Supplemental Digital Content is available in the text
Keywords: collaborative care intervention, heart failure, meta-analysis, quality of life, systematic review
Abstract
Introduction:
Patients with chronic heart failure (HF) show many symptoms that worsen the quality of life (QoL). Collaborative care intervention (CCI) aims to improve the QoL and symptoms by integrating psychosocial and palliative strategies in chronic care.
Methods:
The PubMed, EMBASE, and Cochrane library databases were searched from inception to September 2018. The included studies were used to determine pooled standard mean differences (SMDs) and associated 95% confidence intervals (CIs). The data were assessed by fixed- and random effects models, respectively.
Results:
Twenty-one studies including 2999 patients with chronic heart failure were included. The results showed significantly improved QoL in the CCI group compared with the routine care group (SMD = 0.60, 95%CI 0.27–0.94, Pheterogeneity < .001, I2 = 94.1%). The patients who received face-to-face interventions experienced a significant improvement (SMD = 0.54, 95%CI 0.24–0.85, Pheterogeneity < .001, I2 = 88.7%) in terms of QoL compared with those administered only telephone interventions. Furthermore, significantly improved anxiety level (SMD = 0.33, 95%CI 0.12–0.55, Pheterogeneity = .612, I2 = 0%) and 6-min walk test (SMD = 0.46, 95%CI 0.29–0.64, Pheterogeneity = .458, I2 = 0%) were found in the CCI group compared with the routine care group.
Conclusion:
These findings confirmed that collaborative care intervention effectively improves the quality of life as well as psychological (anxiety) and physical (6-min walk test) functions in patients with chronic heart failure compared with routine care. Furthermore, face-to-face interventions show a greater improvement of QoL compared with telephone-only interventions.
1. Introduction
Heart failure (HF) is a global public health issue affecting between 1% and 2% of adults, and more than 10% of individuals over the age of 70.[1] Morbidity is expected to continuously rise due to demographic changes and the increasing incidence rates of HF risk factors, including hypertension, ischemic heart disease, smoking and diabetes. HF has similar morbidity and mortality as many types of cancer, conferring a lower quality of life than most chronic diseases. In Europe, HF affects more than 15 million individuals, and global HF prevalence is expected to rise by 25% by 2030.[2] Currently, the prevalence of HF among adults in Europe and the United States ranges from 0.4% to 2.3%.[3] Left ventricular myocardial function, dysfunctions of valves, the endocardium, the pericardium, and the myocardium, and altered heart rhythm are associated with HF. These ailments, together with multiple comorbidities, often lead to chronic HF.
HF is a chronic disease with a wide range of effects, almost affecting every important aspect of a patient's life. Consequently, patients with HF experience impaired quality of life (QoL) as well as psychological distress. Severe depressive disorder is a common manifestation of HF, in coping with pain in the patients.[4] Meanwhile increased QoL impairment and depressive symptoms are associated with adverse HF disease trajectories and poor clinical outcomes. These symptoms tend to persist, although the best guidelines are based on the management of HF, and a symptom-oriented approach to palliative care may be beneficial. Although there are multiple compelling palliative care requirements in HF, the patients are generally not examined by palliative care experts in outpatient clinics, since the number of palliative care experts is limited.[5]
To address these challenges, collaborative care intervention (CCI) was developed based on (1) evidence showing potentially modifiable contributors to QoL[6]; (2) primary concerns and needs of patients and informal caregivers, and preferences for palliative care[7]; (3) a successful model of health care delivery. Currently, the effect of collaborative care intervention in patients with chronic heart failure is largely undefined. Therefore, we performed a systematic review and meta-analysis of published studies to provide a comprehensive assessment of the efficacy of collaborative care intervention in patients with chronic heart failure.
2. Methods
The present meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines. The meta-analysis based on public literature is not applicable for ethical approval.
2.1. Search strategy
Two investigators independently searched for eligible studies assessing the effect of collaborative care intervention in patients with chronic heart failure. Studies published from inception to September 2018 were identified via an electronic search of the PubMed, EmBase, and Cochrane Library databases without language restriction. The keywords used were: (collaborative care intervention or psychosocial intervention or multidisciplinary or comprehensive care) and (chronic heart failure) and randomized controlled trial. The references of all identified publications were also searched for additional eligible studies.
2.2. Inclusion and exclusion criteria
Collaborative care intervention is defined in this study as a collaborative symptom care provided by a nurse and psychosocial care provided by health professionals that is focused on improving the psychologic and/or social aspects of a patient's health. The included studies met the following criteria in the present meta analysis: (1) evaluation of the effect of collaborative care intervention in patients with chronic heart failure, (2) randomized controlled trial, and (3) publication in the English language; (4) sufficient data to determine the level of quality of life. Studies were excluded for the following reasons: (1) absence of control subjects, (2) nonclinical studies, (3) reviews, abstracts, or conference papers, and (4) duplicate publication.
2.3. Data extraction and quality assessment
All available data were extracted from each study by two investigators independently based on the above inclusion criteria. Any disagreement was resolved through discussion with a third investigator. The following information was extracted from the included studies: first author's name, year of publication, country, mean patient age, intervention group, control group, follow-up time, and outcomes assessed. The quality of the randomized controlled trials (RCTs) included was evaluated using the Cochrane Collaboration's tool for assessing the risk of bias.[8] The assessment included the following components: random sequence generation, allocation concealment, blinding of patients and study personnel, blinding of outcome assessment, completeness of outcome data, selective reporting of outcomes, and other bias to validity.
2.4. Data pooling and analyses
The current meta-analysis was conducted with Stata 12 (Stata-Corp, College Station, TX, USA). Standard mean differences (SMDs) and corresponding 95% confidence intervals (CIs) were obtained for continuous data. Heterogeneity across each effect size was evaluated by the Q-statistic and the I2 index. I2 > 50% indicated statistically significant heterogeneity; in this case, the random-effects model was used for analyses. Otherwise, the summary effect was computed using the fixed-effects model. The relative influence of each study on the pooled estimate was assessed by omitting one study at a time for sensitivity analysis. The StataSE 12 software was used to generate funnel plots, and the Begg's and Egger's tests were performed to quantify publication bias. Significant publication bias was defined as two-sided P < .05. The trim and fill method was applied in case of publication bias.
3. Results
3.1. Study selection and trial characteristics
Figure 1 graphically illustrates the study flow chart. The literature search yielded 403 potentially relevant articles. Then, five additional records were found by hand searching of reference lists of other review articles. According to inclusion criteria, 318 studies remained after removing duplicates. After screening the titles and abstracts, 255 articles were excluded because of obvious irrelevance. Of the remaining 63 reports, 27 articles were excluded as letters, reviews, and meta-analyses. After reading the full texts of the 36 remaining articles, 15 reports were excluded for the following reasons: lack of controls (n = 7); no usable data (n = 8). Thus, 21 articles[9–29] (21 independent randomized controlled trials), involving 2999 randomized patients contained sufficient data to be included in the current meta-analysis. The data collected from the included studies are summarized in Table 1. The studies were performed in a variety of countries, and sample sizes ranged from 20 to 282 patients. The mean patient age ranged from 57 to 80.1 years. A summary of the risk of bias for each included study is found in Figure 2. All included studies were randomized, but two studies used restricted randomization.
Figure 1.
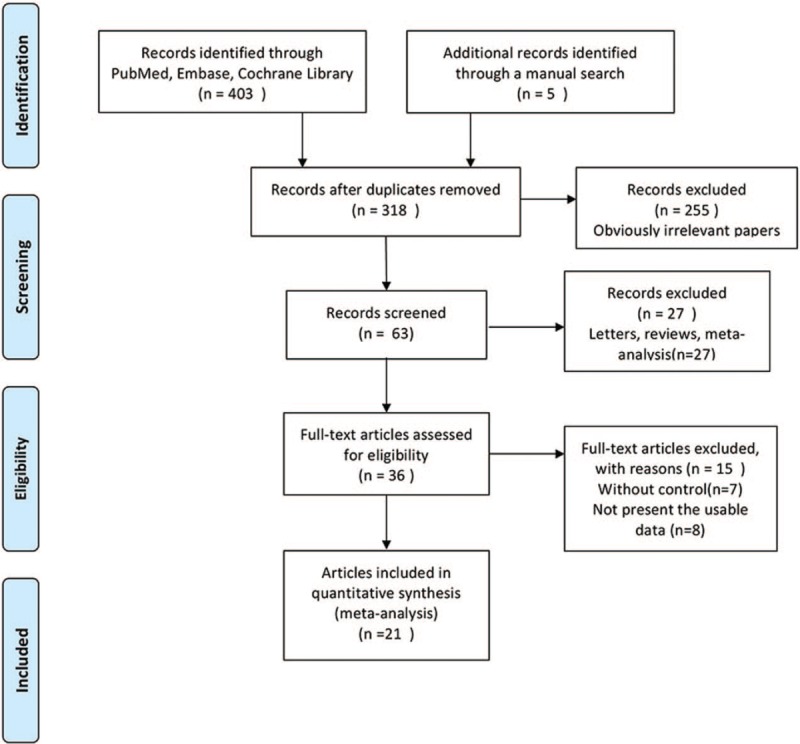
Flow diagram of study identification.
Table 1.
Characteristics of studies included in this meta-analysis.
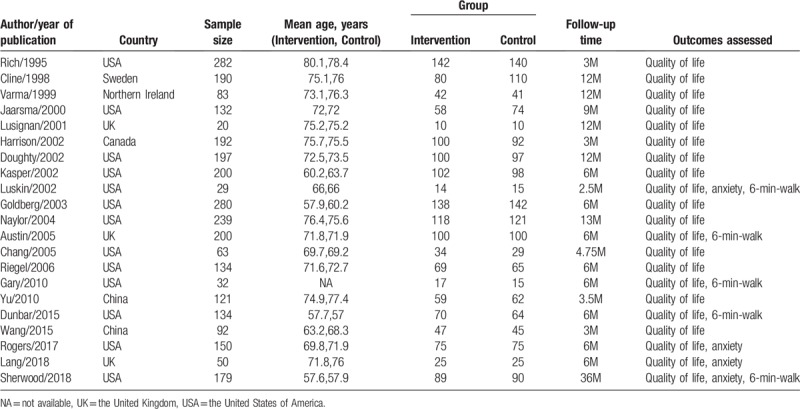
Figure 2.
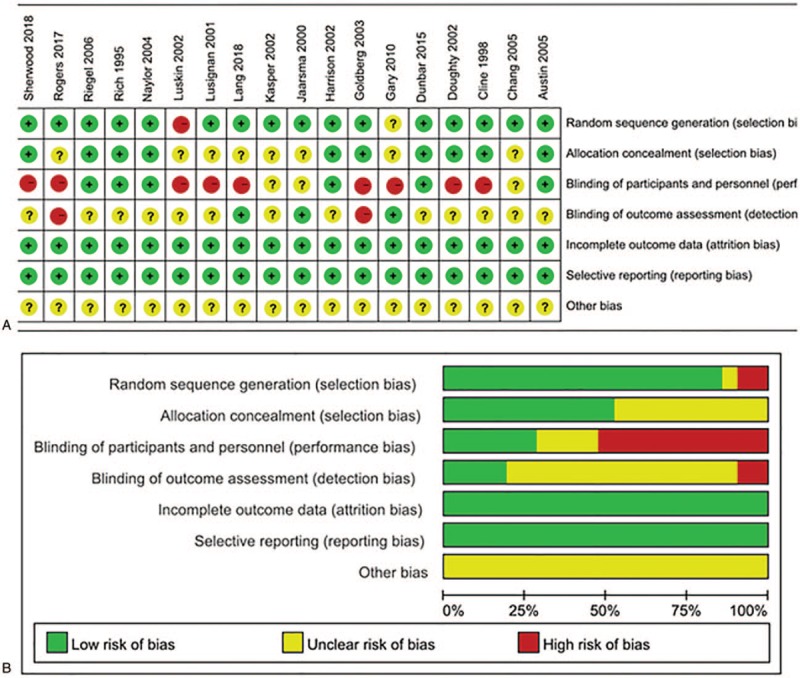
Risk of bias assessment for the randomized trials included in the meta-analysis. (A) Summary; (B) Graph. Symbols: (+), low risk of bias; (?), unclear risk of bias; (-), high risk of bias.
Eleven studies had no blinding of patients or the personnel. Although blinding of outcome assessors was not explicitly indicated, two studies had a risk of detection bias. Allocation concealment was not mentioned in any of the studies, so potential selection bias may be present.
3.2. Quantitative analysis
3.2.1. Quality of life
All 21 studies evaluated the effect of collaborative care intervention on the QoL of patients with chronic heart failure. Pooled analysis was performed with the available data comparing 1489 intervention patients with 1510 controls. Significantly improved QoL was found in the CCI group compared with the routine care group (SMD = 0.60, 95%CI 0.27–0.94, Pheterogeneity < .001, I2 = 94.1%) (Fig. 3A). To assess the sources of heterogeneity, further subgroup analyses were performed. Similarly, significantly improved QoL was found in patients administered multidisciplinary intervention (SMD = 0.63, 95%CI 0.14–1.11, Pheterogeneity < .001, I2 = 92.6%) (Fig. 3B) and those who received only non-multidisciplinary intervention (SMD = 0.59, 95%CI 0.11–1.06, Pheterogeneity < .001, I2 = 95.2%) (Fig. 3B); significant heterogeneity was observed in the two comparison groups. However, patients administered face-to-face interventions experienced a significant improvement (SMD = 0.54, 95%CI 0.24–0.85, Pheterogeneity < .001, I2 = 88.7%) (Fig. 3C) in terms of QoL compared with those who received telephone-only interventions.
Figure 3.
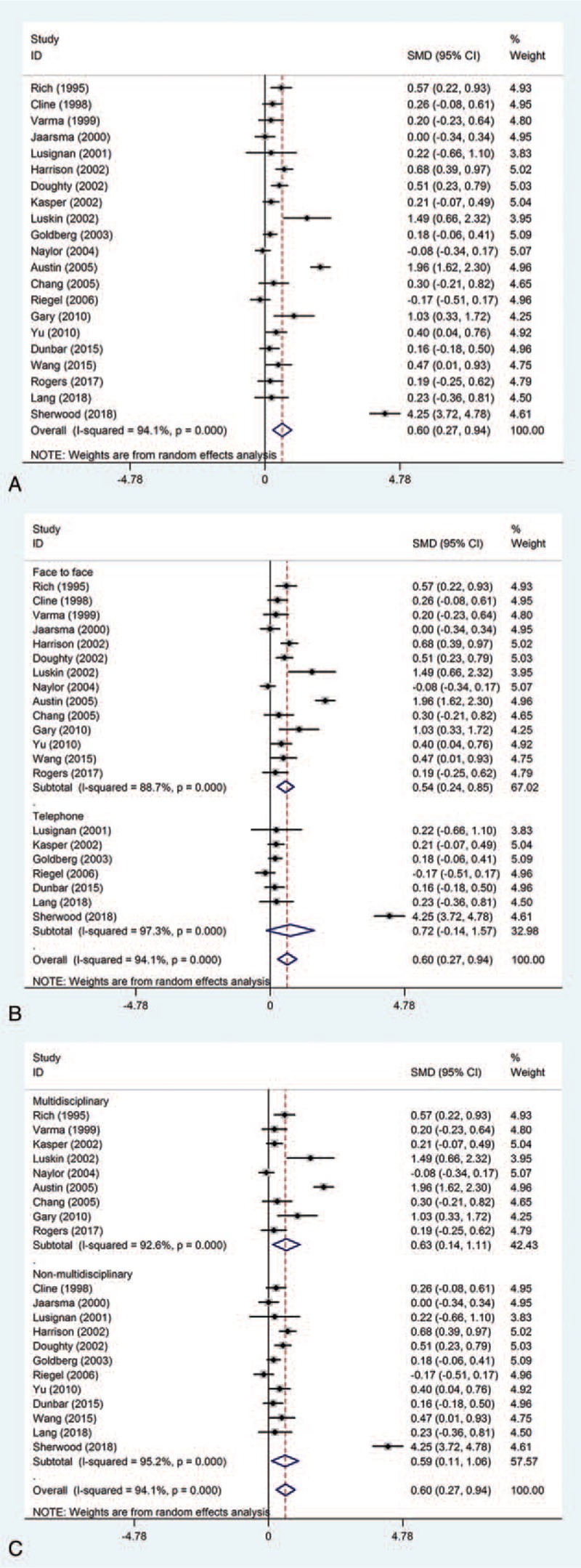
Effect of collaborative care intervention on the QoL of patients with chronic heart failure. (A) Total; (B) Intervention types; (C) Team types.
3.2.2. Anxiety level
Four studies examined the effect of collaborative care intervention on anxiety level in patients with chronic heart failure. Pooled analysis was performed with the available data comparing 203 intervention patients with 205 controls. Significantly improved anxiety level was found in the CCI group compared with the routine care group (SMD = 0.33, 95%CI 0.12–0.55, Pheterogeneity = .612, I2 = 0%) (Fig. 4A).
Figure 4.
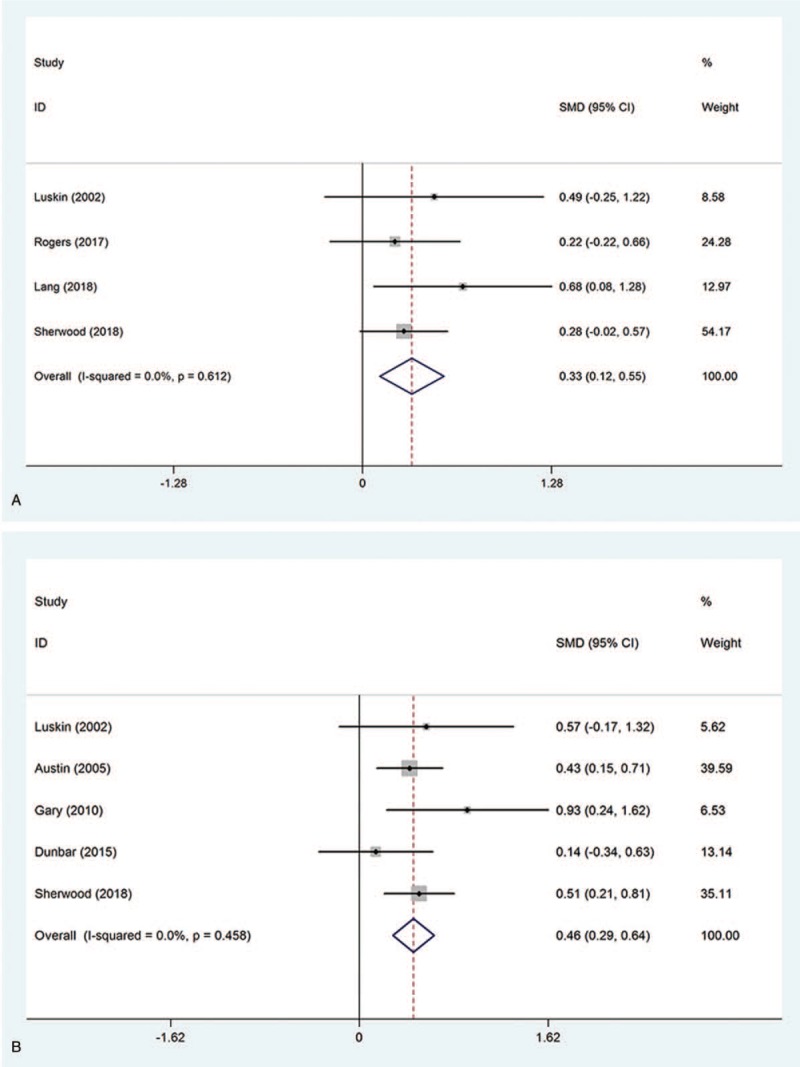
Effect of collaborative care intervention on psychological and physical functions in patients with chronic heart failure. (A) Anxiety level; (B) 6-min walk test.
3.2.3. Six-min walk
Five studies assessed the effect of collaborative care intervention on 6-min walk test in patients with chronic heart failure. Pooled analysis was performed with the available data comparing 290 intervention patients with 284 controls. Significantly improved 6-min walk test was obtained in the CCI group compared with the routine care group (SMD = 0.46, 95%CI 0.29–0.64, Pheterogeneity = .458, I2 = 0%) (Fig. 4B).
3.3. Sensitivity
To evaluate the stability of results, sensitivity analysis was performed, with one study removed from the analysis at a time. As shown in Figure 5, the corresponding pooled results did not significantly change, regardless of which study was removed, suggesting that the results were robust.
Figure 5.
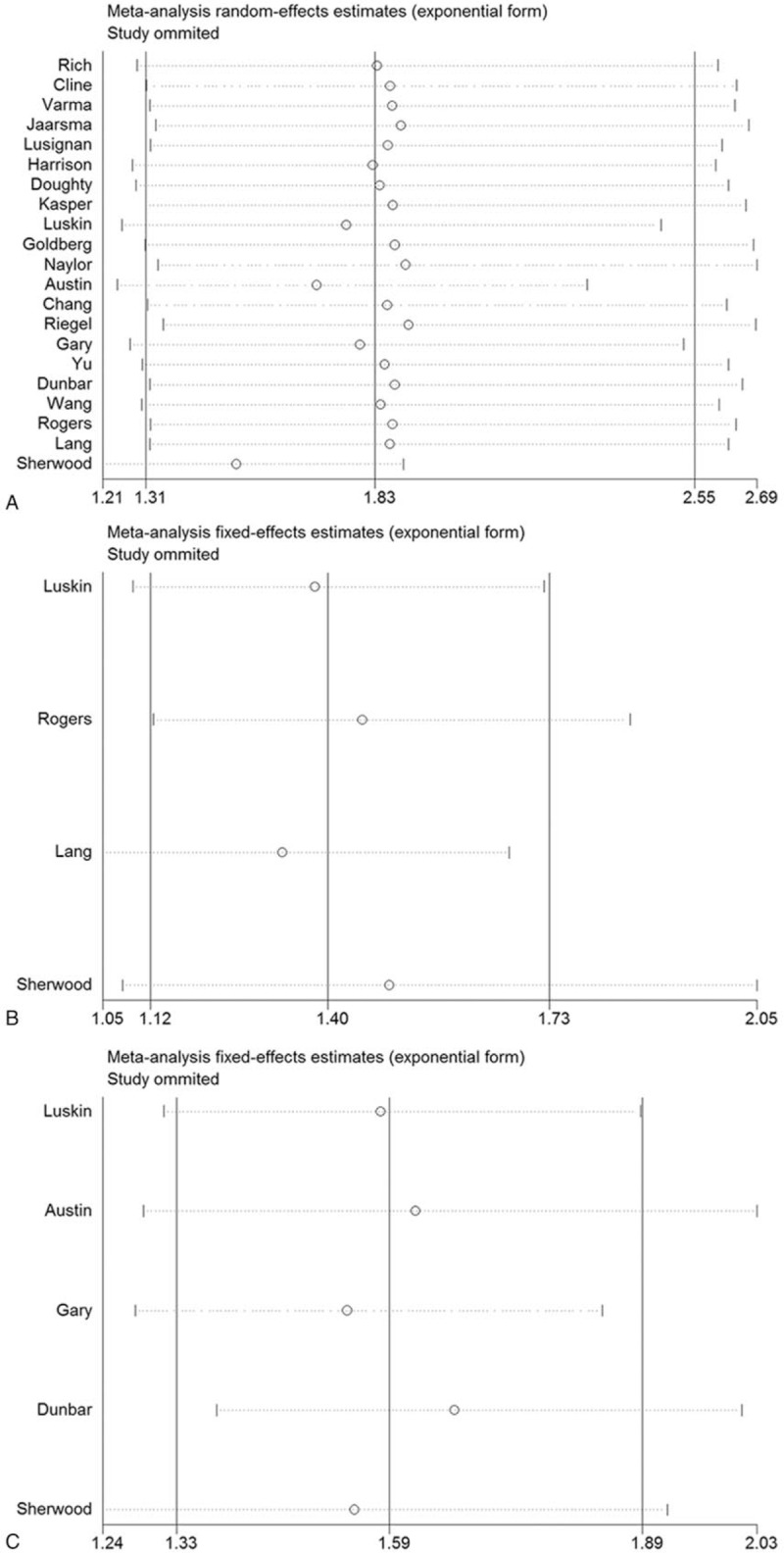
Sensitivity analysis of the effect of collaborative care intervention in patients with chronic heart failure. (A) Quality of life; (B) Anxiety level; (C) 6-min walk test.
3.4. Publication bias
In this meta-analysis, publication bias was assessed by the Begg's and Egger's tests, respectively. The Begg's test revealed the existence of publication bias for QoL (Begg's test P = .027; Egger's test P = .156) (Fig. 6). The cut and fill method showed no need for additional literature (Figure S1).
Figure 6.
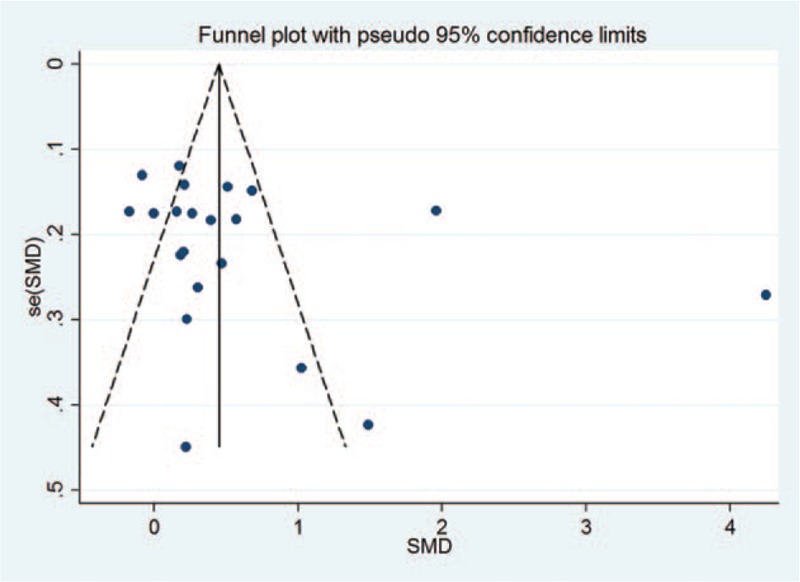
Funnel plot for publication bias assessment. Each point represents a separate study for the indicated association.
4. Discussion
To the best of our knowledge, this is the largest study so far analyzing data from 21 trials with 2999 participants and evaluating the efficacy of collaborative care intervention in patients with chronic heart failure. Our results showed that collaborative care intervention effectively improved the QoL, anxiety level and physical function (6-min walk test) compared with routine care intervention. Furthermore, face-to-face interventions conferred a greater improvement of the QoL compared with telephone-only interventions. The efficacy of psychosocial intervention in patients with chronic heart failure have been assessed in previous meta-analyses. Recently, Samartzis et al.[30] performed a meta-analysis of psychosocial intervention in patients with chronic heart failure, and demonstrated that psychosocial intervention is an efficacious means of QoL improvement. Compared with Samartzis’ work, we included more eligible studies[25–29] and performed a comprehensive analysis of psychological and physical functions, as well as the quality of life, while Samartzis et al. only focused on QoL. Furthermore, Samartzis et al. only consisted of 16 studies, while the current study analyzed data from 21 studies. Our results showed that collaborative care intervention not only improved QoL, but also ameliorated psychological (anxiety) and physical (6-min walk test) functions.
Patients with heart failure often report high levels of mental distress and decreased quality of life (QoL). Therefore, interventions improving their QoL and other positive psychosocial outcomes are needed. Effective interventions include multidisciplinary approaches; repeated face-to-face contact; patient education with a focus on self-care, weight monitoring and medication; and proactive drug optimization rather than relying solely on patient triggers. Structured assessments by telephone or remote monitoring may be inefficient as they mainly focus on HF, which accounts for less than half of all readmissions. This lack of effectiveness of remote support has also been demonstrated in large medical RCTs assessing high-risk HF patients. Strategies including face-to-face assessments may be more effective than remote monitoring in addressing noncardiovascular conditions, which account for about 40% of readmissions. This study also demonstrated that face-to-face interventions resulted in greater improvement of the QoL compared with telephone-only interventions. Anxiety is a common mood disorder in patients with heart failure, which may impair functional ability and aggravate symptoms. This in turn leads to social aggregation, as well as the inability of patients to learn about and care for their disease. About 40% of HF patients suffer from major anxiety. In this study, we found that collaborative care intervention effectively improved anxiety compared with routine care intervention.
Several limitations should be kept in mind while interpreting the current results. Firstly, meta-analyses may be biased when the literature search fails to identify all relevant trials or in case selection criteria for including a trial are applied in a subjective manner. Secondly, language can also introduce a bias. Specifically, we only selected English reports, and other eligible studies were excluded. Thirdly, there is potential publication bias in this study since we did not take into consideration some unpublished papers and abstracts, whose data were not available to us. Fourthly, some clinical items which interfere with QoL, such as age, type of HF (preserved or reduced EF), length of the disease, comorbidities, etc. Finally, several studies had small sample sizes and short follow-up periods, which might reduce the statistical power of this meta-analysis.
In conclusion, despite its limitations, this meta-analysis confirmed that collaborative care intervention effectively improved the quality of life, and psychological (anxiety) and physical (6-min walk test) functions in patients with chronic heart failure compared with routine intervention. Furthermore, face-to-face interventions resulted in a greater improvement of the QoL compared with telephone-only interventions.
Author contributions
Conceptualization: Xiaoting Cui.
Data curation: Wenyi Dong, Hongxiao Zheng, Haiyan Li.
Formal analysis: Xiaoting Cui, Wenyi Dong, Haiyan Li.
Investigation: Wenyi Dong, Haiyan Li.
Methodology: Xiaoting Cui, Wenyi Dong, Hongxiao Zheng, Haiyan Li.
Project administration: Xiaoting Cui, Wenyi Dong.
Resources: Xiaoting Cui.
Software: Xiaoting Cui, Haiyan Li.
Supervision: Xiaoting Cui.
Validation: Xiaoting Cui, Hongxiao Zheng.
Visualization: Hongxiao Zheng.
Writing – original draft: Xiaoting Cui, Wenyi Dong.
Writing – review & editing: Xiaoting Cui, Wenyi Dong, Hongxiao Zheng, Haiyan Li.
Supplementary Material
Footnotes
Abbreviations: CCI = Collaborative care intervention, CIs = confidence intervals, HF = heart failure, QoL = quality of life, RCTs = randomized controlled trials, SMDs = standard mean differences.
XC and WD contributed equally to this work.
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this study.
Supplemental Digital Content is available for this article.
References
- [1].Bleumink GS, Knetsch AM, Sturkenboom MC, et al. Quantifying the heart failure epidemic: prevalence, incidence rate, lifetime risk and prognosis of heart failure: the Rotterdam Study. Eur Heart J 2004;25:1614–9. [DOI] [PubMed] [Google Scholar]
- [2].Go AS, Mozaffarian D, Roger VL, et al. Executive summary: heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation 2014;129:399–410. [DOI] [PubMed] [Google Scholar]
- [3].Mozaffarian D, Benjamin EJ, Go AS, et al. Heart Disease and Stroke Statistics—2016 Update: A Report From the American Heart Association. Circulation 2016;133:e38. [DOI] [PubMed] [Google Scholar]
- [4].Rutledge T, Reis VA, Linke SE, et al. Depression in heart failure: a meta-analytic review of prevalence, intervention effects, and associations with clinical outcomes. J Am Coll Cardiol 2006;48:1527–37. [DOI] [PubMed] [Google Scholar]
- [5].Pantilat SZ, Steimle AE. Palliative care for patients with heart failure. JAMA 2004;292:2476–82. [DOI] [PubMed] [Google Scholar]
- [6].Bekelman DB, Havranek EP, Becker DM, et al. Symptoms, depression, and quality of life in patients with heart failure. J Cardiac Fail 2007;13:643–8. [DOI] [PubMed] [Google Scholar]
- [7].Bekelman DB, Nowels CT, Retrum JH, et al. Giving voice to patients’ and family caregivers’ needs in chronic heart failure: implications for palliative care programs. J Palliat Med 2011;14:1317–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Rich MW, Beckham V, Wittenberg C, et al. A multidisciplinary intervention to prevent the readmission of elderly patients with congestive heart failure. N Engl J Med 1995;333:1190–5. [DOI] [PubMed] [Google Scholar]
- [10].Cline C, Israelsson B, Willenheimer R, et al. Cost effective management programme for heart failure reduces hospitalisation. Heart 1998;80:442–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Varma S, McElnay JC, Hughes CM, et al. Pharmaceutical care of patients with congestive heart failure: interventions and outcomes. Pharmacotherapy 1999;19:860–9. [DOI] [PubMed] [Google Scholar]
- [12].Jaarsma T, Halfens R, Tan F, et al. Self-care and quality of life in patients with advanced heart failure: the effect of a supportive educational intervention. Heart Lung 2000;29:319–30. [DOI] [PubMed] [Google Scholar]
- [13].de Lusignan S, Wells S, Johnson P, et al. Compliance and effectiveness of 1 year's home telemonitoring. The report of a pilot study of patients with chronic heart failure. Eur J Heart Fail 2001;3:6.723–30. [DOI] [PubMed] [Google Scholar]
- [14].Harrison MB, Browne GB, Roberts J, et al. Quality of life of individuals with heart failure: a randomized trial of the effectiveness of two models of hospital-to-home transition. Med Care 2002;271-282: [DOI] [PubMed] [Google Scholar]
- [15].Doughty R, Wright S, Pearl A, et al. Randomized, controlled trial of integrated heart failure management. The Auckland Heart Failure Management Study. Eur Heart J 2002;23:139–46. [DOI] [PubMed] [Google Scholar]
- [16].Kasper EK, Gerstenblith G, Hefter G, et al. A randomized trial of the efficacy of multidisciplinary care in heart failure outpatients at high risk of hospital readmission. J Am Coll Cardiol 2002;39:471–80. [DOI] [PubMed] [Google Scholar]
- [17].Luskin F, Reitz M, Newell K, et al. A controlled pilot study of stress management training of elderly patients with congestive heart failure. Prevent Cardiol 2002;5:168–74. [DOI] [PubMed] [Google Scholar]
- [18].Goldberg LR, Piette JD, Walsh MN, et al. Randomized trial of a daily electronic home monitoring system in patients with advanced heart failure: the Weight Monitoring in Heart Failure (WHARF) trial. Am Heart J 2003;146:705–12. [DOI] [PubMed] [Google Scholar]
- [19].Naylor MD, Brooten DA, Campbell RL, et al. Transitional care of older adults hospitalized with heart failure: a randomized, controlled trial. J Am Geriatr Soc 2004;52:675–84. [DOI] [PubMed] [Google Scholar]
- [20].Austin J, Williams R, Ross L, et al. Randomised controlled trial of cardiac rehabilitation in elderly patients with heart failure. Eur J Heart Fail 2005;7:411–7. [DOI] [PubMed] [Google Scholar]
- [21].Chang B-H, Hendricks A, Zhao Y, et al. A relaxation response randomized trial on patients with chronic heart failure. J Cardiopulm Rehabil Prevent 2005;25:149–57. [DOI] [PubMed] [Google Scholar]
- [22].Riegel B, Carlson B, Glaser D, et al. Randomized controlled trial of telephone case management in Hispanics of Mexican origin with heart failure. J Cardiac Fail 2006;12:211–9. [DOI] [PubMed] [Google Scholar]
- [23].Gary RA, Dunbar SB, Higgins MK, et al. Combined exercise and cognitive behavioral therapy improves outcomes in patients with heart failure. J Psychosom Res 2010;69:119–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Yu DS, Lee DT, Woo J. Improving health-related quality of life of patients with chronic heart failure: effects of relaxation therapy. J Adv Nurs 2010;66:392–403. [DOI] [PubMed] [Google Scholar]
- [25].Dunbar SB, Reilly CM, Gary R, et al. Randomized clinical trial of an integrated self-care intervention for persons with heart failure and diabetes: quality of life and physical functioning outcomes. J Card Fail 2015;21:719–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Wang TC, Huang JL, Ho WC, et al. Effects of a supportive educational nursing care programme on fatigue and quality of life in patients with heart failure: a randomised controlled trial. Eur J Cardiovasc Nurs 2016;15:157–67. [DOI] [PubMed] [Google Scholar]
- [27].Rogers JG, Patel CB, Mentz RJ, et al. Palliative Care in Heart Failure: The PAL-HF Randomized, Controlled Clinical Trial. J Am Coll Cardiol 2017;70:331–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].2018;Lang CC, Smith K, Wingham J. A randomised controlled trial of a facilitated home-based rehabilitation intervention in patients with heart failure with preserved ejection fraction and their caregivers: the REACH-HFpEF Pilot Study. 8:e019649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Sherwood A, Blumenthal JA, Koch GG, et al. Effects of coping skills training on quality of life, disease biomarkers, and clinical outcomes in patients with heart failure: a randomized clinical trial. Circ Heart Fail 2017;10:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Samartzis L, Dimopoulos S, Tziongourou M, et al. Effect of psychosocial interventions on quality of life in patients with chronic heart failure: a meta-analysis of randomized controlled trials. J Card Fail 2013;19:125–34. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


