Abstract
Rationale:
Gorham–Stout disease (GSD) is a rare disorder characterized by multiple osteolytic lesions, sometimes complicated by chylothorax. The aim of this case report is to introduce a very rare case of Gorham–Stout syndrome, which involved several bones along with chylous pericardial and pleural effusions detected by 99mTc-sulfur colloid (SC) lymphoscintigraphy and single photon emission computed tomography/computed tomography (SPECT/CT).
Patient concerns:
A 15-year-old girl presented to our hospital complaining of shortness of breath and bone pain.
Diagnosis:
The CT showed multiple osteolytic lesions, left-sided pleural effusion, and pericardial effusion. 99mTc-SC lymphoscintigraphy showed discontinuation of thoracic duct and tracer accumulation on the left side chest. SPECT/CT revealed increased radioactivity uptake in pleural, pericardial effusions, and some thoracolumbar spines. Diagnostic thoracentesis to identify the nature of pleural effusion and histopathology of biopsy in the right femoral to that of the bone lesion were performed. Based on the clinical information, histopathologic, and radiographic findings, the diagnosis of GSD was made.
Interventions:
The patient received thoracic duct ligation and bisphosphonates treatment.
Outcomes:
After receiving thoracic duct ligation and bisphosphonates treatment, the patient's symptoms of bone pain and dyspnea were relieved, and the pericardial and pleural fluid was diminished dramatically. At the 3-month and 9-month follow-up visit, the patient had nearly complete remission without any complication.
Lessons:
The 99mTc-SC lymphoscintigraphy and SPECT/CT could provide significant value assessing the lymphatic abnormity and evaluating the extent of disease, therefore aiding to guide decision making in the clinic.
Keywords: chylothorax, Gorham–Stout disease, single photon emission computed tomography/computed tomography, 99mTc-lymphoscintigraphy
1. Introduction
Gorham–Stout disease (GSD), also known as vanishing bone disease or massive osteolysis, is a rare disorder of hyperplasia of vascular or lymphatic capillaries within bone.[1–3] The 1st description of “vanishing bone disease” was reported by Jackson in 1838. In 1954, Gorham described a case report with the condition of “disappearing bones.”[4,5] As the endothelial cells proliferate, they aggressively yet painlessly invade the surrounding bone as results of resorption and replacement with angiomatous tissue.[6] This disease can affect multiple skeletal regions, such as spine, pelvis, ribs, scapula, and the facial skeleton.[7]
The extraosseous condition is characterized by cortical erosion and surrounding soft-tissue involvement.[8,9] The chylous pericardial and pleural effusions can complicate the disease and lead to death in severe cases.[10–12] The medical treatment for GSD includes anti-osteoclastic medications (bisphosphonates), radiotherapy, thoracic duct ligation, interferon, and cement augmentation.[13] In this report, we show a case of Gorham disease involving thoracic spine with chylous pericardial and pleural effusions detected by 99mTc-sulfur colloid (SC) single photon emission computed tomography/computed tomography (SPECT/CT).
2. Case report
We present a 15-year-old girl complaining of chest pain, fever, dyspnea for 10 years, lower limb pain, and lameness for 1 year. Laboratory tests in our hospital showed that parathyroid hormone was normal, beta-C-terminal telopeptide was elevated, and all kinds of bacteria, fungi, and other infections were negative. CT examination showed multiple bone destructions in cervicothoracic, lumbosacral, clavicular, scapula, ribs, iliac, and femoral bones, accompanying with pleural and pericardial effusion (Fig. 1A). She received diagnostic thoracentesis and the analysis of pleural effusion identified chylothorax. Pathologic and histologic examination of right femoral indicated that osseous tissue was replaced by hyperplastic fibrous connective tissue. The result of the biopsy was consistent with the research of Johnson, which implied intraosseous capillary proliferation being replaced by fibrous tissue in the later stage of GSD.[14] Based on the clinical information, histopathologic and radiographic findings, the diagnosis of GSD was established. Then, 99mTc-SC lymphoscintigraphy and SPECT/CT were performed to assess the doubtable lymphatic abnormity. Lymphoscintigraphy of the chest was obtained at 90 minutes after subcutaneous injection of 111 MBq/0.5 mL 99mTc-labeled SC into the 1st interdigital spaces of both feet. Lymphoscintigraphy of the chest (90 minutes) showed discontinuation of the thoracic duct and abnormal tracer accumulation on the left side chest, suggesting the injury to the thoracic duct leading to the chylothorax (Fig. 1B). SPECT/CT revealed increased radioactivity uptake in pleural, pericardial effusions, several ribs, and thoracolumbar spines (Fig. 1C). The patient received the treatment of bisphosphonates and thoracic duct ligation surgery after the diagnosis of GSD was clear, then the pleural and pericardial effusion disappeared, and the symptoms of dyspnea and bone pain were also relieved. At the 3-month and 9-month follow-up visits, the patient had nearly complete remission without any complication.
Figure 1.
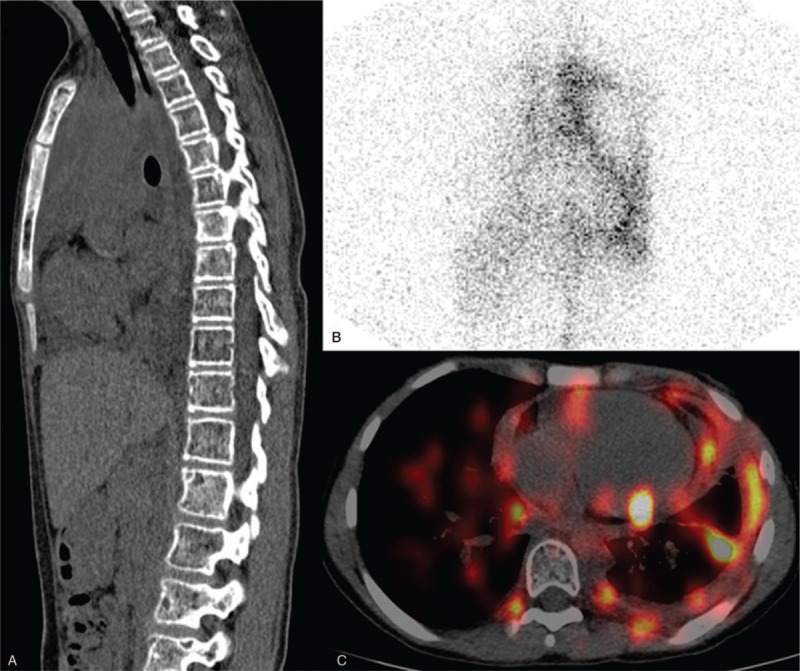
99mTc-sulfur colloid (SC) lymphoscintigraphy and single photon emission computed tomography/computed tomography (SPECT/CT). (A) Sagittal CT showed multiple osteolytic lesions. (B) Anterior spot view of chest by 90 minutes revealed abnormal increase of 99mTc-SC uptake in the left hemithorax and discontinuation of the thoracic duct. (C) Axial SPECT/CT image confirmed 99mTc-SC accumulating in the pericardial space and left thoracic cavity.
3. Discussion
The GSD is a rare disorder characterized by massive osteolysis, with the cause attributed to bone-replacing abnormal capillary proliferation early in the disease or fibrous hyperplasia in the later stage.[15,16] The diagnosis of Gorham disease mainly depended on radiologic findings of affected bones and histopathologic results.[17] Primary osteolysis was classified into 5 sorts: hereditary multicentric osteolysis (HMS) with dominant transmission, HMS with recessive transmission, non-HMS with nephropathy, Winchester disease, and GSD.[18,19] Different from the other previously mentioned primary osteolytic diseases, GSD is neither nephropathy nor associated with hereditary.[20,21] The clinical presentation and complications of GSD depend on the affected bone and invasion to surrounding tissues.[22]
Only about 200 cases of GSD have been reported to date. Although all bones in the body can be affected, the most commonly seen localizations are the skull, pelvis, shoulder girdle, lower, and upper extremities.[8,20] Occurrences in the spine, jawbone, ribs, and sternum have also been reported.[19,23] Involvement of the thoracic skeleton such as thoracic spine, ribs, and scapula can lead to chylous pericardial and pleural effusions (as was the case for our patient), resulting in the associated symptoms such as dyspnea and chest pain. The formation mechanism of chylothorax might be a direct extension of lymphangiectasia into the pleural cavity or invasion of the thoracic duct.[24] The mortality rate of GSD is high and the prognosis is poor in patients complicated with chylothorax.[12] The appearance of chylothorax was always fatal with death resulting from respiratory insufficiency, malnutrition, lymphopenia, and superimposed infection.[25]
The conjunction of clinical information, radiologic findings, and pathologic result led to a diagnosis of GSD. 99mTc-SC lymphoscintigraphy combining with SPECT/CT was then performed to evaluate the possible lymphatic malformation, which other radiologic examinations failed to identify. Lymphoscintigraphy revealed distinct discontinuation of thoracic duct, and radioactivity uptake in left-side chest, which further confirmed the existence of chylothorax. To acquire more information about the chest and bone abnormalities, SPECT/CT was conducted. Besides radioactivity accumulation in pleural and pericardial effusions, multiple foci of increased tracer uptake distributed in pleura, several ribs, and vertebras were seen on SPECT/CT imaging. The results of lymphoscintigraphy and SPECT/CT hinted that the occlusion of thoracic duct and possible lymphatic hyperplasia in pleura and thoracic bones could be responsible for the generation of chylothorax. Deveci et al reported a case with multiple osteolytic lesions and bilateral chylothorax, the patient's lymphatic dysplasia was identified by MRI. Thoracic duct ligation and pleurectomy were then performed, while the patient poorly responded to these therapies and died of respiratory failure and sepsis.[11] Noda et al described a case with chylothorax and successfully detect the lymphatic leakage with classical lymphangiography using lipiodol. After receiving supradiaphragmatic ligation of the thoracic duct, the patient was relieved of pleural effusions and there was no sign of recurrence in the follow-up.[26] Compared to the oil-soluble nature of lipiodol used for lymphangiography, the radiotracer used for lymphoscintigraphy is water soluble and cannot cause pulmonary embolism.[27] To our knowledge, this is the 1st case reporting the use of 99mTc-SC lymphoscintigraphy and SPECT/CT in evaluating the lymphatic anatomy abnormality, which is significantly important for the determination of therapy strategy. We believe that 99mTc-SC lymphoscintigraphy combined with SPECT/CT was a valuable method in evaluation of disease extension and especially identifying the lymphatic malformation.
As mentioned earlier, chylothorax is a very serious complication which has an adverse impact on both prognosis and survival.[10] At present, treatment options for GSD complicated with chylothorax mainly includes radiation therapy, thoracic duct ligation, pleurectomy, and bisphosphanates.[11] The numbers of radiosensitive proliferating endothelial cells could be decreased dramatically when escalating doses of radiation was used. Duffy et al reported a case of Gorham syndrome with chylothorax treated successfully with radiation. Patients whose source of chylous leakage was not clear were appropriate candidates for radiation therapy, as the case Duffy et al described.[28] In our case, the range of disease was wide, the application of radiation therapy may cause many long-term side effects which lower the quality of patient life. After 1 round of bisphosphanates treatment, fever and cough were under control, while symptoms associated with chylothorax and bone pains still remained. Then the patient received a thoracic duct ligation surgery, after which chylous pericardial and pleural effusions soon disappeared. In the study of Tie et al, 7 of 11 patients with chylous pleural effusions were cured after having thoracic duct ligation surgery.[24] Surgical intervention is very essential, without which approximately 64% patients with chylothorax will progress to death ultimately.[28]
4. Conclusion
We reported a case of GSD with multiple bone destructions, chylous pericardial, and pleural effusions. 99mTc-SC lymphoscintigraphy and SPECT/CT played a crucial role in the identification of lymphatic malformation and disease extension, aiding the management decision making in the clinic. To our knowledge, this is the 1st report of Gorham–Stout disease with chylous pericardial and pleural effusions detected by 99mTc-SC SPECT/CT lymphoscintigraphy.
Author contributions
Funding acquisition: Wuying Cheng.
Project administration: Wuying Cheng.
Writing – original draft: Yuanyuan Jiang, Guozhu Hou.
Writing – review & editing: Yuanyuan Jiang, Guozhu Hou.
Footnotes
Abbreviations: GSD = Gorham–Stout disease, HMS = hereditary multicentric osteolysis, SPECT/CT = single photon emission computed tomography/computed tomography, 99mTc-SC = 99mTc-sulfur colloid.
YJ and GH contributed equally to this work.
This study was supported by the National Natural Science Foundation of China (numbers: 81101074 and 81371588).
Ethical approval was waived by the Ethics Committee of our hospital because this study is a retrospective report.
Patient has provided informed consent for publication of the case.
The authors have no conflicts of interest to disclose.
References
- [1].Foster BL, Ramnitz MS, Gafni RI, et al. Rare bone diseases and their dental, oral, and craniofacial manifestations. J Dent Res 2014;93:7S–19S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Liu S, Zhou X, Song A, et al. Successful treatment of Gorham-Stout syndrome in the spine by vertebroplasty with cement augmentation: a case report and literature review. Medicine 2018;97:e11555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Leite I, Hernandez-Martin A, Colmenero I, et al. Invasive lymphatic malformation (Gorham-Stout) of the pelvis with prominent skin involvement. Pediatr Dermatol 2013;30:374–8. [DOI] [PubMed] [Google Scholar]
- [4].Gorham LW, Wright AW, Shultz HH, et al. Disappearing bones: a rare form of massive osteolysis; report of two cases, one with autopsy findings. Am J Med 1954;17:674–82. [DOI] [PubMed] [Google Scholar]
- [5].Gorham LW, Stout AP. Massive osteolysis (acute spontaneous absorption of bone, phantom bone, disappearing bone); its relation to hemangiomatosis. J Bone Joint Surg Am 1955;37-A:985–1004. [PubMed] [Google Scholar]
- [6].Bruch-Gerharz D, Gerharz CD, Stege H, et al. Cutaneous lymphatic malformations in disappearing bone (Gorham-Stout) disease: a novel clue to the pathogenesis of a rare syndrome. J Am Acad Dermatol 2007;56:S21–5. [DOI] [PubMed] [Google Scholar]
- [7].Aizawa T, Sato T, Kokubun S. Gorham disease of the spine: a case report and treatment strategies for this enigmatic bone disease. Tohoku J Exp Med 2005;205:187–96. [DOI] [PubMed] [Google Scholar]
- [8].Liu Y, Zhong DR, Zhou PR, et al. Gorham-Stout disease: radiological, histological, and clinical features of 12 cases and review of literature. Clin Rheumatol 2016;35:813–23. [DOI] [PubMed] [Google Scholar]
- [9].Liu M, Liu W, Qiao C, et al. Mandibular Gorham-Stout disease: a case report and literature review. Medicine 2017;96:e8184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Paez Codeso FM, Morillo Dominguez MC, Dorado Galindo A. A rare case of chylothorax. Gorham-Stout syndrome. Arch Bronconeumol 2017;53:640. [DOI] [PubMed] [Google Scholar]
- [11].Deveci M, Inan N, Corapcioglu F, et al. Gorham-Stout syndrome with chylothorax in a six-year-old boy. Indian J Pediatr 2011;78:737–9. [DOI] [PubMed] [Google Scholar]
- [12].Kotaru AC, Rajput AK. Chylothorax from Gorham-Stout disease. J Bronchol Interv Pulmonol 2018;25:340–2. [DOI] [PubMed] [Google Scholar]
- [13].Klein C, Haraux E, Gouron R. Gorham-Stout disease: hand involvement in an 8-year-old child. J Pediatr 2017;191:277. [DOI] [PubMed] [Google Scholar]
- [14].Johnson PM, Mc CJ. Observations on massive osteolysis; a review of the literature and report of a case. Radiology 1958;71:28–42. [DOI] [PubMed] [Google Scholar]
- [15].Liu SZ, Zhou X, Song A, et al. Gorham-Stout syndrome. QJM 2018;111:911–2. [DOI] [PubMed] [Google Scholar]
- [16].Galiay L, Simon F, Levy R, et al. Temporomandibular joint anomalies in pediatric craniofacial Gorham-Stout disease. J Craniomaxillofac Surg 2018;46:1179–84. [DOI] [PubMed] [Google Scholar]
- [17].Tateda S, Aizawa T, Hashimoto K, et al. Successful management of Gorham-Stout disease in the cervical spine by combined conservative and surgical treatments: a case report. Tohoku J Exp Med 2017;241:249–54. [DOI] [PubMed] [Google Scholar]
- [18].Nikolaou VS, Chytas D, Korres D, et al. Vanishing bone disease (Gorham-Stout syndrome): a review of a rare entity. World J Orthop 2014;5:694–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Posadas MD, Viejo Stuart S, Romano O, et al. Gorham-Stout syndrome: a case report. Eur Rev Med Pharmacol Sci 2014;18:81–3. [PubMed] [Google Scholar]
- [20].Lala S, Mulliken JB, Alomari AI, et al. Gorham-Stout disease and generalized lymphatic anomaly--clinical, radiologic, and histologic differentiation. Skeletal Radiol 2013;42:917–24. [DOI] [PubMed] [Google Scholar]
- [21].Shimizu T, Sato K, Yoshida T, et al. A case report of Gorham-Stout syndrome remission. J Orthop Sci 2012;17:199–204. [DOI] [PubMed] [Google Scholar]
- [22].Johnstun J, Brady L, Simstein R, et al. Chronic recurrent Gorham-Stout syndrome with cutaneous involvement. Rare Tumors 2010;2:e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kai B, Ryan A, Munk PL, et al. Gorham disease of bone: three cases and review of radiological features. Clin Radiol 2006;61:1058–64. [DOI] [PubMed] [Google Scholar]
- [24].Tie ML, Poland GA, Rosenow EC., 3rd Chylothorax in Gorham's syndrome. A common complication of a rare disease. Chest 1994;105:208–13. [DOI] [PubMed] [Google Scholar]
- [25].Riantawan P, Tansupasawasdikul S, Subhannachart P. Bilateral chylothorax complicating massive osteolysis (Gorham's syndrome). Thorax 1996;51:1277–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Noda M, Endo C, Hoshikawa Y, et al. Successful management of intractable chylothorax in Gorham-Stout disease by awake thoracoscopic surgery. Gen Thorac Cardiovasc Surg 2013;61:356–8. [DOI] [PubMed] [Google Scholar]
- [27].Yoshida RY, Kariya S, Ha-Kawa S, et al. Lymphoscintigraphy for imaging of the lymphatic flow disorders. Tech Vasc Interv Radiol 2016;19:273–6. [DOI] [PubMed] [Google Scholar]
- [28].Duffy BM, Manon R, Patel RR, et al. A case of Gorham's disease with chylothorax treated curatively with radiation therapy. Clin Med Res 2005;3:83–6. [DOI] [PMC free article] [PubMed] [Google Scholar]


