Abstract
Telomeres are transcribed into long, noncoding telomeric repeat-containing RNAs (TERRA) that have been implicated in the regulation of telomerase, the enzyme that lengthens telomeres, in heterochromatin formation at telomeres, and in telomere stability. This study aimed to evaluate the correlation between TERRA expression and long-term oncologic outcomes in colorectal cancer (CRC).
We evaluated 18p TERRA expression and telomere length using quantitative real-time PCR in 60 patients who underwent surgical resection for CRC between June 2008 and November 2010.
Patients were grouped according to 18p TERRA expression, with 29 (48.3%) and 31 (51.7%) patients in the low and high TERRA expression groups, respectively. The median follow-up period was 80 months (range 2–103). The 18p TERRA expression was marginally significantly associated with preoperative carcinoembryonic antigen (CEA; P = .082) and was significantly associated with telomere length (P < .05). Multivariate analysis revealed that preoperative CEA (hazard ratio [HR], 2.728; 95% confidence interval [CI], 0.832–8.944, P = .098) and 18p TERRA expression (HR, 0.113; 95% CI, 0.011–1.126, P = .071) were marginally significant independent prognostic factors for overall survival (OS), whereas preoperative CEA (HR, 4.254; 95% CI, 1.394–12.985, P = .011) and 18p TERRA expression (HR, 0.108; 95% CI, 0.011–1.037, P = .054) were significant independent prognostic factors for disease-free survival (DFS). According to our prognostic model with 2 prognostic factors, the OS and DFS rate increased to 76.2% and 80.63%, respectively, in patients with high 18p TERRA expression and CEA levels ≤5 (P = .178, P = .057, respectively).
18p TERRA expression was marginally significantly associated with preoperative CEA and significantly associated with telomere length, rendering it a potential prognostic factor for long-term oncologic outcomes in CRC.
Keywords: colonic neoplasms, long noncoding, prognosis, RNA, telomere, telomere homeostasis
1. Introduction
Colorectal cancer (CRC) is one of the most common types of cancer in Korea and other developed countries.[1,2] The carcinogenesis of CRC is driven by genetic and epigenetic changes in tumor cells and is also influenced by tumor–host interactions.[3–5] Previous studies have reported the potential association of molecular markers such as BRAF, KRAS, and mismatch repair with prognosis in patients with CRC.[6,7] Identification of predictors of poor prognosis and disease recurrence is important for the successful treatment of these patients and for drug discovery.
Telomeres are the ends of linear chromosomes that serve as a protective cap to avoid permanent proliferation arrest and are essential for maintaining genome stability, preventing chromosome ends from being recognized as double-strand breaks, and assuring proper replication of chromosomes.[8] Telomeres are transcribed into long, noncoding telomeric repeat-containing RNAs (TERRA), which have been implicated in the regulation of telomerase, the enzyme that lengthens telomeres, in the formation of heterochromatin at telomeres, and in telomere stability.[9]
Previous studies on TERRA expression have demonstrated its downregulation in advanced stages of human tumors of the larynx, colon, and lymph node compared with normal tissues, suggesting that telomeric transcription is downregulated in advanced tumors. However, the prognostic role of TERRA expression in CRC is unclear. This study aimed to evaluate the correlation between TERRA expression and long-term oncologic outcomes in CRC.
2. Methods
2.1. Patients
From our prospectively collected database, we evaluated the expression of 2p and 18p TERRA along with telomere length using quantitative real-time PCR in 60 patients who underwent surgical resection for CRC between June 2008 and November 2010. Tumor specimens and corresponding nonmalignant colorectal tissue were immediately frozen in liquid nitrogen and stored at −80°C until DNA and RNA isolation. Tissue samples were provided from Keimyung Human Bio-resource Bank, Korea. All patients were explained the study purpose, and informed consent was obtained from each study participant. The protocols were approved by the Institutional Review Board of Keimyung University Dongsan Medical Center (approval #12–41). Clinicopathologic data including age, preoperative carcinoembryonic antigen (CEA), pathologic tumor and nodal stage, tumor differentiation, lymphovascular invasion, perineural invasion, and survival data were obtained. An experienced pathologist reviewed all cases and its clinicopathological characteristics were presented (Fig. 1).
Figure 1.
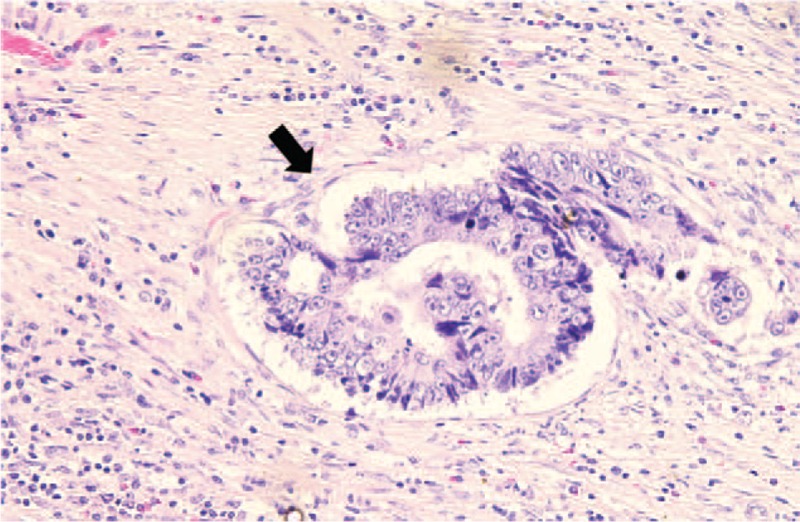
Representative histological finding of lymphovascular invasion (arrow) in colorectal cancers (Hematoxylin-Eosin stain, ×400). Tumor embolus was observed within an endothelial cell-lined space.
2.2. Preoperative staging and follow-up
Preoperative staging evaluation was performed using chest X-ray and abdominal and chest computed tomography (CT) scan. Postoperative chemotherapy (5-FU/leucovorin regimen or FOLFOX) was administered at 3 to 4 weeks after surgery for 6 months. All patients underwent postoperative clinical examinations, measurement of serum CEA levels, chest X-rays every 3 months, and a chest/abdominal CT every 6 months over a period of 3 years. After 3 years, the follow-up interval was changed to 6 months.
2.3. DNA isolation and telomere length analysis
Genomic DNA was isolated from samples using the QIAamp DNA mini kit (Qiagen, Inc., Valencia, CA). Telomere length was analyzed using quantitative PCR (qPCR). For the quantitative determination of telomere length relative to nuclear DNA (nDNA), specific primers for telomere (T) and nDNA-encoded β-globin (S) were selected, according to a previous study.[10] qPCR was carried out using the specific primers and SYBR GREEN Premix (Toyobo, Japan) on a LightCycler 480 II system (Roche Diagnostics, Basel, Switzerland). The relative telomere length was determined by calculating T/S values using the following formula: T/S = 2−ΔCq, where ΔCq = mean CqT − mean CqS. β-globin was used as a housekeeping gene for normalization, and a nontemplate sample was used as a negative control. Each measurement was repeated in triplicate and 5 serially diluted control samples were included in each experiment.
2.4. RNA isolation and TERRA expression analysis
Total cellular RNA was extracted from tissues using the TRIzol reagent (Molecular Research Center Inc., Cincinnati, OH). RNA was quantified using Nanodrop 1000 (Thermo Scientific, Wilmington, Denmark). Each cDNA sample was synthesized from 2 μg of total RNA using M-MLV reverse transcriptase (Promega, Madison, WI) according to the manufacturer's protocol. Transcript levels of 2p and 18p TERRA were measured by qPCR as described previously.[11] The primers used for 2p TERRA are as follows: F: 5′-TAAGCCGAAGCCTAACTCGTGTC-3′ and R: 5′-GTAAAGGCGAAGCAGCATTCTCC-3′; and those for 18p-TERRA are as follows: F: 5′-CCTAACCCTCACCCTTCTAAC-3′ and R: 5′-TACCTCGCTTTGGGACAAC-3′. Relative quantities were determined in relation to the levels of 36B4 (reference single copy gene) and further analysis was performed as described above. Previous study about telomere status routinely uses 36B4, which encodes the acidic ribosomal phosphoprotein P0.
2.5. Evaluation parameters and statistical analyses
The classification system of the American Joint Committee on Cancer 7th edition was used to determine the pathological tumor depth, the number of metastasized lymph nodes, and cancer stage. Recurrence was defined as the presence of a histologically and/or radiologically confirmed tumor. Overall survival (OS) time was calculated from the date of surgery to the date of the latest follow-up visit or the date of death due to any cause, and disease-free survival (DFS) time was defined as the time from surgery to any type of recurrence. Patients who died from other causes or were alive without progression or recurrence at the most recent follow-up were treated as censored in the analysis of DFS time. The 5-year survival rate was determined by the Kaplan–Meier method, and the log-rank test was used to compare survival rates among subgroups. The log-rank test was used for univariate analysis and all variables were found to be associated with survival. Independent prognostic factors were identified by multivariate analysis using the Cox proportional hazards model to calculate hazard ratios (HRs). The results of the Cox model analysis are reported using HR and 95% confidence intervals (CI). All statistical tests were performed using the Statistical Package for Social Sciences software (version 21.0, SPSS, Chicago, IL). Chi-squared test, the Mann–Whitney U test, and simple correlation analysis were used to analyze the associations between the variables. Data are expressed as means with standard deviation. P values <.05 were defined to indicate a statistically significant difference.
3. Results
3.1. Patient and tumor characteristics
Both 2p and 18p TERRA expression were successfully analyzed in all 60 cases of CRC. Both 2p and 18p TERRA expression in the CRC and paired noncancerous tissues were similar (P = .34). The average 2p and 18p TERRA expression in the CRC tissue was 1.33 ± 0.90 and 1.77 ± 0.92, respectively, which contrasted with its expression in normal tissues. To further explore the correlation between TERRA expression and the clinicopathological parameters of CRC, patients were categorized into 2 subgroups according to the average values of the tumor/nontumor ratio. Expression of 18p TERRA showed more significance in clinicopathological characteristics of CRC than 2p TERRA. Therefore, we focused on 18p TERRA expression in CRCs. 18p TERRA expression was categorized as low in 29 (48.3%) patients and high in 31 (51.7%) patients. The patient and tumor characteristics of the study cohort stratified by 18p TERRA status are shown in Table 1. The demographic characteristics between the 2 groups were similar for age, preoperative CEA levels, tumor stage, nodal stage, histology, lymphovascular invasion, and perinodal extension, but high 18p TERRA expression was significantly associated with high expression of 2p TERRA (P < .05) and long telomere length (P < .05), and was marginally significantly associated with low preoperative CEA levels (P = .082). A quantitative correlation analysis also showed that CEA level was negatively associated with 2p TERRA expression (r = −0.326, P = .011) and 18p TERRA expression (r = −0.277, P = .032) (Fig. 2).
Table 1.
Characteristics of patients with colorectal cancer according to 18p TERRA expression.
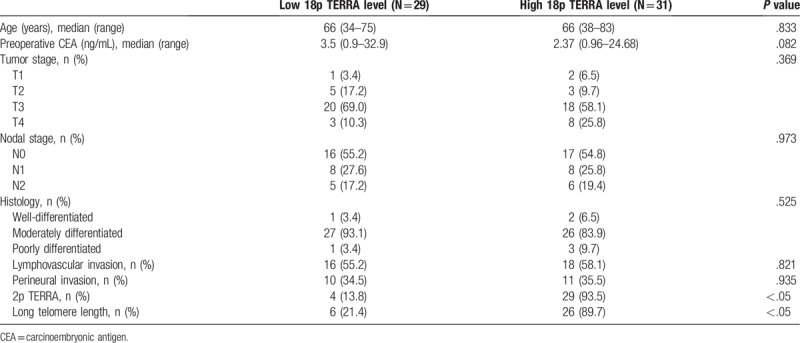
Figure 2.
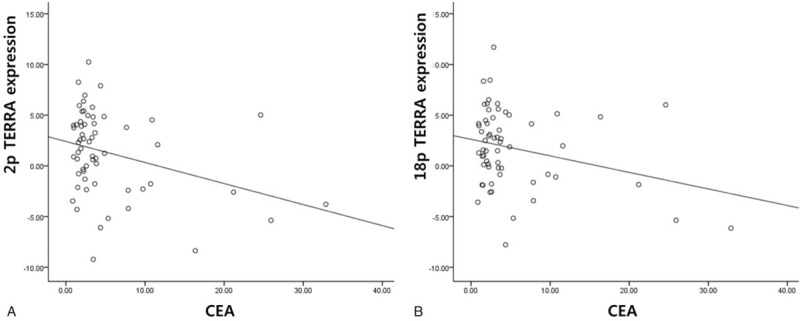
Correlation between TERRA expression and CEA level in colorectal cancers (A) 2p TERRA and CEA level (B) 18p TERRA and CEA level. CEA = preoperative carcinoembryonic antigen, TERRA = telomeric repeat-containing RNAs.
3.2. Oncologic outcomes according to TERRA and telomere length
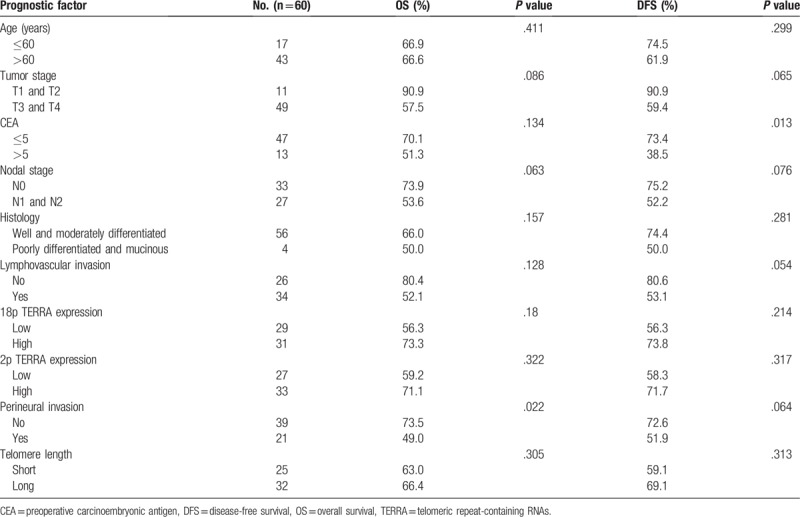
The median follow-up period was 80 months (range 2–103). The OS and DFS rates were not significantly different between the high and low 18p TERRA expression groups (OS: 73.8% vs. 56.3%, P = .180; DFS: 73.8% vs. 56.3%, P = .214) (Fig. 3A and B). The OS and DFS rates were not significantly different between the high and low 2p TERRA expression groups (OS: 71.1% vs. 59.2%, P = .322; DFS: 71.7% vs. 58.3%, P = .317). The OS and DFS rates were not significantly different between the long and short telomere length groups (OS: 66.4% vs. 63.0%, P = .305; DFS: 69.1% vs. 59.1%, P = .313) (Table 2). CEA level showed a prognostic value for not OS (70.1% vs. 51.3%, P = .134) but DFS (73.4% vs. 38.5%, P = .013) (Fig. 3C and D).
Figure 3.
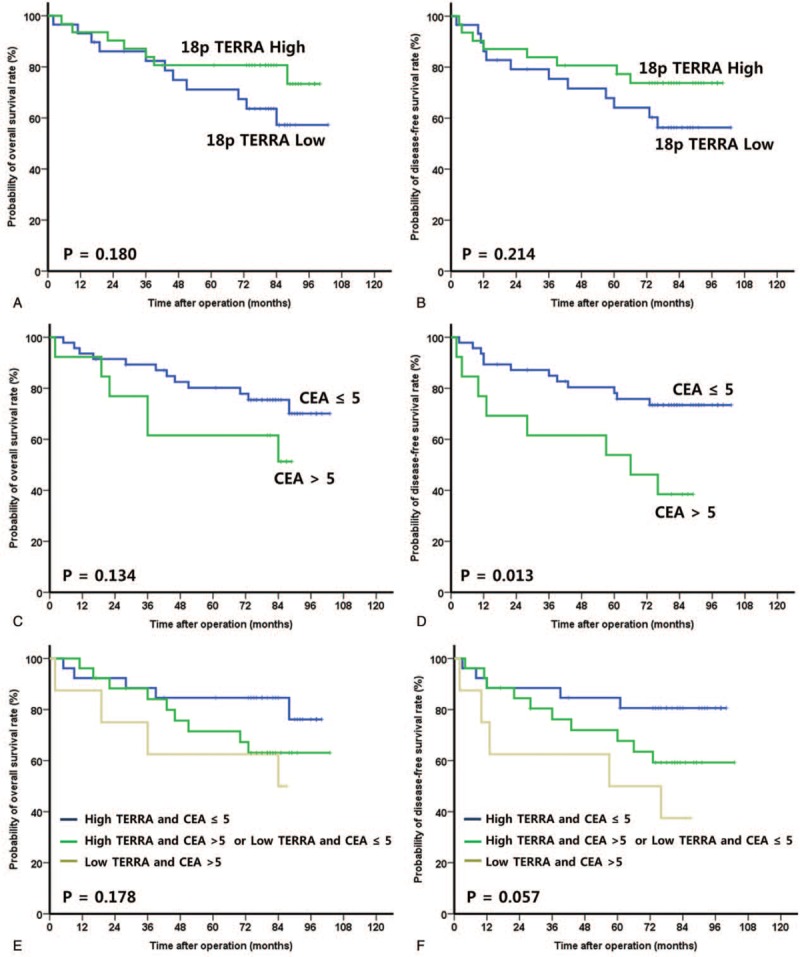
Survival analysis in colorectal cancers. (A) Overall survival according to 18p TERRA expression. (B) Disease-free survival according to 18p TERRA expression. (C) Overall survival according to CEA. (D) Disease-free survival according to CEA. (E) Overall survival according to 18p TERRA expression and CEA. (F) Disease-free survival according to 18p TERRA expression and CEA. CEA = preoperative carcinoembryonic antigen, TERRA = telomeric repeat-containing RNAs.
Table 2.
Prognostic factors of survival in univariate analysis.
3.3. Univariate and multivariate survival analyses of prognostic factors
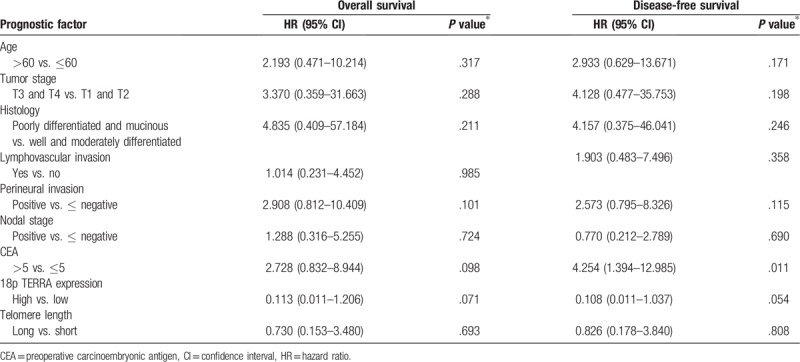
Univariate analyses revealed that perineural invasion was significantly associated with OS and preoperative CEA was significantly associated with DFS (Table 2). Multivariate analysis showed that preoperative CEA (HR, 2.728; 95% CI, 0.832–8.944, P = .098) and 18p TERRA expression (HR, 0.113; 95% CI, 0.011–1.126, P = .071) were tentatively significant independent prognostic factors for OS and preoperative CEA (HR, 4.254; 95% CI, 1.394–12.985, P = .011) and that 18p TERRA expression (HR, 0.108; 95% CI, 0.011–1.037, P = .054) was a significant independent prognostic factor for DFS (Table 3).
Table 3.
Prognostic factors of survival in multivariate analysis.
3.4. Survival analyses according to the prognostic model
According to our prognostic model with 2 prognostic factors, the OS and DFS rate increased to 76.2% and 80.6%, respectively, in patients with high 18p TERRA expression and a CEA level ≤5 (P = .178, P = .057, respectively) (Fig. 3E and F). The 5-year OS and DFS rates of patients with low 18p TERRA expression and CEA level ≤5 or high 18p TERRA expression and CEA level >5 were 63.1% and 59.3%, respectively. The 5-year OS and DFS rates of patients with low 18p TERRA expression and CEA levels >5 were 50.0% and 37.5%, respectively.
4. Discussion
The current study highlights a correlation between TERRA expression and long-term oncologic outcomes in CRC suggesting that TERRA levels may be proposed as a novel and potential molecular prognostic marker for CRC. We found that high tumor TERRA expression was independently associated with preoperative CEA and telomere length in human colorectal carcinoma tissue, and these molecular features have been associated with long-term oncologic outcomes in CRC after controlling for potential confounders, including pathologic outcomes.
Previous studies support the hypothesis that TERRA plays an essential role in telomere maintenance and that deregulation of TERRA synthesis causes genomic instability and telomeric defects.[12–16] A study using astrocytoma cell lines and human samples demonstrated that 2p TERRA was downregulated in anaplastic astrocytoma, whereas 18p TERRA showed no correlation with tumor grade. Pfeiffer et al[17] suggested that TERRA expression was significantly downregulated in advanced stages of different types of human cancer including colon cancer compared to normal tissues, suggesting that downregulation of these RNAs is associated with undifferentiated cell stages. In our study, 18p TERRA expression seemed to be a tentatively significant independent prognostic factor for long-term oncologic outcomes, even though 18p and 2p TERRA showed no correlation with tumor grade.
Telomere length decreases with age, and some studies have demonstrated that TERRA is a critical regulatory factor that controls telomeric length.[17–19] Pfeiffer et al[17] demonstrated that TERRA can promote telomere shortening by inhibiting telomerase activity, promoting Exo1-dependent resection, and that TERRA plays a negative role in telomere length maintenance by interacting with the Exo1-inhibiting Ku70/80 complex. Sampl et al[11] report that TERRA levels are downregulated following tumor grades and that TERRA expression is related to telomerase activity and telomere length in human astrocytoma cell lines. In our study, high 18p TERRA expression was associated with long telomere length (P < .05). Our present data are also consistent with previous reports and further studies of the mechanisms by which TERRA regulates telomere length may provide new molecular targets for the diagnosis and therapy of telomere associated diseases.[20]
Preoperative serum levels of the tumor marker CEA, which represents serum tumor burden, are of prognostic significance. CEA levels ≥5.0 ng/mL have an adverse effect on survival independent of tumor stage. In the current study, 18p TERRA expression was marginally significantly associated with preoperative CEA (P = .082) and elevated preoperative CEA level, and low 18p TERRA expression was identified to be an independent risk predictor for DFS. Additionally, our prognostic model with 2 prognostic factors including preoperative CEA and 18p TERRA expression showed that TERRA expression could be used as a possible prognostic marker in CRC patients. Therefore, further studies with a larger number of patients and additional data should be performed to clarify the precise mechanism of TERRA expression in CRC.
There are several limitations to this study, including a single institutional analysis, a small sample size, the lack of investigation of other molecular biomarkers such as telomerase activity status, KRAS and BRAF mutations, and a retrospective analysis. A possible association between telomere regulation and CRC was suggested, however, telomere may be affected by lifestyle, and various factors, such as lifestyle, obesity, food intake, alcohol, and smoking. Therefore, a study with subdivided patients group should be designed carefully. Further studies with a large population are thus needed to determine the biomarkers for predicting the long-term prognosis and its molecular mechanism. In conclusion, 18p TERRA expression was marginally significantly associated with preoperative CEA and significantly associated with telomere length, rendering it a potential prognostic factor for long-term oncologic outcomes in CRC.
Author contributions
Conceptualization: Sung Uk Bae, Jae-Ho Lee.
Data curation: Won-Jin Park, Woon Kyung Jeong, Seong Kyu Baek, Hye-Won Lee.
Funding acquisition: Jae-Ho Lee.
Investigation: Won-Jin Park.
Methodology: Sung Uk Bae, Won-Jin Park, Seong Kyu Baek, Hye-Won Lee.
Project administration: Sung Uk Bae.
Resources: Sung Uk Bae, Woon Kyung Jeong, Seong Kyu Baek.
Software: Won-Jin Park.
Supervision: Jae-Ho Lee.
Validation: Won-Jin Park, Woon Kyung Jeong.
Visualization: Seong Kyu Baek.
Writing – original draft: Sung Uk Bae, Seong Kyu Baek.
Writing – review & editing: Woon Kyung Jeong, Jae-Ho Lee.
Jae-Ho Lee orcid: 0000-0002-5562-0720.
Footnotes
Abbreviations: CEA = carcinoembryonic antigen, CI = confidence interval, CRC = colorectal cancer, CT = computed tomography, DFS = disease-free survival, HR = hazard ratio, nDNA = nuclear DNA, OS = overall survival, TERRA = telomeric repeat-containing RNAs.
SUB and WJP contributed equally to this work.
This study was supported by grants from the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education (NRF-2014R1A6A3A04058057), and by the Korean Government (MSIP) (No. 2014R1A5A2010008).
The authors have no conflicts of interest to disclose.
References
- [1].Shin A, Kim KZ, Jung KW, et al. Increasing trend of colorectal cancer incidence in Korea, 1999–2009. Cancer Res Treat 2012;44:219–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Arnold M, Sierra MS, Laversanne M, et al. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017;66:683–91. [DOI] [PubMed] [Google Scholar]
- [3].Colussi D, Brandi G, Bazzoli F, et al. Molecular pathways involved in colorectal cancer: implications for disease behavior and prevention. Int J Mol Sci 2013;14:16365–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Di Caro G, Marchesi F, Laghi L, et al. Immune cells: plastic players along colorectal cancer progression. J Cell Mol Med 2013;17:1088–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ogino S, Galon J, Fuchs CS, et al. Cancer immunology: analysis of host and tumor factors for personalized medicine. Nat Rev Clin Oncol 2011;8:711–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Zaanan A, Flejou JF, Emile JF, et al. Defective mismatch repair status as a prognostic biomarker of disease-free survival in stage III colon cancer patients treated with adjuvant FOLFOX chemotherapy. Clin Cancer Res 2011;17:7470–8. [DOI] [PubMed] [Google Scholar]
- [7].Sinicrope FA, Shi Q, Smyrk TC, et al. Molecular markers identify subtypes of stage III colon cancer associated with patient outcomes. Gastroenterology 2015;148:88–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Samassekou O, Gadji M, Drouin R, et al. Sizing the ends: normal length of human telomeres. Ann Anat 2010;192:284–91. [DOI] [PubMed] [Google Scholar]
- [9].Palm W, de Lange T. How shelterin protects mammalian telomeres. Annu Rev Genet 2008;42:301–34. [DOI] [PubMed] [Google Scholar]
- [10].Park WJ, Bae SU, Heo YR, et al. Telomere shortening in non-tumorous and tumor mucosa is independently related to colorectal carcinogenesis in precancerous lesions. Int J Mol Epidemiol Genet 2017;8:53–8. [PMC free article] [PubMed] [Google Scholar]
- [11].Sampl S, Pramhas S, Stern C, et al. Expression of telomeres in astrocytoma WHO grade 2 to 4: TERRA level correlates with telomere length, telomerase activity, and advanced clinical grade. Transl Oncol 2012;5:56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Azzalin CM, Reichenbach P, Khoriauli L, et al. Telomeric repeat containing RNA and RNA surveillance factors at mammalian chromosome ends. Science 2007;318:798–801. [DOI] [PubMed] [Google Scholar]
- [13].Deng Z, Norseen J, Wiedmer A, et al. TERRA RNA binding to TRF2 facilitates heterochromatin formation and ORC recruitment at telomeres. Mol Cell 2009;35:403–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Yu TY, Kao YW, Lin JJ. Telomeric transcripts stimulate telomere recombination to suppress senescence in cells lacking telomerase. Proc Natl Acad Sci U S A 2014;111:3377–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Azzalin CM, Lingner J. Telomere functions grounding on TERRA firma. Trends Cell Biol 2015;25:29–36. [DOI] [PubMed] [Google Scholar]
- [16].Rippe K, Luke B. TERRA and the state of the telomere. Nat Struct Mol Biol 2015;22:853–8. [DOI] [PubMed] [Google Scholar]
- [17].Pfeiffer V, Lingner J. TERRA promotes telomere shortening through exonuclease 1-mediated resection of chromosome ends. PLoS Genet 2012;8:e1002747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Luke B, Panza A, Redon S, et al. The Rat1p 5’ to 3’ exonuclease degrades telomeric repeat-containing RNA and promotes telomere elongation in Saccharomyces cerevisiae. Mol Cell 2008;32:465–77. [DOI] [PubMed] [Google Scholar]
- [19].Maicher A, Kastner L, Dees M, et al. Deregulated telomere transcription causes replication-dependent telomere shortening and promotes cellular senescence. Nucleic Acids Res 2012;40:6649–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Wang C, Zhao L, Lu S. Role of TERRA in the regulation of telomere length. Int J Biol Sci 2015;11:316–23. [DOI] [PMC free article] [PubMed] [Google Scholar]


