Abstract
The purpose was to investigate changes in neuropeptide Y (NPY) protein and dipeptidyl peptidase IV (DPP-IV) activity in the plasma and saliva in normally cycling women and women after menopause. We recruited 7 cycling women and 7 postmenopausal women for a cross-sectional, prospective pilot study. Blood via venipuncture and saliva samples were taken at each point in the menstrual cycle (premenopausal) or once per week (postmenopausal) for 2 months. Blood and saliva were analyzed for estrogen, NPY using ELISA and DPP-IV activity using a fluorometric assay. Plasma β-estradiol was an average of 96.45 ± 57.04 pg/mL over 2 cycles in the premenopausal group and 1.72 ± 0.35 pg/mL over 2 months in the postmenopausal group (P < .05). In the cycling group, there were no significant differences in saliva or plasma NPY or DPP-IV over the cycle. For the postmenopausal group, salivary NPY and DPP-IV did not change over 2 months. Plasma NPY was lowest in the middle 2 weeks (average: 0.52 ± 0.10 ng/mL) compared to the first and fourth weeks (average of week 1 and 4: 0.60 ± 0.14 ng/mL; P < .05). Plasma NPY in postmenopausal women was higher overall (0.56 ± 0.13 ng/mL) compared to cycling women (0.30 ± 0.11 ng/mL; P < .05). Plasma DPP-IV activity was unchanged by time in the postmenopausal group. Saliva DPP-IV and saliva NPY in the cycling group had a significant negative correlation (R = −0.95; P < .05). We found that saliva measures of NPY and DPP-IV activity appear to be poor estimates of plasma concentrations and activities, but a larger sample size is required to conform this. Differences in plasma NPY concentrations between the groups and the relationship between salivary NPY and DPP-IV suggests that there may be some unique differences between these groups.
Keywords: estrogen, plasma, saliva
1. Introduction
Neuropeptide Y (NPY) is a complex peptide that is involved in blood flow, satiety, stress, metabolism, immune function, and reproduction.[1] Dipeptidyl peptidase IV (DPP-IV) is a nonclassical serine protease that is present in most tissues in the body. One of its many targets is NPY and DPP-IV acts to cleave NPY1–36 into NPY3–36. This results in a change in the receptors that NPY can activate and changes the functions of NPY.[2]
Several studies in animals have shown that NPY is involved in regulating luteal hormone production.[3–7] In normally cycling humans, there is no evidence for menstrual cycle changes in NPY.[8,9] However, NPY does decline with amenorrhea.[10,11] It is unclear whether NPY changes with menopause.[8] NPY has diverse effects in the body[12] and changes in this system may help explain changes in women following menopause including body composition changes,[13] blood pressure changes,[14] and blood flow.[15,16]
Previous work suggests that about 27% of the neuropeptides and enzymes found in the saliva come from the blood,[17] therefore, saliva may be a less invasive method of sampling peptides and proteins in the blood. Previous work by this laboratory suggested that DPP-IV in the saliva and plasma have a poor correlation[18] in a gender mixed group, but there are few studies investigating NPY.
The purpose of this study was to investigate the relationship between NPY and DPP-IV in normally cycling and postmenopausal women. We also wished to determine if saliva measures of NPY and DPP-IV were estimates of plasma NPY and DPP-IV.
2. Methods
2.1. Study methodology
Seven normally cycling young women that were not on oral contraceptives for 3 consecutive months and 7 postmenopausal women that were not taking hormone supplements and had been in menopause for more than 2 years were enrolled in this cross-sectional, prospective study. The postmenopausal group had all progressed through natural menopause (no hysterectomy or reproductive disease). To establish cycle length each cycling participant was asked to provide the date of onset for her last 2 menstrual cycles. If a participant was unable to provide the dates she was asked to track the next 2 menstrual cycles before data collection. Once a cycle length for the participant was established, the window of each phase was determined using an ovulation calculator (http://www.webmd.com/baby/healthtool-ovulation-calculator) that takes into account cycle length and cycle start date. The participants were given a 2 to 6 day window that encompassed each phase of the menstrual cycle targeted in this study. Because the ovulatory cycle is only 48 hours in length, our goal was to schedule the participant during this period of time. Ovulation test strips (Ovulation test strips, Wondfo USA Co, Ltd., Willowbrook, IL) designed to detect an luteinizing hormone surge were given to each participant the day before the ovulatory period so they could determine the point of their luteinizing hormone surge at home. When the participant tested positive for the luteinizing hormone surge (>25 mIU/mL) they were scheduled within 24 hours after detection. For example, a person with a 28 day cycle would be given the following time frames: follicular: days 6 to 11; ovulatory: days 13 to 15; luteal: days 18 to 23; and ischemic: days 26 to 28. For each of the menstrual cycle phases described above, participants completed all experimental tasks.
Postmenopausal women were scheduled to come in once per week for 2 months. All participants were scheduled at approximately the same time of day for data collection. To determine the appropriate sample size we used means and standard deviations for plasma NPY from Lewandowski et al[8] and Coiro et al[10] to calculate the Cohen's D. The sample size was determined using G∗Power 3.1.9.2 for Windows.[19] We estimated that we needed a minimum N = 6 for an alpha = 0.05. The last date of follow-up data for this analysis was July 15, 2017. Reporting was conducted in accordance with the STROBE guidelines.
2.2. Study population
All parts of the following study were reviewed and approved by the Institutional Review Board at Auburn University before beginning the study and conformed to the standards set by the latest revision of the Declaration of Helsinki. For this study, participants were recruited locally from the Auburn, Alabama area, and a signed informed consent was obtained from each participant before beginning any data collection. Participants were required to complete a health questionnaire. They were excluded if they had any health conditions including: diabetes, allergic rhinitis, reflux, respiratory problems, are an active smoker at time of investigation, have neurological problems, have a hormonal imbalance, pregnancy, or have been breast-feeding over the preceding 6 months.
2.3. Outcomes
2.3.1. Blood and saliva collection
Participants came to the laboratory where plasma samples were collected by drawing blood using standard venipuncture in the antecubital vein. Blood was centrifuged at 1000 g and 4°C for 10 minutes to separate plasma, which was drawn off and stored at −80°C until analysis.
Saliva was collected using the passive drool method and a saliva collection aid (Salimetrics, LLC, Carlsbad, CA) each stage of the cycle for 2 months. The postmenopausal group came to the laboratory every week for 2 months for blood and saliva collection. The saliva was stored at −80°C until analysis.
2.3.2. Assays
Saliva and plasma samples were analyzed for DPP-IV activity by the use of a fluorometric assay developed by Scharpe et al.[20] DPP-IV samples and assay components were brought to room temperature before analysis. Fifty mmol/L Tris HCl incubation buffer (Sigma-Aldrich, St Louis, MO; pH 8.3), 20 mmol/L glycyl-L-proline-4-methoxy-2-naphthylamide (Sigma-Aldrich) substrate solution, and saliva sample were added to the sample wells of a white 96-well microplate. Standard wells contained 50 mmol/L 4-methoxy-2-naphthylamine (Bachem, Torrance, CA) standard solution, 100 mmol/L citrate stopping solution (Sigma-Aldrich; pH 4.0), substrate solution, and incubation buffer. The microplate incubated for 30 minutes on a heating block set at 37°C, and stopping solution was added to the sample wells to end the reaction. Fluorescence was excited at 340 nm and measured at 435 nm in a Synergy H2 Hybrid Reader (BioTek Instruments, Winooski, VT). Salivary NPY1–36 protein content was determined using a peptide EIA (S-1145, Peninsula Laboratories International, San Carlos, CA) per manufacturer's instructions (Protocol III for saliva analysis). The same assay was used for plasma NPY protein content using protocol V. Plasma was also analyzed for estradiol (estradiol ELISA catalog number 582251; Cayman Chemical, Ann Arbor, MI) using the standard protocol.
2.4. Data analysis
Data are reported as mean ± standard deviation. A Shapiro–Wilkes normality test was run and if the data passed, a 2-way mixed model ANOVA (GraphPad Prism, version 5.01, GraphPad Software Inc, La Jolla, CA) were used to analyze potential differences between groups (pre vs. postmenopausal) and within a cycle (follicular, ovulation, luteal, and ischemic). If appropriate, a Bonferroni post hoc test was used. Alpha was set a priori at 0.05. A Pearson Product Moment correlation was used to determine if there were any significant relationships among the variables.
3. Results
3.1. Participant characteristics
This data is a subset of a larger data set. We use participants that had a complete set of salivary and plasma NPY and DPP-IV data for at least 1 cycle or month. Plasma β-estradiol was an average of 96.45 ± 57.04 pg/mL over 2 cycles in the premenopausal group and 1.72 ± 0.35 pg/mL over 2 months in the postmenopausal group (P < .05). The cycling group was aged 24 ± 5 years and the postmenopausal group was 59 ± 5 years. In the cycling group, there were 5 white, non-Hispanic; 1 Hispanic, and 1 black or African American participant. The postmenopausal group was all white, non-Hispanic.
3.2. Cycling group
Figure 1A (white bars) shows saliva NPY concentrations over the menstrual cycle. In the cycling group (n = 5), there were no significant differences over the cycle (Cohen's D effect size based on time = 0.43). The average saliva NPY over cycle 1 was 0.35 ± 0.33 ng/mL (n = 7) and for cycle 2 was 0.67 ± 0.25 ng/mL (n = 7). The coefficient of variation for cycle 1 and 2 was 25%.
Figure 1.
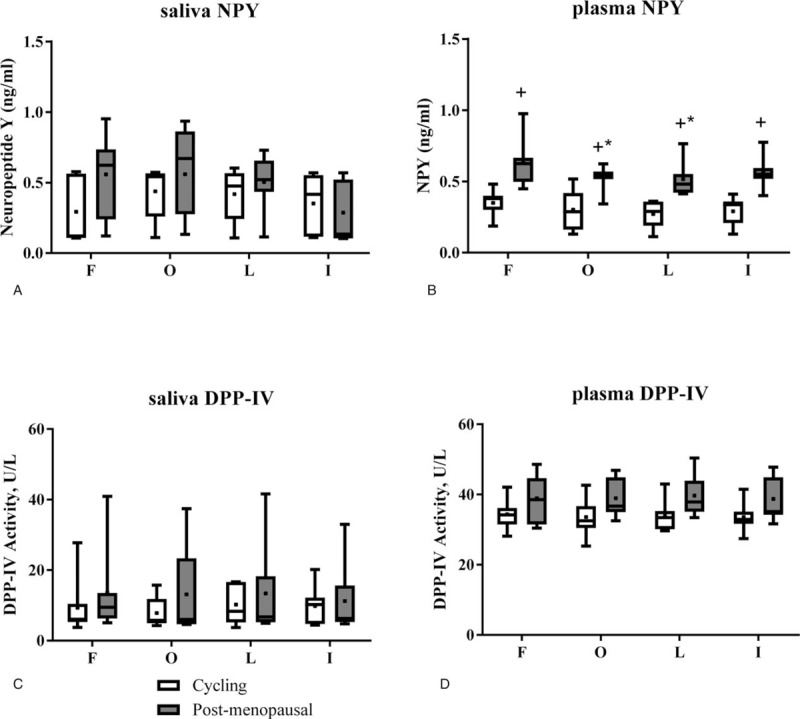
Saliva and plasma concentrations for NPY and activity of DPP-IV. (A) There was no difference within or between groups for saliva NPY. (B) Plasma NPY was higher at all time points in the postmenopausal group. In the postmenopausal group, plasma NPY was lower in the middle 2 weeks compared to week 1 over a 2-month period. (C) The activity of saliva DPP-IV was not different among time points or between groups. (D) There was no difference within or between groups for plasma DPP-IV. +P < .05 different from the cycling group; ∗P < .05 different from week 1 in the postmenopausal group. The box extends to the 25th and 75th percentiles, the line within the box is the median, and the whiskers are the range of the data. The mean is indicated by a plus/square symbol. DPP-IV = dipeptidyl peptidase IV, NPY = neuropeptide Y.
Figure 1B is the average (of 2 cycles) for plasma NPY at each menstrual phase. There was no effect of the cycle phase on plasma NPY (Cohen's D effect size based on time = 1.53). The average plasma NPY for cycle 1 was 0.31 ± 0.10 ng/mL and for cycle 2 was 0.29 ± 0.11 ng/mL.
Salivary DPP-IV (Fig. 1C) and plasma DPP-IV (Fig. 1D) showed no effect of phase of the menstrual cycle in the cycling group. The average salivary DPP-IV was 9.29 ± 5.48 U/L (n = 7) and the plasma DPP-IV was 39.06 ± 6.00 U/L (n = 7). The coefficient of variability over 2 cycles for the salivary DPP-IV was 30%, but the coefficient of variation for plasma DPP-IV was 5%.
3.3. Postmenopausal group
For the postmenopausal group (n = 7), salivary NPY (Fig. 1A, grey bars) did not change over 2 months. The average for month 1 was 0.46 ± 0.17 ng/mL (n = 7) and for month 2 was 0.49 ± 0.20 ng/mL (n = 5). The coefficient of variation was 47%. However, plasma NPY (Fig. 1B, grey bars) was lowest in the second 2 weeks of the average of months 1 and 2 (P < .05). Plasma NPY in postmenopausal women was also higher than plasma NPY seen in the cycling women (P < .05). The coefficient of variation was 25% over the 2 months tested in postmenopausal women.
There was no effect of time of the month for saliva (Fig. 1C, grey bars) or plasma DPP-IV (Fig. 1D, grey bars). For saliva DPP-IV, the average for month 1 was 13.26 ± 10.77 U/L (n = 7) and for month 2 was 13.69 ± 14.32 U/L (n = 6). The coefficient of variation over the 2 months was 29%. For plasma DPP-IV, the average for month 1 was 39.4 ± 6.21 U/L (n = 7) and for month 2 was 38.72 ± 5.90 U/L (n = 7). The coefficient of variation for the 2 months was 5%.
3.4. Correlations
Using the averaged data over 2 cycles or months, we found that saliva DPP-IV and saliva NPY in the cycling group had a significant negative correlation (R = −0.95; P = .01). No significant correlations were found for other variables in the cycling group or in the postmenopausal group.
4. Discussion
This is the first study to look at salivary and plasma NPY and DPP-IV in pre and postmenopausal women over time. We found no differences between cycling and postmenopausal women in salivary NPY, salivary DPP-IV, and plasma DPP-IV. However, we did find that postmenopausal women had higher plasma NPY compared to cycling women. Postmenopausal women had a significant decline in plasma NPY in the middle of the month. There was no correlation between salivary and plasma NPY or DPP-IV, but saliva DPP-IV and saliva NPY had a significant negative correlation. These data suggest that saliva measures may be a poor estimate of plasma concentrations and activities, but a larger sample size is required to confirm.
In normally cycling females not on birth control, we found that there was no cyclic variation in plasma NPY. This is in contrast to rat studies that showed that there is a surge in NPY before luteinizing hormone release.[4,5] However, some human studies showed no change in plasma NPY from the follicular to luteal phase.[8,9]
In women that were postmenopause, we found a higher plasma NPY and a significant decline in plasma NPY that occurred at weeks 2 and 3 of the month. This difference among cycling and postmenopausal does not appear to be directly related to estrogen levels since Coiro et al[10] reported that amenorrheic women athletes had lower plasma NPY, compared to their normally cycling counterparts. This was also reported by Meczekalski et al[11] who found lower plasma NPY in weight loss-related amenorrhea. One reason why the Coiro et al[10] and the Meczekalski et al[11] studies may have seen lower NPY could have been due to stress. Cho et al[7] reported that women had lower serum NPY at weeks 4 and 8 of an intensive physical training program. A number of studies have reported a negative correlation between NPY levels and stress.[21] However, Lewandowski et al[8] reported no difference between ovariectomized women compared to normally cycling women of the same age. It is possible that the contrast between the current study and Lewandowski et al[8] is the difference in age in the current study. Our postmenopausal participants underwent normal menopause and were approximately 36 years older than the cycling group, while Lewandowski et al[8] used women that had early menopause due to uterine myomas. It is possible that age and not sex hormones lead to the differences among groups.
DPP-IV activity in the plasma also did not change with time or by group. DPP-IV is an important enzyme that breaks down full length NPY (1–36) into NPY3–36. This cleaving of NPY results in a change in the actions of NPY. DPP-IV has not previously been measured in women over the cycle or in postmenopausal women over time. Plasma DPP-IV comes from a variety of sources including the gut, kidneys, T-cells, muscle, and fat cells. As a result, it is difficult to cause a change in plasma DPP-IV with exercise[22] or food intake.[18,23,24] Plasma DPP-IV is stable over a 2 month period in cycling and postmenopausal women, but salivary values are quite variable.
Salivary NPY content and DPP-IV activity were also measured to see if it was possible to estimate their concentrations and activities in the plasma. Salivary NPY and DPP-IV were not different between groups or over time. There was no correlation between salivary NPY and plasma NPY, suggesting that salivary NPY may be a poor estimate for plasma NPY, but a larger sample size is required to confirm. Haririan et al[25] also found that saliva NPY was a poor estimate of serum NPY. They found that periodontal disease results in higher saliva NPY. We previously showed that DPP-IV in the saliva is not a good estimate of DPP-IV in the plasma using a group of young men and women.[18] However, salivary NPY had a strong negative correlation with salivary DPP-IV in the cycling group, but not in the postmenopausal group. Previous work showed that there are Y2 receptors in the mouth that send information regarding satiation.[26] This is significant because Y2 receptors are sensitive to NPY3–36, but not the full length NPY. If this relationship also occurs in humans, it would explain why there is a strong negative correlation between NPY and DPP-IV. A poor relationship between DPP-IV and NPY in the postmenopausal group may indicate a disruption in the regulation between DPP-IV and NPY in the mouth and may, in part, explain weight gain in postmenopausal women.
In conclusion, we found no menstrual cycle-based changes in salivary NPY, salivary DPP-IV, and plasma DPP-IV. Postmenopausal women may have higher plasma NPY compared to cycling women and postmenopausal women may have a decline in plasma NPY in the middle of the month. Saliva DPP-IV and saliva NPY had a significant negative correlation in the cycling group, but not in the postmenopausal group. These data suggest that saliva measures may be a poor estimate of plasma concentrations and activities, but a larger sample size is required to confirm. Women after menopause may experience some changes in the NPY system.
Acknowledgments
The authors thank the following students for their technical assistance during this project: Summer Myrick, Will Boswell, Kathryn Williams, and Anna La Mantia.
Author contributions
Conceptualization: Heidi A. Kluess, Leslie E. Neidert, Mary J. Sandage, Laura W. Plexico.
Data curation: Heidi A. Kluess, Leslie E. Neidert, Mary J. Sandage, Laura W. Plexico.
Formal analysis: Heidi A. Kluess, Leslie E. Neidert.
Funding acquisition: Heidi A. Kluess, Mary J. Sandage, Laura W. Plexico.
Investigation: Heidi A. Kluess, Leslie E. Neidert, Mary J. Sandage, Laura W. Plexico.
Methodology: Heidi A. Kluess, Leslie E. Neidert, Mary J. Sandage, Laura W. Plexico.
Project administration: Heidi A. Kluess, Leslie E. Neidert, Mary J. Sandage, Laura W. Plexico.
Resources: Heidi A. Kluess, Laura W. Plexico.
Validation: Heidi A. Kluess, Leslie E. Neidert, Laura W. Plexico.
Visualization: Heidi A. Kluess.
Writing – original draft: Heidi A. Kluess, Leslie E. Neidert.
Writing – review & editing: Heidi A. Kluess, Leslie E. Neidert, Mary J. Sandage, Laura W. Plexico.
HEIDI A KLUESS orcid: 0000-0002-9008-2200.
Footnotes
Abbreviations: DPP-IV = dipeptidyl peptidase IV, NPY = neuropeptide Y.
L.W.P. is principal investigator.
The work presented in this manuscript was supported by Award Number 1R03DC013664–01A1 from the National Institute on Deafness and Other Communication Disorders (NIDCD). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIDCD or the National Institutes of Health.
The authors have no conflicts of interest to disclose.
References
- [1].Farzi A, Reichmann F, Holzer P. The homeostatic role of neuropeptide Y in immune function and its impact on mood and behaviour. Acta Physiol (Oxf) 2015;213:603–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Mulvihill EE, Drucker DJ. Pharmacology, physiology, and mechanisms of action of dipeptidyl peptidase-4 inhibitors. Endocr Rev 2014;35:992–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Turi GF, Liposits Z, Moenter SM, et al. Origin of neuropeptide Y-containing afferents to gonadotropin-releasing hormone neurons in male mice. Endocrinology 2003;144:4967–74. [DOI] [PubMed] [Google Scholar]
- [4].Sutton SW, Toyama TT, Otto S, et al. Evidence that neuropeptide-Y (NPY) released into the hypophysial-portal circulation participates in priming gonadotropes to the effects of gonadotropin-releasing hormone (Gnrh). Endocrinology 1988;123:1208–10. [DOI] [PubMed] [Google Scholar]
- [5].Leupen SM, Besecke LM, Levine JE. Neuropeptide Y Y1-receptor stimulation is required for physiological amplification of preovulatory luteinizing hormone surges. Endocrinology 1997;138:2735–9. [DOI] [PubMed] [Google Scholar]
- [6].Crown A, Clifton DK, Steiner RA. Neuropeptide signaling in the integration of metabolism and reproduction. Neuroendocrinology 2007;86:175–82. [DOI] [PubMed] [Google Scholar]
- [7].Cho GJ, Han SW, Shin JH, Kim T. Effects of intensive training on menstrual function and certain serum hormones and peptides related to the female reproductive system. Medicine (Baltimore) 2017;96:e6876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lewandowski J, Pruszczyk P, Elaffi M, et al. Blood pressure, plasma NPY and catecholamines during physical exercise in relation to menstrual cycle, ovariectomy, and estrogen replacement. Regul Pept 1998;75-76:239–45. [DOI] [PubMed] [Google Scholar]
- [9].Pohl A, Nordin C. Body mass index influences plasma concentration of neuropeptide Y in healthy female volunteers: a pilot study. Gynecol Endocrinol 2003;17:409–12. [DOI] [PubMed] [Google Scholar]
- [10].Coiro V, Chiodera P, Melani A, et al. Different plasma neuropeptide Y concentrations in women athletes with and without menstrual cyclicity. Fertil Steril 2006;85:767–9. [DOI] [PubMed] [Google Scholar]
- [11].Meczekalski B, Genazzani AR, Genazzani AD, et al. Clinical evaluation of patients with weight loss-related amenorrhea: neuropeptide Y and luteinizing hormone pulsatility. Gynecol Endocrinol 2006;22:239–43. [DOI] [PubMed] [Google Scholar]
- [12].Yi M, Li H, Wu Z, et al. A promising therapeutic target for metabolic diseases: neuropeptide Y receptors in humans. Cell Physiol Biochem 2018;45:88–107. [DOI] [PubMed] [Google Scholar]
- [13].Thurston RC, Sowers MR, Sternfeld B, et al. Gains in body fat and vasomotor symptom reporting over the menopausal transition: the study of women's health across the nation. Am J Epidemiol 2009;170:766–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hallajzadeh J, Khoramdad M, Izadi N, et al. Metabolic syndrome and its components in premenopausal and postmenopausal women: a comprehensive systematic review and meta-analysis on observational studies. Menopause 2018;25:1155–64. [DOI] [PubMed] [Google Scholar]
- [15].Olver TD, Hiemstra JA, Edwards JC, et al. Loss of female sex hormones exacerbates cerebrovascular and cognitive dysfunction in aortic banded miniswine through a neuropeptide Y-Ca(2+)-activated potassium channel-nitric oxide mediated mechanism. J Am Heart Assoc 2017;6: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Holwerda SW, Restaino RM, Fadel PJ. Adrenergic and non-adrenergic control of active skeletal muscle blood flow: implications for blood pressure regulation during exercise. Auton Neurosci 2015;188:24–31. [DOI] [PubMed] [Google Scholar]
- [17].Zolotukhin S. Metabolic hormones in saliva: origins and functions. Oral Dis 2013;19:219–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Neidert LE, Babu JR, Kluess HA. Dipeptidyl peptidase-IV activity and neuropeptide Y in the mouth: the relationship with body composition and the impact of sucrose and aspartame. J Nutr Biol 2016;1:61–72. [Google Scholar]
- [19].Faul F, Erdfelder E, Lang AG, Buchner A. G∗Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 2007;39:175–91. [DOI] [PubMed] [Google Scholar]
- [20].Scharpe S, De Meester I, Vanhoof G, et al. Assay of dipeptidyl peptidase IV in serum by fluorometry of 4-methoxy-2-naphthylamine. Clin Chem 1988;34:2299–301. [PubMed] [Google Scholar]
- [21].Kautz M, Charney DS, Murrough JW. Neuropeptide Y, resilience, and PTSD therapeutics. Neurosci Lett 2017;649:164–9. [DOI] [PubMed] [Google Scholar]
- [22].Neidert LE, Mobley CB, Kephart WC, et al. The serine protease, dipeptidyl peptidase IV as a myokine: dietary protein and exercise mimetics as a stimulus for transcription and release. Physiol Rep 2016;4:e12827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ryskjaer J, Deacon CF, Carr RD, et al. Plasma dipeptidyl peptidase-IV activity in patients with type-2 diabetes mellitus correlates positively with HbAlc levels, but is not acutely affected by food intake. Eur J Endocrinol 2006;155:485–93. [DOI] [PubMed] [Google Scholar]
- [24].Meneilly GS, Demuth HU, McIntosh CH, et al. Effect of ageing and diabetes on glucose-dependent insulinotropic polypeptide and dipeptidyl peptidase IV responses to oral glucose. Diabet Med 2000;17:346–50. [DOI] [PubMed] [Google Scholar]
- [25].Haririan H, Andrukhov O, Bottcher M, et al. Salivary neuropeptides, stress and periodontitis. J Periodontol 2018;89:9–18. [DOI] [PubMed] [Google Scholar]
- [26].Hurtado MD, Acosta A, Riveros PP, et al. Distribution of Y-receptors in murine lingual epithelia. PLoS One 2012;7:e46358. [DOI] [PMC free article] [PubMed] [Google Scholar]


