Supplemental Digital Content is available in the text
Keywords: acute lung injury, lung neoplasms, nonsmall cell lung cancer, postoperative pulmonary complication, pulmonary surgical procedures
Abstract
Acute lung injury following lung resection surgery is not rare and often related to mortality. Although it has been a significant clinical and economic impact associated with increased intensive care unit (ICU) utilization, length of hospital stay, and associated cost, it is unpredictable. Aims of this study were to identify the modifiable risk factors of postoperative acute lung injury (PALI) following lung cancer surgery.
We retrospectively analyzed medical records of 354 cases of lung cancer surgery in the tertiary university hospital from January 2012 to December 2015. PALI was defined as bilateral diffuse pulmonary infiltration on chest radiography, oxygenation failure (PaO2/FiO2 < 300), and absence of sign of left ventricular failure within a week from operation. We classified patients into either PALI group or non-PALI group and compared clinical characteristics of two groups. Logistic regression model was fitted to evaluate the risk factor of PALI.
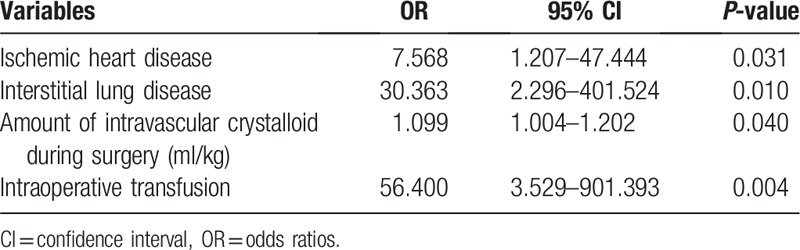
Among 354 cases of lung cancer surgeries, 287 lobectomies were analyzed. The overall incidence of PALI was 2.79% (8/287); four patients developed pneumonia with acute respiratory distress syndrome, and four patients developed ALI without clinical infection sign. There was no difference in baseline characteristics between PALI group and non-PALI group, but in operative parameters, a larger amount of fluid infusion was observed in PALI group. Logistic regression model showed underlying ischemic heart disease (OR 7.67, 95% CI 1.21–47.44, P = .03), interstitial lung disease (OR 30.36, 95% CI 2.30–401.52, P = .01), intravascular crystalloid fluid during surgery (OR 1.10, 95% CI 1.00–1.20, P = .04), and intraoperative transfusion (OR 56.4, 95% CI 3.53–901.39, P < .01) were risk factors of PALI. PALI increases ICU admission, use of mechanical ventilator, duration of hospital stay, and mortality.
The clinical impact of PALI is marked. Significant independent risk factors have been identified in underlying ischemic heart disease, interstitial lung disease, intravascular crystalloid fluid, and transfusion during surgery.
1. Introduction
Lung cancer is currently a leading cause of death worldwide, because patients with lung cancer present at a later stage and hardly have chance of curative surgery. Surgical resection is generally accepted as the most effective treatment for early stage of nonsmall cell lung cancer.[1] As advances in surgical technique and perioperative management, postoperative complications related to thoracic surgery have steadily decreased, and 30-day mortality of lobectomy for lung cancer is reported as about 1%.[2] Nevertheless, incidence of postoperative pulmonary complications following lung cancer surgery still is reported as 12% to 40%.[3–5] Among the postoperative complications, acute lung injury is one of the most serious pulmonary complications increasing needs for admission to intensive care unit (ICU) and mortality.[6]
Although postoperative acute lung injury (PALI) is directly associated with mortality and its incidence is not rare, there are few data regarding its risk factors and clinical outcome. A larger tidal volume and higher airway pressure during one-lung ventilation, excessive fluid infusion, preoperative alcohol abuse were reported as risk factors.[7,8] Otherwise, age, duration of the operation, and preoperative standard spirometry were not useful predictors to develop lung injury following thoracotomy.[9] However, these risk factors are still a matter of debate[10–12] because multiple factors are engaged complexly in development of lung injury and the exact mechanism has not been fully understood.[13]
Lung injury has been recognized as a potential complication of lung resection reported as postpneumonectomy pulmonary edema.[14] Clinical impact of PALI after pneumonectomy is serious because of limited reserved pulmonary capacity and life threatening.[10] PALI was accordingly investigated mainly in the view of postpneumonectomy syndrome. However, lung injury also appears after lobectomy even after sublobar resection in real practice and its clinical impact is important considering curative intention of the surgery. Moreover, incidence of lobectomy is much more than that of pneumonectomy, and lobectomy is the standard surgery of the nonsmall cell lung cancer.
Therefore, analysis of the data about PALI after lobectomy surgery is important. This study aims to assess the incidence and clinical impact of PALI following lobectomy for lung cancer and to identify potentially modifiable risk factors and preclude it.
2. Materials and methods
2.1. Patients and study design
We reviewed the medical records of patients aged 18 years or older, who had undergone lung cancer surgery between January 2012 and December 2015. The cases of metastatic lung cancer or combined surgery or the patients who had previous lung resection history were excluded. Patient data included baseline demographic characteristics, comorbidities, body mass index (BMI), smoking status, preoperative pulmonary function test, and American society of anesthesia score. Ischemic heart disease (IHD) was included, the patients who had been diagnosed coronary heart disease by doctor or through preoperative examinations and interstitial lung disease (ILD) was defined that thoracic special radiologist reports interstitial lung disease on preoperative chest computed tomography (CT) scan or already diagnosed and treated with ILD. Congestive heart failure (CHF) was defined as decreased ejection fraction in preoperative echocardiography or the patients who had been previously diagnosed and taken medications. Chronic obstructive pulmonary disease was defined as FEV1/FVC ratio was less than 0.7 in preoperative pulmonary function test.
2.2. Operation-related factors
During operation, all patients were cared using volume-type ventilator care. Ideal body weight was calculated by formula in the patients whose height were above 60 inches: Ideal body weight (men) = 50 kg + 2.3 kg (Height (inch) – 60), for women 45.5 kg + 2.3 kg (Height (inches) – 60).[15] For individuals less than 60 inches in height, actual body weight was used for tidal volume calculation. During anesthesia, mean tidal volume was calculated by the formula: mean tidal volume = ∑ (tidal volume) × (applied time) / (total anesthesia time).
Parameters related with operation were gathered including duration of anesthesia, extent of resection, mean tidal volume under anesthesia, peak inspiratory pressure, video-assisted thoracoscopic surgery or conventional thoracotomy, amount of intraoperative intravascular fluid (crystalloid and colloid), intraoperative transfusion, and pathologic stage.
2.3. Postoperative acute lung injury (PALI) and pneumonia
PALI was defined as the presence of: (1) severe oxygenation failure (PaO2/FiO2 < 300 mm Hg); (2) diffuse pulmonary infiltrates on chest radiography; and (3) the absence of signs of left heart failure within the first postoperative week.[13] Pneumonia was defined as newly infiltration or aggravation the lesion on chest radiograph or chest CT scan and combined fever or respiratory symptoms such as cough, sputum, and dyspnea. Pneumonia was accepted when microbiologic confirmation was not certified but infection was suspected.
2.4. Clinical outcomes after PALI
We compared clinical outcomes between PALI group and non-PALI group composed with duration of hospital stay, ICU admission, requirement of mechanical ventilator and mortality. Duration of hospital stay was the length of days from operation day to discharge or death. ICU admission was defined unexpected ICU admission after operation in case of unstable vital sign, application of mechanical ventilator care, etc. In case of mechanical ventilator care, we implemented lung protective ventilator strategies.
2.5. Statistical analyses
Descriptive statistics included frequencies and percentages for categorical variables and means and standard deviations (SDs). To compare continuous variables, a Mann–Whitney test was used. To analyze categorical variables, a χ2 analysis or Fisher's exact test was used. Multivariable logistic regression models were fitted to investigate risk factors of PALI. Adjusted odds ratios (OR) and their 95% confidence intervals (95% CI) were calculated. Data were analyzed using the SPSS (IBM SPSS Statistics 22, IBM SPSS Inc., Chicago, IL). All tests were 2-tailed, and a P-value less than .05 was considered to indicate statistical significance.
2.6. Ethic statement
The present study was approved by the Kyungpook National University Hospital Institutional Review Board (KNUH 2016-04-017), and patient data and information were confidential.
3. Results
3.1. Baseline characteristics
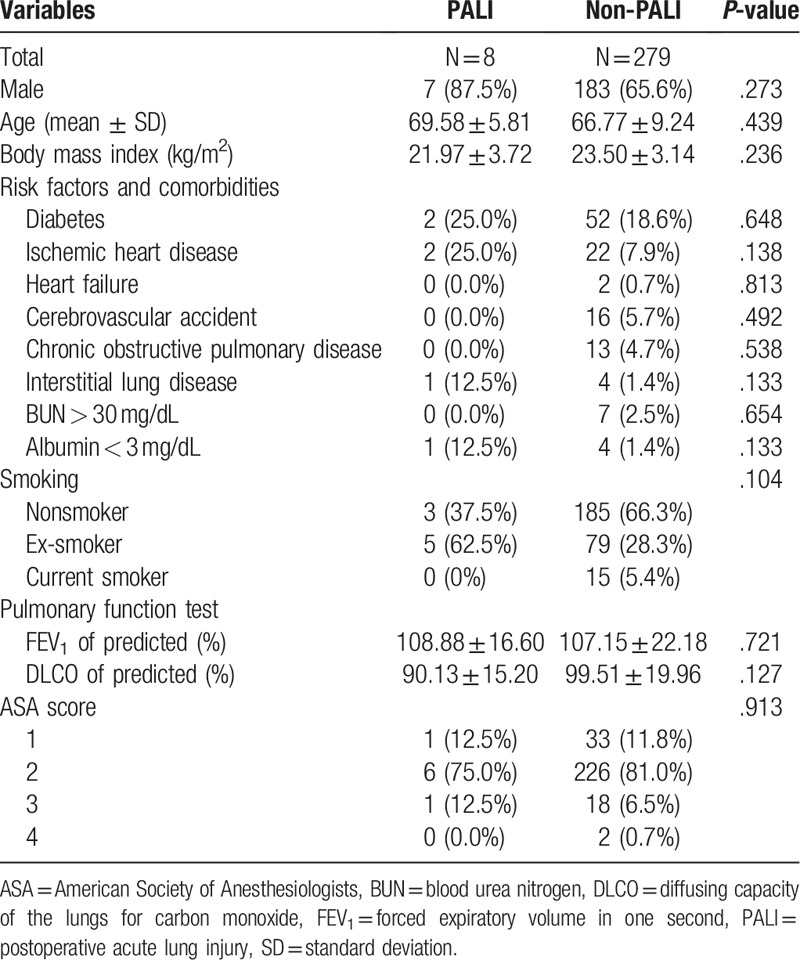
We screened consecutive 354 patients who underwent primary lung cancer surgery during study period. Fifteen patients were excluded because of previous lung resection history, three combined surgeries, three missing anesthesia records, and 11 pressure controlled ventilator care during anesthesia were excluded. Then, 13 sublobar resections, 15 bilobectomies, and 7 pneumonectomies were excluded. Finally, 287 data were included for analysis (Fig. 1). Among them, PALI was developed in eight patients, and the others had no evidence of ALI after lung resection. The incidence of PALI following lobectomy was 2.8% (8/287). Baseline characteristics of both groups are present at Table 1. The proportion of underlying disease was similar between PALI group and non-PALI group. Smoking status and pulmonary function were also similar.
Figure 1.
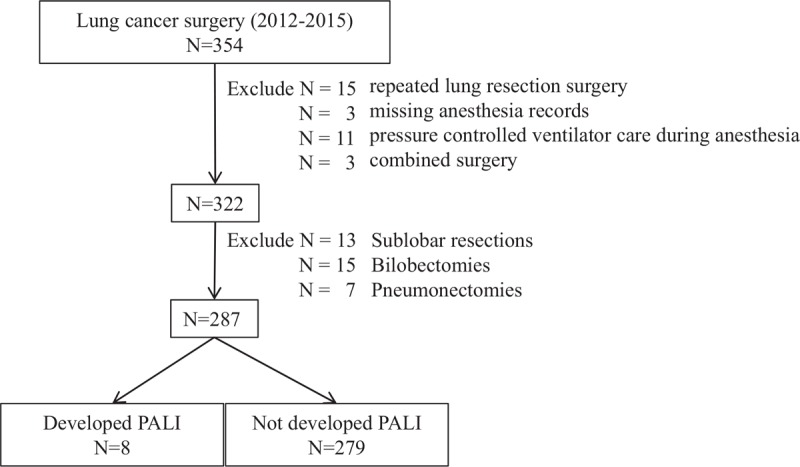
Flow diagram.
Table 1.
Baseline characteristics of enrolled patients.
3.2. Operation-related factors
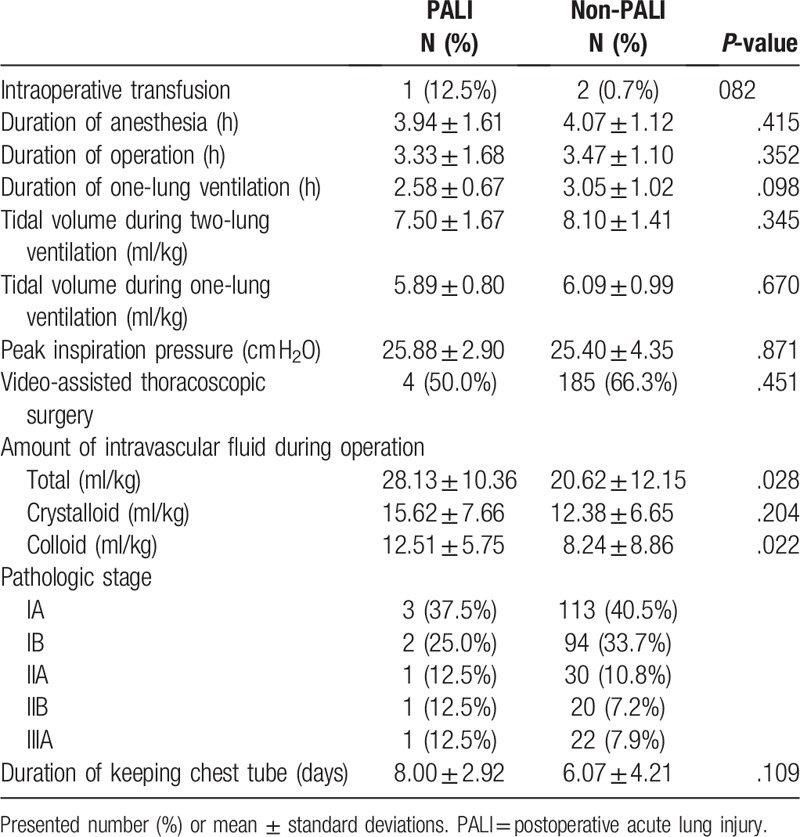
Duration of operation and anesthesia time were not different between PALI group and non-PALI group (Table 2). Tidal volume during one-lung ventilation and two-lung ventilation were similar. However, total amount of intravascular fluid during lung resection was larger in PALI group than in non-PALI groups (28.13 ± 10.36 ml/kg vs. 20.62 ± 12.15 ml/kg, P = .028). Both of crystalloid and colloid fluid volume were larger in PALI group, but colloid fluid volume was statistically significantly larger (12.51 ± 5.75 ml/kg vs. 8.24 ± 8.86 ml/kg, P = 0.022). The incidence of PALI was lower in VATS group than in conventional thoracotomy group (2.12% vs. 4.08%, P = 0.561).
Table 2.
Operation factors.
3.3. Risk factors of PALI following lung cancer surgery
Univariable binary logistic regression model showed that BMI, ischemic heart disease, interstitial lung disease, serum albumin below 3 mg/dL, DLCO (% of predicted), conventional thoracotomy, amount of intravascular fluid (total, crystalloid, colloid), intraoperative transfusion, and duration of keeping chest tube were candidates of risk factors of PALI (see Table, Supplemental Content, which illustrates the result of univariable binary logistic regression model). After stepwise elimination of multivariable logistic regression, ischemic heart disease (OR 7.57, 95% CI 1.21–47.44, P = .031), interstitial lung disease (OR 30.36, 95% CI 2.30–401.52, P = .011), amount of intravascular crystalloid fluid during surgery (OR 1.10, 95% CI 1.00–1.20, P = .040), and intraoperative transfusion (OR 56.40, 95% CI 3.53–901.39, P = .004) were risk factors of PALI (Table 3).
Table 3.
Logistic regression to evaluate risk factors.
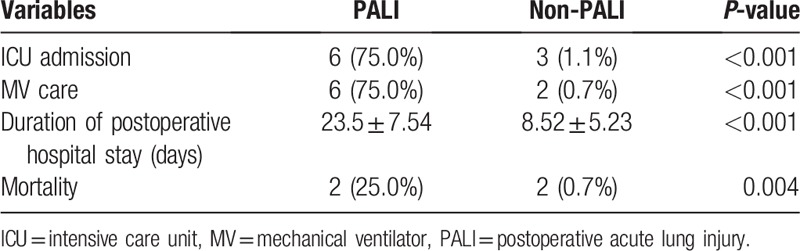
3.4. Clinical outcomes after PALI
Six of the eight (75.0%) in PALI group and three of the 287 (1.1%) in non-PALI group had admitted to the ICU (P < .01) (Table 4). All of the patients in PALI group who had admitted to the ICU required mechanical ventilator care support. The causes of ICU admission of the three patients in non-PALI group were acute stroke, postoperative acute kidney injury acquiring continuous renal replacement therapy, and multiorgan failure. In non-PALI group, two of three patients admitted to the ICU were related to mortality. Duration of the hospital day was longer in PALI group than in non-PALI group (23.5 ± 7.54 days vs. 8.52 ± 5.23 days, P < .01). Mortality was statistically increased in PALI group than in non-PALI groups (25.0% vs. 0.7%, P < .01). In the cases of mortality, death has occurred on postoperative days 24 and 35 in PALI group and on days 33 and 57 in non-PALI group.
Table 4.
Clinical outcomes following PALI.
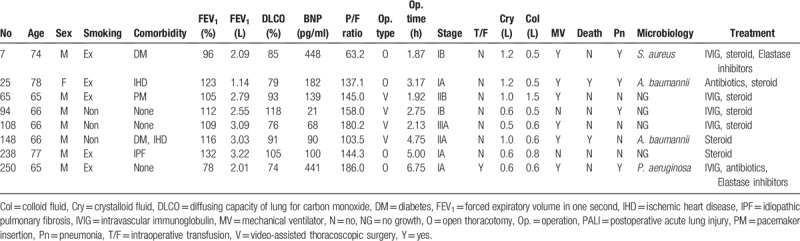
In PALI group, there was no microbiologic confirmation of lung infection, and most patients were treated according to the guideline of acute respiratory distress syndrome (ARDS) using antibiotics and steroids. Clinical characteristics of the patients who had PALI in this study are presented in Table 5.
Table 5.
Clinical characteristics of the patients developed PALI.
4. Discussion
This retrospective observation study showed that underlying ischemic heart disease, interstitial lung disease, amount of intraoperative crystalloid fluid infusion, and intraoperative transfusion were statistical significantly associated with development of PALI following lobectomy for lung cancer. Clinical impact of PALI was distinctly increasing of ICU utilization, duration of hospitalization, and mortality.
The incidence of acute lung injury after pulmonary resection was reported wide range from 3.9% to 12%.[7,9,16,17] Compared to other studies, the incidence of PALI in present study (2.8%) was relatively low. It might be the reason that our study included elective cancer surgeries except in the cases of predisposing infectious condition such as empyema or lung abscess and excluded pneumonectomies.
Ischemic heart disease was one of the predisposing factors of PALI in this study. Newly developed bilateral pulmonary infiltration on chest radiograph should be distinguished from hydrostatic pulmonary edema to diagnose acute lung injury, especially in the patients who had systolic dysfunction or renal problem. In this study, we excluded hydrostatic pulmonary edema using echocardiography or serum BNP level and got advice from pulmonary specialists. The prevalence of concomitant ischemic heart disease in patients who had operable lung cancer was 8.4% (24/287) in this study and the burden of both diseases would be increasing.[18] Therefore, these results have clinical impact, and further researches about its pathogenesis and mechanisms are inquired.
In this study, baseline DLCO was not different between PALI and non-PALI groups; however, interstitial lung disease was a risk factor of PALI. Based on this finding, it is necessary for radiologists to report the parenchymal changes other than lung cancer lesion in preoperative chest CT scan. In addition, the clinicians should monitor the patients carefully who had interstitial lung disease pattern without decline DLCO.
Excessive fluid in the perioperative period might precipitate PALI has been suggested previously,[11,16] but not all studies.[10] Restrictive fluid therapy in lung resection surgery raised concerns over hypovolemia, hypoperfusion, and the risk of postoperative acute kidney injury. According to recent investigations, it is useful to use extravascular lung water index or fluid responsiveness monitoring for guiding proper fluid management and better pulmonary outcome.[19,20] Intraoperative fluid management is likely to affect pulmonary outcome of the thoracic surgery; however, the debate over the type of fluid and infusion rate have not been settled.[21] Although this study presented a larger amount of crystalloid fluid infusion was a risk factor of PALI and several studies already showed colloid infusions appeared to have a modest benefit on pulmonary mechanics, but no overall survival benefit. Well-designed randomized control trials are necessary to solve this problem.
Intraoperative transfusion was one of the risk factors of the PALI in this study. Amount of transfusion in three people was less than 300 ml in each patient, and the amount of intraoperative fluid was not higher than average. Transfusion-related lung injury occurs within 6 h, usually within 1 or 2 h,[22] and onset time could make it different in this study. With regard to immune response, intraoperative transfusion could be a trigger factor of postoperative lung injury. While increased pulmonary microvascular permeability with increased protein in the edema fluid is common in transfusion-related lung injury and PALI, onset time of lung injury make it possible to distinguish between two different clinical syndromes. In this study, bilateral pulmonary infiltration had appeared in three days after operation, and we could exclude transfusion-related acute lung injury.
Previously named postpneumonectomy syndrome or noncardiogenic pulmonary edema, PALI shares similar clinical and radiological characteristics with ARDS.[9,23] General management strategy for ARDS including lung protective mechanical ventilation strategy based on low tidal volume and plateau pressure less than 30 cm H2O, proper antibiotics for infection control and empirical high-dose steroid treatment was applied to the patients with PALI in this study.[24] Some patients showed clinical improvement, however, the others did not. Both are difficult to exclude pulmonary infection and to confirm microbiologic infection in situation of PALI; therefore, proper empirical antibiotics treatment is important. In the present study, A. baumannii and methicillin-resistant S. aureus were cultured in the sputum of patients with PALI; however, it was regarded as colonizer.
The pathogenesis of PALI is not fully elucidated, and it might be multifactorial.[25] Clinical data and animal experimentation showed tidal volume and airway pressure contributed to the lung injury.[26] It is explained by reperfusion injury after surgery.[6] In our study, the diagnosis of PALI was dependent on clinical manifestations, not applied bronchoscopic alveolar lavage or biopsy to exclude other disease or characterize the pathology.
This study has several strengths and limitations. First, this retrospective observation study included only lobectomy surgeries of nonsmall cell lung cancer patients. Previous study reported that the wider extensive resections, the more frequent incidence of PALI.[12,17] Matching the extent of the resection and limitation of the disease as nonsmall cell lung cancer could make the comparison other clinical factors in relatively homogenous baseline. Second, operators and anesthesiologists participated in lung cancer surgery did not change during study period, and PALI was confirmed by experienced pulmonologist based on objective clinical evidence such as echocardiography, serum BNP level, and chest CT scan. That is, operation and anesthesia factor were relatively under controlled condition. Otherwise, the results from small study population participated in one center could not be universalized. While alcohol abuse was cited as preoperative risk factor of acute lung injury after thoracic surgery,[8,27] the variable could not help excluding due to lack of the data. We used the variable of total infusion volume during the operation divided by body weight in this study. However, it could be more relevant to use the variables that reflect the patients’ volume status such as stroke volume variation or pulse pressure variations.[20] In addition, we excluded the patients supported pressure controlled ventilator mode during anesthesia, and we did not set the ventilator during operation equally. Previously mentioned, it is a limitation of this study that bronchoscoalveolar lavage or lung biopsy was not achieved for diagnostic confirmation.
Clinical impact of PALI was significant in terms of increased ICU utilization, duration of hospitalization, and mortality. ALI following lung cancer surgery is associated with a high mortality. In this study, the mortality of PALI groups was 33.3%.
In conclusion, close observation is necessary for the patients with ischemic heart disease or interstitial lung disease to recognize development of acute lung injury after lung cancer surgery and optimize the intraoperative fluid and transfusion.
Author contributions
Conceptualization: Hyun Jung Kim.
Data curation: Gun-Jik Kim.
Formal analysis: Hyun Jung Kim.
Investigation: Hyun Jung Kim, Seung Ick Cha, Chang-Ho Kim, Jaehee Lee, Joon Yong Cho, Youngok Lee, Deok Heon Lee.
Methodology: Gun-Jik Kim.
Resources: Gun-Jik Kim, Deok Heon Lee.
Writing - Original Draft: Hyun Jung Kim.
Writing - Review & Editing: Hyun Jung Kim, Gun-Jik Kim, Deok Heon Lee.
Deok Heon Lee orcid: 0000-0001-6206-4745.
Supplementary Material
Footnotes
Abbreviations: ALI = acute lung injury, ARDS = acute respiratory distress syndrome, BMI = body mass index, CHF = congestive heart failure, CT = computed tomography, DLCO = diffusing capacity for carbon monoxide, FEV1 = forced expiratory volume in one second, FVC = forced vital capacity, IHD = ischemic heart disease, ILD = interstitial lung disease, PALI = postoperative acute lung injury.
Conflicts of interest: The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
The authors of this work have nothing to disclose.
References
- [1].Ettinger DS, Akerley W, Borghaei H, et al. Non-small cell lung cancer, version 2.2013. J Natl Compr Cancer Netw 2013;11:645–53. quiz 653. [DOI] [PubMed] [Google Scholar]
- [2].Laursen LØ, Petersen RH, Hansen HJ, et al. Video-assisted thoracoscopic surgery lobectomy for lung cancer is associated with a lower 30-day morbidity compared with lobectomy by thoracotomy. Eur J Cardiothorac Surg 2016;49:870–5. [DOI] [PubMed] [Google Scholar]
- [3].Lugg ST, Agostini PJ, Tikka T, et al. Long-term impact of developing a postoperative pulmonary complication after lung surgery. Thorax 2016;71:171–6. [DOI] [PubMed] [Google Scholar]
- [4].Pagni S, McKelvey A, Riordan C, et al. Pulmonary resection for malignancy in the elderly: is age still a risk factor? Eur J Cardiothorac Surg 1998;14:40–5. [DOI] [PubMed] [Google Scholar]
- [5].Myrdal G, Gustafsson G, Lambe M, et al. Outcome after lung cancer surgery. Factors predicting early mortality and major morbidity. Eur J Cardiothorac Surg 2001;20:694–9. [DOI] [PubMed] [Google Scholar]
- [6].Williams E, Quinlan G, Goldstraw P, et al. Postoperative lung injury and oxidative damage in patients undergoing pulmonary resection. Eur Respir J 1998;11:1028–34. [DOI] [PubMed] [Google Scholar]
- [7].Jeon K, Yoon JW, Suh GY, et al. Risk factors for post-pneumonectomy acute lung injury/acute respiratory distress syndrome in primary lung cancer patients. Anaesth Intensive Care 2009;37:14–9. [DOI] [PubMed] [Google Scholar]
- [8].Licker M, De Perrot M, Spiliopoulos A, et al. Risk factors for acute lung injury after thoracic surgery for lung cancer. Anesth Analg 2003;97:1558–65. [DOI] [PubMed] [Google Scholar]
- [9].Hayes JP, Williams EA, Goldstraw P, et al. Lung injury in patients following thoracotomy. Thorax 1995;50:990–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Turnage WS, Lunn JJ. Postpneumonectomy pulmonary edema. A retrospective analysis of associated variables. Chest 1993;103:1646–50. [DOI] [PubMed] [Google Scholar]
- [11].Slinger P. Fluid management during pulmonary resection surgery. Ann Card Anaesth 2002;5:220–4. [PubMed] [Google Scholar]
- [12].Licker M, Diaper J, Villiger Y, et al. Impact of intraoperative lung-protective interventions in patients undergoing lung cancer surgery. Crit Care 2009;13:R41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Eichenbaum KD, Neustein SM. Acute lung injury after thoracic surgery. J Cardiothorac Vasc Anesth 2010;24:681–90. [DOI] [PubMed] [Google Scholar]
- [14].Zeldin RA, Normandin D, Landtwing D, et al. Postpneumonectomy pulmonary edema. J Thorac Cardiovasc Surg 1984;87:359–65. [PubMed] [Google Scholar]
- [15].Pai MP, Paloucek FP. The origin of the “ideal” body weight equations. Ann Pharmacother 2000;34:1066–9. [DOI] [PubMed] [Google Scholar]
- [16].Parquin F, Marchal M, Mehiri S, et al. Post-pneumonectomy pulmonary edema: analysis and risk factors. Eur J Cardiothorac Surg 1996;10:929–32. discussion 933. [DOI] [PubMed] [Google Scholar]
- [17].Kutlu CA, Williams EA, Evans TW, et al. Acute lung injury and acute respiratory distress syndrome after pulmonary resection. Ann Thorac Surg 2000;69:376–80. [DOI] [PubMed] [Google Scholar]
- [18].Maki M, Tsubochi H, Endo T, et al. [Management of patients with ischemic heart disease in lung cancer resection]. Kyobu Geka/Jpn J Thorac Surg 2015;68:271–7. [PubMed] [Google Scholar]
- [19].Chau EH, Slinger P. Perioperative fluid management for pulmonary resection surgery and esophagectomy. Semin Cardiothorac Vasc Anesth 2014;18:36–44.23719773 [Google Scholar]
- [20].Benes J, Chytra I, Altmann P, et al. Intraoperative fluid optimization using stroke volume variation in high risk surgical patients: results of prospective randomized study. Crit Care 2010;14:R118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].van der Heijden M, Verheij J, van Nieuw Amerongen GP, et al. Crystalloid or colloid fluid loading and pulmonary permeability, edema, and injury in septic and nonseptic critically ill patients with hypovolemia. Crit Care Med 2009;37:1275–81. [DOI] [PubMed] [Google Scholar]
- [22].Toy P, Popovsky MA, Abraham E, et al. Transfusion-related acute lung injury: definition and review. Crit Care Med 2005;33:721–6. [DOI] [PubMed] [Google Scholar]
- [23].Force ADT, Ranieri VM, Rubenfeld GD, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA 2012;307:2526–33. [DOI] [PubMed] [Google Scholar]
- [24].Bein T, Grasso S, Moerer O, et al. The standard of care of patients with ARDS: ventilatory settings and rescue therapies for refractory hypoxemia. Intensive Care Med 2016;42:699–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Jordan S, Mitchell JA, Quinlan GJ, et al. The pathogenesis of lung injury following pulmonary resection. Eur Respir J 2000;15:790–9. [DOI] [PubMed] [Google Scholar]
- [26].Slinger P. Pro: low tidal volume is indicated during one-lung ventilation. Anesth Analg 2006;103:268–70. [DOI] [PubMed] [Google Scholar]
- [27].Fernandez-Perez ER, Sprung J, Afessa B, et al. Intraoperative ventilator settings and acute lung injury after elective surgery: a nested case control study. Thorax 2009;64:121–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


