Abstract
Numerous studies have investigated the association between ALDH2 gene rs671G>A polymorphism and various cancer type in Asians. However, the results remain inconclusive.
We conducted a comprehensive meta-analysis including 63 articles with 66 studies containing 25,682 cases and 47,455 controls retrieved by searching PubMed and Embase electronic databases up to March 5, 2018.
Pooled results indicated that ALDH2 gene rs671 polymorphism was significantly associated with the overall cancer risk in Asians (homozygous model: odds ratio [OR] = 0.85, 95% confidence interval [CI] = 0.72–0.99, P = .042; heterozygous model: OR = 1.32, 95% CI = 1.14–1.52, P < .001; recessive model: OR = 0.73, 95% CI = 0.60–0.88, P = .001; dominant model: OR = 1.32, 95% CI = 1.16–1.51, P < .001; and allele comparison model: OR = 1.11, 95% CI = 1.03–1.19, P = .004), especially in esophageal cancer and among the Chinese and the Japanese.
Our results suggest that ALDH2 rs671 polymorphism is associated with the overall cancer risk in Asians. Well-designed prospective studies with more information about gene–environment interaction, such as drinking, should be conducted to validate our findings.
Keywords: ALDH2, cancer, meta-analysis, polymorphism, risk, rs671
1. Introduction
Cancer has been a major killer worldwide, with an estimation of 18.1 million new cases and 9.6 million deaths occurring in 2018.[1] In China, cancer has also been the leading cause of death with the increasing incidence and mortality since 2010. In 2015, 4,292,000 new cancer cases and 2,814,000 cancer deaths were projected to occur in China, that is, about 12,000 new cancer cases and 7500 cancer deaths on average per day.[2] Cancer is a multifactorial disease. Besides the internal/inherited factors, the environmental factors have been considered to play an important role in cancer development including tobacco, alcohol, diet, obesity, infectious agents, and radiation.[3] Approximately 3.6% of all cancers and 3.5% of all cancer deaths are attributable to alcohol consumption.[4] Meanwhile, the effect of alcohol consumption on the risk of cancer is on the rise, and the trend seems to continue.[3]
Alcohol itself is not carcinogenic, but its metabolite, acetaldehyde, is known to interfere DNA synthesis and repairmen and consequently increase the risk of cancer. Alcohol is mainly metabolized by 2 key NAD-dependent enzymes, alcohol dehydrogenase (ADH) and aldehyde dehydrogenase (ALDH). Alcohol is 1st conversed into acetaldehyde by ADH. Acetaldehyde is then oxidized to harmless acetate by ALDH.[5] Aldehyde dehydrogenase-2 (ALDH2) is the major enzyme to eliminate most of the acetaldehyde.[6]
The ALDH2 gene, located at chromosome 12q24, is composed of 13 exons with 46,031 base pairs, and its polymorphisms affect the blood acetaldehyde concentrations after alcohol consumption.[7,8] Rs671G>A, the most commonly studied polymorphism, is reported to an amino acid substitution from glutamine to lysine (Glu487Lys).[9] The 487Lys allele encodes a catalytically inactive subunit and finally affects the status of the ALDH2 enzyme, with only 6.25% of normal 487Glu activity.[10] Such a polymorphism inactivates ALDH2 and leads a high concentration of blood acetaldehyde after drinking alcohol, which could contribute to susceptibility to carcinogenesis.[5] Increasing evidence suggests that the rs671 polymorphism may be associated with many types of cancer, such as head and neck cancer, esophageal cancer, liver cancer, breast cancer, colorectal cancer and gastric cancer.[11–73] However, the results remain inconclusive. Hence, we conducted a comprehensive meta-analysis to assess the association of ALDH2 rs671 polymorphism and the overall cancer risk in Asians.
2. Materials and methods
2.1. Search strategy
PubMed and Embase electronic databases were searched to retrieve all the relevant studies up to March 5, 2018. The following search terms were used: “alcohol dehydrogenase 2 or ALDH2” or “polymorphism or variant or variation” or “cancer or tumor or neoplasm or carcinoma.” Additionally, all the eligible studies and reviews were checked to avoid the missing of any relevant studies. However, only the largest or the latest study would be included in our meta-analysis. As this article is a meta-analysis of the previous published studies, hence patients consent and approval of the ethics committee were not required.
2.2. Inclusion and exclusion criteria
The studies included in our meta-analysis must meet the following criteria: evaluating the association between the ALDH2 rs671 polymorphism and cancer risk in Asians; case–control design; written in English and Chinese; and sufficient information for estimation of odds ratios (ORs) and their 95% confidence intervals (CIs). The exclusion criteria were as follows: case only studies; duplicate articles; and reviews, meta-analyses, and comments. Studies with genotype frequencies in the controls departure from Hardy–Weinberg equilibrium (HWE) were also excluded, unless further evidence indicated that another polymorphism was in HWE.
2.3. Data extraction
Two investigators assessed and extracted the information from all the eligible publications independently. The following information was collected: the surname of authors, year of publication, country of origin, cancer type, source of control, and alleles and genotypes distribution. In case of any disagreement, the issue was resolved by discussion with a third investigator until consensus was reached.
2.4. Statistical analysis
Goodness-of-fit Chi-squared test was employed to evaluate HWE in the control subjects of each study. P < .05 was considered significant and indicated the study departed from HWE. Crude ORs and their corresponding 95% CIs were applied to assess the strength of association between the ALDH2 rs671 polymorphism and the overall cancer risk in Asians under the five genetic models: homozygous model (AA vs GG), heterozygous model (GA vs GG), recessive model (AA vs GA + GG), dominant model (GA + AA vs GG), and allele comparison model (A vs G). Stratification analyses were also conducted to assess the association regarding country, cancer type, and source of control. Q value was performed to test the heterogeneity among studies. If no significant heterogeneity was observed with P-value more than .10, a fixed-effects model (the Mantel–Haenszel method) was adopted. Otherwise, the random-effects model (the DerSimonian and Laird method) was applied.[74,75] Moreover, we used Egger linear regression to detect the symmetry of funnel plots and the potential publication bias.[76] All the statistical analyses were performed with STATA 12.0 (STATA Corporation, College Station, TX). All the P-values were 2-sided, and P < .05 was considered statistically significant.
3. Results
3.1. Eligible studies
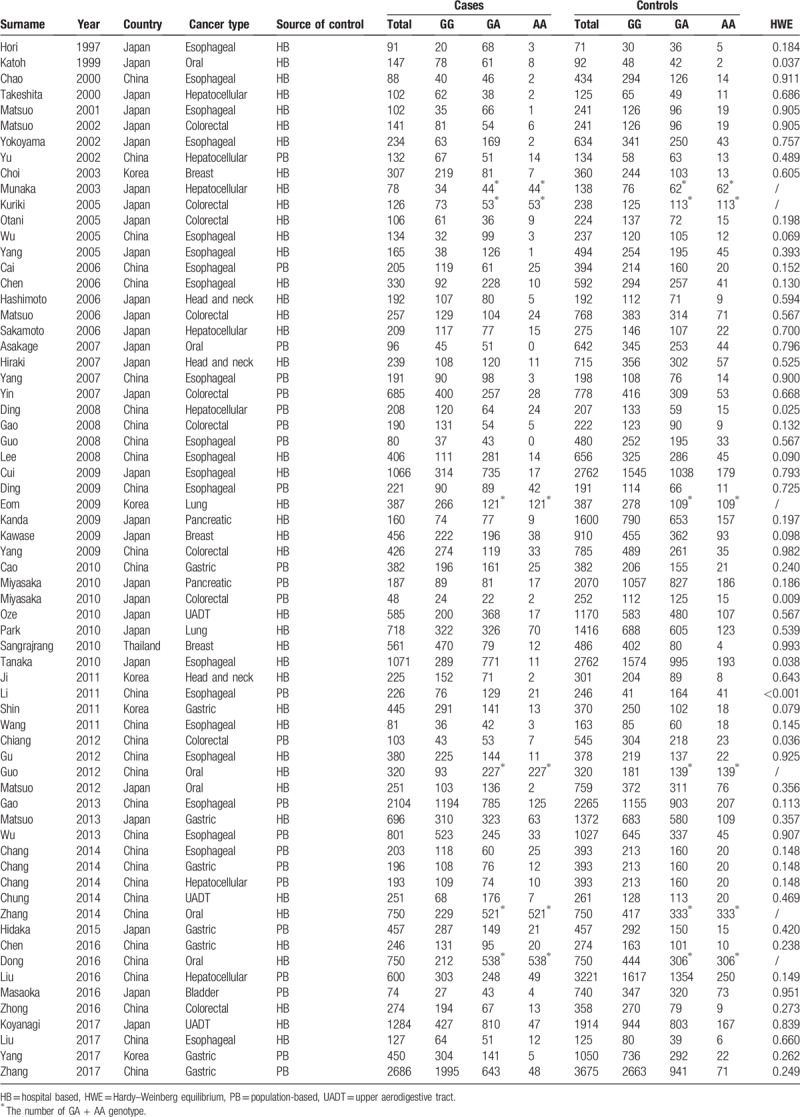
A total of 381 relevant articles were retrieved from PubMed and Embase electronic databases using search terms described in the materials and methods part. After title and abstract screening, 286 articles were excluded for not dealing with the association between the ALDH2 rs671 polymorphism and any cancer risk in Asians. Only 95 articles remained for further assessment, among which, 21 were excluded for being irrelevant; 9 without sufficient information; 6 overlapped with others and 1 departed from HWE.[77] On the contrary, 5 additional articles were found manually. Ultimately, 63 articles with 66 studies comprising 25,682 cases and 47,455 controls were subject to the final meta-analysis,[11–73] and the characteristics of all the original studies are shown in Table 1. The studies were conducted on various types of cancer, such as esophageal cancer (21 studies), oral cancer (6), hepatocellular cancer (7), colorectal cancer (10), breast cancer (3), head and neck cancer (3), lung cancer (2), pancreatic cancer (2), gastric cancer (8), bladder cancer (1) and upper aerodigestive tract cancer (UADT) (3). Among them, 31 were conducted in China, 29 in Japan, 5 in Korea, and 1 in Thailand. Out of the 66 studies selected, 42 were hospital based (HB) and 24 were population based (PB).
Table 1.
Characteristics of studies included in ALDH2 rs671 polymorphism and cancer risk.
3.2. Meta-analysis results
Between-study heterogeneity was found for all the 5 genetic models. Therefore, we used random-effects model to calculate the ORs and their 95% CIs. A significant association between ALDH2 rs671 polymorphism and overall cancer risk in Asians was observed under all the 5 genetic models (Table 2): homozygous model (AA vs GG): OR = 0.85, 95% CI = 0.72 to 0.99, P = .042; heterozygous model (GA vs GG): OR = 1.32, 95% CI = 1.14 to 1.52, P < .001; recessive model (AA vs GA + GG): OR = 0.73, 95% CI = 0.60 to 0.88, P = .001 (Fig. 1); dominant model (GA + AA vs GG): OR = 1.32, 95% CI = 1.16 to 1.51, P < .001; and allele comparison model (A vs G): OR = 1.11, 95% CI = 1.03 to 1.19, P = .004 (Fig. 2). In the stratification analyses by country, the similar results were found in Japanese. However, ALDH2 rs671 polymorphism was associated with the increased cancer risk in Chinese: heterozygous model (GA vs GG): OR = 1.21, 95% CI = 1.02 to 1.44, P = .029; dominant model (GA + AA vs GG): OR = 1.35, 95% CI = 1.11 to 1.64, P = .003; and allele comparison model (A vs G): OR = 1.12, 95% CI = 1.01 to 1.25, P = .030; and with the decreased cancer risk in Korean: homozygous model (AA vs GG): OR = 0.57, 95% CI = 0.35 to 0.91, P = .018; recessive model (AA vs GA + GG): OR = 0.55, 95% CI = 0.34 to 0.88, P = .013. In terms of the stratification analyses by cancer type, there was significant association between ALDH2 rs671 polymorphism and esophageal cancer: heterozygous model (GA vs GG): OR = 1.79, 95% CI = 1.28 to 2.49, P = .001; recessive model (AA vs GA + GG): OR = 0.53, 95% CI = 0.35 to 0.81, P = .004; dominant model (GA + AA vs GG): OR = 1.69, 95% CI = 1.25 to 2.29, P = .001; and allele comparison model (A vs G): OR = 1.28, 95% CI = 1.08 to 1.50, P = .003], oral cancer, head and neck cancer, and UADT. Meanwhile, in the stratification analyses by source of control, a significant association was only found in HB: homozygous model (AA vs GG): OR = 0.76, 95% CI = 0.62 to 0.94, P = .011; heterozygous model (GA vs GG): OR = 1.59, 95% CI = 1.32 to 1.92, P < .001; recessive model (AA vs GA + GG): OR = 0.58, 95% CI = 0.45 to 0.76, P < .001; dominant model (GA + AA vs GG): OR = 1.55, 95% CI = 1.32 to 1.82, P < .001; and allele comparison model (A vs G): OR = 1.19, 95% CI = 1.09 to 1.29, P < .001.
Table 2.
Stratified analyses of the ALDH2 rs671 polymorphism and cancer risk.
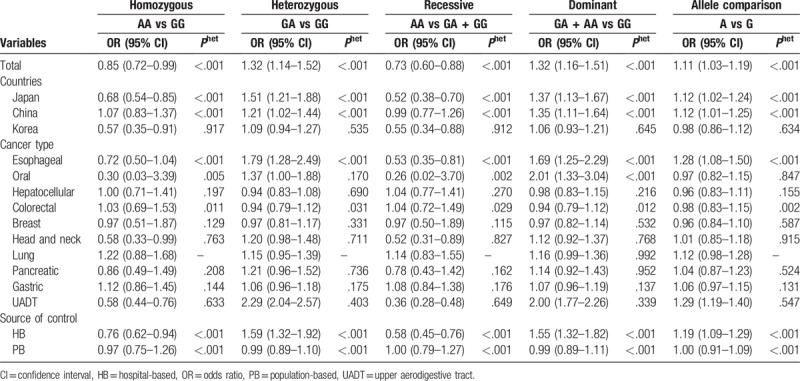
Figure 1.

Forest plot of the association between ALDH2 rs671 polymorphism and overall cancer risk in Asians in the recessive model.
Figure 2.
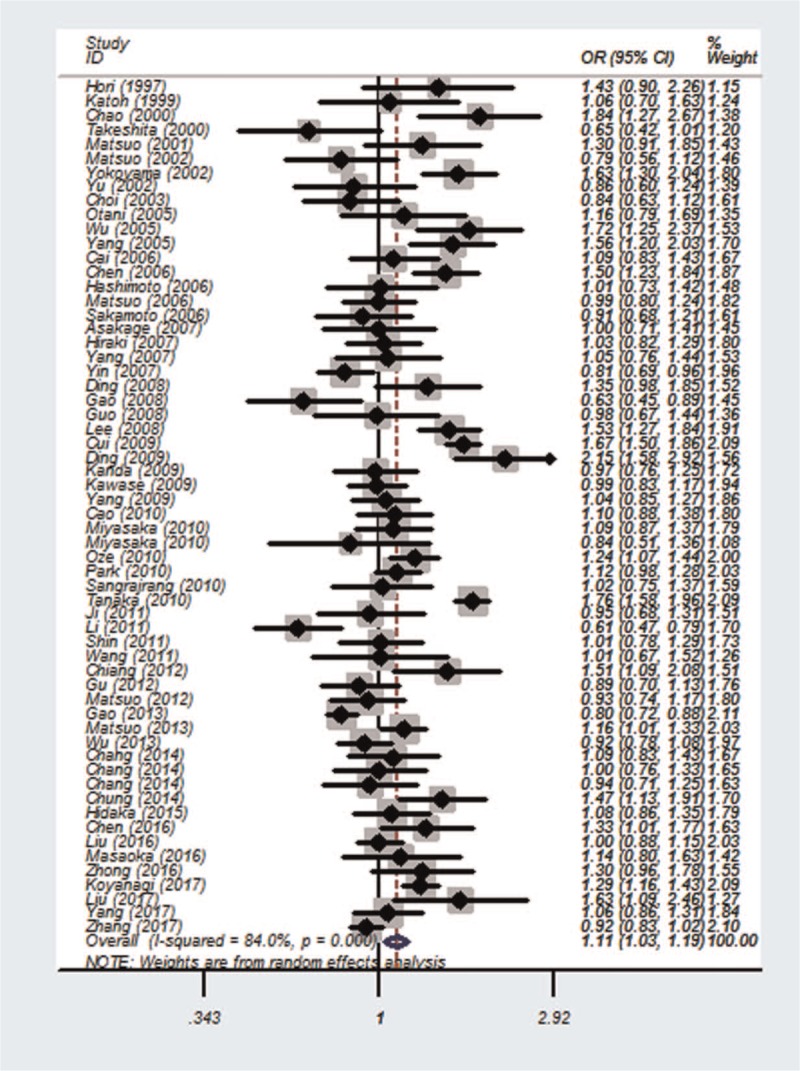
Forest plot of the association between ALDH2 rs671 polymorphism and overall cancer risk in Asians in the allele comparison model.
3.3. Heterogeneity and sensitivity analyses
Heterogeneities were detected among all the studies investigating the association between ALDH2 rs671 polymorphism and the overall cancer risk in Asians (homozygous model, P < .001; heterozygous model, P < .001; recessive model, P < .001; dominant model, P < .001; and allele comparison model, P < 0.001). Therefore, we used the random-effects model to calculate the pooled ORs and their 95% CIs. Moreover, the leave-one-out sensitivity analyses indicated that no single study was able to change the results of our final meta-analysis.
3.4. Publication bias
The funnel plot and Egger linear regression analysis were used to evaluate the publication bias, and no evidence of publication bias was observed (homozygous model, P = .405; heterozygous model, P = .683; recessive model, P = .219; dominant model, P = .841; and allele comparison model, P = .569).
4. Discussion
ALDH2 gene, located at chromosome 12q24, is composed of 13 exons with 46,031 base pairs. ALDH2 is one of the major enzymes which plays a key role in alcohol metabolism. Alcohol is 1st conversed into acetaldehyde by ADH, and acetaldehyde is then oxidized to harmless acetate by ALDH.[5] Therefore, the blood acetaldehyde concentrations after alcohol consumption are mainly dependent on the enzyme activity of ALDH2. However, acetaldehyde could interfere DNA synthesis and repair and consequently results in more than 200 diseases, including alcohol-related cancer.[78] Many studies have investigated the role of ALDH2 gene polymorphisms and their relationship with acetaldehyde elimination. Among them, ALDH2 rs671G>A polymorphism was found dramatically reducing the activity of ALDH2 enzyme and maintaining the high blood acetaldehyde concentrations after alcohol consumption.[9] Drinking the same amount of alcohol, the blood acetaldehyde concentrations in individuals with ALDH2 GA and AA genotype were 6 and 19 folds higher than that with ALDH2 GG genotype, respectively.[79] The accumulation and continuous exposure of acetaldehyde may contribute to the development of cancer.
To the best of our knowledge, this is the 1st meta-analysis to investigate the association between ALDH2 rs671 polymorphism and the overall cancer risk in Asians. In the present meta-analysis, 63 articles with 66 studies containing 25,682 cases and 47,455 controls were retrieved. A significant association between ALDH2 rs671 polymorphism and the overall cancer risk in Asians was observed. Further stratification analyses indicated that a significant association was also found in esophageal cancer, oral cancer, head and neck cancer, and UADT, while the evidence for other cancers is less, as colorectal cancer and hepatocellular cancer. All of the studies investigated colorectal cancer and hepatocellular cancer have a small sample size that resulted in insufficient statistical power.[5] In a latest meta-analysis, Cai et al explored whether the polymorphism was associated with the overall cancer risk in all the ethnicity, and retrieved only 51 studies (49 studies in Asians) with a total of 16,774 cases and 32,060 controls before 2014.[80] In turn, in our meta-analysis, at least 15 studies were published evaluating the association between ALDH2 rs671 polymorphism and cancer risk in Asians. Moreover, Cai et al evaluated the association only under 2 genetic models, the dominant model (GA + AA vs GG) and the allele comparison model (A vs G), and ALDH2 rs671 polymorphism was observed to be associated with the increased overall cancer risk under the dominant model instead of the allele comparison model.
In the general population, the prevalence of the variant 487Lys allele varies in different Asian populations, with about 45% of Japanese, 31% of Chinese, and 29% of Koreans.[81] In the stratification analyses by country, Cai et al also found that ALDH2 rs671 polymorphism was associated with the increased cancer risk in Japanese under the dominant model rather than the allele comparison model.[80] However, the results could not be repeated in the Chinese population. In our meta-analysis, ALDH2 rs671 polymorphism was associated with the increased overall cancer risk in Asians, especially in Japanese and Chinese, not only under the dominant model but also the allele comparison model. Cai et al included only 18 studies with 6193 cases and 8581 controls in Chinese populations in 2014. While, 31 studies with 13,284 cases and 20,449 controls in Chinese populations were retrieved in our meta-analysis. Moreover, unlike this previous article, the studies with genotype frequencies in the controls departure from HWE were not included, unless further evidence indicated that another polymorphism was in HWE. To data, ours is the largest meta-analysis with the strongest statistical power among the existing studies of the same kind.
In our meta-analysis, we found that ALDH2 rs671 polymorphism was associated with an increased overall cancer risk in Asians, especially with esophageal cancer and among the Chinese and the Japanese. Interestingly, individuals with ALDH2 rs671 AA genotype were associated with a reduced risk of esophageal cancer and among the Japanese (Table 2). Such associations were also found in the previous esophageal cancer-specific meta-analysis.[82] The AA individuals tend to avoid alcohol intake due to the development of a severe reaction after small amounts of alcohol drinking, which then leads to be associated with a reduced risk of esophageal cancer and among the Japanese.[5,82] However, among the alcohol consumption group, the AA individuals still suffer highly increased esophageal cancer risk (OR = 3.87, 95% CI = 1.67–8.96) compared to the GG individuals.[82]
Several possible limitations should be acknowledged in our present meta-analysis. First, in the stratification analysis of cancer type and country, the sample sizes of the oral cancer and the head and neck cancer, as well as the sample sizes with the Korean, are relatively small, which might diminish statistical power to evaluate the association. Second, due to the lack of the original information, our meta-analysis was based on unadjusted ORs for other confounding factors, for example, alcohol consumption, which might affect our findings. Third, heterogeneity was observed among the 5 genetic models, and the random-effects model was applied to estimate the association, which might present unstable results. Lastly, we just chose the studies written in English and Chinese, which might overlook the publications in other languages.
In conclusion, our meta-analysis indicated that ALDH2 rs671G>A polymorphism may be associated with the overall cancer risk in Asians, especially in esophageal cancer and among the Chinese and the Japanese. Well-designed prospective studies with more information about gene–environment interaction, such as drinking, should be conducted to validate our findings.
Author contributions
Data curation: Wei Zuo, Zhenyu Zhan.
Methodology: Wei Bai.
Project administration: Zhenyu Zhan.
Software: Wei Zuo, Wei Bai.
Validation: Lin Ma.
Writing – original draft: Shanggan Zeng.
Writing – review & editing: Shanggan Zeng.
Footnotes
Abbreviations: ADH = alcohol dehydrogenase, ALDH = aldehyde dehydrogenase, ALDH2 = aldehyde dehydrogenase-2, CI = confidence interval, HB = hospital based, HWE = Hardy–Weinberg equilibrium, OR = odds ratio, PB = population based.
WZ and ZZ contributed equally to this work and should be considered as co-first authors.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- [2].Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115–32. [DOI] [PubMed] [Google Scholar]
- [3].Anand P, Kunnumakkara AB, Sundaram C, et al. Cancer is a preventable disease that requires major lifestyle changes. Pharm Res 2008;25:2097–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Boffetta P, Hashibe M, La Vecchia C, et al. The burden of cancer attributable to alcohol drinking. Int J Cancer 2006;119:884–7. [DOI] [PubMed] [Google Scholar]
- [5].Chang JS, Hsiao JR, Chen CH. ALDH2 polymorphism and alcohol-related cancers in Asians: a public health perspective. J Biomed Sci 2017;24:19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Liu P, Wang X, Hu CH, et al. Bioinformatics analysis with graph-based clustering to detect gastric cancer-related pathways. Genet Mol Res 2012;11:3497–504. [DOI] [PubMed] [Google Scholar]
- [7].Eriksson CJ. The role of acetaldehyde in the actions of alcohol (update 2000). Alcohol Clin Exp Res 2001;25Suppl ISBRA:15S–32S. [DOI] [PubMed] [Google Scholar]
- [8].Hsu LC, Bendel RE, Yoshida A. Direct detection of usual and atypical alleles on the human aldehyde dehydrogenase-2 (ALDH2) locus. Am J Hum Genet 1987;41:996–1001. [PMC free article] [PubMed] [Google Scholar]
- [9].Yoshida A, Huang IY, Ikawa M. Molecular abnormality of an inactive aldehyde dehydrogenase variant commonly found in Orientals. Proc Natl Acad Sci U S A 1984;81:258–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Crabb DW, Edenberg HJ, Bosron WF, et al. Genotypes for aldehyde dehydrogenase deficiency and alcohol sensitivity. The inactive ALDH2(2) allele is dominant. J Clin Invest 1989;83:314–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zhang LQ, Song X, Zhao XK, et al. Association of genotypes of rs671 withinALDH2 with risk for gastric cardia adenocarcinoma in the Chinese Han population in high- and low-incidence areas. Cancer Biol Med 2017;14:60–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Yang S, Lee J, Choi IJ, et al. Effects of alcohol consumption, ALDH2 rs671 polymorphism, and Helicobacter pylori infection on the gastric cancer risk in a Korean population. Oncotarget 2017;8:6630–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Liu P, Zhao HR, Li F, et al. Correlations of ALDH2 rs671 and C12orf30 rs4767364 polymorphisms with increased risk and prognosis of esophageal squamous cell carcinoma in the Kazak and Han populations in Xinjiang province. J Clin Lab Anal 2018;32: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Koyanagi YN, Ito H, Oze I, et al. Development of a prediction model and estimation of cumulative risk for upper aerodigestive tract cancer on the basis of the aldehyde dehydrogenase 2 genotype and alcohol consumption in a Japanese population. Eur J Cancer Prev 2017;26:38–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Zhong Q, Wu RR, Zeng ZM. Association of ADH1B Arg47His and ALDH2 Glu487Lys polymorphisms with risk of colorectal cancer and their interaction with environmental factors in a Chinese population. Genet Mol Res 2016;15: [DOI] [PubMed] [Google Scholar]
- [16].Masaoka H, Ito H, Soga N, et al. Aldehyde dehydrogenase 2 (ALDH2) and alcohol dehydrogenase 1B (ADH1B) polymorphisms exacerbate bladder cancer risk associated with alcohol drinking: gene-environment interaction. Carcinogenesis 2016;37:583–8. [DOI] [PubMed] [Google Scholar]
- [17].Liu J, Yang HI, Lee MH, et al. Alcohol drinking mediates the association between polymorphisms of ADH1B and ALDH2 and hepatitis B-related hepatocellular carcinoma. Cancer Epidemiol Biomarkers Prev 2016;25:693–9. [DOI] [PubMed] [Google Scholar]
- [18].Dong TT, Wang LJ, Liu LZ, et al. Susceptibility to oral squamous cell carcinoma: correlation with variants of CYP1A1-MspI, GSTT1, GSTM1, ALDH2, EC-SOD and Lifestyle factors. Balkan J Med Genet 2016;19:61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Chen ZH, Xian JF, Luo LP. Analysis of ADH1B Arg47His, ALDH2 Glu487Lys, and CYP4502E1 polymorphisms in gastric cancer risk and interaction with environmental factors. Genet Mol Res 2016;15: [DOI] [PubMed] [Google Scholar]
- [20].Hidaka A, Sasazuki S, Matsuo K, et al. Genetic polymorphisms of ADH1B, ADH1C and ALDH2, alcohol consumption, and the risk of gastric cancer: the Japan Public Health Center-based prospective study. Carcinogenesis 2015;36:223–31. [DOI] [PubMed] [Google Scholar]
- [21].Zhang C, Guo L, Shi S. Correlation between drinking behavior and polymorphisms of extracellular superoxide dismutase, aldehyde dehydrogenase 2 genes, and oral squamous cell carcinoma [in Chinese]. Hua Xi Kou Qiang Yi Xue Za Zhi 2014;32:119–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Chung CS, Lee YC, Liou JM, et al. Tag single nucleotide polymorphisms of alcohol-metabolizing enzymes modify the risk of upper aerodigestive tract cancers: HapMap database analysis. Dis Esophagus 2014;27:493–503. [DOI] [PubMed] [Google Scholar]
- [23].Chang SC, Chang PY, Butler B, et al. Single nucleotide polymorphisms of one-carbon metabolism and cancers of the esophagus, stomach, and liver in a Chinese population. PLoS One 2014;9:e109235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Wu M, Chang SC, Kampman E, et al. Single nucleotide polymorphisms of ADH1B, ADH1C and ALDH2 genes and esophageal cancer: a population-based case-control study in China. Int J Cancer 2013;132:1868–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Matsuo K, Oze I, Hosono S, et al. The aldehyde dehydrogenase 2 (ALDH2) Glu504Lys polymorphism interacts with alcohol drinking in the risk of stomach cancer. Carcinogenesis 2013;34:1510–5. [DOI] [PubMed] [Google Scholar]
- [26].Gao Y, He Y, Xu J, et al. Genetic variants at 4q21, 4q23 and 12q24 are associated with esophageal squamous cell carcinoma risk in a Chinese population. Hum Genet 2013;132:649–56. [DOI] [PubMed] [Google Scholar]
- [27].Matsuo K, Rossi M, Negri E, et al. Folate, alcohol, and aldehyde dehydrogenase 2 polymorphism and the risk of oral and pharyngeal cancer in Japanese. Eur J Cancer Prev 2012;21:193–8. [DOI] [PubMed] [Google Scholar]
- [28].Guo LK, Zhang CX, Guo XF. Association of genetic polymorphisms of aldehyde dehydrogenase-2 and cytochrome P450 2E1-RsaI and alcohol consumption with oral squamous cell carcinoma. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 2012;34:390–5. [DOI] [PubMed] [Google Scholar]
- [29].Gu H, Gong D, Ding G, et al. A variant allele of ADH1B and ALDH2, is associated with the risk of esophageal cancer. Exp Ther Med 2012;4:135–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Chiang CP, Jao SW, Lee SP, et al. Expression pattern, ethanol-metabolizing activities, and cellular localization of alcohol and aldehyde dehydrogenases in human large bowel: association of the functional polymorphisms of ADH and ALDH genes with hemorrhoids and colorectal cancer. Alcohol 2012;46:37–49. [DOI] [PubMed] [Google Scholar]
- [31].Wang Y, Ji R, Wei X, et al. Esophageal squamous cell carcinoma and ALDH2 and ADH1B polymorphisms in Chinese females. Asian Pac J Cancer Prev 2011;12:2065–8. [PubMed] [Google Scholar]
- [32].Shin CM, Kim N, Cho SI, et al. Association between alcohol intake and risk for gastric cancer with regard to ALDH2 genotype in the Korean population. Int J Epidemiol 2011;40:1047–55. [DOI] [PubMed] [Google Scholar]
- [33].Li QD, Li H, Wang MS, et al. Multi-susceptibility genes associated with the risk of the development stages of esophageal squamous cell cancer in Feicheng County. BMC Gastroenterol 2011;11:74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Ji YB, Tae K, Ahn TH, et al. ADH1B and ALDH2 polymorphisms and their associations with increased risk of squamous cell carcinoma of the head and neck in the Korean population. Oral Oncol 2011;47:583–7. [DOI] [PubMed] [Google Scholar]
- [35].Tanaka F, Yamamoto K, Suzuki S, et al. Strong interaction between the effects of alcohol consumption and smoking on oesophageal squamous cell carcinoma among individuals with ADH1B and/or ALDH2 risk alleles. Gut 2010;59:1457–64. [DOI] [PubMed] [Google Scholar]
- [36].Sangrajrang S, Sato Y, Sakamoto H, et al. Genetic polymorphisms in folate and alcohol metabolism and breast cancer risk: a case-control study in Thai women. Breast Cancer Res Treat 2010;123:885–93. [DOI] [PubMed] [Google Scholar]
- [37].Park JY, Matsuo K, Suzuki T, et al. Impact of smoking on lung cancer risk is stronger in those with the homozygous aldehyde dehydrogenase 2 null allele in a Japanese population. Carcinogenesis 2010;31:660–5. [DOI] [PubMed] [Google Scholar]
- [38].Oze I, Matsuo K, Hosono S, et al. Comparison between self-reported facial flushing after alcohol consumption and ALDH2 Glu504Lys polymorphism for risk of upper aerodigestive tract cancer in a Japanese population. Cancer Sci 2010;101:1875–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Miyasaka K, Hosoya H, Tanaka Y, et al. Association of aldehyde dehydrogenase 2 gene polymorphism with pancreatic cancer but not colon cancer. Geriatr Gerontol Int 2010;10Suppl 1:S120–6. [DOI] [PubMed] [Google Scholar]
- [40].Cao HX, Li SP, Wu JZ, et al. Alcohol dehydrogenase-2 and aldehyde dehydrogenase-2 genotypes, alcohol drinking and the risk for stomach cancer in Chinese males. Asian Pac J Cancer Prev 2010;11:1073–7. [PubMed] [Google Scholar]
- [41].Yang H, Zhou Y, Zhou Z, et al. A novel polymorphism rs1329149 of CYP2E1 and a known polymorphism rs671 of ALDH2 of alcohol metabolizing enzymes are associated with colorectal cancer in a southwestern Chinese population. Cancer Epidemiol Biomarkers Prev 2009;18:2522–7. [DOI] [PubMed] [Google Scholar]
- [42].Kawase T, Matsuo K, Hiraki A, et al. Interaction of the effects of alcohol drinking and polymorphisms in alcohol-metabolizing enzymes on the risk of female breast cancer in Japan. J Epidemiol 2009;19:244–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Kanda J, Matsuo K, Suzuki T, et al. Impact of alcohol consumption with polymorphisms in alcohol-metabolizing enzymes on pancreatic cancer risk in Japanese. Cancer Sci 2009;100:296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Eom SY, Zhang YW, Kim SH, et al. Influence of NQO1, ALDH2, and CYP2E1 genetic polymorphisms, smoking, and alcohol drinking on the risk of lung cancer in Koreans. Cancer Causes Control 2009;20:137–45. [DOI] [PubMed] [Google Scholar]
- [45].Ding JH, Li SP, Cao HX, et al. Polymorphisms of alcohol dehydrogenase-2 and aldehyde dehydrogenase-2 and esophageal cancer risk in Southeast Chinese males. World J Gastroenterol 2009;15:2395–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Cui R, Kamatani Y, Takahashi A, et al. Functional variants in ADH1B and ALDH2 coupled with alcohol and smoking synergistically enhance esophageal cancer risk. Gastroenterology 2009;137:1768–75. [DOI] [PubMed] [Google Scholar]
- [47].Lee CH, Lee JM, Wu DC, et al. Carcinogenetic impact of ADH1B and ALDH2 genes on squamous cell carcinoma risk of the esophagus with regard to the consumption of alcohol, tobacco and betel quid. Int J Cancer 2008;122:1347–56. [DOI] [PubMed] [Google Scholar]
- [48].Guo YM, Wang Q, Liu YZ, et al. Genetic polymorphisms in cytochrome P4502E1, alcohol and aldehyde dehydrogenases and the risk of esophageal squamous cell carcinoma in Gansu Chinese males. World J Gastroenterol 2008;14:1444–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Gao CM, Takezaki T, Wu JZ, et al. Polymorphisms of alcohol dehydrogenase 2 and aldehyde dehydrogenase 2 and colorectal cancer risk in Chinese males. World J Gastroenterol 2008;14:5078–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Ding J, Li S, Wu J, et al. Alcohol dehydrogenase-2 and aldehyde dehydrogenase-2 genotypes, alcohol drinking and the risk of primary hepatocellular carcinoma in a Chinese population. Asian Pac J Cancer Prev 2008;9:31–5. [PubMed] [Google Scholar]
- [51].Yin G, Kono S, Toyomura K, et al. Alcohol dehydrogenase and aldehyde dehydrogenase polymorphisms and colorectal cancer: the Fukuoka Colorectal Cancer Study. Cancer Sci 2007;98:1248–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Yang SJ, Wang HY, Li XQ, et al. Genetic polymorphisms of ADH2 and ALDH2 association with esophageal cancer risk in southwest China. World J Gastroenterol 2007;13:5760–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Hiraki A, Matsuo K, Wakai K, et al. Gene-gene and gene-environment interactions between alcohol drinking habit and polymorphisms in alcohol-metabolizing enzyme genes and the risk of head and neck cancer in Japan. Cancer Sci 2007;98:1087–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Asakage T, Yokoyama A, Haneda T, et al. Genetic polymorphisms of alcohol and aldehyde dehydrogenases, and drinking, smoking and diet in Japanese men with oral and pharyngeal squamous cell carcinoma. Carcinogenesis 2007;28:865–74. [DOI] [PubMed] [Google Scholar]
- [55].Sakamoto T, Hara M, Higaki Y, et al. Influence of alcohol consumption and gene polymorphisms of ADH2 and ALDH2 on hepatocellular carcinoma in a Japanese population. Int J Cancer 2006;118:1501–7. [DOI] [PubMed] [Google Scholar]
- [56].Matsuo K, Wakai K, Hirose K, et al. A gene-gene interaction between ALDH2 Glu487Lys and ADH2 His47Arg polymorphisms regarding the risk of colorectal cancer in Japan. Carcinogenesis 2006;27:1018–23. [DOI] [PubMed] [Google Scholar]
- [57].Hashimoto T, Uchida K, Okayama N, et al. ALDH2 1510 G/A (Glu487Lys) polymorphism interaction with age in head and neck squamous cell carcinoma. Tumour Biol 2006;27:334–8. [DOI] [PubMed] [Google Scholar]
- [58].Chen YJ, Chen C, Wu DC, et al. Interactive effects of lifetime alcohol consumption and alcohol and aldehyde dehydrogenase polymorphisms on esophageal cancer risks. Int J Cancer 2006;119:2827–31. [DOI] [PubMed] [Google Scholar]
- [59].Cai L, You NC, Lu H, et al. Dietary selenium intake, aldehyde dehydrogenase-2 and X-ray repair cross-complementing 1 genetic polymorphisms, and the risk of esophageal squamous cell carcinoma. Cancer 2006;106:2345–54. [DOI] [PubMed] [Google Scholar]
- [60].Yang CX, Matsuo K, Ito H, et al. Esophageal cancer risk by ALDH2 and ADH2 polymorphisms and alcohol consumption: exploration of gene-environment and gene-gene interactions. Asian Pac J Cancer Prev 2005;6:256–62. [PubMed] [Google Scholar]
- [61].Wu CF, Wu DC, Hsu HK, et al. Relationship between genetic polymorphisms of alcohol and aldehyde dehydrogenases and esophageal squamous cell carcinoma risk in males. World J Gastroenterol 2005;11:5103–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Otani T, Iwasaki M, Hanaoka T, et al. Folate, vitamin B6, vitamin B12, and vitamin B2 intake, genetic polymorphisms of related enzymes, and risk of colorectal cancer in a hospital-based case-control study in Japan. Nutr Cancer 2005;53:42–50. [DOI] [PubMed] [Google Scholar]
- [63].Kuriki K, Hamajima N, Chiba H, et al. Relation of the CD36 gene A52C polymorphism to the risk of colorectal cancer among Japanese, with reference to with the aldehyde dehydrogenase 2 gene Glu487Lys polymorphism and drinking habit. Asian Pac J Cancer Prev 2005;6:62–8. [PubMed] [Google Scholar]
- [64].Munaka M, Kohshi K, Kawamoto T, et al. Genetic polymorphisms of tobacco- and alcohol-related metabolizing enzymes and the risk of hepatocellular carcinoma. J Cancer Res Clin Oncol 2003;129:355–60. [DOI] [PubMed] [Google Scholar]
- [65].Choi JY, Abel J, Neuhaus T, et al. Role of alcohol and genetic polymorphisms of CYP2E1 and ALDH2 in breast cancer development. Pharmacogenetics 2003;13:67–72. [DOI] [PubMed] [Google Scholar]
- [66].Yu SZ, Huang XE, Koide T, et al. Hepatitis B and C viruses infection, lifestyle and genetic polymorphisms as risk factors for hepatocellular carcinoma in Haimen, China. Jpn J Cancer Res 2002;93:1287–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Yokoyama A, Kato H, Yokoyama T, et al. Genetic polymorphisms of alcohol and aldehyde dehydrogenases and glutathione S-transferase M1 and drinking, smoking, and diet in Japanese men with esophageal squamous cell carcinoma. Carcinogenesis 2002;23:1851–9. [DOI] [PubMed] [Google Scholar]
- [68].Matsuo K, Hamajima N, Hirai T, et al. Aldehyde dehydrogenase 2 (ALDH2) genotype affects rectal cancer susceptibility due to alcohol consumption. J Epidemiol 2002;12:70–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Matsuo K, Hamajima N, Shinoda M, et al. Gene-environment interaction between an aldehyde dehydrogenase-2 (ALDH2) polymorphism and alcohol consumption for the risk of esophageal cancer. Carcinogenesis 2001;22:913–6. [DOI] [PubMed] [Google Scholar]
- [70].Takeshita T, Yang X, Inoue Y, et al. Relationship between alcohol drinking, ADH2 and ALDH2 genotypes, and risk for hepatocellular carcinoma in Japanese. Cancer Lett 2000;149:69–76. [DOI] [PubMed] [Google Scholar]
- [71].Chao YC, Wang LS, Hsieh TY, et al. Chinese alcoholic patients with esophageal cancer are genetically different from alcoholics with acute pancreatitis and liver cirrhosis. Am J Gastroenterol 2000;95:2958–64. [DOI] [PubMed] [Google Scholar]
- [72].Katoh T, Kaneko S, Kohshi K, et al. Genetic polymorphisms of tobacco- and alcohol-related metabolizing enzymes and oral cavity cancer. Int J Cancer 1999;83:606–9. [DOI] [PubMed] [Google Scholar]
- [73].Hori H, Kawano T, Endo M, et al. Genetic polymorphisms of tobacco- and alcohol-related metabolizing enzymes and human esophageal squamous cell carcinoma susceptibility. J Clin Gastroenterol 1997;25:568–75. [DOI] [PubMed] [Google Scholar]
- [74].DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- [75].Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 1959;22:719–48. [PubMed] [Google Scholar]
- [76].Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Boonyaphiphat P, Thongsuksai P, Sriplung H, et al. Lifestyle habits and genetic susceptibility and the risk of esophageal cancer in the Thai population. Cancer Lett 2002;186:193–9. [DOI] [PubMed] [Google Scholar]
- [78].Rehm J, Shield KD. Global alcohol-attributable deaths from cancer, liver cirrhosis, and injury in 2010. Alcohol Res 2013;35:174–83. [PMC free article] [PubMed] [Google Scholar]
- [79].Haas SL, Ye W, Lohr JM. Alcohol consumption and digestive tract cancer. Curr Opin Clin Nutr Metab Care 2012;15:457–67. [DOI] [PubMed] [Google Scholar]
- [80].Cai Q, Wu J, Chen EZ, et al. Association between Glu504Lys polymorphism of ALDH2 gene and cancer risk: a meta-analysis. PLoS One 2015;10:e0117173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Gu JY, Li LW. ALDH2 Glu504Lys polymorphism and susceptibility to coronary artery disease and myocardial infarction in East Asians: a meta-analysis. Arch Med Res 2014;45:76–83. [DOI] [PubMed] [Google Scholar]
- [82].Zhao T, Wang C, Shen L, et al. Clinical significance of ALDH2 rs671 polymorphism in esophageal cancer: evidence from 31 case-control studies. Onco Targets Ther 2015;8:649–59. [DOI] [PMC free article] [PubMed] [Google Scholar]


