Abstract
Objective:
To assess the factors that contributed to the successful completion of recruitment for the largest clinical trial ever conducted in Australia, the Aspirin in Reducing Events in the Elderly (ASPREE) study.
Design:
Enrolment of GPs; identification of potential participants in general practice databases; screening of participants.
Setting, participants:
Selected general practices across southeast Australia (Tasmania, Victoria, Australian Capital Territory, New South Wales, South Australia).
Major outcomes:
Numbers of patients per GP screened and randomised to participation; geographic and demographic factors that influenced screening and randomising of patients.
Results:
2717 of 5833 GPs approached (47%) enrolled to recruit patients for the study; 2053 (76%) recruited at least one randomised participant. The highest randomised participant rate per GP was for Tasmania (median, 5; IQR, 1–11), driven by the high rate of participant inclusion at phone screening. GPs in inner regional (adjusted odds ratio [aOR], 1.45; 95% CI, 1.14–1.84) and outer regional areas (aOR, 1.86; 95% CI, 1.19–2.88) were more likely than GPs in major cities to recruit at least one randomised participant. GPs in areas with a high proportion of people aged 70 years or more were more likely to randomise at least one participant (per percentage point increase: aOR, 1.10; 95% CI, 1.05–1.15). The number of randomised patients declined with time from GP enrolment to first randomisation.
Conclusion:
General practice can be a rich environment for research when barriers to recruitment are overcome. Including regional GPs and focusing efforts in areas with the highest proportions of potentially eligible participants improves recruitment. The success of ASPREE attests to the clinical importance of its research question for Australian GPs.
Large clinical trials without effective recruitment strategies fail to meet their recruitment goals, which can lead to increased costs or termination of the study. In contrast to the rich literature describing trial design and analysis, few detailed accounts of recruitment strategy have been published, resulting in a weak evidence base for effective approaches, especially for primary care studies.1
Australian general practice should be a productive environment for research. More than 80% of Australians visit a general practitioner at least once a year,2 and patients are more likely to agree to participate in research if invited by their usual doctor.3 The second Australian National Blood Pressure (ANBP2) study, until recently the largest trial in Australian general practice, facilitated the recruitment of 6081 community-dwelling participants in the 1990s by enrolling GPs as co-investigators.4,5
However, more recent reports have highlighted barriers to recruiting participants through general practices,6–9 including time constraints for GPs and workforce shortages,1,10 lack of remuneration,10 not recognising GPs as investigators,10 and lack of interest in the research question.11 Further, there is little infrastructure support for GPs participating in data collection and research.12,13
It was against this backdrop that the landmark Aspirin in Reducing Events in the Elderly (ASPREE) study was conducted in Australia and the United States.14 In Australia, participants were recruited through general practices with the ANBP2 strategy of enrolling GPs as co-investigators. The initial Australian target of 12 500 participants was increased to 16 500 during the final stages of recruitment to offset lower than expected recruitment in the US. Overall, ASPREE enrolled 2717 Australian GPs, 2053 of whom facilitated the recruitment of 16 035 participants. An additional 668 participants, recruited directly from the community, increased the Australian total to 16 703 participants, exceeding the Australian recruitment target and providing a representative sample.14 In this article we outline and assess the factors that contributed to the successful recruitment of participants for ASPREE.
Method
The methods and results of the ASPREE clinical trial are described in detail elsewhere.14,15 Briefly, ASPREE was a randomised placebo-controlled trial in Australia and the US of low dose (100 mg) aspirin in 19 114 healthy people aged 70 years or more (65 or more for US minority populations) who did not have a history of cardiovascular disease.14 The study commenced in March 2010 and concluded in June 2017, with follow-up of participants for a median 4.7 years. The primary outcome was disability-free survival; ASPREE found no benefit of low dose aspirin treatment for older people.15
Recruitment methods
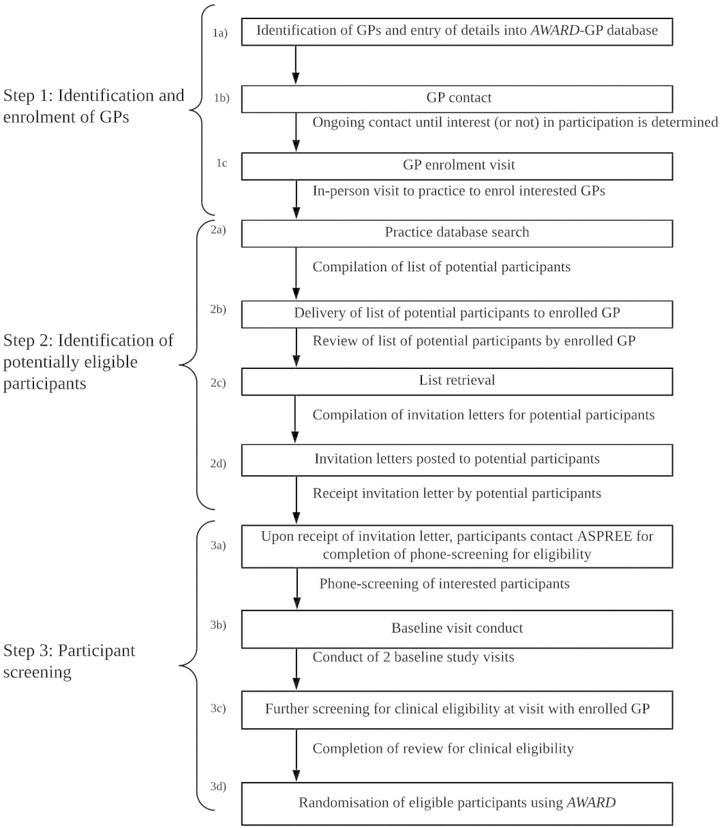
In Australia, general practice-based recruitment was divided into three steps: identifying and enrolling GPs; identifying potentially eligible participants in general practice databases; and screening participants (Box 1; Supporting information, table 1). To reduce time commitments and the burden for GPs, study staff were trained to undertake administrative tasks on their behalf, including searching databases, compiling lists, and sending invitation letters (Box 1, step 2). Additionally, practices were reimbursed for the use of their facilities with one-time payments of $100 per randomised participant, and enrolled GPs who recruited randomised participants were recognised as co-investigators.
Box 1.The three stages of recruitment of general practitioners and participants for the ASPREE study ASPREE = Aspirin in Reducing Events in the Elderly.
An information technology system (AWARD-GP), custom built to track recruitment activity, was added to the ASPREE Web Accessible Relational Database (AWARD) suite in 2011. Study staff recorded in this system their interactions with each GP, and detailed reports facilitated continuous monitoring of recruitment activity. GP characteristics such as sex, number of years of practice, qualifications, Royal Australian College of General Practitioners (RACGP) membership and the number of other GPs at the practice have been found to be of limited value for predicting recruitment outcomes,16,17 and were therefore not collected.
Divisions of General Practice were initially requested to provide introductions to local GPs, but this approach was unsuccessful because of the logistic challenge of coordinating communication with multiple divisions in different states and territories. Consequently, a list of GPs and practices was collated manually by ASPREE staff from online practitioner registers and the Yellow Pages business telephone directory, and by personal identification of clinics in the community. A minimum dataset — including the name of the GP, their practice name, address, and phone number, and ASPREE catchment area — was entered into AWARD-GP to facilitate communication.
Expansion of ASPREE catchment areas according to population demographic features
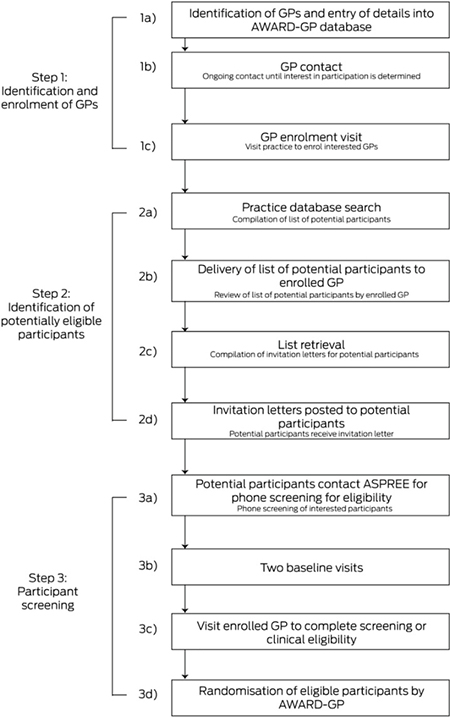
When the study commenced in 2010, three investigator-led hubs were established in the Australian Capital Territory, Tasmania, and Victoria, each with a geographic catchment area. By January 2011, an average of six participants per GP were being randomised rather than the 17 expected on the basis of the ASPREE pilot study.18 At this rate, the final number of randomised participants was projected to be 8000 instead of the targeted 12 500. Data from the Primary Health Care Research and Information Service (PHCRIS) at Flinders University in Adelaide and the Australian Bureau of Statistics (ABS) indicated that the mean proportion of the population in the original catchment areas who were aged 70 years or more was 9%. As enrolling GPs in areas with higher proportions of older people was expected to increase the number of randomised participants recruited per GP, the catchments were expanded to include areas in which more than 10% of the population were aged 70 years or more. This resulted in an additional hub in Adelaide, expansion of the ACT hub into southern New South Wales, and the addition of six new regional sites in Victoria, New South Wales and South Australia (Box 2).
Box 2. Timeline of site expansion and cumulative numbers of randomised participants.
Analysis and statistical methods
Three key outcomes were assessed: the number of participants who were screened by phone; the number who were included after phone screening; and the number who were randomised to participation in the trial. Phone screening (step 3a in Box 1) was specifically investigated because most participants who dropped out did so at this step (Supporting information, figure 1).
Socio-economic status percentiles were assigned to each practice postcode according to the ABS Index of Relative Socio-economic Advantage and Disadvantage (IRSAD)19 and grouped in high (75–100%), moderate (40–74%), and low socio-economic status bands (< 40%). Remoteness was defined according to Australian Statistical Geography Standard categories.20 The number of GPs and the proportion of the population aged 70 years or more in each catchment area were estimated on the basis of publicly available ABS and PHCRIS data.
As recruitment data were skewed and zero-inflated, differences in recruitment outcomes between states were reported as descriptive statistics and analysed in Kruskal–Wallis rank equality of populations tests. Associations between demographic factors and recruitment outcomes were analysed by logistic regression, adjusted for state-related variation. Enrolled GPs were grouped according to whether they had recruited at least one randomised participant. To identify the demographic features most useful for optimising recruitment for future projects, only variables for GPs with publicly available data — state, socio-economic status percentile and remoteness of postcode, and proportion of residents in area aged 70 years or more — were included in the model. The relationship between the timeliness of recruitment activity and total number of randomisations was assessed in unequal variance t tests.
The recruitment cost per randomised participant was calculated and expressed in Australian dollars.
All data were analysed in Stata 13 (StataCorp).
Ethics approval
Primary ethics approval was granted by the Monash University Human Research Ethics Committee (HREC) (reference, CF07/3730–2006/745MC). Ethics approval was also granted by the RACGP National Research and Evaluation Ethics Committee (reference, NREEC 02/022b), the University of Tasmania HREC (reference, H0008933), the ACT Health HREC (reference, ETH.11.07.997), the Goulburn Valley Health HREC (reference, GVH 21/07), and the University of Adelaide HREC (reference, H-250–2011).
Results
A median 13 patients per GP (interquartile range [IQR], 2–34) responded to written invitations to participate in ASPREE and were screened by phone, of whom a median of six patients per GP (IQR, 3–13) were identified as potential participants (Box 1, step 3); a median of three patients per GP (IQR, 0–8) were randomised to participation. The median numbers of patients included at phone screening (9; IQR, 5–16) and of randomised participants (5; IQR, 1–11) were highest in Tasmania; the lowest median numbers at phone screening (5; IQR 2–12) and at randomisation (2, IQR, 0–6) were in NSW. The total number of participants randomised was largest in Victoria (10 850). Of 2717 GPs enrolled in the study, 664 (24%) did not recruit a randomised participant (Box 3). Overall recruitment progression is depicted in the Supporting information, figure 1.
Box 3. Enrolment of general practitioners and recruitment of participants, by state.
| ACT | NSW | SA | Tasmania | Victoria | Total | P* | |
|---|---|---|---|---|---|---|---|
| Number of GPs (PCHRIS data) | 463 | 704 | 2140 | 594 | 6394 | 10 295 | |
| GPs contacted | 97 (21%) | 277 (39%) | 259 (12%) | 395 (66%) | 4805 (75%) | 5833 (57%) | |
| GPs enrolled | 91 (20%) | 251 (36%) | 245 (11%) | 238 (40%) | 1892 (30%) | 2717 (27%) | |
| Phone screens per GP, median (IQR)† | 13 (2–40) | 9 (1–29) | 15 (4–33) | 13 (3–29) | 14 (3–36) | 13 (2–34) | 0.035 |
| Patients included after phone screen per GP, median (IQR)† | 8 (4–17) | 5 (2–12) | 7 (3–12) | 9 (5–16) | 6 (3–12) | 6 (3–13) | < 0.001 |
| Randomised participants per GP, median (IQR)† | 3 (1–10) | 2 (0–6) | 3 (1–7) | 5 (1–11) | 2 (0–7) | 3 (0–8) | < 0.001 |
| Randomised participants (proportion of population)‡ | 679 (3%) | 1064 (1%) | 1365 (1%) | 2077 (5%) | 10 850 (2%) | 16 035 (2%) | |
| Enrolled GPs with at least one randomised participant | 71 (78%) | 168 (67%) | 1408 (74%) | 206 (87%) | 200 (82%) | 2053 (76%) |
IQR = interquartile range; PHCRIS = Primary Health Care Research and Information Service.
Differences between states: Kruskal–Wallis test.
Median number per enrolled GP per practice; 205 GPs were enrolled at more than one practice.
668 participants were randomised without a linked enrolled GP (4% of all randomisations).
Compared with GPs in Victoria, GPs in NSW were significantly less likely (odds ratio [OR], 0.60; 95% confidence interval [CI], 0.44–0.84) and GPs in Tasmania significantly more likely (OR, 1.91; 95% CI, 1.24–2.95) to recruit at least one randomised patient; the odds for GPs in the ACT (OR, 1.14; 95% CI, 0.67–1.95) and South Australia (OR, 1.23; 95% CI, 0.87–1.73) were not significantly different from those for Victoria.
In the logistic regression model, GPs in inner regional (adjusted OR [aOR], 1.45; 95% CI, 1.14–1.84) and outer regional areas (aOR, 1.86; 95% CI, 1.19–2.88) were more likely than GPs in major cities to recruit at least one randomised participant. Each increase of one percentage point in the proportion of the population aged 70 years or more was associated with a 10% increase (95% CI, 5–15%) in the likelihood of a GP having recruited a randomised participant. Compared with GPs in low socio-economic status areas, GPs in moderate (aOR, 1.30; 95% CI, 1.03–1.65) and high socio-economic status areas (aOR, 1.83; 95% CI, 1.36–2.43) were more likely to have recruited a randomised participant (Box 4).
Box 4. Results of general practitioner enrolment and participant recruitment, by population demographic characteristics.
| Patients per GP, median (IQR)* | At least one randomised patient recruited by GP | |||||
|---|---|---|---|---|---|---|
| GPs enrolled | Screened by phone | Included after phone screen | Randomised participants | Adjusted odds ratio (95% CI)† | P | |
| Remoteness classification (ASGS)‡ | ||||||
| Major cities of Australia | 1637 | 14 (6–30) | 5 (2–11) | 3 (1–7) | 1 | |
| Inner regional Australia | 864 | 14 (5–30) | 8 (4–15) | 4 (1–10) | 1.45 (1.14–1.84) | 0.002 |
| Outer regional Australia | 205 | 15 (5–32) | 8 (4–18) | 5 (1–13) | 1.86 (1.19–2.88) | 0.006 |
| Remote | 0 | NA | NA | NA | NA | |
| Age (per percentage point of local population aged 70 years or more) | 1.10 (1.05–1.15) | < 0.001 | ||||
| Proportion of population ages 70 years or more | ||||||
| < 8% | 627 | 12 (5–25) | 5 (2–10) | 2 (0–5) | ||
| 8–9% | 735 | 12 (4–25) | 6 (3–12) | 2 (0–7) | ||
| 10% | 657 | 18 (7–35) | 8 (3–14) | 4 (1–9) | ||
| ≥ 11% | 917 | 16 (6–34) | 7 (3–14) | 3 (1–8) | ||
| Socio-economic status of practice postcode (IRSAD), by percentile group§ | ||||||
| Low (< 40%) | 807 | 15 (3–36) | 7 (3–13) | 3 (1–9) | 1 | |
| Moderate (40–74%) | 845 | 14 (4–36) | 6 (3–12) | 3 (1–8) | 1.30 (1.03–1.65) | 0.025 |
| High (75–100%) | 889 | 18 (5–39) | 7 (3–13) | 4 (1–9) | 1.83 (1.36–2.43) | < 0.001 |
ASGS = Australian Statistical Geography Standard; IQR = interquartile range; IRSAD = Index of Relative Socio-economic Advantage and Disadvantage.
Median number per enrolled GP per practice; 205 GPs were enrolled at more than one practice.
Adjusted for state.
Data missing for 179 GPs.
Data missing for 175 GPs.
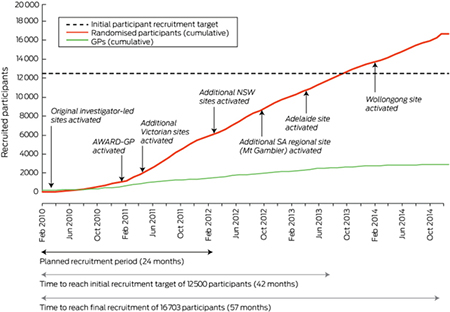
The mean number of randomised patients per GP decreased substantially with time to first randomisation. Compared with the reference group (0–3 months to first randomisation), the mean number of randomised patients per GP at 3–6 months was 1.8 (95% CI, 2.8–0.9), at 6–9 months 2.2 (95% CI, 3.3–1.0), and after 9 months 4.5 (95% CI, 5.8–3.4) fewer (each P < 0.001) (Box 5).
Box 5. Change in mean number of randomised patients per enrolled general practitioner, by time to first randomisation*.
* The reference group comprises enrolled GPs who recruited at least one participant within 3 months of their enrolment (mean number of randomised participants for this group: 9.5; standard deviation, 9.7 [skewed distribution]).
Costs of recruitment
The estimated total cost of recruitment was $830 per randomised participant, including $480 for the baseline screening visits, $100 for GP practice reimbursement, $80 for travel and other costs, and $170 for GP enrolment and participant phone screening. The development of AWARD-GP cost $120 980.
Discussion
We found that Australian general practice is a productive environment for recruiting participants for large scale clinical trials when key barriers are mitigated by reducing GP time requirements, reimbursing practices for the costs of participating, and recognising GPs as associate investigators.
Previous studies have found that interest in the research question is the key motivation for GPs participating in research.10,21,22 Some GPs report unease with participant randomisation6 and researchers must therefore justify equipoise, “a state of genuine uncertainty … regarding the comparative therapeutic merits of each arm in a trial”.23 The proportion of approached GPs who agreed to participate in the study (47%) was higher than for ANBP2 (17%)5 and other studies,9 indicating that there was significant interest in the research question examined by ASPREE, an interpretation confirmed by a large amount of anecdotal feedback from GPs.
We found that the odds of GPs in high socio-economic status postcodes recruiting a randomised participant were 83% higher than for those in low socio-economic status areas, in contrast to reports of greater recruitment in low socio-economic status areas.16
Recruitment for ASPREE was assisted by a demographic analysis of Divisions of General Practice that identified areas with higher proportions of older people. Had ASPREE recruited only from these areas, the randomisation target could have been achieved with as few as 2290 enrolled GPs, a 15% reduction in the number of enrolments. However, the population proportion of people aged 70 years or more reached 10% in only 36% of Australian divisions active in 2011, and including them all in recruitment efforts would have required expansion into Western Australia, Queensland and regional NSW. Even were this financially feasible, the numbers of GPs in these areas were insufficient to reach ASPREE recruitment goals.
We found that including GPs from regional areas is particularly valuable because they were more likely to recruit eligible participants than their metropolitan counterparts. Another study noted higher levels of research interest among GPs in outer suburban or rural practices than in metropolitan practices;17 in ASPREE, GPs from regional areas were 45% more likely to recruit a randomised participant than GPs in major cities. However, regional populations alone may not be sufficient for reaching recruitment targets, and strategies based on a combination of regional and city engagement are therefore recommended for large trials.
Differences between states indicate that further factors influence recruitment. The odds of a GP in Tasmania recruiting a randomised participant were significantly higher than in Victoria after adjusting for remoteness and demographic characteristics. Interest in research may be greater in Tasmania; alternatively, the difference might be explained by fewer studies being conducted in Tasmania, a higher population-to-GP ratio, or a stronger relationship between patients and GPs. The low participant randomisation rate in NSW was related to Wollongong being the final hub to begin recruitment; post-enrolment activity required 3–6 months, and GPs in Wollongong may not have had sufficient time to complete this activity before recruitment ended. Although our analysis cannot fully explain differences between states, investigators should be aware of the possibility and adjust their recruitment models accordingly.
Recruitment activities should be completed as rapidly as possible after a GP has committed themselves to participating (Box 5); that is, while their motivation is strongest, as the mean number of randomisations per GP was significantly lower when post-enrolment activity took longer than 9 months. Future primary care studies may consider ending active follow-up of enrolled GPs who have not recruited a randomised participant within 9 months of their own enrolment.
Continuous monitoring of recruitment with appropriate technology was crucial to the success of ASPREE. Without the assistance of AWARD-GP, ASPREE could not have identified and modelled the impact of lower than expected recruitment outcomes. Additionally, the availability of PCHRIS population and GP demographic data allowed ASPREE to identify potential hotspots for recruitment and to undertake targeted expansion. AWARD-GP was expensive, but necessary, as there was no existing central resource or network for engaging with research-willing GPs.
Opportunities for improvement
ASPREE ultimately exceeded its recruitment target in Australia, but the process took more than 4 years and required substantial resources. Establishing the initial recruitment sites required months of lead-in time for staff recruitment, study centre fit-out, ethics approval, identification of GPs, and development of AWARD-GP, and recruitment during 2010 was consequently slow (Box 2). Similar lead-in times preceded the establishment of subsequent hubs, and logistic factors prohibited the simultaneous establishment of new sites. Practice-based research networks (PBRNs) reduce recruitment times overseas by enabling concurrent recruitment at multiple sites.24,25 The utility of Australian PBRNs has long been recognised,12,26 but their funding is limited and their future uncertain.13 Investing in national PBRNs that provide ASPREE-equivalent infrastructure and access to relevant population demographic data would assist other studies implement the strategies employed by ASPREE, while avoiding costly delays.
Conclusion
Australian general practice is a productive environment for large scale clinical trials when barriers to participation in research are mitigated. To maximise recruitment, investigators should include regional areas, focus on areas with larger proportions of the target population groups, and ensure that their research question is of clinical importance for GPs. The success of ASPREE attests to both the interest of Australian GPs in research and the value of engaging GP co-investigators in large scale clinical trials. Investing in primary care research infrastructure would assist smaller studies implement these strategies.
Supplementary Material
Figure 1:
The three-step GP and participant recruitment pipeline.
Summary box.
The known:
Enrolling general practitioners as co-investigators has been a successful approach to recruiting community-dwelling patients for longitudinal research.
The new:
ASPREE reduced barriers to recruitment by easing the burden on GPs, remunerating practices for their involvement, and recognising GPs as associate investigators. Participants were recruited most efficiently in regional locations and areas with higher proportions of people in the target demographic group. By analysing GP and population data, ASPREE was able to adapt its strategy, successfully reaching its recruitment goals.
The implications:
General practice is a rich environment for clinical research, and could be better exploited by establishing a national general practice-based research network.
Acknowledgements:
The ASPREE study, including the design and implementation of the recruitment strategy, was supported by the National Institute on Aging and the National Cancer Institute at the National Institutes of Health in the United States (U01AG029824), the National Health and Medical Research Council (334047 and 1127060), Monash University, and the Victorian Cancer Agency. Bayer provided aspirin and the matching placebo. We acknowledge the dedicated and skilled staff in Australia and the Unites States who undertook the ASPREE trial. We are also most grateful to the ASPREE participants who so willingly volunteered for this study, and the general practitioners and medical clinics who supported the participants in the ASPREE study.
Footnotes
Competing interests: No relevant disclosures.
Trial registration (ASPREE): International Standard Randomized Controlled Trial Number Register (ISRCTN83772183) and clinicaltrials.gov (NCT01038583).
References
- 1.Foster JM, Sawyer SM, Smith L, et al. Barriers and facilitators to patient recruitment to a cluster randomized controlled trial in primary care: lessons for future trials. BMC Med Res Methodol 2015; 15: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Australian Bureau of Statistics. 4839.0. Patient experiences in Australia: summary of findings, 2015–16. Nov 2017. http://www.abs.gov.au/ausstats/abs@.nsf/mf/4839.0 (viewed Oct 2017).
- 3.Newington L, Metcalfe A. Factors influencing recruitment to research: qualitative study of the experiences and perceptions of research teams. BMC Med Res Methodol 2014; 14: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wing LMH, Reid CM, Ryan P, et al. A comparison of outcomes with angiotensin-converting-enzyme inhibitors and diuretics for hypertension in the elderly. N Engl J Med 2003; 348: 583–592. [DOI] [PubMed] [Google Scholar]
- 5.Reid CM, Ryan P, Nelson M, et al. General practitioner participation in the second Australian national blood pressure study (ANBP2). Clin Exp Pharmacol Physiol 2001; 28: 663–667. [DOI] [PubMed] [Google Scholar]
- 6.Hunt CJ, Shepherd LM, Andrews G. Do doctors know best? Comments on a failed trial. Med J Aust 2001; 174: 144–146. [DOI] [PubMed] [Google Scholar]
- 7.Goodyear-Smith F, York D, Petousis-Harris H, et al. Recruitment of practices in primary care research: the long and the short of it. Fam Pract 2009; 26: 128–136. [DOI] [PubMed] [Google Scholar]
- 8.Page MJ, French SD, McKenzie JE, et al. Recruitment difficulties in a primary care cluster randomised trial: investigating factors contributing to general practitioners’ recruitment of patients. BMC Med Res Methodol 2011; 11: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paul CL, Piterman L, Shaw JE, et al. Poor uptake of an online intervention in a cluster randomised controlled trial of online diabetes education for rural general practitioners. Trials 2017; 18: 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yallop JJ, McAvoy BR, Croucher JL, et al. Primary health care research — essential but disadvantaged. Med J Aust 2006; 185: 118–120. [DOI] [PubMed] [Google Scholar]
- 11.Askew DA, Clavarino AM, Glasziou PP, Del Mar CB. General practice research: attitudes and involvement of Queensland general practitioners. Med J Aust 2002; 177: 74–77. [DOI] [PubMed] [Google Scholar]
- 12.Zwar NA, Weller DP, McCloughan L, Traynor VJ. Supporting research in primary care: are practice-based research networks the missing link? Med J Aust 2006; 185: 110–113. [DOI] [PubMed] [Google Scholar]
- 13.Winzenberg TM, Gill GF. Prioritising general practice research. Med J Aust 2016; 205: 55–57. [DOI] [PubMed] [Google Scholar]
- 14.McNeil JJ, Woods RL, Nelson MR, et al. Baseline characteristics of participants in the ASPREE (ASPirin in Reducing Events in the Elderly) study. J Gerontol A Biol Sci Med Sci 2017; 72: 1586–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McNeil JJ, Woods RL, Nelson MR, et al. Effect of aspirin on disability-free survival in the healthy elderly. N Engl J Med 2018; 379: 1499–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Williams CM, Maher CG, Hancock MJ, et al. Recruitment rate for a clinical trial was associated with particular operational procedures and clinician characteristics. J Clin Epidemiol 2014; 67: 169–175. [DOI] [PubMed] [Google Scholar]
- 17.Silagy CA, Carson NE. Factors affecting the level of interest and activity in primary care research among general practitioners. Fam Pract 1989; 6: 173–176. [DOI] [PubMed] [Google Scholar]
- 18.Nelson MR, Reid CM, Ames D, et al. Feasibility of conducting a primary prevention trial of low-dose aspirin for major adverse cardiovascular events in older people in Australia: results from the ASPirin in Reducing Events in the Elderly (ASPREE) pilot study. Med J Aust 2008; 189: 105–109. [DOI] [PubMed] [Google Scholar]
- 19.Australian Bureau of Statistics. 2033.0.55.001. Census of population and housing: Socio-Economic Indexes for Areas (SEIFA), Australia, 2016. IRSAD. http://www.abs.gov.au/ausstats/abs@.nsf/Lookup/by%20Subject/2033.0.55.001~2016~Main%20Features~IRSAD~20 (viewed Oct 2018).
- 20.Australian Bureau of Statistics. 1270.0.55.005. Australian Statistical Geography Standard (ASGS): Volume 5 — remoteness structure, July 2016. Mar 2018. http://www.abs.gov.au/ausstats/abs@.nsf/Latestproducts/1270.0.55.005Main%20Features15July%202016?opendocument&tabname=Summary&prodno=1270.0.55.005&issue=July%202016&num=&view (viewed Oct 2018).
- 21.Williamson MK, Pirkis J, Pfaff JJ, et al. Recruiting and retaining GPs and patients in intervention studies: the DEPS-GP project as a case study. BMC Med Res Methodol 2007; 7: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Borgiel AEM, Dunn EV, Lamont CT, et al. Recruiting family physicians as participants in research. Fam Pract 1989; 6: 168–172. [DOI] [PubMed] [Google Scholar]
- 23.Equipoise Freedman B. and the ethics of clinical research. N Engl J Med 1987; 317: 141–145. [DOI] [PubMed] [Google Scholar]
- 24.Nuttall J, Hood K, Verheij TJ, et al. Building an international network for a primary care research program: reflections on challenges and solutions in the set-up and delivery of a prospective observational study of acute cough in 13 European countries. BMC Fam Pract 2011; 12: 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peterson KA, Lipman PD, Lange CJ, et al. Supporting better science in primary care: a description of practice-based research networks (PBRNs) in 2011. J Am Board Fam Med 2012; 25: 565–571. [DOI] [PubMed] [Google Scholar]
- 26.Gunn JM. Should Australia develop primary care research networks? Med J Aust 2002; 177: 63–66. https://www.mja.com.au/journal/2002/177/2/should-australia-develop-primary-care-research-networks [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.