Supplemental Digital Content is available in the text
Keywords: combination therapy, epidermal growth factor receptor, erlotinib, gefitinib
Abstract
Background:
Combination therapy based on epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) is an emerging trend in cancer treatment, but the clinical value of EGFR-TKIs combination therapy remains controversial. Thus, we conducted a comprehensive analysis of randomized controlled trials (RCTs) comparing EGFR-TKIs combination therapies with monotherapies, aiming to evaluate the safety and efficacy of EGFR-TKIs based combination therapy and to find a more beneficial combination strategy.
Methods:
We searched for clinical studies that evaluated EGFR-TKIs combination therapy in cancer. We extracted data from these studies to evaluate the relative risk (RR) of overall response rate (ORR) and grade 3/4 treatment-related adverse events (AEs), the hazard ratios (HRs) of overall survival (OS), and progression-free survival (PFS).
Results:
Fourteen RCTs were identified (n = 3774). Treatments included combinations of EGFR-TKIs and chemotherapy, combinations of EGFR-TKIs and radiotherapy, and combinations of EGFR-TKIs and bevacizumab. EGFR-TKIs combination therapies showed higher ORR [RR: 1.62; 95% confidence interval (95% CI):1.16–2.26; P = .005], PFS (HR: 0.76; 95% CI: 0.64–0.89; P = .001), and OS (HR: 0.88; 95% CI: 0.79–0.97; P = .013) values than monotherapies. However, higher grade 3/4 treatment-related AEs (RR: 1.79; 95% CI: 1.02–3.15; P = .000) were observed in combination therapy than in monotherapy.
Conclusion:
Our pooled analysis and subgroup analysis results showed that the addition of chemotherapy to EGFR-TKIs better benefits PFS and safety. Adding bevacizumab was associated with better ORR and OS. The efficacy and safety of a bevacizumab-EGFR-TKIs-chemotherapy combination should be investigated further.
1. Introduction
Personalized therapy is a novel idea and has becoming an emerging trend in the treatment of cancer. The epidermal growth factor receptor (EGFR) and its downstream signaling, including the Ras-Raf-MAP, PI3K, and Akt pathways, play a vital role in the regulation of cell proliferation, survival, and differentiation.[1–3] Therefore, targeting EGFR has become a focus of investigation, including research in EGFR tyrosine kinase inhibitors (EGFR-TKIs). EGFR-TKIs can inhibit enzyme tyrosine kinase that involved in signal transduction cascade and downstream activation of many proteins.[4] EGFR-TKIs such as gefitinib and erlotinib have been approved by the US Food and Drug Administration (FDA).[5,6]
Gefitinib, a selective EGFR (ErbB1) tyrosine kinase inhibitor, can also inhibit the growth of some ErbB2-overexpressing tumor cells.[7,8] Gefitinib has been shown to improve OS in patients with nonsmall cell lung cancer (NSCLC) after failure of chemotherapies.[7] Erlotinib is a potent and selective inhibitor of the EGFR tyrosine kinase.[9] It can block cell cycle progression in the G1 phase.[10] Erlotinib has been shown to reduce symptoms and increase survival in patients with advanced stage III or IV NSCLC.[11] In addition, Erlotinib has also been reported to be associated with alleviation of tumor-related symptoms in patients who were positive for ErbB1.[12]
EGFR tyrosine kinase inhibitors combination therapy has gained popularity and there are several preclinical trials on EGFR-TKIs combination therapies, as well as phase I and phase II studies. However, the clinical value of EGFR-TKIs combination therapy remains under debate. Recent studies have begun to examine EGFR-TKIs combination therapy, though whether the benefits outweigh potential toxicities remains uncertain.
This study aims to evaluate the safety and efficacy of EGFR-TKIs combination therapy, and to investigate an appropriate combination type.
2. Methods
2.1. Study design and search strategy
All relevant information was identified from published trials that compared combination of EGFR-TKIs therapies with monotherapy to evaluate the clinical value of EGFR-TKIs based combination therapy. We searched for the trials based on the following computerized bibliographic databases: PubMed/Medline, Embase, the Cochrane Central Register of Controlled Trials (CENTRAL), and Google Scholar without any language restrictions. The following Keywords were included: combination therapy, EGFR-TKIs, gefitinib, and erlotinib. In order to find potential publications, we reviewed a reference list of related articles for further analysis.
2.2. Selection criteria
We identified eligible studies according to the following four criteria: Phase II or phase III Trials for cancer; An intervention that combined EGFR-TKIs with other treatments, such as chemotherapy or radiotherapy; Evaluating the clinical value or adverse events (AEs) of treatment; and Comparing combination therapy with monotherapy.
The exclusion criteria were studies were not related to EGFR-TKIs or not clinical trials; Conference documents; and Studies lacked necessary data.
2.3. Data extraction
Three reviewers (RX, HS, JZ) independently extracted data with a standardized form. We reviewed all studies and extracted the following information from the studies: the first author, published year, intervention in the experimental groups and control groups, progression-free survival (PFS), overall survival (OS), overall response rate (ORR), and grade 3/4 treatment-related AEs. ORR was collected directly or calculated according to CRR and PRR. Initially, reviewers measured ORR and OS. Secondary reviewers assessed PFS and grade 3/4 treatment-related AEs.
2.4. Assessment of the study quality and risk of bias
Cochrane Collaboration's tool was used to evaluate the risk of bias, and any controversies were resolved by mutual discussion. We assessed the study quality based on the latest 2009 version of the initial Stroke Therapy Academic Industry Roundtable (STAIR) standard, which includes sample-size calculation, inclusion and exclusion criteria, allocation concealment, blinded assessment of outcome, reporting of patients excluded from analysis, and reporting potential conflicts of interest and study funding. All reviewers assessed the qualities in all included studies. The “unclear” means that the quality was not clear. Details on the risk of bias in 14 studies are illustrated in Supporting Information Figure S4.
2.5. Statistical analysis
Statistical analysis, forest plots, sensitivity analysis, and detection of publication bias were calculated by Stata/SE 12.0 (Stata Corp, College Station, TX), and we used Review Manager (RevMan5.3; The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark) to assess the risk of bias. In addition, we used an Excel spreadsheet developed by Matthew Sydes and Jayne Tierney of the MRC Clinical Trials Unit, London, UK, to evaluate the ln (HR) values and se(ln(HR)) values.[13,14] Relative risk (RR) and hazard ratios (HRs) were used for evaluation. Publication bias was assessed by Egger test and Begg test. All analyses (OS, ORR, PFS, and grade 3/4 treatment-related AEs) were performed using a random-effects model (M–H heterogeneity).[15] In addition, we calculated 95% confidence intervals (95% CIs) for each estimate.
2.6. Data availability
All data generated during and/or analyzed in this study are included in this published article (and its supplementary information files).
2.7. Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
2.8. Informed consent
For this type of study, formal consent is not required.
3. Results
3.1. Search results and study characteristics
A total of 533 potentially relevant studies were retrieved from initial database search from PubMed/Medline, Embase, CENTRAL, and Google Scholar, of which 14 articles met all inclusion criteria.[16–29] All the studies evaluated the efficacy of EGFR-TKIs combination therapies, combining EGFR-TKIs with other therapies. We found several types of combination trials: 7 combinations of EGFR-TKIs with chemotherapies (n = 2615), 4 combinations of EGFR-TKIs with radiotherapies (n = 453), and 3 combinations of EGFR-TKIs with bevacizumab (n = 706). The detailed studies selection process is illustrated in “PRISMA Flow Diagram.”
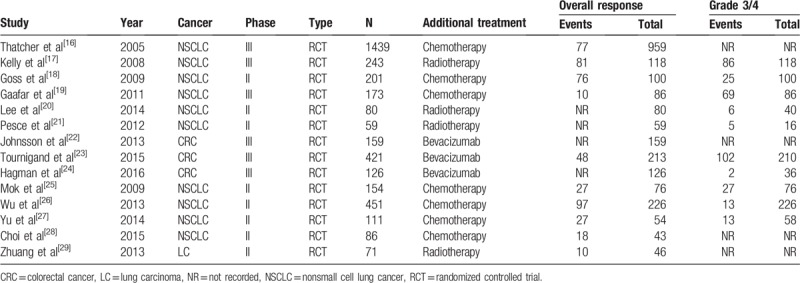
A total of 3774 patients were included in the analysis, of whom 2858 had nonsmall-cell lung cancer, 71 had lung carcinoma, and 706 had colorectal cancer. The safety analysis included 2109 of these patients. In total, 2146 patients received combination therapies, including 7 combinations of EGFR-TKIs with chemotherapies (n = 1544), 4 combinations of EGFR-TKIs with radiotherapies (n = 247), and 3 combinations of EGFR-TKIs with bevacizumab (n = 355). Patients receiving monotherapy served as the controls (n = 1628). Main characteristics of those trials are available in Table 1.
Table 1.
Characteristics of the included studies in the meta-analysis.
3.2. Efficacy outcomes
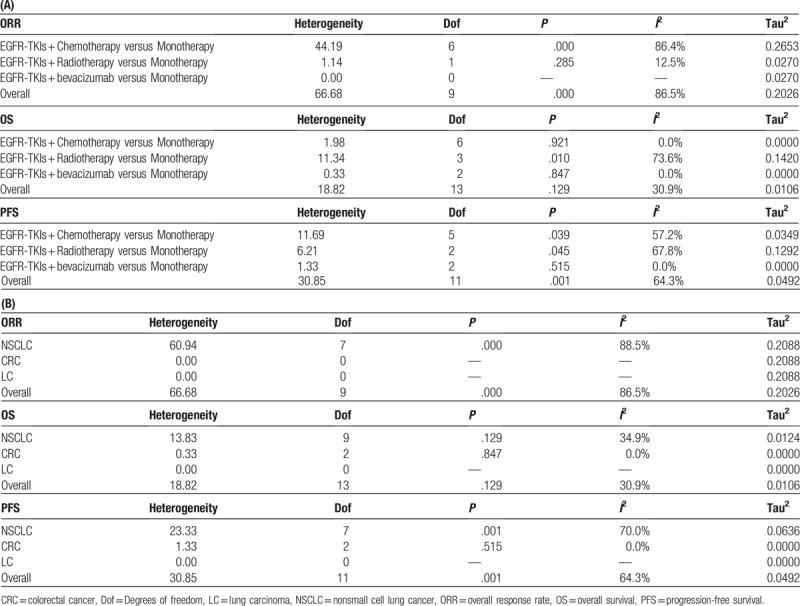
The OS was reported in all 14 trails.[16–29] The ORR was reported in 10 of the trails.[16–19,23,25–29] The PFS was reported in 12 of the trails.[17–20,22–29] The ORR, OS, and PFS of combination therapies were significantly higher than monotherapies (Fig. 1). Combination of EGFR-TKIs and other therapies was associated with significantly higher ORRs than monotherapies (RR: 1.62, 95% CI: 1.16–2.26, P = .005) (Fig. 1A). Compared with monotherapies, combination therapy significantly prolonged PFS (HR: 0.76, 95% CI: 0.64–0.89, P = .001) (Fig. 1B). In addition, the OS of combination therapies was also significantly higher than monotherapies (HR: 0.88, 95% CI: 0.79–0. 97, P = .013) (Fig. 1C). There was high heterogeneity in the ORR (I2 = 86.5%) and PFS (I2 = 64.3%) analyses, while heterogeneity in OS (I2 = 30.9%) was low. Subgroup analysis of combination type and cancer type are shown in Figs. 2 and 3 and Table 2. Detailed heterogeneity analyses are shown in Supporting Information Figure S1. Subgroup analysis of combination type showed that combination of bevacizumab and EGFR-TKIs can better benefit ORR and OS (Fig. 2A,C), and combination of chemotherapy and EGFR-TKIs can better benefit PFS (Fig. 2B).
Figure 1.
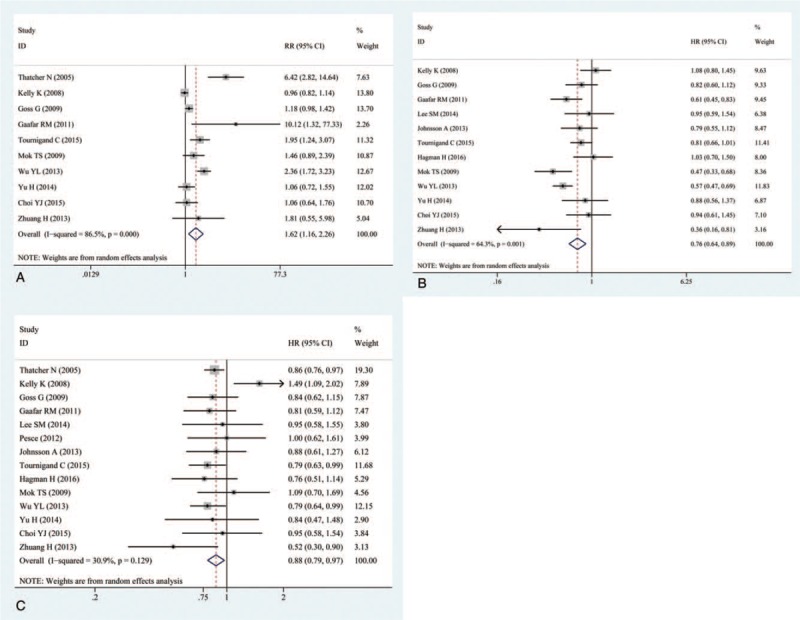
Pooled analysis of overall response rate (ORR), progression-free survival (PFS), and overall survival (OS). (A) overall response rate (ORR); (B) progression-free survival (PFS); (C) overall survival (OS).
Figure 2.
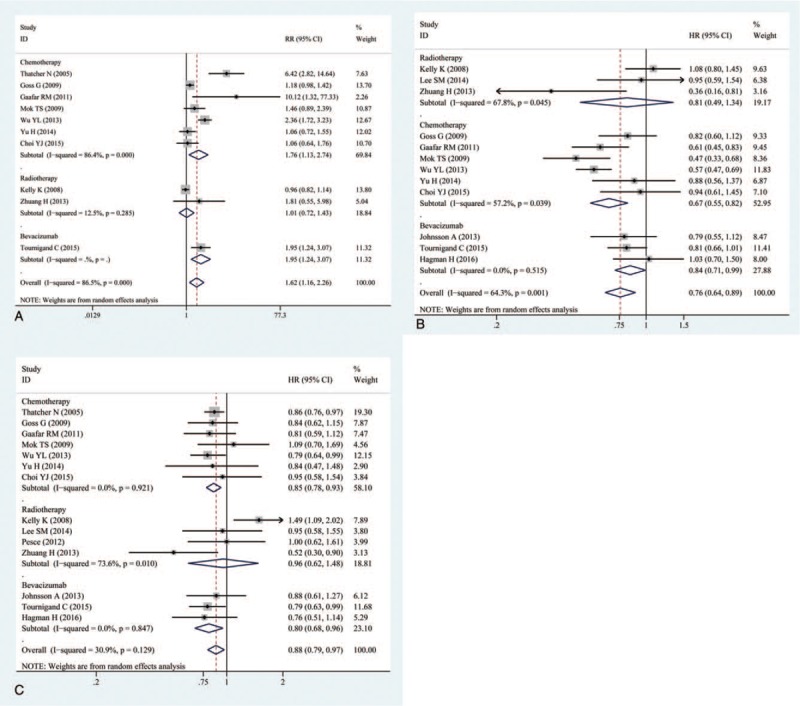
(A) Subgroup analysis of overall response rate (ORR); (B) subgroup analysis of progression-free survival (PFS); (C) subgroup analysis of overall survival (OS) by type of combination.
Figure 3.
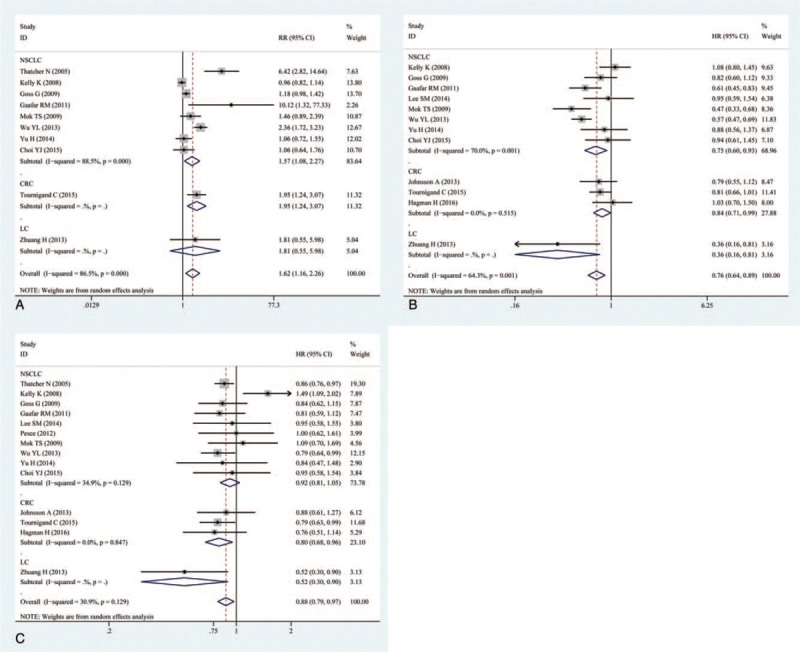
(A) Subgroup analysis of overall response rate (ORR); (B) subgroup analysis of progression-free survival (PFS); (C) subgroup analysis of overall survival (OS) by cancer type.
Table 2.
Subgroup analysis by type of EGFR-TKIs combination and cancer type.
3.3. Safety outcomes
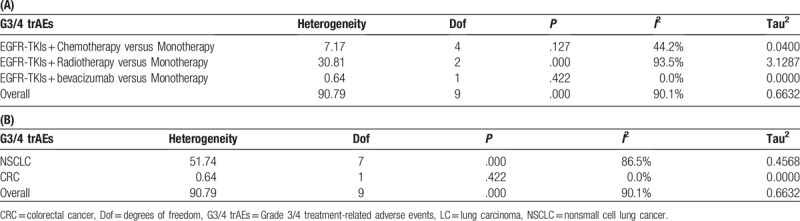
The grade 3/4 treatment-related AEs of EGFR-TKIs combination therapies were reported in ten trials.[17–21,23–27] Analysis shows significantly high rate of grade 3/4 AEs in combination therapies (RR: 1.79, 95% CI: 1.02–3.15, P = .000) (Fig. 4A), with high heterogeneity (I2 = 90.1%). Tests of heterogeneity and subgroup analyses by type of combination and cancer are shown in Fig. 4B, C and Table 3. Combination of EGFR-TKIs and bevacizumab showed the highest rate of grade 3/4 treatment-related AEs (RR: 5.02, 95% CI: 3.29–7.66) (Fig. 4B). The grade 3/4 treatment-related AEs of EGFR-TKIs combination therapies in CRC are higher than that in NSCLC (Fig. 4C).
Figure 4.

(A) Pooled analysis of grade 3/4 treatment-related adverse events; (B) subgroup analysis of grade 3/4 treatment-related adverse events by type of combination (C) subgroup analysis of grade 3/4 treatment-related adverse events by cancer type.
Table 3.
Grade 3/4 treatment-related adverse events, analyzed by type of EGFR-TKIs combination and by cancer type.
3.4. Sensitivity analysis and publication bias
All sensitivity analysis performed in this study indicated a stable result. No sensitivity analysis shows positive results. Detailed sensitivity analysis is shown in Supporting Information Figure S2. Our publication bias was based on both Begg test and Egger test. In Begg test, the P values were .210 for ORR, .474 for grade 3/4 treatment-related AEs, .837 for PFS, and .743 for OS. In Egger test, the P values were .042 for ORR, .680 for grade 3/4 treatment-related AEs, .627 for PFS, and .933 for OS. The Begg graphs are shown in Fig. 5, and the Egger graphs are shown in Supporting Information Figure S3.
Figure 5.
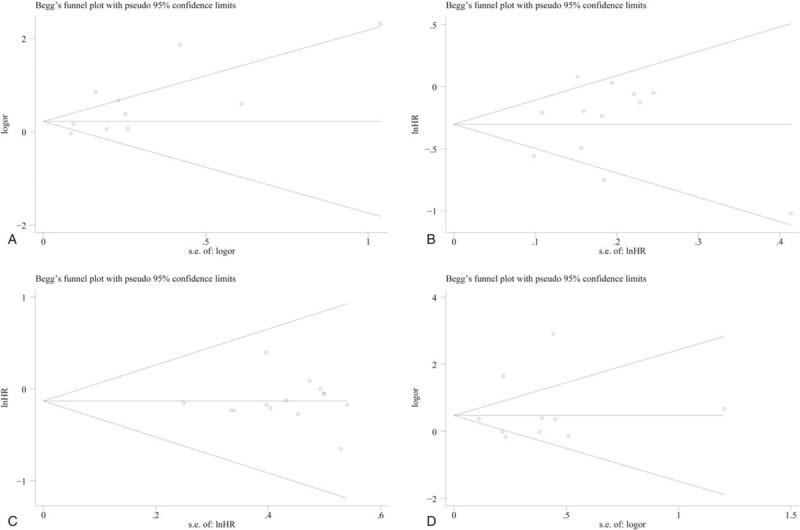
Publication bias assessed by Begg test. (A) ORR; (B) PFS; (C) OS; (D) AEs.
4. Discussion
To our knowledge, this is the first comprehensive analysis with RCTs to assess the efficacy and safety of EGFR-TKIs combination therapies. The current trials have some limitations, but we think that outcomes can still provide insights into EGFR-TKIs combination therapies. Outcomes of studies on EGFR-TKIs combination therapies, including chemotherapy, radiotherapy, and bevacizumab have been published, but efficacy and safety of combination therapies are still under debate. Outcomes of several trials did not improve the clinical outcome of cancer patients.[27,28] Therefore, we performed this comprehensive analysis to evaluate the value and toxic effects of EGFR-TKIs combination therapies; in addition, subgroup analyses were warranted to evaluate the optimal combination strategies.
The pooled analyses showed that EGFR-TKIs combination therapies led to significantly improved ORR, OS, and PFS in comparison with monotherapies. However, most combinations were associated with higher rate of grade 3/4 treatment-related AEs. After all EGFR-TKIs combination therapies were evaluated, we found that combining EGFR-TKIs with bevacizumab yielded the best ORR, combining EGFR-TKIs with chemotherapy improved ORR. However, combining EGFR-TKIs with radiotherapies did not improve ORR compared with monotherapies. Improvements in PFS were documented in all combinations. Both combining EGFR-TKIs with chemotherapy and combining EGFR-TKIs with bevacizumab led to improved OS significantly. While combinations of EGFR-TKIs and radiotherapies were associated with only slight OS improvement. In terms of toxicity, we found that combining EGFR-TKIs with bevacizumab led to the highest rate of grade 3/4 treatment-related AEs, and combinations of EGFR-TKIs with chemotherapy showed the lowest rate of grade 3/4 treatment-related AEs.
Our study indicates that combining EGFR-TKIs with bevacizumab showed more benefits in ORR and OS among all the combinations, but this combination also showed high toxicity. In addition, combining EGFR-TKIs with chemotherapy led to significant benefits in PFS, and this combination showed the lowest toxicity. The efficacy and safety of bevacizumab-EGFR-TKIs-chemotherapy combination therapy should be investigated further for its potential to extend the clinical success.
Previous studies have assessed the efficacy of EGFR-TKIs.[30–36] Some of them showed that EGFR-TKIs can improve the clinical outcome, but they only evaluate 1 or 2 clinical outcomes.[31] One analysis compared efficacy and toxicity in different EGFR-TKIs treatment, suggesting a high efficacy-moderate toxicity pattern of erlotinib and a medium efficacy-moderate toxicity pattern of gefitinib.[36] As far as we know, no other analysis assessed the added benefits against the toxicity of different combination types.
Some certain limitations of our study should be mentioned. Our analysis did not exclude publication bias; in addition, some studies have reported only short-term follow-up and lack of long-term outcomes. Lastly, the ORR, PFS, and grade 3/4 treatment-related AEs were not available in some of the reports. More study investigation is required in future.
5. Conclusion
Our results indicated that combining EGFR-TKIs with bevacizumab showed more benefits in ORR and OS among all the combinations, but this combination also showed high toxicity. In addition, combining EGFR-TKIs with chemotherapies led to significant benefits in PFS, and this combination showed the lowest toxicity. The efficacy and safety of bevacizumab-EGFR-TKIs-chemotherapy combination therapy should be investigated further for its potential to extend the clinical success.
Acknowledgment
The authors thank the data provided by the authors of included trials.
Author contributions
Conceptualization: Ran Xu, Hong Shao, Jing Zhu, Hui Shi.
Data curation: Ran Xu, Hong Shao, Jing Zhu.
Formal analysis: Ran Xu, Zhu Jing, Hui Shi
Investigation: Ran Xu, Jing Zhu.
Methodology: Ran Xu, Hong Shao, Jing Zhu, Hui Shi.
Project administration: Ran Xu, Hong Shao, Hui Shi
Resources: Ran Xu, Shao Hong, Zhu Jing.
Software: Ran Xu, Hong Shao, Qianqian Ju, Hui Shi.
Supervision: Ran Xu.
Validation: Ran Xu, Jing Zhu, Hui Shi.
Visualization: Ran Xu.
Writing – original draft: Ran Xu, Hong Shao, Jing Zhu, Hui Shi.
Writing – review & editing: Ran Xu, Shao Hong, Qianqian Ju, Zhu Jing, Hui Shi.
Supplementary Material
Footnotes
Abbreviations: CI = confidence interval, CRC = colorectal cancer, EGFR = epidermal growth factor receptor, HR = hazard ratio, LC = lung carcinoma, NSCLC = non-small cell lung cancer, ORR = overall response rate, OS = overall survival, PFS = progression free survival, RCT = randomized controlled trial, RR = relative risk, TKIs = tyrosine kinase inhibitors.
These authors contributed equally to this work.
All authors gave final approval for submission of the manuscript.
The authors report no conflicts of interest.
The author(s) of this work have nothing to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Spano JP, Fagard R, Soria JC, et al. Epidermal growth factor receptor signaling in colorectal cancer: preclinical data and therapeutic perspectives. Ann Oncol 2005;16:189–94. [DOI] [PubMed] [Google Scholar]
- [2].Heinemann V, Stintzing S, Kirchner T, et al. Clinical relevance of EGFR- and KRAS-status in colorectal cancer patients treated with monoclonal antibodies directed against the EGFR. Cancer Treat Rev 2009;35:262–71. [DOI] [PubMed] [Google Scholar]
- [3].Bertotti A, Papp E, Jones S, et al. The genomic landscape of response to EGFR blockade in colorectal cancer. Nature 2015;526:263–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Normanno N, Pergameno M, Lannaccone A, et al. Mechanisms of action of EGFR inhibitors. EGFR Inhibitors in Cancer Treatment: Future Medicine Ltd 2012;6–17. [Google Scholar]
- [5].Cohen MH, Williams GA, Sridhara R, et al. FDA drug approval summary: gefitinib (ZD1839) (Iressa) tablets. Oncologist 2003;8:303–6. [DOI] [PubMed] [Google Scholar]
- [6].Cohen MH, Johnson JR, Chen YF, et al. FDA drug approval summary: erlotinib (Tarceva) tablets. Oncologist 2005;10:461–6. [DOI] [PubMed] [Google Scholar]
- [7].Thomas SM, Grandis JR. Pharmacokinetic and pharmacodynamic properties of EGFR inhibitors under clinical investigation. Cancer Treat Rev 2004;30:255–68. [DOI] [PubMed] [Google Scholar]
- [8].Moulder SL, Yakes FM, Muthuswamy SK, et al. Epidermal growth factor receptor (HER1) tyrosine kinase inhibitor ZD1839 (Iressa) inhibits HER2/neu (erbB2)-overexpressing breast cancer cells in vitro and in vivo. Cancer Res 2001;61:8887–95. [PubMed] [Google Scholar]
- [9].Ranson M. Epidermal growth factor receptor tyrosine kinase inhibitors. Br J Cancer 2004;90:2250–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Moyer JD, Barbacci EG, Iwata KK, et al. Induction of apoptosis and cell cycle arrest by CP-358,774, an inhibitor of epidermal growth factor receptor tyrosine kinase. Cancer Res 1997;57:4838–48. [PubMed] [Google Scholar]
- [11].Perez-Soler R. The role of erlotinib (Tarceva, OSI 774) in the treatment of non-small cell lung cancer. Clin Cancer Res 2004;10:4238s–40s. [DOI] [PubMed] [Google Scholar]
- [12].Herbst RS. Erlotinib (Tarceva): an update on the clinical trial program. Semin Oncol 2003;303 suppl 7:34–46. [PubMed] [Google Scholar]
- [13].Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med 1998;17:2815–34. [DOI] [PubMed] [Google Scholar]
- [14].Williamson PR, Smith CT, Hutton JL, et al. Aggregate data meta-analysis with time-to-event outcomes. Stat Med 2002;21:3337–51. [DOI] [PubMed] [Google Scholar]
- [15].DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- [16].Thatcher N, Chang A, Parikh P, et al. Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer). Lancet 2005;366:1527–37. [DOI] [PubMed] [Google Scholar]
- [17].Kelly K, Chansky K, Gaspar LE, et al. Phase III trial of maintenance gefitinib or placebo after concurrent chemoradiotherapy and docetaxel consolidation in inoperable stage III non-small-cell lung cancer: SWOG S0023. J Clin Oncol 2008;26:2450–6. [DOI] [PubMed] [Google Scholar]
- [18].Goss G, Ferry D, Wierzbicki R, et al. Randomized phase II study of gefitinib compared with placebo in chemotherapy-naive patients with advanced non-small-cell lung cancer and poor performance status. J Clin Oncol 2009;27:2253–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Gaafar RM, Surmont VF, Scagliotti GV, et al. A double-blind, randomised, placebo-controlled phase III intergroup study of gefitinib in patients with advanced NSCLC, non-progressing after first line platinum-based chemotherapy (EORTC 08021/ILCP 01/03). Eur J Cancer 2011;47:2331–40. [DOI] [PubMed] [Google Scholar]
- [20].Lee SM, Lewanski CR, Counsell N, et al. Randomized trial of erlotinib plus whole-brain radiotherapy for NSCLC patients with multiple brain metastases. J Natl Cancer Inst 2014;106: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Pesce GA, Klingbiel D, Ribi K, et al. Outcome, quality of life and cognitive function of patients with brain metastases from non-small cell lung cancer treated with whole brain radiotherapy combined with gefitinib or temozolomide. A randomised phase II trial of the Swiss Group for Clinical Cancer Research (SAKK 70/03). Eur J Cancer 2012;48:377–84. [DOI] [PubMed] [Google Scholar]
- [22].Johnsson A, Hagman H, Frodin JE, et al. A randomized phase III trial on maintenance treatment with bevacizumab alone or in combination with erlotinib after chemotherapy and bevacizumab in metastatic colorectal cancer: the Nordic ACT Trial. Ann Oncol 2013;24:2335–41. [DOI] [PubMed] [Google Scholar]
- [23].Tournigand C, Chibaudel B, Samson B, et al. Bevacizumab with or without erlotinib as maintenance therapy in patients with metastatic colorectal cancer (GERCOR DREAM; OPTIMOX3): a randomised, open-label, phase 3 trial. Lancet Oncol 2015;16:1493–505. [DOI] [PubMed] [Google Scholar]
- [24].Hagman H, Frodin JE, Berglund A, et al. A randomized study of KRAS-guided maintenance therapy with bevacizumab, erlotinib or metronomic capecitabine after first-line induction treatment of metastatic colorectal cancer: the Nordic ACT2 trial. Ann Oncol 2016;27:140–7. [DOI] [PubMed] [Google Scholar]
- [25].Mok TS, Wu YL, Yu CJ, et al. Randomized, placebo-controlled, phase II study of sequential erlotinib and chemotherapy as first-line treatment for advanced non-small-cell lung cancer. J Clin Oncol 2009;27:5080–7. [DOI] [PubMed] [Google Scholar]
- [26].Wu YL, Lee JS, Thongprasert S, et al. Intercalated combination of chemotherapy and erlotinib for patients with advanced stage non-small-cell lung cancer (FASTACT-2): a randomised, double-blind trial. Lancet Oncol 2013;14:777–86. [DOI] [PubMed] [Google Scholar]
- [27].Yu H, Zhang J, Wu X, et al. A phase II randomized trial evaluating gefitinib intercalated with pemetrexed/platinum chemotherapy or pemetrexed/platinum chemotherapy alone in unselected patients with advanced non-squamous non-small cell lung cancer. Cancer Biol Ther 2014;15:832–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Choi YJ, Lee DH, Choi CM, et al. Randomized phase II study of paclitaxel/carboplatin intercalated with gefitinib compared to paclitaxel/carboplatin alone for chemotherapy-naive non-small cell lung cancer in a clinically selected population excluding patients with non-smoking adenocarcinoma or mutated EGFR. BMC Cancer 2015;15:763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Zhuang H, Yuan Z, Wang J, et al. Phase II study of whole brain radiotherapy with or without erlotinib in patients with multiple brain metastases from lung adenocarcinoma. Drug Des Devel Ther 2013;7:1179–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Li F, Zhang SH, Pang LM. Meta-analysis of efficacy and adverse events of erlotinib-based targeted therapies for advanced/metastatic non-small cell lung cancer. Oncotarget 2017;8:86816–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Lee CK, Davies L, Wu YL, et al. Gefitinib or erlotinib vs chemotherapy for EGFR mutation-positive lung cancer: individual patient data meta-analysis of overall survival. J Natl Cancer Inst 2017;109: [DOI] [PubMed] [Google Scholar]
- [32].Yang Z, Hackshaw A, Feng Q, et al. Comparison of gefitinib, erlotinib and afatinib in non-small cell lung cancer: a meta-analysis. Int J Cancer 2017;140:2805–19. [DOI] [PubMed] [Google Scholar]
- [33].Burotto M, Manasanch EE, Wilkerson J, et al. Gefitinib and erlotinib in metastatic non-small cell lung cancer: a meta-analysis of toxicity and efficacy of randomized clinical trials. Oncologist 2015;20:400–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Shi L, Tang J, Tong L, et al. Risk of interstitial lung disease with gefitinib and erlotinib in advanced non-small cell lung cancer: a systematic review and meta-analysis of clinical trials. Lung Cancer 2014;83:231–9. [DOI] [PubMed] [Google Scholar]
- [35].Yang ZY, Yuan JQ, Di MY, et al. Gemcitabine plus erlotinib for advanced pancreatic cancer: a systematic review with meta-analysis. PLoS One 2013;8:e57528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Zhang Y, Sheng J, Yang Y, et al. Optimized selection of three major EGFR-TKIs in advanced EGFR-positive non-small cell lung cancer: a network meta-analysis. Oncotarget 2016;7:20093–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated during and/or analyzed in this study are included in this published article (and its supplementary information files).


