Abstract
There are many preclinical and epidemiological reports suggesting a correlation between 3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMG-CoAR) or HMG-CoAR inhibitor (statin) treatment and prognosis in breast cancer. This study aimed to investigate the expression of HMG-CoAR in Korean patients with breast cancer.
The expression of HMG-CoAR on tissue microarrays from 191 patients who underwent resection from 2005 to 2006 in the Pusan National University Hospital was assessed by immunohistochemistry (IHC). The IHC assessment by a board-certified pathologist included areas of both carcinoma and peritumoral tissue of the breast. The scores of cancer-specific staining were adjusted by the scores of peritumoral staining.
The patients were followed for a median 9.1 years. Disease-free survival (DFS) was shorter in patients with a positive adjusted HMG-CoAR score by log-rank test (not reached vs 11.6 years, P = .011). After adjusting for age, T stage, N stage, pathological grade, perioperational chemotherapy, adjuvant radiotherapy, estrogen receptor positivity, progesterone receptor positivity, human epidermal growth factor receptor-2 positivity, and high Ki-67 (>10%), a positive adjusted HMG-CoAR IHC score was also associated with shorter DFS (hazard ratio = 2.638, 95% confidence interval [CI] 1.112–6.262, P = .028).
The expression of HMG-CoAR might be an independent prognostic factor in breast cancer. There are established drugs targeting HMG-CoAR, and further studies on its potential as a predictive marker are needed.
Keywords: breast cancer, prognosis, statins, targeted therapy, triple negative breast cancer
1. Introduction
Breast cancer is the most common cancer in women worldwide and the 2nd most common in Korean women, after thyroid cancer. The incidence of breast cancer has continuously increased in Korea.[1] Breast cancer is a heterogenous disease entity and can be categorized into subtypes based on expression of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor-2 (Her2).[2,3] Determining therapeutic targets other than ER, PR, and Her2 that can be used for triple-negative breast cancer (TNBC) is vital.
There are many preclinical and epidemiological reports that suggest the rate-limiting enzyme in the mevalonate pathway, 3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMG-CoAR), or HMG-CoAR inhibitor (statin) treatment are associated with prognosis in breast cancer. Kotamraju et al reported that statin-induced antiproliferative and proapoptotic effects on breast cancer cells are mediated by enhanced nitric oxide levels.[4] Borgquist et al demonstrated that HMG-CoAR was differentially expressed in breast cancer in a Swedish cohort and that high expression was associated with prognostically favorable tumor parameters.[5]
Whether expression of HMG-CoAR correlates with prognosis or tumor characteristics in Korean breast cancer patients has not been well investigated. We aimed to investigate the expression of HMG-CoAR and its association with prognosis in Korean breast cancer patients with long-term follow up.
2. Materials and methods
2.1. Patients
Two hundred and three consecutive patients who had been diagnosed with primary breast cancer and underwent operation in the Pusan National University Hospital from 2005 to 2006 were included in this retrospective study. Patients who were initially diagnosed with stage IV breast cancer or another malignancy besides breast cancer were excluded because we intended to study the course of curable primary breast cancers. Stage IV breast cancers have extremely various disease courses according to the subtypes and provide progression-free survivals rather than disease-free survivals of stage I, II, III diseases.
Patients were followed up through their medical records. Clinical information on age, treatment, staging, tumor characteristics, and breast cancer-related events was obtained from of patient charts. Information about tumor characteristics, such as ER, PR, Her2, or Ki-67 status, was acquired from pathologic reports.
2.2. Ethical statement
The study was approved by the Pusan National University Hospital Institutional Review Board (2011.5.9.). All patients provided informed consent about the use of human-derived materials at the time of surgery.
2.3. Tissue microarray construction
Surgical specimens preserved in paraffin-embedded tissue blocks after pathologic diagnosis from the included patients were collected for constructing a tissue microarray. A representative tumor block including peritumoral breast tissue was selected from each patient. Two 5-mm–diameter cores were punched out and mounted in a recipient tissue microarray paraffin block.
2.4. Immunohistochemistry
Tissue microarray blocks were sectioned at a 4-μm–thickness and mounted on glass slides. The sections were stained by hand with T1500 rabbit polyclonal anti-HMG-CoAR antibody (EPITOMICS, 863 Mitten Road, Suite 103 Burlingame, California 94010-1303) diluted 1:50. Immunohistochemistry (IHC) staining assessment by board-certified pathologists included areas of both carcinoma and peritumoral normal breast tissue. We examined the intensity of staining and fraction of positive cells in carcinoma and background normal tissues. Two pathologists assessed the HMG-CoAR–stained microarray slides. The cytoplasmic positivity of staining in invasive tumor cell components and the adjacent normal acini was assessed. The intensity of each core was categorized into 4 categories: none, mild, moderate, or intense. The positive percentage of each core was semi-quantitatively scored, with the categories of 0%, 0 to 25%, 25 to 50%, and >50%. Scoring was synthetically performed with 2 tumor cores for each patient. The pathologists were blinded to demographic and survival data. They discussed about the cases of disagreement and made consensus.
The staining positivity was finally scored from 0 to 3 (negative = 0, weak = 1, moderate = 2, and strong = 3). We scored no intensity as 0, weak intensity with <25% positivity as 1; weak intensity with 25 to 50% positivity or moderate intensity with 1 to 25% positivity as 2; and weak intensity with >50% positivity, moderate intensity with >25% positivity, or any strong intensity as 3. Scores >1 were considered positive. Adjusted staining scores were calculated by subtracting the score of peritumoral tissue from the score of invasive carcinoma tissue. A positive adjusted score of HMG-CoAR IHC indicated that the staining score was higher in the invasive carcinoma than in the peritumoral breast tissue.
2.5. Statistics
The statistical analyses were performed using SPSS Statistics 22 (IBM, Chicago, IL). Chi-square tests were used to identify correlated variables. Time to recurrence and death were calculated from the date of surgery until failure and death from any cause, respectively. Disease-free survival (DFS) was defined as survival without disease recurrence at the time of censoring. Overall survival (OS) was defined as time to death, regardless of the cause, from the date of surgery. The survival curves were obtained by the Kaplan–Meier method. The univariable survival analyses were performed by log-rank test, and Cox proportional hazard regression model was used for multivariable tests. P-values < .05 were considered statistically significant. Non-informative censoring was minimally conducted due to loss to follow-up in the survival analysis.
3. Results
3.1. Patient characteristics
Among 203 patients who underwent breast surgery from primary breast cancer, 7 patients who had metastatic lesions at the time of operation and 5 patients who had another malignancy besides breast cancer were excluded. The median age of included 191 patients at diagnosis was 49.4 years (25–80 years), and the median follow-up was 9.1 years (0.5–13.8 years). All 191 patients were women. One hundred forty-one patients (73.1%) received radical mastectomy and 50 patients (25.9%) underwent breast conserving surgery. The pathologic staging after surgery revealed that 78 patients (40.4%) had stage 1 cancer and 79 patients (40.9%) had stage 2 cancer. Thirty-four patients (17.6%) had stage 3 breast cancer (Table 1). Seventy patients (36.6%) received neoadjuvant chemotherapy before surgery and 105 patients (55%) received post-operative chemotherapy. One hundred nine patients (56.5%) received post-operative radiotherapy.
Table 1.
Patient characteristics.
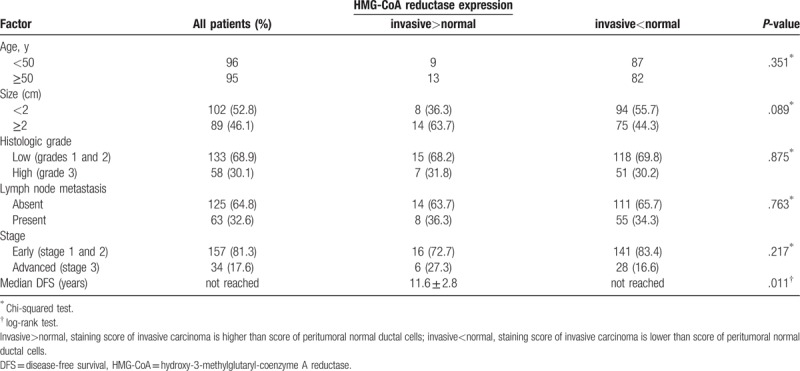
3.2. Tumor characteristics
The ER, PR, Her2 data were available in all patients. One hundred twenty-two patients (63.2%) had luminal A subtype (ER+ and/or PR+, Her2-) and 25 patients (13%) had luminal B subtype (ER+ and/or PR+, Her2+). The Her2-positive cancer accounted for 44 patients (22.8%), and TNBC comprised 22 (13%) of patients (Table 2). The DFS and OS according to breast cancer subtype are shown in Figure 1. The luminal A and B subtypes showed similar survival. The Her2-positive subtype had the worst survival, and the TNBC subtype showed worse survival than luminal A and B.
Table 2.
Tumor characteristics.
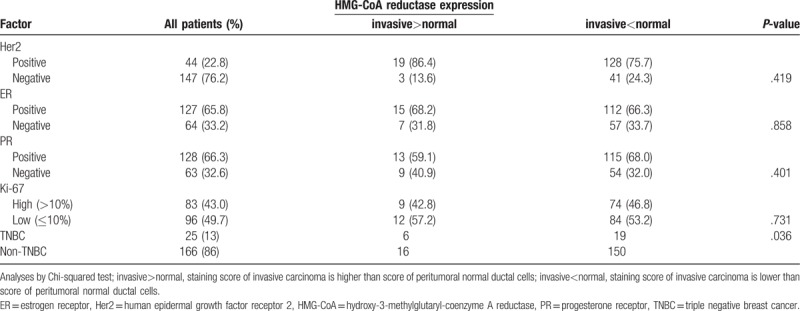
Figure 1.
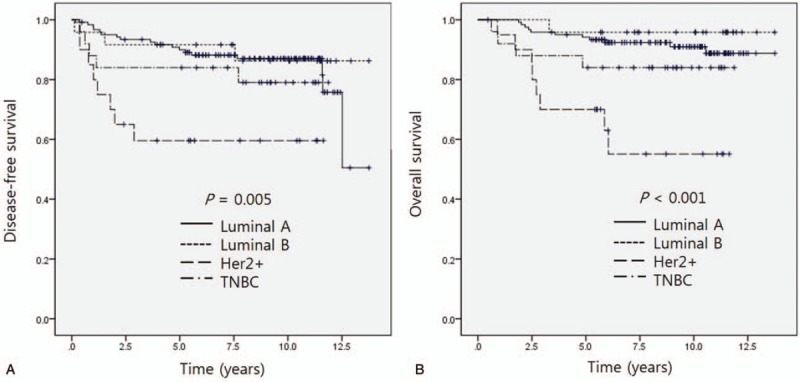
(A) Disease-free survival according to breast cancer subtype. (B) Overall survival according to breast cancer subtype. Her2 = human epidermal growth factor receptor 2, TNBC = triple negative breast cancer.
The staining score varied in invasive carcinoma and peritumoral normal breast tissues. Seventeen patients had no slides with peritumoral normal breast ductal cells; their data were processed as missing data for scoring for normal and had no adjustment. Most peritumoral normal ductal cells had a diffuse pattern with more than 50% positivity. Staining in invasive carcinoma samples were more varied. Figure 2 shows representative immunohistochemical staining of HMG-CoAR in invasive carcinoma and peritumoral breast tissues.
Figure 2.
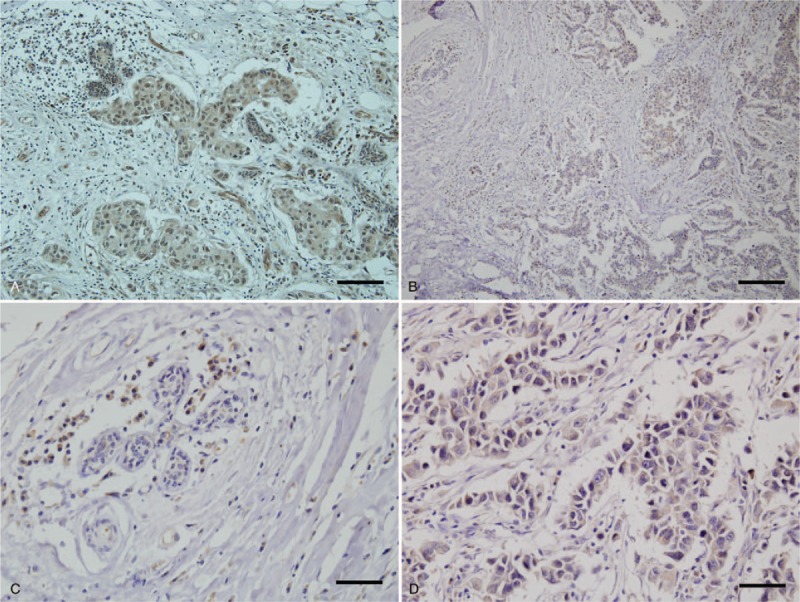
Representative immunohistochemistry of HMG-CoA reductase expression. (A) Moderately positive expression in invasive carcinoma and normal breast tissue (×200, scale bar = 20 μm). (B) Weakly positive expression in invasive carcinoma and no expression in normal breast tissue (×100, scale bar = 10 μm). (C) No expression in normal breast tissue (×400, scale bar = 5 μm). (D) Weakly positive expression in invasive carcinoma (×400, scale bar = 5 μm). HMG-CoA = hydroxy-3-methylglutaryl-coenzyme A reductase.
The invasive cancer sample of 114 patients (59.1%) was positive for HMG-CoAR, and the peritumoral tissue of 110 patients (63.2%) was positive. The adjusted HMG-CoAR staining scores were positive in 22 patients (11.4%). A positive adjusted score was correlated with TNBC (Table 2). Patients with a positive adjusted staining score for HMG-CoAR had a 2.961-times higher risk for TNBC (95% confidence interval [CI] = 1.033–8.483, P = .036).
3.3. Survival
Kaplan–Meier survival analysis did not show significant differences in DFS or OS according to the HMG-CoAR staining score of invasive carcinoma tissue (Fig. 3A, C). The score of peritumoral normal breast tissue was also not significantly correlated with DFS or OS (Fig. 3B, D). The DFS of patients with a positive adjusted score of HMG-CoAR IHC was shorter than that of the non-positive score group by log-rank test (11.6 years vs not reached, P = .011) (Fig. 4A). In TNBC, the positive adjusted HMG-CoAR IHC score group also had a shorter DFS (0.8 years vs not reached, P < .001) (Fig. 4B). Kaplan–Meier analysis indicated that the positive adjusted HMG-CoAR IHC score group had a shorter OS; however, statistical significance was not identified (Fig. 4C). In TNBC, the positive adjusted HMG-CoAR IHC score group had a shorter OS with statistical significance by Kaplan–Meier analysis (1.7 years vs not reached, P = .004) (Fig. 4D). The Her2-positive, luminal A and B subtypes did not show significantly different survivals according to adjusted HMG-CoAR IHC scores.
Figure 3.
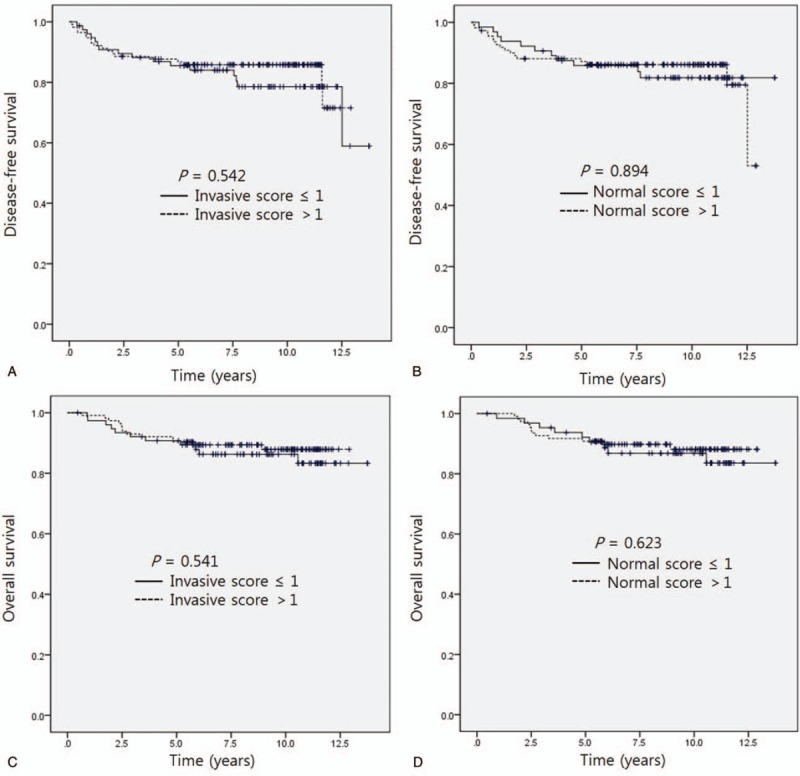
Disease-free survival according to hydroxy-3-methylglutaryl-coenzyme A reductase (HMG-CoAR) staining in invasive carcinoma tissue (A) and normal tissue (B). Overall survival according to HMG-CoAR score in invasive carcinoma tissue (C) and normal tissue (D).
Figure 4.
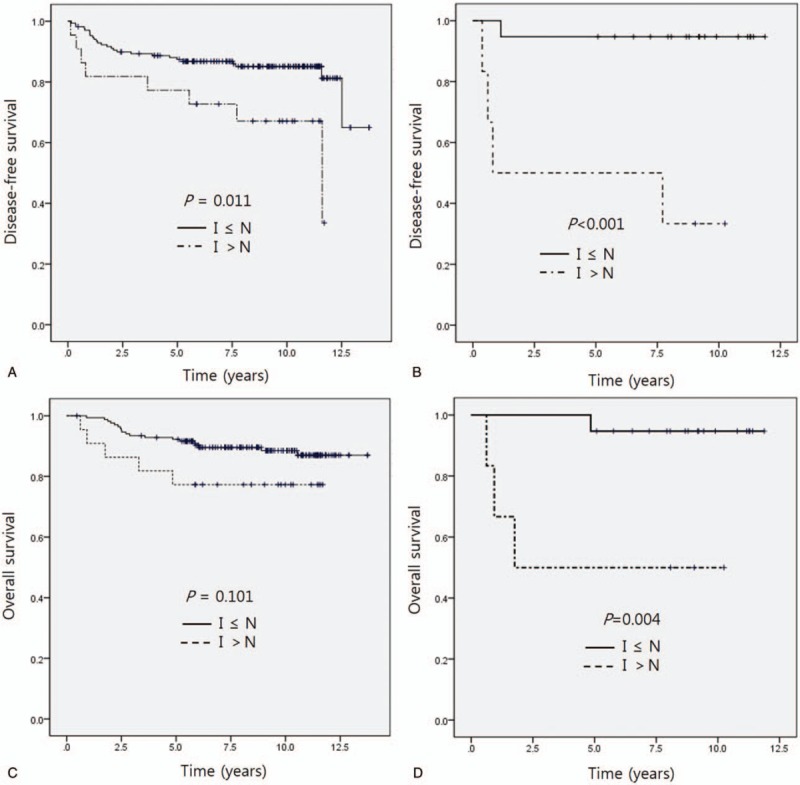
(A) Disease-free survival according to the differential intensity of immunohistochemical staining of HMG-CoAR. (B) Disease-free survival according to the differential intensity of immunohistochemical staining of HMG-CoAR in TNBC. (C) Overall survival according to the differential intensity of HMG-CoAR. (D) Overall survival according to the differential intensity of HMG-CoAR in TNBC. TNBC = triple-negative breast cancer, I = immunohistochemistry staining score of invasive carcinoma, N = immunohistochemistry staining score of normal tissue adjacent to the carcinoma.
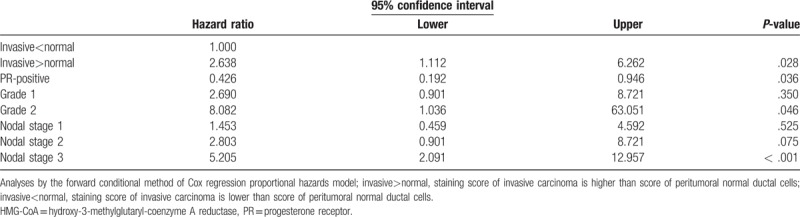
In a Cox regression proportional hazards model with adjustments for age, T stage, N stage, pathological grade, perioperative chemotherapy, adjuvant radiotherapy, ER positivity, PR positivity, Her2 positivity, and high Ki-67 (>10%), a positive adjusted HMG-CoAR IHC score was associated with shorter DFS (hazard ratio [HR] = 2.638, 95% CI 1.112–6.262, P = .028). The analysis also showed that N stage correlated with shorter DFS. The N3 stage had an HR of 5.2 compared with N0 (P < .001). The PR positivity showed an HR of 0.426, indicating a longer DFS in patients with PR-positive cancer compared to those with PR-negative cancer (P = .036). Table 3 shows the multivariable analysis for DFS in relation to HMG-CoAR expression. The OS did not show a statistically significant correlation with HMG-CoAR expression. In TNBC, the Cox regression proportional hazards model did not identify statistically significant correlations with HMG-CoAR expression.
Table 3.
Multivariable analysis for disease-free survival in relation of HMG-CoAR expression.
4. Discussion
This study suggests that HMG-CoAR expression can be an independent prognostic factor in breast cancer. There have been reports that HMG-CoAR expression correlated with favorable prognosis of breast cancer and the ER-positive subtype in a Swedish population.[5,6] Brennan et al performed western blotting and polymerase chain reaction (PCR) to measure HMG-CoAR protein and ribonucleic acid (RNA), respectively, in tumors.[6] Gustbée et al reported that moderate or strong expression of HMG-CoAR was associated with less aggressive tumor characteristics in breast cancer.[7] However, the study did not show correlation with survival, unlike previous studies, as they had a short follow-up time. These studies all quantified tumor-specific HMG-CoAR protein or RNA.
In the present study, we determined that a positive score with adjustment for HMG-CoAR staining was associated with TNBC and shorter DFS in Korean patients. This result is quite different from those of previous studies. Previous studies about HMG-CoAR staining as a prognostic marker were mostly reported from a Swedish group, although an American research group found a statin-induced decrease in ER-negative breast cancer and suggested the potential of statins to convert breast cancer phenotype.[8] However, the American group did not investigate any potential markers. We might be able to speculate that ER-negative breast cancer could have sensitivity to statins. The ER-negative population of the present study showed significantly different DFS and OS according to HMG-CoAR staining score with adjustment. If patients with ER-negative breast cancer have sensitivity to statins, expression of HMG-CoAR might be a potential marker.
We adjusted the staining scores for HMG-CoAR based on the staining of peritumoral normal breast ductal cells. This adjustment might be regarded as an arbitrary manipulation; however, cancer metabolism, such as that controlled by the mevalonate pathway, can be significantly affected by the tumor microenvironment. The mevalonate pathway has a homeostatic feedback system that could been described at the molecular level. When levels of sterol are high in the cells, the endoplasmic reticula hold back cytoplasmic transcription factors, known as sterol regulatory element-binding proteins (SREBPs).[9] When levels of sterol decrease, cells release activated SREBPs from the membrane. This response, which has many downstream molecular reactions, induces the transcription of target genes like HMG-CoAR and the low-density lipoprotein (LDL) receptor. This homeostatic feedback could affect normal and cancer cells, and the expression of HMG-CoAR in normal tissue could reflect the patient's baseline metabolic dynamics.
The HMG-CoAR is basally expressed in various organs, and is highly expressed in the cerebral cortex, hippocampus, appendix, gallbladder, gastrointestinal tract, epididymis, seminal vesicle, and placenta.[10] The breast is considered to have medium expression of HMG-CoAR. We found that the peritumoral breast ductal cells in some patients were stained by HMG-CoAR antibody. We consider that each individual patient's tumor metabolism would differ, and race and diet could substantially contribute to the different results from previous reports with expression of HMG-CoAR in breast cancer.
Statins are HMG-CoAR inhibitors and are clinically used to treat cardiovascular disease. However, some studies have examined their use in cancer patients. The antiproliferative activity of statins was demonstrated in the early 1990 s.[11] It has been reported that statins trigger G1 arrest by modulation of the activity, expression, and distribution of cyclin-dependent kinase 2, p21, and p27 in breast cancer.[12–14] So-called pleiotropic effects of statins include not only antiproliferative effects, but also anti-inflammatory, antioxidant, and immunomodulatory effects.[11] There have also been clinical studies investigating the anti-cancer efficacy of statins in breast cancer. Most studies published around 2000 examined statins for cardiovascular events or safety.[15–17] A meta-analysis of seven randomized clinical studies and nine observational studies through 2005 did not show a protective effect against breast cancer.[18]
Despite this finding, lipophilic statins were known to decrease the incidence or recurrence of breast cancer by inhibiting both intrahepatic and extrahepatic HMG-CoAR activity.[19] It was suggested that hydrophilic statins only inhibit intrahepatic HMG-CoAR and lead to positive feedback, promoting the upregulation of extrahepatic HMG-CoAR, unlike lipophilic statins. Bjarnadottir et al conducted a phase 2 clinical trial with the lipophilic statin atorvastatin in 50 Swedish patients with breast cancer in the neoadjuvant setting.[20] They analyzed pre- and post-statin paired tumor samples for Ki-67 and HMG-CoAR IHC expression. They noted upregulation of HMG-CoAR and decrease of Ki-67 expression after statin treatment in paired tumor samples. Moreover, the decrease in Ki-67 expression was more prominent in tumors expressing HMG-CoAR before statin treatment. This study shed light on the potential of HMG-CoAR as a predictive marker for statin treatment in breast cancer. It also suggested the potential of statins for treatment of breast cancer.
Some studies have tried to find specific breast cancer populations who might benefit from statin treatment. A team from the University of Texas demonstrated in a mouse model that simvastatin might prevent skeletal metastasis of breast cancer by an antagonistic interplay between p53 and CD44.[21] Brewer et al reported that hydrophilic statin administration was associated with significantly improved progression-free survival in inflammatory breast cancer.[22] They believe that the hydrophilic statin is pharmacologically much more potent than the lipophilic statin, which is why hydrophilic statins, but not lipophilic statins, showed a survival benefit. There might be certain patient subgroups, such as those with skeletal metastasis or inflammatory breast cancer, which are more likely to benefit from statins. In this study, TNBC was associated with a positive adjusted staining score for HMG-CoAR and the positive scores were related with shorter DFS and OS in TNBC (Fig. 4B, D). The ER-negative patients showed the same result with TNBC and other subtypes did not show different survivals.
There are some limitations in this study. The assessment of HMG-CoAR expression was examined only by IHC. Moreover, this retrospective cohort study from a single center population could have some bias in scoring for IHC staining. Although it is a relatively long follow-up study, the number of patients is relatively small, and it is difficult to calculate a difference between subgroups.
Markers related to cancer metabolism, such as HMG-CoAR, could be influenced by dietary habits, lifestyle, medication, race, and other factors. Although IHC was the only method used to assess HMG-CoAR expression in the present study, combining more sophisticated methods, like PCR for RNA quantification, with a larger cohort would help to clarify the role of HMG-CoAR in breast cancer. Some mutations in related target genes, rather than expression of HMG-CoAR, might also be predictive markers for statins. More targets for targeted treatment of breast cancers, especially cancers with poor prognosis like TNBCs, are urgently needed. We identified that the positive adjusted scores of HMG CoAR IHC were related with poor prognosis in Korean patients with breast cancer. This study could suggest that HMG-CoAR expression considering peritumoral condition might be a predictive or prognostic marker in breast cancer, especially TNBC.
Author contributions
Conceptualization: Hyojeong Kim, Youngtae Bae, Young Jin Choi.
Data curation: Hyojeong Kim, Young Mi Seol, Nari Shin, Ahrong Kim, Jee Yeon Kim, Keun Young Kim, Youngtae Bae.
Formal analysis: Nari Shin, Ahrong Kim, Jee Yeon Kim, Keun Young Kim.
Funding acquisition: Hyojeong Kim.
Investigation: Hyojeong Kim, Young Mi Seol, Nari Shin, Keun Young Kim.
Methodology: Nari Shin, Ahrong Kim, Jee Yeon Kim, Keun Young Kim.
Project administration: Keun Young Kim.
Resources: Young Mi Seol.
Software: Hyojeong Kim.
Supervision: Young Mi Seol, Ho-Jin Shin, Joo Seop Chung, Jee Yeon Kim, Youngtae Bae, Young Jin Choi.
Validation: Ho-Jin Shin, Joo Seop Chung.
Visualization: Nari Shin, Ahrong Kim.
Writing – original draft: Hyojeong Kim.
Writing – review & editing: Ho-Jin Shin, Joo Seop Chung, Young Jin Choi.
Footnotes
Abbreviations: DFS = Disease-free survival, ER = estrogen receptor, Her2 = human epidermal growth factor receptor-2, HMG-CoAR = 3-hydroxy-3-methylglutaryl-coenzyme A reductase, HR = hazard ratio, IHC = immunohistochemistry, LDL = low-density lipoprotein, OS = overall survival, PCR = polymerase chain reaction, PR = progesterone receptor, RNA = ribonucleic acid, SREBP = sterol regulatory element-binding protein, TNBC = triple-negative breast cancer.
The authors have no conflicts of interest to disclose.
This study was supported by Biomedical Research Institute Grant (2017-32), Pusan National University Hospital.
Statistical consultation
Jinmi Kim, Ph.D./Research Professor
Department of Biostatistics, Clinical Trial Center, Biomedical Research Institute, Pusan National University Hospital
References
- [1].Park EH, Min SY, Kim Z, et al. Basic facts of breast cancer in Korea in 2014: the 10-year overall survival progress. J Breast Cancer 2017;20:1–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Badve S, Dabbs DJ, Schnitt SJ, et al. Basal-like and triple-negative breast cancers: a critical review with an emphasis on the implications for pathologists and oncologists. Mod Pathol 2011;24:157–67. [DOI] [PubMed] [Google Scholar]
- [3].Sotiriou C, Pusztai L. Gene-expression signatures in breast cancer. N Engl J Med 2009;360:790–800. [DOI] [PubMed] [Google Scholar]
- [4].Kotamraju S, Williams CL, Kalyanaraman B. Statin-induced breast cancer cell death: role of inducible nitric oxide and arginase-dependent pathways. Cancer Res 2007;67:7386–94. [DOI] [PubMed] [Google Scholar]
- [5].Borgquist S, Djerbi S, Pontén F, et al. HMG-CoA reductase expression in breast cancer is associated with a less aggressive phenotype and influenced by anthropometric factors. Int J Cancer 2008;123:1146–53. [DOI] [PubMed] [Google Scholar]
- [6].Brennan DJ, Laursen H, O’Connor DP, et al. Tumor-specific HMG-CoA reductase expression in primary premenopausal breast cancer predicts response to tamoxifen. Breast Cancer Res 2011;13:R12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Gustbée E, Tryggvadottir H, Markkula A, et al. Tumor-specific expression of HMG-CoA reductase in a population-based cohort of breast cancer patients. BMC Clin Pathol 2015;15:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kumar AS, Benz CC, Shim V, et al. Estrogen receptor-negative breast cancer is less likely to arise among lipophilic statin users. Cancer Epidemiol Biomarkers Prev 2008;17:1028–33. [DOI] [PubMed] [Google Scholar]
- [9].Brown MS, Goldstein JL. Cholesterol feedback: from Schoenheimer's bottle to Scap's MELADL. J Lipid Res 2009;50Suppl:S15–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Thul PJ, Åkesson L, Wiking M, et al. A subcellular map of the human proteome. Science 2017;356: pii: eaal3321. doi: 10.1126/science.aal3321. [DOI] [PubMed] [Google Scholar]
- [11].Clendening JW, Penn LZ. Targeting tumor cell metabolism with statins. Oncogene 2012;31:4967–78. [DOI] [PubMed] [Google Scholar]
- [12].Gray-Bablin J, Rao S, Keyomarsi K. Lovastatin induction of cyclin-dependent kinase inhibitors in human breast cells occurs in a cell cycle-independent fashion. Cancer Res 1997;57:604–9. [PubMed] [Google Scholar]
- [13].Wachtershauser A, Akoglu B, Stein J. HMG-CoA reductase inhibitor mevastatin enhances the growth inhibitory effect of butyrate in the colorectal carcinoma cell line Caco-2. Carcinogenesis 2001;22:1061–7. [DOI] [PubMed] [Google Scholar]
- [14].Rao S, Lowe M, Herliczek TW, et al. Lovastatin mediated G1 arrest in normal and tumor breast cells is through inhibition of CDK2 activity and redistribution of p21 and p27, independent of p53. Oncogene 1998;17:2393–402. [DOI] [PubMed] [Google Scholar]
- [15].Lovastatin 5-year safety and efficacy study. Lovastatin Study Groups I through IV. Arch Intern Med. 1993; 153:1079–87. [PubMed] [Google Scholar]
- [16].Sacks FM, Pfeffer MA, Moye LA, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. N Engl J Med 1996;335:1001–9. [DOI] [PubMed] [Google Scholar]
- [17].Blais L, Desgagne A, LeLorier J. 3-Hydroxy-3-methylglutaryl coenzyme A reductase inhibitors and the risk of cancer: a nested case-control study. Arch Intern Med 2000;160:2363–8. [DOI] [PubMed] [Google Scholar]
- [18].Bonovas S, Filioussi K, Tsavaris N, et al. Use of statins and breast cancer: a meta-analysis of seven randomized clinical trials and nine observational studies. J Clin Oncol 2005;23:8606–12. [DOI] [PubMed] [Google Scholar]
- [19].Duncan RE, El-Sohemy A, Archer MC. Statins and cancer development. Cancer Epidemiol Biomarkers Prev 2005;14:1897–8. [DOI] [PubMed] [Google Scholar]
- [20].Bjarnadottir O, Romero Q, Bendahl PO, et al. Targeting HMG-CoA reductase with statins in a window-of-opportunity breast cancer trial. Breast Cancer Res Treat 2013;138:499–508. [DOI] [PubMed] [Google Scholar]
- [21].Mandal CC, Ghosh-Choudhury N, Yoneda T, et al. Simvastatin prevents skeletal metastasis of breast cancer by an antagonistic interplay between p53 and CD44. J Biol Chem 2011;286:11314–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Brewer TM, Masuda H, Liu DD, et al. Statin use in primary inflammatory breast cancer: a cohort study. Br J Cancer 2013;109:318–24. [DOI] [PMC free article] [PubMed] [Google Scholar]


