Abstract
Novel D2-symmetric chiral amidoporphyrins with alkyl bridges across two chiral amide units on both sides of the porphyrin plane (designated as “HuPhyrin”) have been effectively constructed in a modular fashion to permit variation of bridge length. The Co(II) complexes of HuPhyrin, [Co(HuPhyrin)], represent new-generation metalloradical catalysts where the metal-centered d-radical is situated inside a cavity-like ligand with more rigid chiral environment and enhanced hydrogen-bonding capability. As demonstrated with cyclopropanation and aziridination as model reactions, the bridged [Co(HuPhyrin)] function notably different from the open catalysts, exhibiting significant enhancement in both reactivity and stereoselectivity. Furthermore, the length of the distal alkyl bridge can have a remarkable influence on the catalytic property.
Keywords: radical reaction, metalloradical catalysis, chiral porphyrins, cobalt, catalyst engineering
Graphical Abstract

Novel D2-symmetric chiral amidoporphyrins with alkyl bridges across two chiral amide units on both sides of the porphyrin plane have been effectively constructed in a modular fashion. The length of the distal bridge can significantly influence the reactivity and stereoselectivity of Co(II)-based metalloradical catalysis.
Homolytic radical chemistry has been increasingly explored for the development of alternative tools for bond-breaking and bond-forming that may shape the state of the art of organic synthesis.[1,2] Despite significant advancements in this endeavor, general strategies for addressing the enduring issues of controlling reactivity and selectivity in radical reactions, particularly enantioselectivity, remain to be uncovered. Among recent progresses,[3] metalloradical catalysis (MRC), which explores the use of metalloradical complexes as open-shell catalysts for catalytically generating organic radical intermediates as well as controlling subsequent homolytic radical transformations, has emerged as a conceptually new approach.[4,5] In this context, Co(II) complexes of porphyrins, as 15e stable metalloradicals, have exhibited unusual efficiency in radical activation of diazo compounds to generate the fundamentally new α-Co(III)-alkyl radicals A1 (Scheme 1A).[6] The resulting Co-stabilized C-centered radical intermediates can undergo radical addition to alkenes for generation of γ-Co(III)-alkyl radicals A2, which subsequently proceed intramolecular homolytic radical substitution (3-exo-tet radical cyclization) to produce cyclopropanes upon regeneration of Co(II)-based metalloradicals. The outcome of this Co(II)-based metalloradical catalysis (Co(II)-MRC) is the revelation of an unprecedented catalytic pathway for olefin cyclopropanation that operates via a stepwise radical mechanism (Scheme 1A).[7] In parallel, the application of Co(II)-MRC with organic azides has resulted in the disclosure of a new radical pathway for catalytic olefin aziridination that involves α-Co(III)-aminyl radicals B1[8] and γ-Co(III)-alkyl radicals B2 as key intermediates (Scheme 1B).[9] Despite their underlying radical mechanisms, catalytic transformations via Co(II)-MRC can be rendered stereoselective because the radical intermediates involved are no longer “free” but controlled by the catalysts.[7,9–10] In practice, the formidable challenge associated with controlling stereoselectivity of radical reactions can essentially be translated to a solvable problem of catalyst design and development.
Scheme 1.
Radical pathways for cyclopropanation and aziridination.
Since first introduced in 2004[7a] and later expanded,[7c-d] the family of D2-symmetric chiral amidoporphyrins (D2-Por*) have proved particularly effective in controlling reactivity as well as stereoselectivity of various radical transformations via Co(II)-MRC.[7],[9–10] In additon to the pocket-like chiral environment with tunable electronic and steric properties, the effectiveness of this family of ligands in supporting Co(II)-MRC is also attributed to the postulated H-bonding interactions between N–H units of the amides on the amidoporphyrin ligand as the HB-donors and the substituents on the C-or N-centered radical moiety as the HB-acceptors, as illustrated (Scheme 1C) with the common metalloradical catalyst [Co(P1)] (P1: 3,5-DitBu-ChenPhyrin).[7a] While existing D2-symmetric chiral amidoporphyrins have been successfully applied for a number of catalytic radical processes, the need to design the next generation of MRC catalysts with improved catalytic properties has become increasingly evident. In addition to addressing the limitations of existing systems, it may open windows for discovering new catalytic radical transformations. To this end, we envisioned new D2-Por* with bridges across two chiral amide units on both sides of the porphyrin plane where the metal-centered radicals are situated inside a cavity-like chiral environment (Figure 1).[11–12] Besides offering additional dimension for fine-tuning steric, electronic and chiral properties, the bridged-amidoporphyrins are expected to possess enhanced hydrogen-bonding capability as a result of the rigidification of the chiral amide units. Herein, we wish to report the first construction of such porphyrin ligands (designated as “HuPhyrin”) having different alkyl bridges (Figure 1). As initial demonstration with asymmetric radical cyclopropanation and aziridination as model reactions, Co(II) complexes of HuPhyrin ([Co(HuPhyrin)]) exhibit notably different catalytic properties from the Co(II) catalysts supported by the open analog of D2-Por*.
Figure 1.

Creation of cavity-like chiral environment via bridging.
For construction of the bridges, 3,5-DitBu-Tao(tBu)Phyrin (3) (Scheme 2), a new D2-symmetric chiral amidoporphyrin that carries tert-butyl ester moieties, was selected as the scaffold structure, considering that the ester functionalities in 3 may serve as convenient handles for building the bridges. Following the previously established procedure,[7a] 3 was prepared in 88% yield through Pd-catalyzed quadruple amidation reaction of tetrabromoporphyrin 1 with the optically pure chiral amide 2[7b] (Scheme 2). The tert-butyl esters in 3 could readily undergo transesterification involving the first generation of the corresponding porphyrin carboxylic acids by hydrolysis and then subsequent O-alkylation with alkyl halides or tosylates. For example, the use of ethyl tosylate afforded 3,5-DitBu-Tao(Et)Phyrin (P2) in 82% yield for the two-step transformation, which was metallated to form Co(II) complex [Co(P2)] in 93% yield. When the transesterification operation was carried out with allyl bromide and homoallyl tosylate, 4a and 4b were formed in 80% and 89% yields, respectively. With the second-generation Grubbs catalyst, 4a and 4b underwent ring-closing metathesis to form olefin-bridged porphyrins as a non-consequential mixture of cis and trans-isomers. They were directly hydrogenated to form the alkyl-bridged porphyrins P3 (3,5-DitBu-Hu(C4)Phyrin) and P4 (3,5-DitBu-Hu(C6)Phyrin) in 76% and 85% yields, respectively. The five-step synthesis was accomplished in high overall yields (54% for P3 and 67% for P4). Metallation gave Co(II) complexes [Co(P3)] and [Co(P4)] in 94% and 90% yields, respectively, on the scale of hundreds of milligrams.
Scheme 2.
Synthesis of D2-symmetric chiral bridged-amidoporphyrins and cobalt(II) complexes.
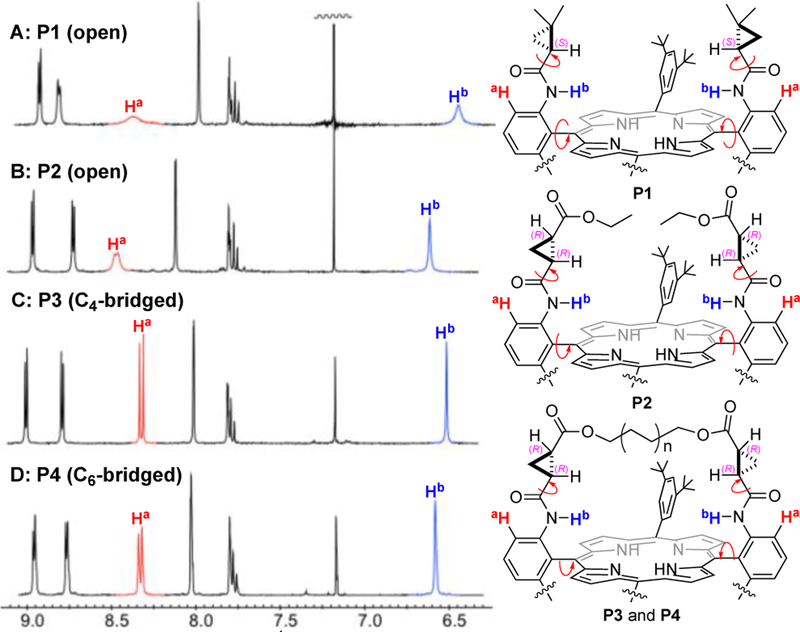
To examine the bridging effect on the conformation of D2-Por*, the 1H-NMR spectra of C4-bridged P3 and C6-bridged P4 were analyzed in comparison with the open counterparts P2 and P1. As illustrated by the low-field region of their 1H-NMR spectra (Figure 2), both the line width and chemical shift of the signals corresponding to the aromatic protons Ha and the amide protons Hb vary significantly. Although existing D2-Por*, as represented by P1, have a relatively-defined configuration that directs the ortho-chiral amide units toward the center of the porphyrin where the Co(II) ion is situated, they bear a certain degree of conformational flexibility owing to the existence of rotational freedom between the meso-phenyl rings and the porphyrin plane as well as between the trans-amides and the cyclopropyl groups (Figure 2). This rotational freedom is manifested by the broad Ha and Hb signals at 8.45 and 6.52 ppm, respectively (Figure 2A). Due to steric effects of -CO2Et, the Ha and Hb signals in P2 became less broad and shifted to the lower field at 8.55 and 6.69 ppm, respectively, (Figure 2B) As a result of the bridging, both Ha (8.41 ppm) and Hb (6.60 ppm) signals in P3 were notably sharpened, signifying the rigidification of the conformational freedom (Figure 2C). Similar but slightly less sharpening of the Ha (8.44 ppm) and Hb (6.66 ppm) signals in P4 indicates relatively reduced rigidification in conformation upon elongation of the bridge from C4 to C6 (Figure 2D). Consequently, the more rigid conformation in P3 and P4 should enhance H-bonding capability of the chiral amide units for stabilizing the catalytic intermediates.
Figure 2.
Low-field region 1H-NMR spectra of chiral amidoporphyrins.
X-ray diffraction analysis unveiled the structural details of P3 and P4, including the double alkyl-bridges and associated dual cavities (Figure 3). The 38-and 42-membered macrocyclic structures in P3 and P4 created by the double ring-closing olefin metathesis are bisected by the porphyrin core. In addition to the porphyrin ring, the macrocycles consist of multiple small rings (2 benzenes and 4 cyclopropanes) and functional groups (4 amides and 4 esters). As shown, C6-bridged P4 contains a significantly larger cavity than C4-bridged P3. The observation that the cavity size can be varied by simple change of the bridge length suggests a new dimension for catalyst engineering.
Figure 3.

X-ray structures of bridged-amidoporphyrins. The distances between N1 and N2 and between C1 and C2 are averaged values of those on both sides of the porphyrin plane.
The bridging effect on the catalytic performance of [Co(D2-Por*)] was demonstrated with cyclopropanation[13] and aziridination[14] as two model reactions. The catalytic reactions by bridged catalysts [Co(P3)] and [Co(P4)] were conducted in direct comparison with the open catalysts [Co(P1)] and [Co(P2)] (Scheme 3). Asymmetric cyclopropanation of styrene with ethyl diazoacetate (EDA) (eq. 1) was carried out at room temperature using 1 mol % catalyst in the absence of additives with the alkene as the limiting reagent without slow addition of the diazo reagent,[15] a practical condition that is atypical for other catalytic systems. Within only 1 h, [Co(P3)] could catalyze the efficient formation of cyclopropane 5 in high yield (93%) with high diastereoselectivity (90% de) and excellent enantioselectivity (96% ee). By comparing the results with [Co(P1)][15] and [Co(P2)] under the same conditions, it is evident that [Co(P3)] is a superior catalyst for the reaction in terms of both reactivity and stereoselectivity, indicating the positive bridging effect on catalytic performance. While [Co(P4)] could also catalyze the reaction effectively, it differed in both reactivity (84% yield vs 93% yield) and enantioselectivity (88% ee vs 96% ee) from [Co(P3)]. Enantioselective radical aziridination of styrene with trichloroethoxysulfonyl azide (TcesN3) (eq. 2) was carried out at 0 ºC for 24 h with 2 mol % catalyst loading in the absence of additives with the alkene as the limiting reagent.[16] While both [Co(P1)] and [Co(P2)] could generate the desired aziridine 6 in high yields, the enantioselectivities were inferior.[16] Under the same conditions, the use of [Co(P3)] resulted in dramatic improvement in enantioselectivity (85% ee) but a considerable decrease in reactivity (24% yield). Switching from [Co(P3)] to [Co(P4)] led to further improvement in enantioselectivity (92% ee) while significantly enhancing reactivity (94% yield). Considering that [Co(P3)] and [Co(P4)] differs only in the length of the distal bridge by merely two methylene units, the observed ligand effect is truly remarkable and may have an important implication in catalyst design and development.
Scheme 3.

Bridging effect on Co(II)-catalyzed radical cyclopropanation and aziridination.
In summary, we have introduced the new-generation D2-Por* that contain alkyl bridges across the two chiral amide units on both sides of the porphyrin plane. The bridged D2-Por* can be efficiently constructed in a modular fashion via five-step synthesis featuring double ring-closing olefin metathesis in high overall yields. They have more rigid, cavity-like chiral environments, the shape and size of which can be adjusted by variation of the bridge length. As demonstrated with two model reactions, bridged [Co(D2-Por*)] exhibit notably different catalytic reactivity and stereoselectivity from the existing non-bridged catalysts. Furthermore, our results indicate subtle alteration on the distal bridge can have a profound effect on catalytic performance, signifying the importance of cavity manipulation in catalyst engineering. This study opens the door to further applications of Co(II)-MRC for the development of new stereoselective radical reactions
Supplementary Material
Acknowledgements
We are grateful for financial support by NIH (R01-GM102554) and in part by NSF (CHE-1624216).
References
- [1].Curran DP, Porter NA, Giese B, Stereochemistry of Radical Reactions: Concepts, Guidelines, and Synthetic Applications; Wiley-VCH: Weinheim, Germany, 2008. [Google Scholar]
- [2].a) Plesniak MP, Huang HM, Procter DJ, Nat. Rev. Chem 2017, 1, 0077 [Google Scholar]; b) Kern N, Plesniak MP, McDouall JJW, Procter DJ, Nat. Chem 2017, 9, 1198. [DOI] [PubMed] [Google Scholar]; c) Dénès F, Pichowicz M, Povie G, Renaud P, Chem. Rev 2014, 114, 2587. [DOI] [PubMed] [Google Scholar]; d) Sibi MP, Manyem S, Zimmerman J, Chem. Rev 2003, 103, 3263. [DOI] [PubMed] [Google Scholar]; e) Ollivier C, Renaud P, Chem. Rev 2001, 101, 3415. [DOI] [PubMed] [Google Scholar]
- [3].a) Hashimoto T, Kawamata Y, Maruoka K, Nat. Chem 2014, 6, 702. [DOI] [PubMed] [Google Scholar]; b) Zhang W, Wang F, McCann SD, Wang D, Chen P, Stahl SS, Liu G, Science 2016, 353, 1014. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Meyer D, Renaud P, Angew. Chem. Int. Ed 2017, 56, 10858. [DOI] [PubMed] [Google Scholar]; d) Morrill C, Jensen C, Just-Baringo X, Grogan G, Turner NJ, Procter DJ, Angew. Chem. Int. Ed 2018, 57, 3692; Angew. Chem.2018, 130, 3754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].a) Miyabe H, Kawashima A, Yoshioka E, Kohtani S, Chem. Eur. J 2017, 23, 6225. [DOI] [PubMed] [Google Scholar]; b) Studer A, Curran DP, Angew. Chem. Int. Ed 2016, 55, 58; Angew. Chem. 2016, 128, 58 [DOI] [PubMed] [Google Scholar]; c) Pellissier H, Clavier H, Chem. Rev 2014, 114, 2775. [DOI] [PubMed] [Google Scholar]
- [5].a) Gansäuer A, Fan C-A, Keller F, Keil J, J. Am. Chem. Soc 2007, 129, 3484. [DOI] [PubMed] [Google Scholar]; b) Gansäuer A, Shi L, Otte M, J. Am. Chem. Soc 2010,132,11858. [DOI] [PubMed] [Google Scholar]; c) Hildebrandt S, Gansäuer A, Angew. Chem. Int. Ed 2016, 55, 9719; Angew. Chem. 2016, 128, 9871 [DOI] [PubMed] [Google Scholar]; d) Funken N, Mühlhaus F, Gansäuer A, Angew. Chem. Int. Ed 2016, 55, 12030; Angew. Chem. 2016, 128, 12209 [DOI] [PubMed] [Google Scholar]; e) Zhang Y-Q, Vogelsang E, Qu Z-W, Grimme S; Gansäuer A, Angew. Chem. Int. Ed 2017, 56, 12654; Angew. Chem. 2017, 129, 12828. [DOI] [PubMed] [Google Scholar]
- [6].a) Dzik WI, Xu X, Zhang XP, Reek JNH, de Bruin B, J. Am. Chem. Soc 2010, 132, 10891. [DOI] [PubMed] [Google Scholar]; b) Lu H, Dzik WI, Xu X, Wojtas L, de Bruin B, Zhang XP, J. Am. Chem. Soc 2011, 133, 8518. [DOI] [PubMed] [Google Scholar]
- [7].a) Chen Y, Fields KB, Zhang XP, J. Am. Chem. Soc 2004, 126, 14718. [DOI] [PubMed] [Google Scholar]; b) Chen Y, Ruppel JV, Zhang XP, J. Am. Chem. Soc 2007, 129, 12074. [DOI] [PubMed] [Google Scholar]; c) Zhu S, Ruppel JV, Lu H, Wojtas L, Zhang XP, J. Am. Chem. Soc 2008, 130, 5042. [DOI] [PubMed] [Google Scholar]; d) Xu X, Lu H, Ruppel JV, Cui X, Lopez de Mesa S, Wojtas L, Zhang XP, J. Am. Chem. Soc 2011, 133, 15292. [DOI] [PubMed] [Google Scholar]; e) Fantauzzi S, Gallo E, Rose E, Raoul N, Caselli A, Issa S, Ragaini F, Cenini S, Organometallics 2008, 27, 6143 [Google Scholar]; f) Wang Y, Wen X, Cui X, Wojtas L, Zhang XP, J. Am. Chem. Soc 2017, 139, 1049. [DOI] [PMC free article] [PubMed] [Google Scholar]; g) Reddy AR, Hao F, Wu K, Zhou C-Y, Che CM, Angew. Chem. Int. Ed 2016, 55, 1810; Angew. Chem. 2016, 128, 1842 [DOI] [PubMed] [Google Scholar]; h) Chirila A, Das BG, Paul ND, de Bruin B, ChemCatChem 2017, 9, 1413. [DOI] [PMC free article] [PubMed] [Google Scholar]; i) Roy S, Das SK, Chattopadhyay B, Angew. Chem. Int. Ed 2018, 57, 2238; Angew. Chem. 2018, 130, 2260. [DOI] [PubMed] [Google Scholar]
- [8].Lyaskovskyy V, Olivos Suarez AI, Lu H, Jiang H, Zhang XP, de Bruin B, J. Am. Chem. Soc 2011, 133, 12264. [DOI] [PubMed] [Google Scholar]
- [9].a) Subbarayan V, Ruppel JV, Zhu S, Perman JA, Zhang XP, Chem. Commun 2009, 4266. [DOI] [PubMed]; b) Jin L-M, Xu X, Lu H, Cui X, Wojtas L, Zhang XP, Angew. Chem. Int. Ed 2013, 52, 5309; Angew. Chem. 2013, 125, 5417 [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Jiang H, Lang K, Lu H, Wojtas L, Zhang XP, J. Am. Chem. Soc 2017, 139, 9164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].a) Wang Y, Wen X, Cui X, Zhang XP, J. Am. Chem. Soc 2018. 140, 4792. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Karns AS, Goswami M, de Bruin B, Chem. Eur. J 2018, 24, 5253. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Lu HJ, Jiang HL, Wojtas L, Zhang XP, Angew. Chem. Int. Ed 2010, 49, 10192; Angew. Chem. 2010, 122, 10390 [DOI] [PubMed] [Google Scholar]; d) Villanueva O, Weldy NM, Blakey SB, MacBeth CE, Chem. Sci 2015, 6, 6672; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].a) Boitrel B, Lecas A, Renko Z, Rose E, J. Chem. Soc., Chem. Commun 1985, 1820; b) Rose E, Lecas A, Quelquejeu M, Kossanyi A, Boitrel B, Coord. Chem. Rev 1998, 178, 1407 [Google Scholar]; c) Boitrel B, Baveux-Chambenoît V, Richard P, Eur. J. Inorg. Chem 2002, 1666; d) Even P, Boitrel B, Coord. Chem. Rev 2006, 250, 519 [Google Scholar]; e) Gallo E, Rose E, Boitrel B, Legnani L, Toma L, Organometallics 2014, 33, 608 [Google Scholar]; f) Carminati DM, Intrieri D, Caselli A, Le Gac S, Boitrel B, Toma L, Legnani L, Gallo E, Chem. Eur. J 2016, 22, 13599. [DOI] [PubMed] [Google Scholar]
- [12].Raynal M, Ballester P, Vidal-Ferran A, van Leeuwen PWNM, Chem. Soc. Rev 2014, 43, 1734. [DOI] [PubMed] [Google Scholar]
- [13].a) Lebel H, Marcoux J-F, Molinaro C, Charette AB, Chem. Rev 2003, 103, 977. [DOI] [PubMed] [Google Scholar]; b) Doyle MP, Forbes DC, Chem. Rev 1998, 98, 911. [DOI] [PubMed] [Google Scholar]
- [14].a) Degennaro L, Trinchera P, Luisi R, Chem. Rev 2014, 114, 7881. [DOI] [PubMed] [Google Scholar]; b) Müller P, Fruit C, Chem. Rev 2003, 103, 2905. [DOI] [PubMed] [Google Scholar]
- [15]. While [Co(P1)] was previously demonstrated to be effective for cyclopropanation with different diazo compounds, including tert-butyl diazoacetate (t-BDA) (ref 7), the use of more common ethyl diazoacetate (EDA) was less efficient even in longer reaction time (20 h) and in the presence of DMAP (0.5 equiv) as additive, forming cyclopropane 5 in 82% yield with 78% ee and 94% de (ref 7a).
- [16]. The Co(II) complex of 2,6-DiMeO-ZhuPhyrin was previously reported to catalyze the reaction, but required higher catalyst loading (5 mol %), longer reaction time (48 h), and excess amount of styrene (5 equiv) as well as the use of 5 mol % Pd(OAc)2 as an additive (ref. 9a). Under the current more desired condition, it only led to aziridine 6 in 10% yield with 80% ee.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.