Supplemental Digital Content is available in the text
Keywords: antimalarial, meta-analysis, serum lipids, systemic lupus erythematosus
Abstract
Background:
Dyslipidemia is a common disorder in systemic lupus erythematosus (SLE) patients. It is still inconclusive whether antimalarial drugs could affect the serum lipids in SLE patients, therefore we conducted a systematic review and meta-analysis of available data to address this issue.
Methods:
We comprehensively searched the databases of PubMed, EMBASE and Cochrane Library from date of inception to Sep 2018 for both randomized controlled trials (RCTs) and observational studies. Review Manager 5.3 software was used for analysis. We performed meta-analysis using random-effects model and weighted the mean difference (WMD) and its 95% confidence interval (CI). The Q test was used to assess the presence of heterogeneity and the I2 index was used to quantify the extent of heterogeneity.
Results:
In total, 8 studies met our selection criteria including 2 RCTs, 2 cohort studies, and 4 case-control studies. There were 717 patients (336 patients in CQ (chloroquine) or HCQ (hydroxychloroquine) group, and 381 patients in control group (SLE patients without the therapy of AM)). Compared with the control group, TC, TG, LDL-C, VLDL-C were associated with a significant decrease, respectively (WMD = −21.40 mg/dL, 95% CI −27.62 to −15.18, P < .00001), (WMD = −29.07 mg/dL, 95% CI −45.28 to −12.86, P = .0004), (WMD = −16.25 mg/dL, 95% CI −28.82 to −3.68, P = .01), (WMD = −6.41 mg/dL, 95% CI −12.39 to 0.44, P = .04), however the change of HDL-C did not reach statistically significance (WMD = 4.42 mg/dL, 95% CI −1.21 to 10.06, P = .12).
Conclusions:
CQ or HCQ can infect the serum lipids in SLE patients. However, these results should be interpreted with cautions since lacking sufficient RCTs.
1. Introduction
SLE is a chronic autoimmune disease with multiple system impairments that can be severe and threaten patients’ life.[1–3] The morbidity of SLE was 8.3/100,000/year for females and 1.4/100,000/year for males.[4] Premature atherosclerosis and subsequent progressions have become the leading cause of death in patients with SLE.[5–7] Dyslipidemia is one of the traditional risk factors for atherosclerosis,[8,9] and it is a very frequent comorbidity in SLE patients with negative effects in the long term,[10,11] which not only increases the risk of cardiovascular disease but also affects other clinical symptoms of SLE patients, such as accelerating chronic kidney disease process and damaging brain function. There are also some conditions which influence dyslipidemia prevalence, such as auto-antibody in lipoprotein metabolism, renal involvement, disease activity and increased lipid level due to drugs.[6,12,13] Tselios et al reported that the prevalence of dyslipidemia was 36% among newly diagnosed SLE patients, and 60% or even higher after 3 years.[14]
Antimalarials are the old drugs used in clinics. CQ and HCQ are common antimalarials, CQ was introduced in 1953, and HCQ in 1955.[15] These 2 kinds of drugs are both 4-amino-quinolines, HCQ is an analog of CQ. These 2 compounds have many similarities in pharmacological function and biological mechanism.[16] Nowadays, CQ and HCQ are widely used in SLE patients.[17–20]
The use of CQ or HCQ may be associated with lower levels of serum lipid in SLE patients, especially among those who were on steroids. In 1990, Wallace et al had suggested that HCQ therapy had a significantly statistical association with serum lipids reduction in SLE patient.[21] However, Tam et al had reported that HCQ has no significant effect on lipid in Chinese lupus patients in 2000.[22] Since then, there were some studies on this, but the evidences are insufficient and inconclusive. We, therefore, performed a systematic review and meta-analysis to evaluate the impact of CQ or HCQ on serum lipids in SLE patients.
2. Methods
2.1. Search strategy
In order to identify all available studies, the study was performed according to PRISMA (preferred reporting items for systematic review and meta-analysis) guidelines.[23] We searched all literatures in PubMed, Cochrane Databases and EMBASE (up to September 2018). There were no limitations on language or publication date. Literature search was performed using the following search terms in all possible combinations: systemic lupus erythematosus (SLE), lupus erythematosus disseminatus, lupus, SLE, Antimalarials, Hydroxychloroquine, chloroquine, lipid (see search strategy for PubMed in online supplementary file). In addition, all the references in the retrieved literatures were manually reviewed to identify other potentially relevant articles.
2.2. Study selection
The following inclusive selection criteria were applied:
-
1.
study design: RCTs, cohort or case-control studies with detailed data,
-
2.
study population: the diagnosis of patients was fulfilled the American College of Rheumatology (ACR) criteria for SLE, without diseases caused by dyslipidemia and the current taking lipid-lowering drugs,
-
3.
comparison intervention: with and without CQ or HCQ,
-
4.
outcome measure: serum lipids (TC, TG, LDL-C, HDL-C, and VLDL-C). In the case of duplicate data publication (several studies with overlapping samples), we only included the most informative article or complete study to avoid duplication of information.
2.3. Date extraction and quality assessment
In each study, the following data items were extracted:
-
(1)
first author's name,
-
(2)
year of publication,
-
(3)
study design,
-
(4)
population,
-
(5)
number of participants in the CQ or HCQ and control groups,
-
(6)
age, weight of study participants,
-
(7)
duration of SLE,
-
(8)
mean dose of drug,
-
(9)
level of serum lipids.
A systematic assessment of bias in the included RCTs’ studies was performed using the Cochrane criteria.[24] We assessed the authenticity and quality of the included observational studies by Newcastle-Ottawa scales (NOS).[25] The scoring system encompasses a resulting score range between 0 and 9 with a higher score representing a better methodological quality.[26] Two investigators (Chen Yang Tao and Jin Shang) carried out the literature search, study selection, data extraction and quality assessment independently. Any discrepancies were resolved by consensus.
2.4. Statistical analysis and risk of bias assessment
All statistical analysis was conducted by Review Manager 5.3 software. Differences between cases and controls were calculated by using the WMD for continuous variables and were illustrated by a forest plot. Random-effects model was employed to obtain an average effect size and to study heterogeneity. Statistical heterogeneity between studies was assessed with Chi-square heterogeneity statistic Q and then quantified with I2. P < .1 or I2 > 50% revealed significant heterogeneity among studies.[27,28] When standard error of the mean (SEM) was only reported, standard deviation (SD) was estimated using the following formula: SD = SEM × sqrt(n), where n is the number of subjects. A P value less than .05 was considered significant for all statistical tests.
2.5. Sensitivity analyses
Our sensitivity analysis was performed by leave-one-out approach.[29]
2.6. Patient and public involvement
No patients or the public were involved in this study.
3. Results
3.1. Characteristics of included studies
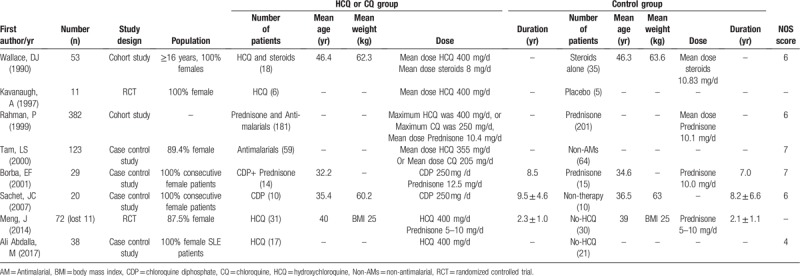
Three hundred twenty-five publications were found during the initial literature search. Of which, 47 were excluded for duplicate studies and 245 papers were excluded by reading their title and abstract (see the detail in Fig. 1). The remaining 8 studies were included in the final review.[21,30–36] The main characteristics of the 8 included studies are shown in Table 1. These studies were published between 1990 and 2017. In the 8 studies, 717 SLE patients were included (336 cases in CQ or HCQ group and 381 cases in control group). Of which, 2 experimental of studies used CQ,[33,34] 2 experimental of studies used HCQ or CQ while there was not detailed statement,[31,32] 4 experimental of studies used HCQ.[21,30,35,36] The overall patients were unbalanced in gender composition with more females than males.
Figure 1.
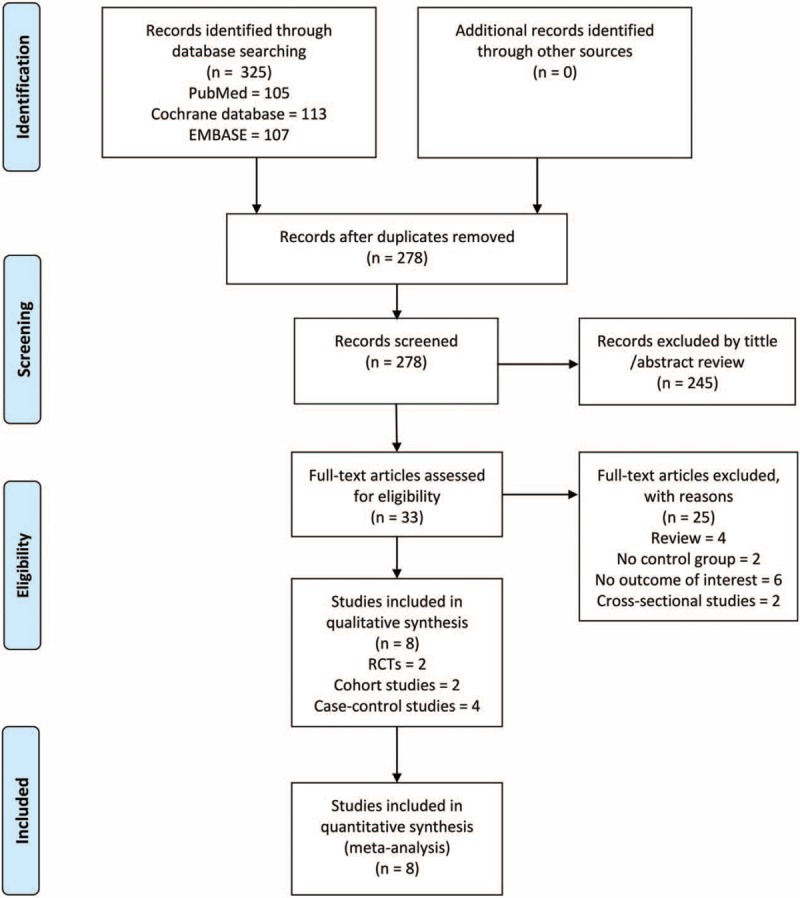
Schematic representation of the literature identification and selection process.
Table 1.
Characteristics of included studies.
3.2. Quality assessment
Figure 2 and Table 1 show a risk-of-bias made for the studies included. There are some unclear items in 2 RCTs. No observational studies obtain the score of 9 stars, 2 studies scored 7, 3 scored 6, 1 scored 4. Therefore, the methodological quality of studies included here was not satisfied.
Figure 2.
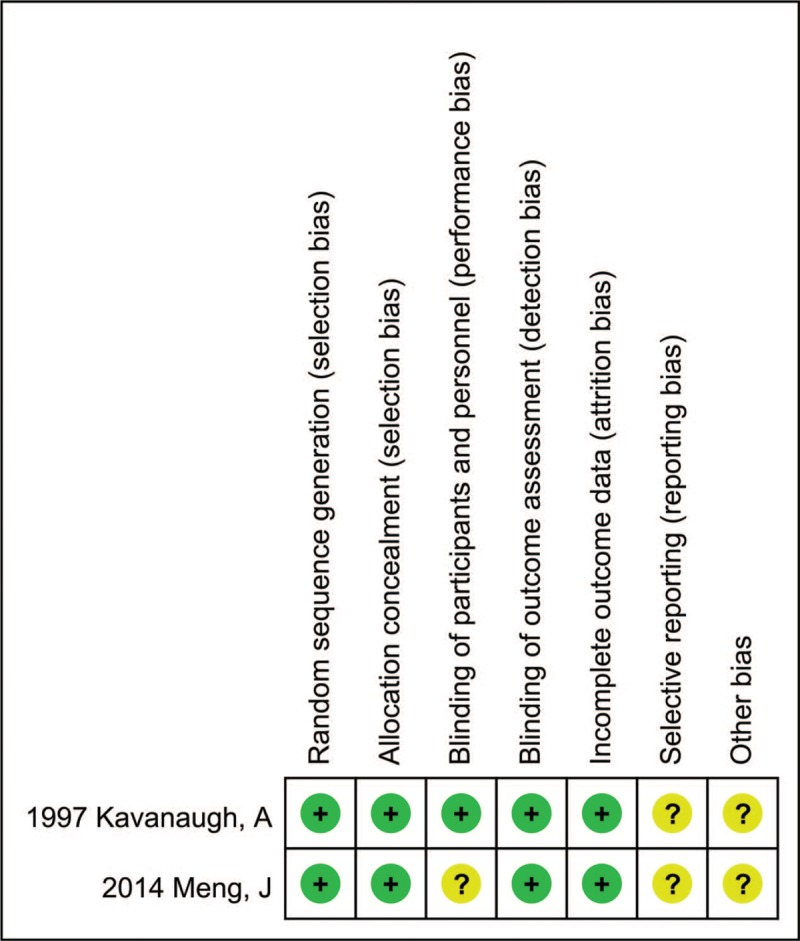
Quality assessment of the included RCTs. RCTs = randomised controlled trials.
3.3. Meta-analysis results
Figure 3 shows the pooled results from the random-effects model combing the WMD for serum lipids. CQ or HCQ's effect on SLE serum lipids was reported in 2 RCTs, 2 cohort studies and 4 case-control studies. Among them, 8 of the studies assessed TC, 7 of the studies assessed TG, LDL-C, HDL-C and 5 of the studies assessed VLDL-C.
Figure 3.
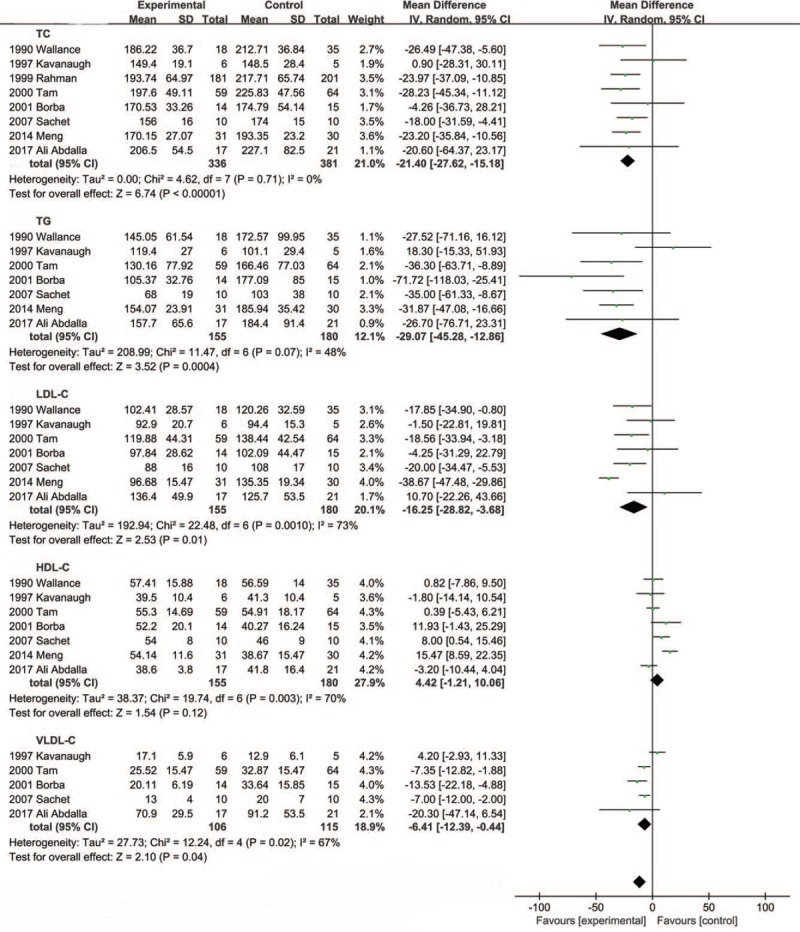
Meta-analysis of the results.
The systematic review and meta-analysis suggested that the CQ or HCQ therapy reduce the level of serum TC (WMD = −21.40 mg/dL, 95% CI −27.62 to −15.18, P < .00001; I2 = 0%, P = .71). TG (WMD = −29.07 mg/dL, 95% CI −45.28 to −12.86, P = .0004; I2 = 48%, P = .07). LDL-C (WMD = −16.25 mg/dL, 95%CI −28.82 to −3.68, P = .01; I2 = 73%, P = .001), HDL-C (WMD = 4.42 mg/dL, 95% CI −1.21 to 10.06, P = .12; I2 = 70%, P = .003). VLDL-C (WMD = −6.41 mg/dL, 95% CI −12.39 to 0.44, P = .04; I2 = 67%, P = .02).
3.4. Subgroup and sensitivity analysis
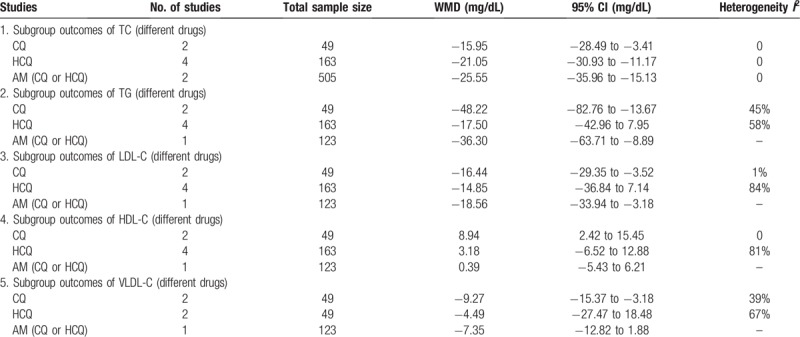
We repeated the primary analyses among subgroups defined by: drugs (RCTs or observational studies). Table 2 shows the results of subgroup analysis for serum lipids. The finding of antimalarials decreased serum lipids (TC, TG, LDL-C, VLDL-C) in SLE patients was consistently found in most subgroup analysis.
Table 2.
The results of subgroup analyses.
Our sensitivity analysis indicates that excluding one study at a time does not make a significant difference on TC, TG, LDL-C, HDL-C. None of the results was significantly altered, confirming that the result was robust.
3.5. Publication bias
There was significant asymmetry in the funnel plot for the effect of CQ or HCQ on TG, LDL-C, HDL-C, VLDL-C, which may be due to publication bias and other causes and no publication bias was found for the effect of CQ or HCQ on TC (Fig. 4).
Figure 4.
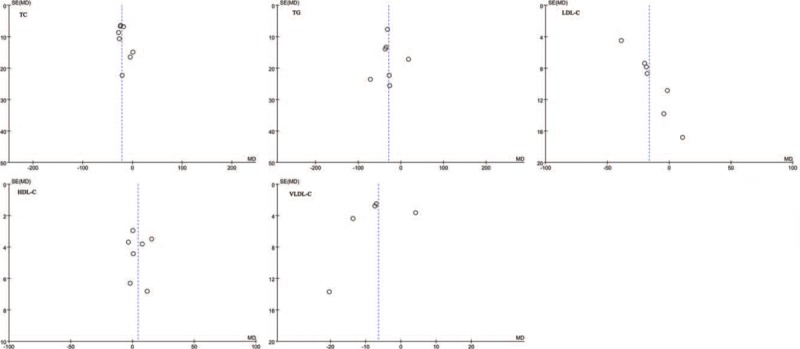
Funnel plots for the effect of CQ or HCQ on serum lipids in SLE patients. CQ = chloroquine, HCQ = hydroxychloroquine, SLE = systemic lupus erythematosus.
4. Discussion
To the best of our knowledge, this is the first meta-analysis to explore the effect of CQ or HCQ on TC, TG, LDL-C, HDL-C and VLDL-C in SLE patients. A total of 8 studies, predominantly reporting low level of evidence (2 RCTs, 2 cohort studies, 4 case-control studies) were included in this study. Overall, we quantitatively summarize all the available evidences and find that HCQ or CQ therapy could significantly decrease the serum TC, TG, LDL-C, VLDL-C by 21.40 mg/dL, 29.07 mg/dL, 16.25 mg/dL, 6.41 mg/dL, respectively, but not HDL-C in comparison of control group. We found that the trend varied a little in subgroup analyses.
Although the treatments of SLE vary, antimalarials were still considered as the basic drugs in SLE patients.[37,38] Some relevant studies have reported the influence of CQ or HCQ on serum lipids in SLE patients. In 1997, Arthur Kavanaugh et al reported a double-blind, randomized, placebo-controlled, pilot study on 17 patients with SLE, the results showed a significant decrease in TC among patients receiving 400 mg/day HCQ (MD = 11.6 mg/dL, P = .03).[30] A recent meta-analysis also reported the decrease of LDL-C after using HCQ, but this study did not include other serum lipids in terms of TC, TG, HDL-C, and VLDL-C, and could not give people a complete acknowledge of the impact of AM on serum lipids in SLE patients. In 2017, Ali Abdalla et al reported a case-control study about the impact of HCQ on serum lipids, this study showed that there was no significant difference between the HCQ group and the control group.[36]
The mechanism of HCQ or CQ decreasing the serum lipid among SLE patients remains unclear. In 1983, Sewell et al reported that after chloroquine administration, the hepatic activities of lysosomal enzymes (N-acetyl-beta-glucosaminidase, beta-glucuronidase, and beta-galactosidase) were increased and cholesterol saturation of bile decreased by 22% in rat.[39] In 1984, Chen et al reported that CQ treatment of rat cells in culture results in the increase of hydroxymethylglutaryl coenzyme A (HMG-CoA) reductase activity,[40] HMG-CoA reductase was a rate limiting enzyme in the process of cholesterol synthetize in hepatocytes. In 2007, Sachet et al reported that CQ can up-regulation LDL-receptor, this is a very efficient mechanism for LDL reduction in SLE.[34] This up-regulation in LDL receptors may be due to the CQ-mediated decrease in cholesterol synthesis.[41] More studies are needed to determine the mechanism of CQ or HCQ decrease serum lipid.
Different studies had different results and conclusions, we conducted a meta-analysis of relevant studies to further confirm the impact. The results showed that CQ or HCQ can decrease the TC, TG, LDL-C, VLDL-C in SLE patients, and our findings are of clinical significance to some extent. CQ or HCQ can decrease the serum lipids, which was the most risk factor results in atherosclerosis and coronary heart disease (CAD). In 2014, Meng et al reported that after 24 months’ using of HCQ, the level of serum lipids reduced, and the left ventricular end diastolic diameter and left ventricular posterior wall thickness decreased as well.[35] We proposed that CQ or HCQ might be a beneficial choice in the prevention of CAD in lupus patients. To our best knowledge, SLE patients have a more prevalent about atherosclerosis and CAD. Once diagnosed with CAD, patients were probably to take several drugs lifelong. HCQ is a multi-functional drug that contains the both benefits of therapy SLE and prevents CAD. In China, SLE patients from the onset to the use of HCQ is still relatively interval a long time, we think that should shorten the time. SLE patients may be more popular to promote the use of HCQ.
Strengths of this meta-analysis included its exhaustive search without language restrictions and validated systematic review methods following the PRISMA guidelines. Several limitations of this meta-analysis merit consideration. First, the characteristics of populations, and the adjusted confounding factor were not strictly described in some studies; however, these factors may result in heterogeneity and have a potential impact on our results. Second, since there were not enough RCTs for this meta-analysis, the conclusion remains questionable for all the SLE population.
5. Strength and limitations
5.1. Strength
This study exhaustive searched without language restrictions and validated systematic review methods following the PRISMA guidelines.
5.2. Limitations
-
(1)
The characteristics of populations and the adjusted confounding factor were not strictly described in some studies. However, these factors may result in heterogeneity and have a potential impact on our results.
-
(2)
There were not enough RCTs for this meta-analysis, the conclusion remains questionable for all the SLE population.
6. Conclusions
Our results suggest that CQ or HCQ has the effect on reducing the serum lipids in patients with SLE; however, these results should be interpreted with cautions due to the significant heterogeneity in the study and limited RCTs. Further RCTs are needed to confirm this conclusion.
Author contributions
Conceptualization: Chen-Yang Tao, Jin Shang.
Data curation: Chen-Yang Tao, Jin Shang, Tao Chen.
Formal analysis: Chen-Yang Tao, Jin Shang, Dahai Yu.
Funding acquisition: Jin Shang, Zhan-Zheng Zhao.
Investigation: Chen-Yang Tao, Jin Shang, Yu-Min Jiang
Methodology: Chen-Yang Tao, Jin Shang, Yu-Min Jiang
Project administration: Chen-Yang Tao, Jin Shang, Gen-Yang Cheng.
Resources: Chen-Yang Tao, Jin Shang, Zhan-Zheng Zhao.
Software: Chen-Yang Tao, Jin Shang, Tao Chen.
Supervision: Dong Liu, Gen-Yang Cheng, Jing Xiao.
Validation: Chen-Yang Tao, Jin Shang, Dong Liu.
Visualization: Chen-Yang Tao, Jin Shang, Jing Xiao.
Writing – Original Draft: Chen-Yang Tao, Jin Shang.
Writing – Review and Editing: Chen-Yang Tao, Jin Shang.
Supplementary Material
Footnotes
Abbreviations: CI = confidence interval, HDL-C = high-density lipoprotein cholesterol, LDL-C = low-density lipoprotein cholesterol, RCTs = randomised controlled trials, SLE = systemic lupus erythematosus, TC = cholesterol, TG = triglyceride, VLDL-C = very low-density lipoprotein cholesterol, WMD = weighted the mean difference.
C-YT and JS contributed equally to this work.
We retrieved all data for the meta-analyses from published material. Therefore, the data are available in the respective articles.
This work was supported by the National Natural Science Foundation of China (Grant Nos. 81570690, 81873611, and 81700633), Science and Technology Innovation Team of Henan (Grant No. 17IRTSTHN020); the Youth Foundation of the First Affiliated Hospital of Zhengzhou University to Jin Shang, Foundation and Frontier Technology Research Program of Henan Province (Grant No. 142300410211).
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Shaikh MF, Jordan N, D’Cruz DP. Systemic lupus erythematosus. Clin Med (London, England) 2017;17:78–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Manson JJ, Rahman A. Systemic lupus erythematosus. Orphanet J Rare Dis 2006;1:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Babary H, Liu X, Ayatollahi Y, et al. Favorable effects of hydroxychloroquine on serum low density lipid in patients with systemic lupus erythematosus: a systematic review and meta-analysis. Int J Rheum Dis 2018;21:84–92. [DOI] [PubMed] [Google Scholar]
- [4].Gordon C, Amissah-Arthur MB, Gayed M, et al. The British Society for Rheumatology guideline for the management of systemic lupus erythematosus in adults. Rheumatology (Oxford, England) 2018;57:e1–45. [DOI] [PubMed] [Google Scholar]
- [5].Tazi Mezalek Z, Harmouche H, Ammouri W, et al. Atherosclerosis in systemic lupus erythematosus. La Presse medicale 2014;43:1034–47. [DOI] [PubMed] [Google Scholar]
- [6].Doria A, Shoenfeld Y, Wu R, et al. Risk factors for subclinical atherosclerosis in a prospective cohort of patients with systemic lupus erythematosus. Ann Rheum Dis 2003;62:1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Abu-Shakra M, Urowitz MB, Gladman DD, et al. Mortality studies in systemic lupus erythematosus. Results from a single center. I. Causes of death. J Rheumatol 1995;22:1259–64. [PubMed] [Google Scholar]
- [8].Manzi S, Meilahn EN, Rairie JE, et al. Age-specific incidence rates of myocardial infarction and angina in women with systemic lupus erythematosus: comparison with the Framingham Study. Am J Epidemiol 1997;145:408–15. [DOI] [PubMed] [Google Scholar]
- [9].Petri M, Perez-Gutthann S, Spence D, et al. Risk factors for coronary artery disease in patients with systemic lupus erythematosus. Am J Med 1992;93:513–9. [DOI] [PubMed] [Google Scholar]
- [10].Wijaya LK, Kasjmir YI, Sukmana N, et al. The proportion of dyslipidemia in systemic lupus erythematosus patient and distribution of correlated factors. Acta Med Indones 2005;37:132–44. [PubMed] [Google Scholar]
- [11].Szabo MZ, Szodoray P, Kiss E. Dyslipidemia in systemic lupus erythematosus. Immunol Res 2017;65:543–50. [DOI] [PubMed] [Google Scholar]
- [12].Fesmire J, Reichlin M, Reichlin M. Effects of autoimmune antibodies anti-LPL, anti-LDL and anti-OXLDL on lipid metabolism and atherosclerosis in systemic lupus erythematosus. Revista Brasil Reumatol 2010;50:539–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Urowitz MB, Ibanez D, Gladman DD, et al. Adjusted framingham risk factor scoring for systemic lupus erythematosus. Arthritis & Rheumatism 2011;63:2262. [Google Scholar]
- [14].Tselios K, Koumaras C, Gladman DD, et al. Dyslipidemia in systemic lupus erythematosus: just another comorbidity? Semin Arthritis Rheum 2016;45:604–10. [DOI] [PubMed] [Google Scholar]
- [15].Lee SJ, Silverman E, Bargman JM. The role of antimalarial agents in the treatment of SLE and lupus nephritis. Nat Rev Nephrol 2011;7:718–29. [DOI] [PubMed] [Google Scholar]
- [16].Furst DE. Pharmacokinetics of hydroxychloroquine and chloroquine during treatment of rheumatic diseases. Lupus 1996;5Suppl 1:S11–15. [PubMed] [Google Scholar]
- [17].Rothfield N. Efficacy of antimalarials in systemic lupus erythematosus. Am J Med 85D 1988:53–6. [DOI] [PubMed] [Google Scholar]
- [18].Meinão IM, Sato EI, Andrade LE, et al. Controlled trial with chloroquine diphosphate in systemic lupus erythematosus. Lupus 1996;5:237–41. [DOI] [PubMed] [Google Scholar]
- [19].Canadian Hydroxychloroquine Study Group. A randomized study of the effect of withdrawing hydroxychloroquine sulfate in systemic lupus erythematosus. N Engl J Med 1991;324:150–4. [DOI] [PubMed] [Google Scholar]
- [20].Tsakonas E, Joseph L, Esdaile JM, et al. A long-term study of hydroxychloroquine withdrawal on exacerbations in systemic lupus erythematosus. The Canadian hydroxychloroquine study group. Lupus 1998;7:80–5. [DOI] [PubMed] [Google Scholar]
- [21].Wallace DJ, Metzger AL, Stecher VJ, et al. Cholesterol-lowering effect of hydroxychloroquine in patients with rheumatic disease: reversal of deleterious effects of steroids on lipids. The Am J Med 1990;89:322–6. [DOI] [PubMed] [Google Scholar]
- [22].Tam LS, Li EK, Lam CW, et al. Hydroxychloroquine has no significant effect on lipids and apolipoproteins in Chinese systemic lupus erythematosus patients with mild or inactive disease. Lupus 2000;9:413–6. [DOI] [PubMed] [Google Scholar]
- [23].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLOS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ (Clin Res ed) 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Zhang L, Hu P, Chen X, et al. Transarterial chemoembolization (TACE) plus sorafenib versus TACE for intermediate or advanced stage hepatocellular carcinoma: a meta-analysis. PloS one 2014;9:e100305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Wang B, Shao X, Wang D, et al. Vaccinations and risk of systemic lupus erythematosus and rheumatoid arthritis: a systematic review and meta-analysis. Autoimmun Rev 2017;16:756–65. [DOI] [PubMed] [Google Scholar]
- [27].DerSimonian R, Laird N. Meta-analysis in clinical trials. Controll Clin Trials 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- [28].Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. Br Med J 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Wu GC, Liu HR, Leng RX, et al. Subclinical atherosclerosis in patients with systemic lupus erythematosus: a systemic review and meta-analysis. Autoimmun Rev 2016;15:22–37. [DOI] [PubMed] [Google Scholar]
- [30].Kavanaugh A, Adams-Huet B, Jain R, et al. Hydroxychloroquine effects on lipoprotein profiles (the HELP trial): a double-blind, randomized, placebo-controlled, pilot study in patients with systemic lupus erythematosus. J Clin Rheumatol 1997;3:3–8. [PubMed] [Google Scholar]
- [31].Rahman P, Gladman DD, Urowitz MB, et al. The cholesterol lowering effect of antimalarial drugs is enhanced in patients with lupus taking corticosteroid drugs. J Rheumatol 1999;26:325–30. [PubMed] [Google Scholar]
- [32].Tam LS, Gladman DD, Hallett DC, et al. Effect of antimalarial agents on the fasting lipid profile in systemic lupus erythematosus. J Rheumatol 2000;27:2142–5. [PubMed] [Google Scholar]
- [33].Borba EF, Bonfa E. Longterm beneficial effect of chloroquine diphosphate on lipoprotein profile in lupus patients with and without steroid therapy. J Rheumatol 2001;28:780–5. [PubMed] [Google Scholar]
- [34].Sachet JC, Borba EF, Bonfa E, et al. Chloroquine increases low-density lipoprotein removal from plasma in systemic lupus patients. Lupus 2007;16:273–8. [DOI] [PubMed] [Google Scholar]
- [35].Meng J, Lu Y, Dong X, et al. Long-term effects of hydroxychloroquine on metabolism of serum lipids and left ventricular structure and function in patients of systemic lupus erythematosus. Nat Med J China 2014;94:965–8. [PubMed] [Google Scholar]
- [36].Ali Abdalla M, Mostafa El Desouky S, Sayed Ahmed A. Clinical significance of lipid profile in systemic lupus erythematosus patients: Relation to disease activity and therapeutic potential of drugs. Egypt Rheumatol 2017;39:93–8. [Google Scholar]
- [37].Tsang ASMW, Bultink IE. Systemic lupus erythematosus: review of synthetic drugs. Expert Opin Pharmacother 2015;16:2793–806. [DOI] [PubMed] [Google Scholar]
- [38].Golder V, Hoi A. Systemic lupus erythematosus: an update. Med J Aust 2017;206:215–20. [DOI] [PubMed] [Google Scholar]
- [39].Sewell RB, Barham SS, LaRusso NF. Effect of chloroquine on the form and function of hepatocyte lysosomes. Morphologic modifications and physiologic alterations related to the biliary excretion of lipids and proteins. Gastroenterology 1983;85:1146–53. [PubMed] [Google Scholar]
- [40].Chen HW, Leonard DA. Chloroquine inhibits cyclization of squalene oxide to lanosterol in mammalian cells. J Biol Chem 1984;259:8156–62. [PubMed] [Google Scholar]
- [41].Beynen AC. Could chloroquine be of value in the treatment of hypercholesterolemia? Artery 1986;13:340–51. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


