous pregnancy (%/n) | 15 (26/170) | 21 (28/135) | 0.22 | 83 (59/71) | <0.01 |
| Exogenous hormone use within the past year (%/n) | 43 (73/170) | 19 (25/135) | <0.01 | 3 (2/71) | <0.01 |
| Regular menstruation (%/n) | 79 (134/170) | 100 (135/135) | <0.01 | 100 (71/71) | <0.01 |
| Current smoking (%/n) | 3 (5/170) | 10 (13/135) | 0.01 | 13 (9/71) | <0.01 |
| MOR (median; IQR) | |||||
| AMH, pg/mLc | 877 (710–1127) | 2982 (2548–3735) | <0.01 | 401 (179–826) | <0.01 |
| FSH, mIU/mLc | 11.1 (10.5–11.5) | 6.5 (5.8–7.1) | <0.01 | 10.8 (9.5–12.1) | 0.30 |
| Estradiol, pg/mLc | 42.6 (35.6–49.6) | 48.4 (44.4–52.1) | 0.16 | 48.5 (47.1–50.2) | <0.01 |
| LH, mIU/mLc | 5.0 (4.1–6.9) | 3.9 (3.6–4.2) | <0.01 | 4.5 (2.5–6.8) | <0.01 |
| Inhibin B, pg/mLc | 27.1 (23.6–32.3) | 39.9 (39.3–41.1) | <0.01 | 32.1 (26.3–37.7) | <0.01 |
| MOV, cm3c | 5.3 (4.6–6.2) | 7.1 (6.2–7.8) | <0.01 | 6.2 (5.7–6.7) | <0.01 |
| AFC, countc | 14 (12.5–17) | 24.5 (22–28) | <0.01 | 9.5 (7.8–11.8) | <0.01 |
| Cancer type (%/n) | |||||
| Breast | 8 (15/170) | ||||
| Leukemia | 29 (52/170) | ||||
| Lymphoma | 29 (52/170) | ||||
| Sarcoma | 15 (26/170) | ||||
| Brain | 1 (2/170) | ||||
| Wilm’s | 6 (11/170) | ||||
| Other | 7 (12/170) | ||||
| Alkylating agent use (%/n) | 85 (144/170) | ||||
| Cyclophosphamide equivalent score (median; IQR) | 6239 (3000–13,196) | ||||
| Pelvic radiation therapy (%/n) | 4 (7/170) | ||||
| Time since cancer therapy, y (median; IQR) | 9.9 (5.1–16.0) | ||||
| Length of follow-up (median mo; IQR) | 37 (18.1–59) | 38 (19.4–63) | 0.45 | 24 (14–36.2) | <0.01 |
| Number of follow-up visits (mean; range) | 4.1 (2–9) | 4.3 (2–9) | 0.67 | 3.0 (2–5) | <0.01 |
Boldface indicates P < 0.05. Abbreviation: IQR, interquartile range.
Comparison between survivors and similar-aged controls.
Comparison between survivors and late reproductive-aged controls.
GM controlled for age, race, BMI, and reported exogenous hormone use within the past year.
At the time of enrollment, levels of AMH and inhibin B, AFC, and MOV were all significantly lower, and FSH was higher in cancer survivors compared with similar-aged controls, even when controlled for age, BMI, and whether the participant reported having used exogenous hormones within the past year. Adjustment for potential confounders, including smoking, income, race, and education, did not change estimates of association and therefore, were not included in the models. In unadjusted analyses, levels of AMH and AFC were significantly higher in the cancer survivors compared with the late reproductive-aged controls, although the MOV in the survivors was lower. There was no difference in FSH levels between cancer survivors and late reproductive-aged controls.
The cancer survivors were a median 9.9 years from the end of their cancer therapy (range 1 to 28 years); 144 (85%) received treatment with alkylators, seven (4%) received pelvic radiation, and 35 (20%) had a history of bone marrow transplant [BMT; 15 with total body irradiation (TBI)]. Median age at diagnosis was 13 years (range 4 months to 36 years of age). Seventy-four (44%) were treated before menarche and 96 (56%) after menarche.
Cancer survivors were followed prospectively with annual visits for a median of 3.1 years (range one to eight), with a mean of 4.1 study visits (range two to nine). There were no statistically significant differences in the mean length of follow-up or number of study visits between the survivors and similar-aged controls. The late reproductive-aged controls were followed for a shorter period of time, median 24 months and three study visits.
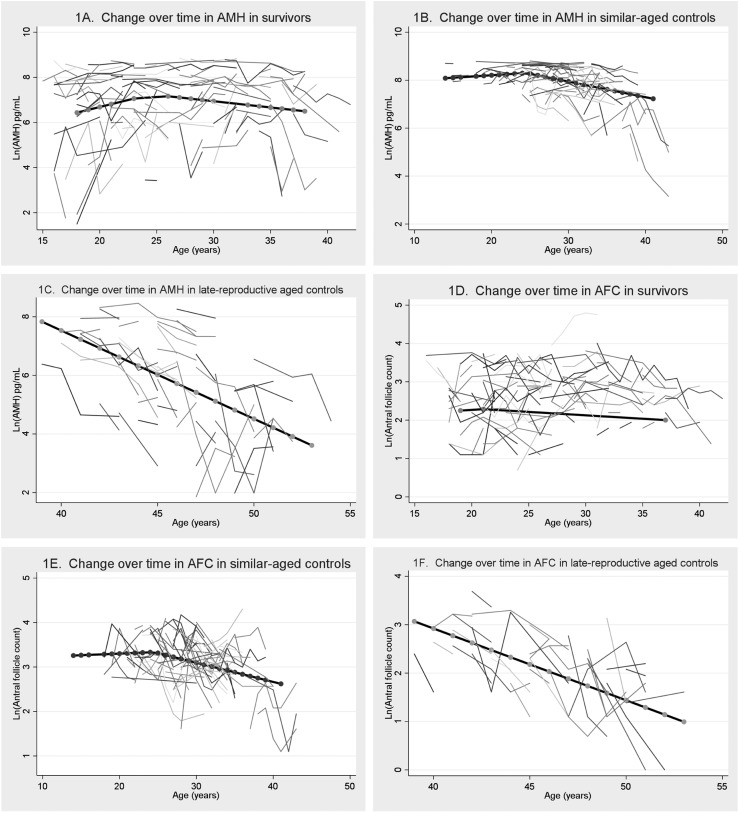
Both the survivors and the similar-aged controls demonstrated rising AMH levels before a point of maximization of age 24.5, followed by a gradual linear decline. However, the absolute levels of AMH and the rate of change over time varied between cancer survivors and controls. At all ages, the absolute levels of AMH were significantly lower than similar-aged controls. For example, the GM of AMH, estimated at age 20, was 3665 pg/mL in controls compared with 801 pg/mL in survivors (P ≤ 0.01) in models that controlled for BMI, time since cancer therapy, and reported exogenous hormone use at some point in the past year. Before age 24.5, AMH rose at a rate of 12.5% per year in survivors compared with 2.0% in similar-aged controls (difference in rate of change P < 0.01) until at age 24.5, controls had a mean AMH of 4019 pg/mL compared with 1406 pg/mL in survivors (P < 0.01).
Beyond age 24.5 years, the absolute levels of AMH remained lower in the survivors compared with the similar-aged controls at all ages. The GM of AMH at age 30 in controls was 2811 pg/mL compared with 1036 pg/mL in survivors (P < 0.01), whereas at age 35, the GM of AMH was 2031 pg/mL for controls compared with 785 pg/mL for survivors (P < 0.01). AMH fell in both groups over time, although the rates were not different; in survivors, AMH decreased at a rate of 5.5% per year compared with a fall of 6.5% per year in controls (P = 0.78). When analysis was restricted to include only the subset of cancer survivors with regular menses who had never taken exogenous hormones, the decrease in AMH over time after age 24.5 was even slower, at 3.1% per year, compared with the 6.5% in controls (P = 0.21). These trends are summarized graphically in Fig. 1A and 1B).
Figure 1.
Change over time of AMH and AFC in cancer survivors, similar-aged controls, and late reproductive-aged controls. (A and B) Change over time in AMH in survivors compared with similar-aged controls. (C) Change over time in AMH in late reproductive-aged controls. (D and E) Change over time in AFC in survivors compared with similar-aged controls. (F) Change over time in AFC in late reproductive-aged controls. Ln, natural log.
We also evaluated the levels and change over time in AMH in a group of late reproductive-aged controls who were still premenopausal (aged 40 to 50 years) to see if perhaps the levels and rates of change of cancer survivors more closely resembled these older women than their similarly-aged counterparts. The levels of AMH were lower in the late reproductive-aged women, and the rate of decline of AMH in the late reproductive-aged controls was significantly faster than the cancer survivors, at 30% per year (P < 0.01). These data are summarized in Fig. 1C.
Similar trends were noted for AFC. The GM of AFC, estimated at age 20, was 27 follicles for similar-aged controls compared with 12 follicles for survivors (P ≤ 0.01). The rate of change of AFC before age 24.5 was an increase of 0.7% per year for controls compared with 1.7% per year for cancer survivors (P = 0.78). Over age 24.5, AFC was also shown to decrease over time, at a rate of 4.3% for controls compared with 2.8% for all survivors (P = 0.37) and a rate of 3.9% for survivors who reported regular menses without exogenous hormones (P = 0.59 compared with controls). The GM of AFC at age 30 was 22 follicles in controls compared with 12 follicles in survivors (P < 0.01), and at age 35, the GM was 18 follicles in controls compared with 10 follicles in survivors (P < 0.01). These trends are summarized graphically in Fig. 1D and 1E). Compared with late reproductive-aged controls, the rate of decline of AFC was significantly slower in survivors, as late-reproductive women’s AFC declined precipitously at 16% per year (P < 0.01). This is summarized in Fig. 1F.
For FSH, there was a statistically significant difference in the levels and the rate of change over time in survivors compared with controls. At age 20, the adjusted GM for FSH was 5.9 mIU/mL in controls compared with 11.2 mIU/mL in survivors (P < 0.01). At age 30, the adjusted GM for FSH was 7.4 mIU/mL in controls compared with 10.9 mIU/mL in survivors (P < 0.01). The rate of change of FSH in controls was an increase of 2.3% per year compared with a relatively static 0.3% per year in survivors (P = 0.04). For the other MOR, no statistically significant differences were noted in the rates of change over time.
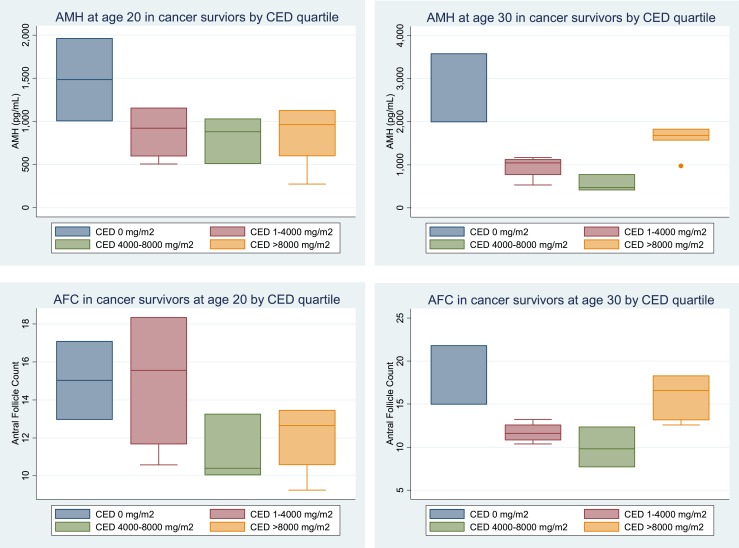
We then investigated whether within the group of cancer survivors, there were any factors that influenced either the level or the rate of change of AMH or AFC over time. Thirty-one percent of the survivors (n = 54) in this cohort were treated with chemotherapy, with a high alkylator score, defined as a score of three or more. For the purposes of analysis, CEDs were used, as these allow for comparison of cancer therapy toxicity in disparate cancer types (26). For those who received alkylating agents, the median CED was 6239 mg/m2, with an interquartile range of 3000 to 13,196 mg/m2. There were no statistically significant differences in the rates of change of AMH over time in those who received high-dose (>4000 CED) vs low-dose (<4000 CED) cancer therapy. The rate of change of AMH per year, before age 24.5, in those receiving low-dose therapy was 10.5% compared with 17.2% in those who received high-dose therapy (P = 0.35). The rate of change of AMH after age 24.5 in those receiving low-dose therapy was −4.1% per year compared with −8.5% per year in those receiving high-dose therapy (P = 0.44). However, there were substantial differences in the absolute levels themselves. At ages 20 and 30, the adjusted AMH GMs by CED quartile received are shown in Fig. 2.
Figure 2.
AMH and AFC at ages 20 and 30 in cancer survivors by CED quartile.
Likewise, the rate of change of AFC per year before age 24.5 in those receiving low-dose therapy is essentially static at 0% per year compared with a rate of 2.8% per year in those receiving high-dose therapy (P = 0.58). After age 24.5, the rate of change of AFC per year in those receiving low-dose therapy was −2.9% per year compared with −3.4% per year in those receiving high-dose therapy (P = 0.87). At ages 20 and 30, the adjusted AMH GMs by CED quartile received are shown in Fig. 2.
In survivors who had received a BMT, again, there was no statistically significant difference in rates of change over time of either of the measures when controlled for CED, but there were differences in the levels themselves. Survivors who had been exposed to BMT had natural log AMH levels at age 30 that were 92% lower (P < 0.01) and natural log AFCs 59% lower (P < 0.01) than survivors not exposed, and this was persistent even when CED was included in the model. Likewise, the 15 survivors who had undergone TBI had AMH levels 93% lower (P < 0.01) and AFC levels 62% lower (P = 0.01) than other survivors, even when controlling for CED and BMT. There were no differences in the absolute levels nor the rates of change over time of AMH or AFC based on receipt of pelvic radiation (n = 7 survivors) compared with survivors who did not.
Within cancer survivors, there was no difference in AMH or AFC levels based on menstrual regularity (ever reported an irregular period vs always regular periods) or whether the survivors reported a previous pregnancy compared with those survivors who had never been pregnant. Within all of the participants, there was no difference in AMH or AFC levels in those who had never been on exogenous hormones compared with those who had discontinued use at least 30 days before the study visit.
Discussion
This prospective longitudinal study compares the rate of change of hormones between cancer survivors remote from treatment with healthy, young controls and late reproductive-aged women. This study demonstrates that both AMH and AFC decline with age after a midreproductive age peak in both groups. However, the absolute levels of AMH and AFC are significantly lower in cancer survivors compared with similar-aged controls at all ages. Notably, however, the rate of decline after age 24.5 in survivors is not significantly different than in controls. This is an important finding for the counseling of cancer survivors, as it indicates that although cancer therapy affects the levels of these MOR, it does not subsequently lead to an accelerated rate of decline over time. The largest prospective study to examine longitudinal changes in AMH over time in healthy women found that at the same age, women with higher AMH initially had a slower rate of decline than women with a lower AMH before age 40, at which point, both high and low groups demonstrated a precipitous decline (21). In our study, however, the cancer survivors had a decline that was not statistically, significantly different than similar-aged controls, which is reassuring, despite their lower absolute levels. This is in line with a prior study that examined rates of change in AMH in cancer survivors over a shorter time period (30). The rate of change of AMH and AFC of the late reproductive-aged controls did demonstrate a significantly accelerated decrease in levels over time, consistent with other normative data (21).
In this study, the absolute levels of AMH and AFC in cancer survivors are between those of unexposed, similar-aged women and unexposed, late reproductive-age women. AMH has been shown to correlate with the primordial follicle number determined histologically (16), and cancer therapy has been shown to cause follicular apoptosis and cortical fibrosis (31) that diminish the number of these follicles. Importantly, however, despite these depleted measures, many of these women are still reporting regular menses and have reported conception, reflecting that these measures may reflect the quantity and not the quality of oocytes within the ovaries.
Importantly, this study supports prior observations of a dose-dependent relationship between cancer therapies and MOR (32–34). Specifically, cancer survivors with greater exposure to alkylating agents, BMT and TBI, had the most impaired ovarian reserve. There was no association with pelvic radiation, but the number of women with this exposure in this study was very small.
This study adds to the dearth of normative longitudinal data available, examining AMH and AFC levels in healthy, reproductive-aged women. Whereas these measures have been shown to be useful in the assessment of natural, reproductive aging and the prediction of response in infertile women undergoing assisted reproductive technology (35–37), the lack of association with outcomes, including the prediction of unassisted conception in a noninfertile population (20), makes these numbers difficult to interpret in isolation. This study demonstrates a rise in levels of AMH and AFC in reproductive-aged women before 24.5, followed by a gradual decline. This is consistent with prior cross-sectional data from a normative population (19). This fluctuation across the reproductive lifespan makes longitudinal analyses challenging, and caution should be used with the interpretation of absolute levels of AMH of AFC in women <24 years of age, as these may not reflect the maximum potential ovarian reserve (19). Of note, the increase before age 24.5 was more notable in cancer survivors compared with controls, which may reflect some underlying physiologic difference in this group, perhaps a differential rate of recruitment of primordial follicles into the follicular pool that may be related to the previous treatment. Additional longitudinal data are necessary to understand how to interpret levels in women in this age demographic.
Strengths of this study include the length of follow-up and the number of interindividual serial hormone measures analyzed. The restriction of the study to nonpregnant, nonlactating females, not using hormones and without other causes of ovarian dysfunction, reduced confounding. Hormone variability was decreased by using only early follicular measures. Cancer diagnoses and treatments were validated with medical records to diminish misclassification bias.
Whereas once thought to be static throughout the menstrual cycle (17, 38), newer studies have demonstrated a decrease in AMH levels in those women using exogenous hormones (39, 40). We did not find that the women who had stopped hormones 1 month before their study visit had AMH or AFC levels different than those who had never been on hormones, indicating that at least in this population, a 4-week “washout” period was sufficient.
Several limitations of this study should be noted. Typical of studies involving childhood and young-adult cancer survivors, subjects with a variety of diagnoses and treatments were included. Therefore, it was not possible to compare the effect of specific chemotherapeutic regimens on ovarian reserve in this study. In addition, whereas this study has a long longitudinal follow-up time period examined in a cancer survivor population, the overall length of follow-up time of several years is relatively short when making conclusions about the reproductive lifespan, and additional follow-up of this cohort is necessary to strengthen these findings. Likewise, data presented in this report are also insufficient to assess the predictive value of MOR or estimate the “reproductive window,” and therefore, these tests should be interpreted with caution by clinicians. Given that there was no difference in hormone levels within survivors menstruating regularly or not or demonstrating previous fecundity or not, this study corroborates previous findings that hormone levels and menstrual function should not be used to predict spontaneous pregnancy rates or need for contraception in cancer survivors (11, 41).
Clinically, the results of this study are important for the counseling of cancer survivors about the need to interpret their MOR with caution in relation to normative populations. In survivors, levels remain relatively static, and a comparatively decreased level of AMH does not necessarily mean that undetectable levels are imminent. Likewise, cancer survivors can achieve unassisted pregnancy with diminished ovarian reserve (11), as can a healthy population (20). Yet, at the same time, the long-term implication of these diminished levels remains a mystery. It is possible that lower levels may lead to an earlier acceleration in decline at the late reproductive-aged stages, and thus, cancer survivors interested in pregnancy should not delay attempts. The prediction of the length of the reproductive window for both cancer survivors and the general population remains an enormous clinical challenge that will only be solved with additional prospective information about large cohorts of women as they progress through life.
Acknowledgments
Financial Support: Support for this work was provided by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (Grants K01 L:1-CA-133839-03 to C.G. and 1R01HD062797 to C.G. and M.D.S.).
Clinical Trial Information: ClinicalTrials.gov no. NCT02467231 (registered 9 June 2015).
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- AFC
antral follicle count
- AMH
anti-Müllerian hormone
- BMI
body mass index
- BMT
bone marrow transplant
- CED
cyclophosphamide equivalent dose
- FSH
follicle-stimulating hormone
- GM
geometric mean
- LH
luteinizing hormone
- MOR
measures of ovarian reserve
- MOV
mean ovarian volume
- TBI
total body irradiation
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. [DOI] [PubMed] [Google Scholar]
- 2. Kinahan KE, Didwania A, Nieman CL. Childhood cancer: fertility and psychosocial implications. Cancer Treat Res. 2007;138:191–200. [DOI] [PubMed] [Google Scholar]
- 3. Schover LR. Psychosocial aspects of infertility and decisions about reproduction in young cancer survivors: a review. Med Pediatr Oncol. 1999;33(1):53–59. [DOI] [PubMed] [Google Scholar]
- 4. Bath LE, Wallace WH, Critchley HO. Late effects of the treatment of childhood cancer on the female reproductive system and the potential for fertility preservation. BJOG. 2002;109(2):107–114. [DOI] [PubMed] [Google Scholar]
- 5. Chemaitilly W, Mertens AC, Mitby P, Whitton J, Stovall M, Yasui Y, Robison LL, Sklar CA. Acute ovarian failure in the childhood cancer survivor study. J Clin Endocrinol Metab. 2006;91(5):1723–1728. [DOI] [PubMed] [Google Scholar]
- 6. Sklar CA, Mertens AC, Mitby P, Whitton J, Stovall M, Kasper C, Mulder J, Green D, Nicholson HS, Yasui Y, Robison LL. Premature menopause in survivors of childhood cancer: a report from the childhood cancer survivor study. J Natl Cancer Inst. 2006;98(13):890–896. [DOI] [PubMed] [Google Scholar]
- 7. Wallace WH, Thomson AB, Saran F, Kelsey TW. Predicting age of ovarian failure after radiation to a field that includes the ovaries. Int J Radiat Oncol Biol Phys. 2005;62(3):738–744. [DOI] [PubMed] [Google Scholar]
- 8. Lie Fong S, Laven JS, Hakvoort-Cammel FG, Schipper I, Visser JA, Themmen AP, de Jong FH, van den Heuvel-Eibrink MM. Assessment of ovarian reserve in adult childhood cancer survivors using anti-Müllerian hormone. Hum Reprod. 2009;24(4):982–990. [DOI] [PubMed] [Google Scholar]
- 9. Dezellus A, Barriere P, Campone M, Lemanski C, Vanlemmens L, Mignot L, Delozier T, Levy C, Bendavid C, Debled M, Bachelot T, Jouannaud C, Loustalot C, Mouret-Reynier MA, Gallais-Umbert A, Masson D, Freour T. Prospective evaluation of serum anti-Müllerian hormone dynamics in 250 women of reproductive age treated with chemotherapy for breast cancer. Eur J Cancer. 2017;79:72–80. [DOI] [PubMed] [Google Scholar]
- 10. Decanter C, Morschhauser F, Pigny P, Lefebvre C, Gallo C, Dewailly D. Anti-Müllerian hormone follow-up in young women treated by chemotherapy for lymphoma: preliminary results. Reprod Biomed Online. 2010;20(2):280–285. [DOI] [PubMed] [Google Scholar]
- 11. Dillon KE, Sammel MD, Ginsberg JP, Lechtenberg L, Prewitt M, Gracia CR. Pregnancy after cancer: results from a prospective cohort study of cancer survivors. Pediatr Blood Cancer. 2013;60(12):2001–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Anderson RA, Cameron DA. Pretreatment serum anti-Müllerian hormone predicts long-term ovarian function and bone mass after chemotherapy for early breast cancer. J Clin Endocrinol Metab. 2011;96(5):1336–1343. [DOI] [PubMed] [Google Scholar]
- 13. Anderson RA, Rosendahl M, Kelsey TW, Cameron DA. Pretreatment anti-Müllerian hormone predicts for loss of ovarian function after chemotherapy for early breast cancer. Eur J Cancer. 2013;49(16):3404–3411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Deb S, Campbell BK, Clewes JS, Pincott-Allen C, Raine-Fenning NJ. Intracycle variation in number of antral follicles stratified by size and in endocrine markers of ovarian reserve in women with normal ovulatory menstrual cycles. Ultrasound Obstet Gynecol. 2013;41(2):216–222. [DOI] [PubMed] [Google Scholar]
- 15. Hehenkamp WJ, Looman CW, Themmen AP, de Jong FH, Te Velde ER, Broekmans FJ. Anti-Müllerian hormone levels in the spontaneous menstrual cycle do not show substantial fluctuation. J Clin Endocrinol Metab. 2006;91(10):4057–4063. [DOI] [PubMed] [Google Scholar]
- 16. Hansen KR, Hodnett GM, Knowlton N, Craig LB. Correlation of ovarian reserve tests with histologically determined primordial follicle number. Fertil Steril. 2011;95(1):170–175. [DOI] [PubMed] [Google Scholar]
- 17. van Rooij IA, Broekmans FJ, Scheffer GJ, Looman CW, Habbema JD, de Jong FH, Fauser BJ, Themmen AP, te Velde ER. Serum antimullerian hormone levels best reflect the reproductive decline with age in normal women with proven fertility: a longitudinal study. Fertil Steril. 2005;83(4):979–987. [DOI] [PubMed] [Google Scholar]
- 18. La Marca A, Spada E, Sighinolfi G, Argento C, Tirelli A, Giulini S, Milani S, Volpe A. Age-specific nomogram for the decline in antral follicle count throughout the reproductive period. Fertil Steril. 2011;95(2):684–688. [DOI] [PubMed] [Google Scholar]
- 19. Kelsey TW, Wright P, Nelson SM, Anderson RA, Wallace WH. A validated model of serum anti-Müllerian hormone from conception to menopause. PLoS One. 2011;6(7):e22024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Steiner AZ, Pritchard D, Stanczyk FZ, Kesner JS, Meadows JW, Herring AH, Baird DD. Association between biomarkers of ovarian reserve and infertility among older women of reproductive age. JAMA. 2017;318(14):1367–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. de Kat AC, van der Schouw YT, Eijkemans MJ, Herber-Gast GC, Visser JA, Verschuren WM, Broekmans FJ. Back to the basics of ovarian aging: A population-based study on longitudinal anti-Mullerian hormone decline. BMC Med. 2016;14(1):151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tehrani FR, Shakeri N, Solaymani-Dodaran M, Azizi F. Predicting age at menopause from serum antimüllerian hormone concentration. Menopause. 2011;18(7):766–770. [DOI] [PubMed] [Google Scholar]
- 23. Freeman EW, Sammel MD, Lin H, Gracia CR. Anti-Mullerian hormone as a predictor of time to menopause in late reproductive age women. J Clin Endocrinol Metab. 2012;97(5):1673–1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Freeman EW, Sammel MD, Lin H, Boorman DW, Gracia CR. Contribution of the rate of change of antimüllerian hormone in estimating time to menopause for late reproductive-age women. Fertil Steril. 2012;98(5):1254–1259.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gracia CR, Sammel MD, Freeman E, Prewitt M, Carlson C, Ray A, Vance A, Ginsberg JP. Impact of cancer therapies on ovarian reserve. Fertil Steril. 2012;97(1):134–140.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Green DM, Nolan VG, Goodman PJ, Whitton JA, Srivastava D, Leisenring WM, Neglia JP, Sklar CA, Kaste SC, Hudson MM, Diller LR, Stovall M, Donaldson SS, Robison LL. The cyclophosphamide equivalent dose as an approach for quantifying alkylating agent exposure: a report from the Childhood Cancer Survivor Study. Pediatr Blood Cancer. 2014;61(1):53–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cameron K, Sammel MD, Prewitt M, Gracia C. Data from: Differential rates of change in measures of ovarian reserve in young cancer survivors across the reproductive lifespan. figshare 2018. Deposited 3 December 2018. https://figshare.com/articles/Supplemental_Figure_1/7413416. [DOI] [PMC free article] [PubMed]
- 28. Muggeo VM. Estimating regression models with unknown break-points. Stat Med. 2003;22(19):3055–3071. [DOI] [PubMed] [Google Scholar]
- 29. Sawa T. Information criteria for discriminating among alternative regression models. Econometrica. 1978;46(6):1273–1291. [Google Scholar]
- 30. van der Kooi AL, van den Heuvel-Eibrink MM, van Noortwijk A, Neggers SJ, Pluijm SM, van Dulmen-den Broeder E, van Dorp W, Laven JS. Longitudinal follow-up in female childhood cancer survivors: no signs of accelerated ovarian function loss. Hum Reprod. 2017;32(1):193–200. [DOI] [PubMed] [Google Scholar]
- 31. Meirow D, Biederman H, Anderson RA, Wallace WH. Toxicity of chemotherapy and radiation on female reproduction. Clin Obstet Gynecol. 2010;53(4):727–739. [DOI] [PubMed] [Google Scholar]
- 32. Damewood MD, Grochow LB. Prospects for fertility after chemotherapy or radiation for neoplastic disease. Fertil Steril. 1986;45(4):443–459. [DOI] [PubMed] [Google Scholar]
- 33. Couto-Silva AC, Trivin C, Thibaud E, Esperou H, Michon J, Brauner R. Factors affecting gonadal function after bone marrow transplantation during childhood. Bone Marrow Transplant. 2001;28(1):67–75. [DOI] [PubMed] [Google Scholar]
- 34. van den Berg MH, Overbeek A, Lambalk CB, Kaspers GJL, Bresters D, van den Heuvel-Eibrink MM, Kremer LC, Loonen JJ, van der Pal HJ, Ronckers CM, Tissing WJE, Versluys AB, van der Heiden-van der Loo M, Heijboer AC, Hauptmann M, Twisk JWR, Laven JSE, Beerendonk CCM, van Leeuwen FE, van Dulmen-den Broeder E; DCOG LATER-VEVO Study Group . Long-term effects of childhood cancer treatment on hormonal and ultrasound markers of ovarian reserve. Hum Reprod. 2018;33(8):1474–1488. [DOI] [PubMed] [Google Scholar]
- 35. Sowers MR, Eyvazzadeh AD, McConnell D, Yosef M, Jannausch ML, Zhang D, Harlow S, Randolph JF Jr. Anti-Mullerian hormone and inhibin B in the definition of ovarian aging and the menopause transition. J Clin Endocrinol Metab. 2008;93(9):3478–3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. van Disseldorp J, Faddy MJ, Themmen AP, de Jong FH, Peeters PH, van der Schouw YT, Broekmans FJ. Relationship of serum antimüllerian hormone concentration to age at menopause. J Clin Endocrinol Metab. 2008;93(6):2129–2134. [DOI] [PubMed] [Google Scholar]
- 37. Ebner T, Sommergruber M, Moser M, Shebl O, Schreier-Lechner E, Tews G. Basal level of anti-Müllerian hormone is associated with oocyte quality in stimulated cycles. Hum Reprod. 2006;21(8):2022–2026. [DOI] [PubMed] [Google Scholar]
- 38. Cook CL, Siow Y, Taylor S, Fallat ME. Serum müllerian-inhibiting substance levels during normal menstrual cycles. Fertil Steril. 2000;73(4):859–861. [DOI] [PubMed] [Google Scholar]
- 39. Kallio S, Puurunen J, Ruokonen A, Vaskivuo T, Piltonen T, Tapanainen JS. Antimüllerian hormone levels decrease in women using combined contraception independently of administration route. Fertil Steril. 2013;99(5):1305–1310. [DOI] [PubMed] [Google Scholar]
- 40. Kristensen SL, Ramlau-Hansen CH, Andersen CY, Ernst E, Olsen SF, Bonde JP, Vested A, Toft G. The association between circulating levels of antimüllerian hormone and follicle number, androgens, and menstrual cycle characteristics in young women. Fertil Steril. 2012;97(3):779–785. [DOI] [PubMed] [Google Scholar]
- 41. Hershlag A, Schuster MW. Return of fertility after autologous stem cell transplantation. Fertil Steril. 2002;77(2):419–421. [DOI] [PubMed] [Google Scholar]