Abstract
Background:
As reported, patients experience less postoperative pain after propofol-based total intravenous anesthesia (TIVA). In the present study, we investigated the postoperative analgesic effects between propofol-based TIVA and desflurane anesthesia after spine surgery.
Methods:
Sixty patients were included who received (surgical time >180 minutes) lumbar spine surgery. Patients were randomly assigned to receive either TIVA (with target-controlled infusion) with propofol/fentanyl-based anesthesia (TIVA group) or desflurane/fentanyl-based anesthesia (DES group), titrated to maintain Bispectral Index values between 45 and 55. All patients received patient-controlled analgesia (PCA) with fentanyl for postoperative pain relief. Numeric pain rating scale (NRS) pain scores, postoperative fentanyl consumption, postoperative rescue tramadol use, and fentanyl-related side effects were recorded.
Results:
The TIVA group patients reported lower NRS pain scores during coughing on postoperative day 1 but not day 2 and 3 (P = .002, P = .133, P = .161, respectively). Less fentanyl consumption was observed on postoperative days 1 and 2, but not on day 3 (375 μg vs 485 μg, P = .032, 414 μg vs 572 μg, P = .033, and 421 μg vs 479 μg, P = .209, respectively), less cumulative fentanyl consumption at postoperative 48 hours (790 μg vs 1057 μg, P = .004) and 72 hours (1210 μg vs 1536 μg, P = .004), and total fentanyl consumption (1393 μg vs 1704 μg, P = .007) when compared with the DES group. No difference was found in rescue tramadol use and fentanyl-related side effects.
Conclusion:
Patients anesthetized with propofol-based TIVA reported less pain during coughing and consumed less daily and total PCA fentanyl after lumbar spine surgery.
Keywords: anesthetics i.v., propofol, postoperative pain, spine surgery
1. Introduction
Intravenous anesthetic propofol, according to its pharmacokinetic profile, is the mainstay for total intravenous anesthesia (TIVA); it has rapid onset and offset with fewer side effects, particularly postoperative nausea and vomiting (PONV).[1] In our previous study, we found that patients recovered quicker after TIVA via a target-controlled infusion (TCI) system, than with volatile anesthesia in lumbar spine surgery.[2] Propofol has long been considered a nonanalgesic intravenous hypnotic. However, studies have been conducted to explore the possible anti-nociceptive mechanisms of propofol and its potential role as an analgesic. In animal studies, propofol has been shown to directly depress dorsal horn neurons in the spinal cord,[3] inhibiting the phosphorylation of the N-methyl-D-aspartate receptor NR1 subunit,[4] and of cannabinoid CB1 and CB2 receptors.[5] In human volunteers, propofol at a hypnotic dose of 3.5 mcg/ml decreased pain-related regional blood flow to the thalamus and anterior cingulate cortex.[6] Propofol was reported to significantly decrease pain scores by 40% and areas of hyperalgesia and allodynia in human volunteers.[7] Propofol's preferential binding to the HCN1 pacemaker channels further reinforces its anti-hyperalgesic effect.[8] Moreover, the anti-inflammatory effects of propofol have been shown both in vitro[9] and in human studies[10]; and they may be responsible for propofol role in postoperative analgesia. Propofol-based anesthesia has been shown to be associated with reduced postoperative pain compared with volatile anesthesia,[11–14] whereas other studies have reported no evidence of the superiority of propofol.[15,16] A recent meta-analysis comparing postoperative pain between inhalational and propofol anesthesia showed no significant differences (P value of .04) probably due to substantial heterogeneity among studies.[17]
The aim of this prospective observer-blinded study was to evaluate the analgesic effect (pain scores at rest and during coughing, and daily and total fentanyl consumption) of propofol-based TIVA compared to inhalational anesthesia in lengthy lumbar spine surgery. Anesthesia-associated side effects were also assessed.
2. Methods
2.1. Patient recruitment
We obtained written informed consent from the patients and approval from the Ethics Committee (TSGHIRB No: 097–05–062) of Tri-Service General Hospital, Taipei, Taiwan and the study was registered at the Chinese Clinical Trial Registry (ChiCTR-INR-1800014805). Sixty patients aged between 19 and 79 years, American Society of Anesthesiologists physical status I–III, undergoing elective lumbar spine surgery (surgical time >180 minutes), including posterolateral fusion and pedicle screw fixation, were recruited. Study recruitment took place between July 12, 2008 and December 2, 2009. Patients were excluded when they had cardiopulmonary, endocrinologic, or immunologic diseases, malignancies, spine deformity, chronic pain management, or there was difficulty in the assessment of postoperative pain (e.g., postoperative mechanical ventilation), early termination of patient-controlled analgesia (PCA) due to deterioration of patient condition, or when the patients required a second operation.
2.2. Randomization and blind-study
Patients were randomly assigned to either a desflurane or propofol group for general anesthesia maintenance. Randomization was performed by 2 independent anesthesiologists using 60 opaque sealed envelopes, 30 for each group, indicating patient group assignment and describing the anesthetic protocol for this particular group. The patients and the acute pain service (APS) team involved in assessing postoperative pain and analgesic consumption and the anesthesiologists involved in data collection and analysis of results, were not aware of group assignment (Lu CH and Lin WL).
2.3. Anesthetic technique
There was no premedication prior to anesthesia induction. Regular monitoring, including electrocardiography (lead II), pulse oximetry, noninvasive blood pressure, respiratory rate, and end-tidal carbon dioxide pressure, was performed.
Anesthesia was induced with fentanyl (2 μg/kg), lidocaine (2%, 1.5 mg/kg), and propofol (2 mg/kg). After loss of consciousness, rocuronium (0.6 mg/kg) was administered for tracheal intubation. Maintenance of anesthesia, in the TIVA group, continuous infusion of propofol (Fresenius 1%) was initiated using a TCI system programmed with the Schneider model (Fresenius Orchestra Primea, Fresenius Kabi AG, Bad Homburg, Germany) at an effective target concentration of 4 mg/ml. In the DES group, desflurane was delivered via pure oxygen at 300 ml/min under a closed system, and the concentration was also monitored (Datex-Ohmeda S/5 Anesthesia Monitor, Helsinki, Finland). Anesthesia depth was adjusted according to Bispectral Index (BIS) at a target of 40 to 60.
To prevent postoperative nausea and vomiting, all patients received 4 mg of IV dexamethasone immediately after induction of anesthesia. The ventilation rate and maximum airway pressure were adjusted to maintain end-tidal carbon dioxide pressure at 35 to 45 mmHg. Repetitive bolus injections of fentanyl were prescribed according to hemodynamic response by the anesthesiologist in charge throughout the procedure. Rocuronium was administered as required by the return of neuromuscular function. At the end of the operation, desflurane or propofol was discontinued, and the lungs were ventilated with 100% oxygen at a fresh gas flow of 6 L/min. After completion of surgery, reversal of neuromuscular blockade was achieved with atropine 0.02 mg/kg and neostigmine 0.05 mg/kg, followed by tracheal extubation.
2.4. Postoperative analgesia and assessment of postoperative pain
At the post anesthesia care unit, PCA (Graseby 3300 Syringe Pump, Smiths Medical, London, UK) was applied at a regimen of fentanyl at 10 μg/bolus with a five-min lockout interval and a maximum dose of 1 to 1.5 μg/kg/h, continuous basal infusion was disabled, to maintain the verbal numerical rating scale (NRS, 0 = no pain, 10 = the worst imaginable pain) at less than 3. Intravenous tramadol injection (50 mg) was prescribed as rescue pain medication. All the patients were visited daily by anesthesiologists from the APS team. The APS team were informed if pain control was inadequate and hourly limit and bolus dose parameters could then be adjusted after assessment. Postoperative pain intensity, measured with the NRS, at rest and during coughing on postoperative days 1 to 3, daily and total PCA fentanyl consumption, the doses of rescue tramadol use at postoperative 72 hours, and side effects were recorded.
2.5. Statistical analysis
The primary end-point of the study was the NRS pain scores at rest and during coughing. The secondary end-point was the postoperative analgesic requirements (cumulative fentanyl consumption during the 72 hours after surgery). Sample size calculation was based on an initial pilot study where the standard deviation within each group was approximately 1.5. To achieve 80% power at a = 0.015 level to detect a two-tailed difference of at least 1.5 NRS points, we required 27 patients in each group. Then, we enrolled 30 patients in each group. All data are expressed as mean (standard deviation) or numbers with percentage unless otherwise indicated. Statistical analyses were performed using the Statistical Package for Social Sciences 12.0 for Windows (SPSS, Inc., Chicago, IL). Means of the 2 groups were compared by Student's t test following conversion of raw data into a logarithmic scale when appropriate. Categorical variables were analyzed by the chi-squared test or Fisher exact test for proportions and continuous variables by 2-tailed unpaired t tests (Bonferroni t test). P values of less than .05 were considered significant.
3. Results
All 60 patients completed the study (30 in each group). No statistically significant differences were observed between the 2 groups in patient demographics (Table 1). NRS pain scores at rest and during coughing for the first 3 postoperative days are presented in Figure 1. There were no significant differences in NRS pain scores at rest between the 2 groups. The patients that received TIVA reported a lower NRS pain score during coughing with a mean of 4.1 (vs 4.7 in the DES group) on postoperative day 1 (P = .002), but no differences between the 2 groups were observed on days 2 and 3.
Table 1.
Patient demographics, duration of surgery, blood loss, and intraoperative fentanyl use.
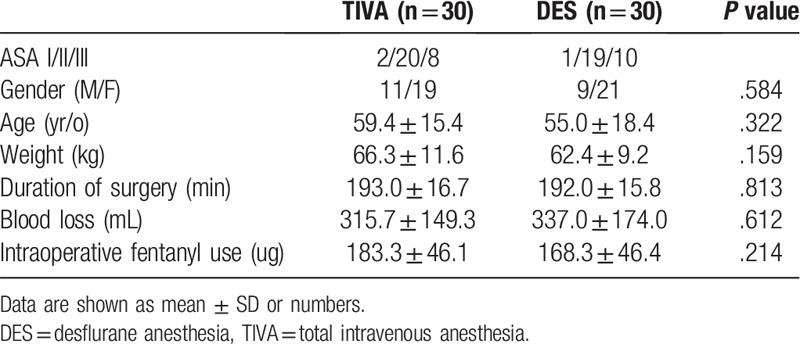
Figure 1.
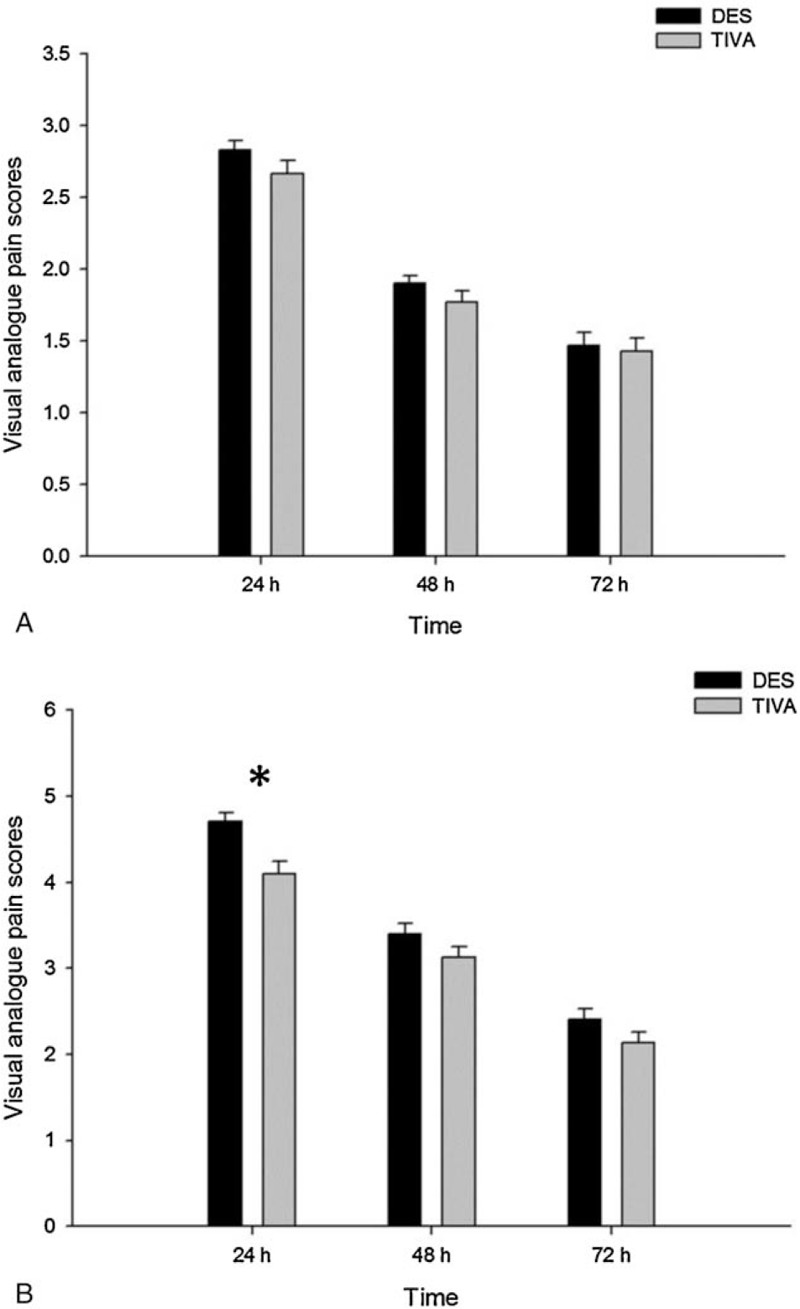
VAS pain scores at rest (A) and during coughing (B). Coughing VAS pain scores were significantly lower at day 1 (D1) after surgery in the TIVA group compared with the DES group (P = .002). DES = desflurane anesthesia, TIVA = total intravenous anesthesia, VAS = visual analogue pain scores.
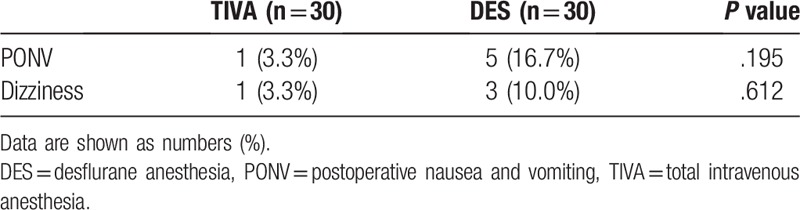
Daily fentanyl consumption on postoperative days 1 to 3, cumulative fentanyl consumption at postoperative 48 hours and 72 hours, and total fentanyl consumption are shown in Table 2. There was lower daily PCA fentanyl consumption in the TIVA group on postoperative days 1 and 2 (375 μg vs 485 μg, P = .032 on day 1 and 414 μg vs 572 μg, P = .033 on day 2) than in the DES group, and no difference was observed on day 3 (421 μg vs 479 μg, P = .209). Mean cumulative fentanyl consumption at postoperative 48 hours and 72 hours was significantly lower in the TIVA than in DES group (790 μg vs 1057 μg, P = .004 at postoperative 48 hours and 1210 μg vs 1536 μg, P = .004 at postoperative 72 hours). Total fentanyl consumption was also lower in the TIVA than in the DES group (1393 μg vs 1704 μg, P = .007). There was no significant differences in the doses of rescue tramadol use at postoperative 72 hours (Table 2) between the 2 groups (TIVA, 25 mg vs DES, 35 mg, P = .118). No difference in side effects, such as PONV and dizziness, was observed between the 2 groups (Table 3).
Table 2.
Daily fentanyl consumption from postoperative day 1 to 3, cumulative fentanyl consumption at postoperative 48 hours and 72 hours, total fentanyl consumption (μg), and rescue tramadol use at postoperative 72 hours (mg).
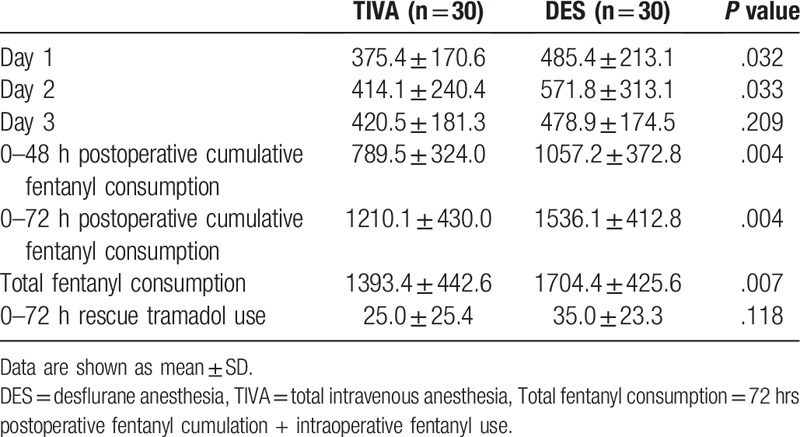
Table 3.
Postoperative side effects.
4. Discussion
Our study showed that patients receiving propofol for maintenance of general anesthesia in lumbar spine surgery reported significantly less pain than patients receiving desflurane/fentanyl-based anesthesia, reflected by lower mean NRS pain scores during coughing at day 1 and less fentanyl consumption up to postoperative day 2. It is important to achieve good acute pain control to prevent progression to chronic pain and facilitate early mobilization, and high postoperative morphine consumption at the first 24 hours is predictive of the development and severity of chronic pain.[18] This study showed that TIVA-propofol anesthesia provided better pain relief with less opioid consumption during the first 2 days after surgery.
Propofol was originally developed as an anesthetic and sedative drug; however, its potential analgesic effect was an interesting and serendipitous discovery.[12] The effect of propofol on noxious stimuli remains controversial; animal studies have reported systemic propofol to have either no effect[19,20] or antinociceptive and/or antihyperalgesic effects.[21,22] Moreover, systemic administration of propofol depressed noxious stimulus–evoked responses of neurons in the spinal cord dorsal[3,23–25] and ventral[26,27] horns and either reduced[28] or had no effect[19] on formalin-evoked spinal neuronal expression of c-fos, a marker of neuronal activity. In contrast, several studies of experimental pain in humans have reported the analgesic effects of subhypnotic doses of propofol.[7,29–31] Some recent clinical studies have reported that surgical patients receiving propofol anesthesia reported less postoperative pain.[11–14] Regarding acute pain, Chan et al. found that patients anesthetized with propofol TIVA reported less pain during coughing and consumed less daily, cumulative, and total morphine after liver surgery than patients anesthetized with sevoflurane.[11] A study by Cheng and colleagues showed that propofol was associated with less postoperative pain and less PCA morphine when compared with isoflurane on the first day after open uterine surgery.[12] Two more studies found that patients undergoing laparoscopic gynecological surgery reported less pain during the immediate postoperative period with propofol than with sevoflurane anesthesia. Li and colleagues studied the pain scores of 90 patients at rest at 0.5 hour and 1 hour postoperatively and found that they were significantly lower in the propofol group than in the sevoflurane group.[13] Tan et al found that pain scores were higher in the sevoflurane group than in the propofol group at postoperative first 4 hours.[14] In contrast to previous studies that measured pain scores from 0.5 to 24 hours postoperatively,[12–14] our study revealed a reduction in pain scores during coughing on day 1, with less daily fentanyl consumption extending to postoperative day 2. This is consistent with Chan et al findings that lower pain scores extended to postoperative day 2 with less daily morphine consumption up to day 3 in propofol-anesthetized patients.[14] This signifies that the reduction in pain scores and morphine consumption within the first 24 hour was probably not due to a sedative effect from residual anesthetics; propofol has a very fast recovery profile.
There are also some controversial reports. A study comparing desflurane, sevoflurane, and propofol found no difference in cumulative opioid consumption and pain scores at rest or after coughing at postoperative 2, 4, 8, and 24 hours after abdominal hysterectomy or myomectomy.[15] A recent meta-analysis[17] reported that the current results are affected by substantial heterogeneity, which precludes any investigation for significant differences (P value of <.01, such as in highly heterogeneous samples among groups or numerous combinations of groups with small sample sizes). However, this meta-analysis showed a possible superiority of propofol anesthesia over inhalational anesthesia with respect to the analgesic effect; propofol use was associated with reduced postoperative pain intensity at rest at 30 minutes, 1 hour, and 12 hours (P = .04) and reduced morphine-equivalent consumption 0 to 24 hours postoperatively (P = .05).[17] Although the effect of propofol on postoperative pain is controversial, and we could not ascertain whether our finding was attributable to the analgesic properties of propofol, the reduction in pain scores during coughing and the decrease in total fentanyl consumption by 18% in our study are both statistically and clinically significant. TIVA-propofol anesthesia potentially provides better postoperative pain relief with less opioid consumption, and it may be considered a good option for reducing postoperative pain and chronic pain development.
Several possible mechanisms may explain the effects of propofol and of volatile agents on acute postoperative pain. Volatile agents are known to suppress the propagation of sensory afferent stimuli to the nervous system at anesthetic concentrations.[32,33] It is worth noting that inhalational anesthetics tend to cause hyperalgesia at 0.1 minimum alveolar concentrations, which may increase pain perception during emergence from anesthesia.[34] This increased sensitivity to pain is mediated by modulation of central adrenergic and cholinergic transmission, as well as by 5-HT3 receptor–mediated currents.[35,36] In contrast, propofol exhibits short-lasting analgesic properties with a trend toward reduced hyperalgesia and allodynia in healthy volunteers.[7] The exact mechanism of propofol action remains unknown, evidence from cell cultures and animal studies suggests that propofol may interact with GABA receptors and exert its anesthetic as well as analgesic effects.[8,12,22] Animal studies employing the delta-opioid antagonist naltrindole have suggested that propofol antinociception is mediated through spinal delta-opioid receptors and through GABA receptors.[22] Other potential mechanisms involved in propofol's analgesic effects[30] may be its anti-inflammatory[37] and antioxidant[38] actions.
With regard to side effect profiles, our results showed no difference in the incidence of PONV or dizziness between the DES and TIVA-propofol groups. However, in our previous retrospective study, the incidence of PONV was significantly reduced in patients receiving TIVA than in patients receiving DES undergoing ophthalmic surgery.[1] This study may not have been powered to detect side effects with a low incidence and the preventive effect of dexamethasone, and it is to be noted that the raw data showed that 16.7% of the DES group vs 3.3% of the TIVA group had PONV.
There are several limitations to our study. First, although we did not measure postoperative sedation scores. There is a possibility that patients may had become more sedated, and hence have consumed less fentanyl; however, residual analgesia was unlikely, as our results showed that the decrease in NRS pain scores during coughing was still apparent on postoperative day 1, and daily fentanyl consumption was also lower, outlasting the therapeutic duration of both desflurane and propofol. Second, pain ratings provided by patients are not objective. However, the best assessment of pain is performed by the patients; our approach is realistic and represents a daily clinical routine. Finally, we did not perform blood sample analysis of the plasma levels of propofol in our patients; we adjusted propofol targets according to target BIS and previous pharmacokinetic data of propofol to derive our pharmacodynamic model.
In conclusion, the patients that underwent lumbar spine surgery using propofol for induction and maintenance of anesthesia had better pain relief with less fentanyl consumption during the first 2 days after surgery than the patients who received desflurane. This anesthetic technique of propofol-based TIVA should be considered as a viable option for reducing postoperative pain.
Author contributions
Conceptualization: Wei-Lin Lin, Chih-Shung Wong, Zhi-Fu Wu, Chueng-He Lu.
Data curation: Wei-Lin Lin, Chueng-He Lu.
Formal analysis: Meei-Shyuan Lee, Wei-Lin Lin, Chueng-He Lu.
Funding acquisition: Zhi-Fu Wu, Wei-Lin Lin, Chueng-He Lu.
Investigation: Wei-Lin Lin, Chih-Shung Wong, Chueng-He Lu.
Methodology: Wei-Lin Lin, Meei-Shyuan Lee, Zhi-Fu Wu, Chueng-He Lu.
Project administration: Wei-Lin Lin, Chueng-He Lu.
Resources: Wei-Lin Lin, Chih-Shung Wong, Shun-Ming Chan, Hou-Chuan Lai, Zhi-Fu Wu, Chueng-He Lu.
Software: Wei-Lin Lin, Meei-Shyuan Lee, Chueng-He Lu.
Supervision: Wei-Lin Lin, Chih-Shung Wong, Zhi-Fu Wu, Chueng-He Lu.
Validation: Wei-Lin Lin, Chueng-He Lu.
Visualization: Wei-Lin Lin, Meei-Shyuan Lee, Chueng-He Lu.
Writing - Original Draft: Wei-Lin Lin, Chueng-He Lu.
Writing - Review & Editing: Wei-Lin Lin, Chueng-He Lu.
WEI LIN LIN orcid: 0000-0001-7774-9878.
Footnotes
Abbreviations: APS = acute pain service, BIS = bispectral index, DES = desflurane anesthesia, NRS = numeric pain rating scale, PCA = patient control analgesia, PONV = postoperative nausea and vomiting, TCI = target-controlled infusion, TIVA = total intravenous anesthesia.
TSGHIRB (Institutional Review Board of Tri-Service General Hospital) Approval Number: 097-05-062. Contact information: Chairman Mu-Hsieh Yu, MD, PhD. Tel: 886-2-87923311 ext. 10552, E-mail: tsghirb@ndmctsgh.edu.tw
This work was supported by grants TSGH-C99-05-S02 from Tri-Service General Hospital, Taipei, Taiwan, Republic of China.
The authors have no conflicts of interest to disclose.
References
- [1].Wu ZF, Jian GS, Lee MS, et al. An analysis of anesthesia-controlled operating room time after propofol-based total intravenous anesthesia compared with desflurane anesthesia in ophthalmic surgery: a retrospective study. Anesth Analg 2014;119:1393–406. [DOI] [PubMed] [Google Scholar]
- [2].Lu CH, Wu ZF, Lin BF, et al. Faster extubation time with more stable hemodynamics during extubation and shorter total surgical suite time after propofol-based total intravenous anesthesia compared with desflurane anesthesia in lengthy lumbar spine surgery. J Neurosurg Spine 2016;24:268–74. [DOI] [PubMed] [Google Scholar]
- [3].Antognini JF, Wang XW, Piercy M, et al. Propofol directly depresses lumbar dorsal horn neuronal responses to noxious stimulation in goats. Can J Anaesth 2000;47:273–9. [DOI] [PubMed] [Google Scholar]
- [4].Kingston S, Mao L, Yang L, et al. Propofol inhibits phosphorylation of N-methyl-D-aspartate receptor NR1 subunits in neurons. Anesthesiology 2006;104:763–9. [DOI] [PubMed] [Google Scholar]
- [5].Guindon J, LoVerme J, Piomelli D, et al. The antinociceptive effects of local injections of propofol in rats are mediated in part by cannabinoid CB1 and CB2 receptors. Anesth Analg 2007;104:1563–9. table of contents. [DOI] [PubMed] [Google Scholar]
- [6].Hofbauer RK, Fiset P, Plourde G, et al. Dose-dependent effects of propofol on the central processing of thermal pain. Anesthesiology 2004;100:386–94. [DOI] [PubMed] [Google Scholar]
- [7].Bandschapp O, Filitz J, Ihmsen H, et al. Analgesic and antihyperalgesic properties of propofol in a human pain model. Anesthesiology 2010;113:421–8. [DOI] [PubMed] [Google Scholar]
- [8].Tibbs GR, Rowley TJ, Sanford RL, et al. HCN1 channels as targets for anesthetic and nonanesthetic propofol analogs in the amelioration of mechanical and thermal hyperalgesia in a mouse model of neuropathic pain. J Pharmacol Exp Ther 2013;345:363–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ma X, Hu YW, Zhao ZL, et al. Anti-inflammatory effects of propofol are mediated by apolipoprotein M in a hepatocyte nuclear factor-1alpha-dependent manner. Arch Biochem Biophys 2013;533:1–0. [DOI] [PubMed] [Google Scholar]
- [10].Samir A, Gandreti N, Madhere M, et al. Anti-inflammatory effects of propofol during cardiopulmonary bypass: a pilot study. Ann Card Anaesth 2015;18:495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Chan AC, Qiu Q, Choi SW, et al. Effects of intra-operative total intravenous anaesthesia with propofol versus inhalational anaesthesia with sevoflurane on post-operative pain in liver surgery: a retrospective case-control study. PLoS One 2016;11:e0149753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Cheng SS, Yeh J, Flood P. Anesthesia matters: patients anesthetized with propofol have less postoperative pain than those anesthetized with isoflurane. Anesth Analg 2008;106:264–9. table of contents. [DOI] [PubMed] [Google Scholar]
- [13].Li M, Mei W, Wang P, et al. Propofol reduces early post-operative pain after gynecological laparoscopy. Acta Anaesthesiol Scand 2012;56:368–75. [DOI] [PubMed] [Google Scholar]
- [14].Tan T, Bhinder R, Carey M, et al. Day-surgery patients anesthetized with propofol have less postoperative pain than those anesthetized with sevoflurane. Anesth Analg 2010;111:83–5. [DOI] [PubMed] [Google Scholar]
- [15].Fassoulaki A, Melemeni A, Paraskeva A, et al. Postoperative pain and analgesic requirements after anesthesia with sevoflurane, desflurane or propofol. Anesth Analg 2008;107:1715–9. [DOI] [PubMed] [Google Scholar]
- [16].Pokkinen SM, Yli-Hankala A, Kalliomaki ML. The effects of propofol vs sevoflurane on post-operative pain and need of opioid. Acta Anaesthesiol Scand 2014;58:980–5. [DOI] [PubMed] [Google Scholar]
- [17].Peng K, Liu HY, Wu SR, et al. Does propofol anesthesia lead to less postoperative pain compared with inhalational anesthesia? A systematic review and meta-analysis. Anesth Analg 2016;123:846–58. [DOI] [PubMed] [Google Scholar]
- [18].Cho AR, Kwon JY, Kim KH, et al. The effects of anesthetics on chronic pain after breast cancer surgery. Anesth Analg 2013;116:685–93. [DOI] [PubMed] [Google Scholar]
- [19].Merrill AW, Barter LS, Rudolph U, et al. Propofol's effects on nociceptive behavior and spinal c-fos expression after intraplantar formalin injection in mice with a mutation in the gamma-aminobutyric acid-type (A) receptor beta3 subunit. Anesth Analg 2006;103:478–83. table of contents. [DOI] [PubMed] [Google Scholar]
- [20].Gilron I, Coderre TJ. Preemptive analgesic effects of steroid anesthesia with alphaxalone in the rat formalin test. Evidence for differential GABA(A) Receptor modulation in persistent nociception. Anesthesiology 1996;84:572–9. [DOI] [PubMed] [Google Scholar]
- [21].Anwar MM, Abdel-Rahman MS. Effect of propofol on perception of pain in mice: mechanisms of action. Comp Biochem Physiol A Mol Integr Physiol 1998;120:249–53. [DOI] [PubMed] [Google Scholar]
- [22].Nadeson R, Goodchild CS. Antinociceptive properties of propofol: involvement of spinal cord gamma-aminobutyric acid (A) receptors. J Pharmacol Exp Ther 1997;282:1181–6. [PubMed] [Google Scholar]
- [23].Barter LS, Mark LO, Jinks SL, et al. Immobilizing doses of halothane, isoflurane or propofol, do not preferentially depress noxious heat-evoked responses of rat lumbar dorsal horn neurons with ascending projections. Anesth Analg 2008;106:985–90. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Sun YY, Li KC, Chen J. Evidence for peripherally antinociceptive action of propofol in rats: behavioral and spinal neuronal responses to subcutaneous bee venom. Brain Res 2005;1043:231–5. [DOI] [PubMed] [Google Scholar]
- [25].Uchida H, Kishikawa K, Collins JG. Effect of propofol on spinal dorsal horn neurons. Comparison with lack of ketamine effects. Anesthesiology 1995;83:1312–22. [DOI] [PubMed] [Google Scholar]
- [26].Kim J, Yao A, Atherley R, et al. Neurons in the ventral spinal cord are more depressed by isoflurane, halothane, and propofol than are neurons in the dorsal spinal cord. Anesth Analg 2007;105:1020–6. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kungys G, Kim J, Jinks SL, et al. Propofol produces immobility via action in the ventral horn of the spinal cord by a GABAergic mechanism. Anesth Analg 2009;108:1531–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Gilron I, Quirion R, Coderre TJ. Pre- versus postinjury effects of intravenous GABAergic anesthetics on formalin-induced Fos immunoreactivity in the rat spinal cord. Anesth Analg 1999;88:414–20. [DOI] [PubMed] [Google Scholar]
- [29].Anker-Moller E, Spangsberg N, Arendt-Nielsen L, et al. Subhypnotic doses of thiopentone and propofol cause analgesia to experimentally induced acute pain. Br J Anaesth 1991;66:185–8. [DOI] [PubMed] [Google Scholar]
- [30].Hand R, Jr, Riley GP, Nick ML, et al. The analgesic effects of subhypnotic doses of propofol in human volunteers with experimentally induced tourniquet pain. AANA J 2001;69:466–70. [PubMed] [Google Scholar]
- [31].Zacny JP, Coalson DW, Young CJ, et al. Propofol at conscious sedation doses produces mild analgesia to cold pressor-induced pain in healthy volunteers. J Clin Anesth 1996;8:469–74. [DOI] [PubMed] [Google Scholar]
- [32].Freye E, Bruckner J, Latasch L. No difference in electroencephalographic power spectra or sensory-evoked potentials in patients anaesthetized with desflurane or sevoflurane. Eur J Anaesthesiol 2004;21:373–8. [DOI] [PubMed] [Google Scholar]
- [33].Yeo ST, Holdcroft A, Yentis SM, et al. Analgesia with sevoflurane during labour: i. Determination of the optimum concentration. Br J Anaesth 2007;98:105–9. [DOI] [PubMed] [Google Scholar]
- [34].Zhang Y, Eger EI, 2nd, Dutton RC, et al. Inhaled anesthetics have hyperalgesic effects at 0.1 minimum alveolar anesthetic concentration. Anesth Analg 2000;91:462–6. [DOI] [PubMed] [Google Scholar]
- [35].Rowley TJ, Daniel D, Flood P. The role of adrenergic and cholinergic transmission in volatile anesthetic-induced pain enhancement. Anesth Analg 2005;100:991–5. [DOI] [PubMed] [Google Scholar]
- [36].Stevens RJ, Rusch D, Davies PA, et al. Molecular properties important for inhaled anesthetic action on human 5-HT3A receptors. Anesth Analg 2005;100:1696–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].An K, Shu H, Huang W, et al. Effects of propofol on pulmonary inflammatory response and dysfunction induced by cardiopulmonary bypass. Anaesthesia 2008;63:1187–92. [DOI] [PubMed] [Google Scholar]
- [38].Tsuchiya M, Asada A, Maeda K, et al. Propofol versus midazolam regarding their antioxidant activities. Am J Respir Crit Care Med 2001;163:26–31. [DOI] [PubMed] [Google Scholar]


