Abstract
Introduction
Intestinal endometriosis is considered the most severe form of deep endometriosis, the rectosigmoid being involved in about 90% of cases of bowel infiltration. Transvaginal sonography (TVS) and magnetic resonance imaging (MRI) have been used for noninvasive diagnosis and preoperative mapping of rectosigmoid endometriosis (RE), but no consensus has been reached so far regarding which method is the most accurate in this setting.
Objective
We aimed at performing a systematic review and meta-analysis to compare the accuracy of TVS versus MRI in the diagnosis of RE in a same population.
Methods
A systematic review was conducted in accordance with the PRISMA guidelines. Studies were identified by searching the MEDLINE, Embase, and LILACS databases, as well the reference lists of retrieved articles, through February 2019. We included all cross-sectional studies that evaluated the accuracy of TVS versus MRI in the diagnosis of RE within a same sample of subjects and that used surgical findings with histological confirmation as the gold standard. The QUADAS-2 instrument was used to evaluate study quality. Sensitivity, specificity, positive likelihood ratios (LR+), and negative likelihood ratios (LR-) for the diagnosis of RE were calculated. This study is registered with PROSPERO, number CRD42017064378.
Results
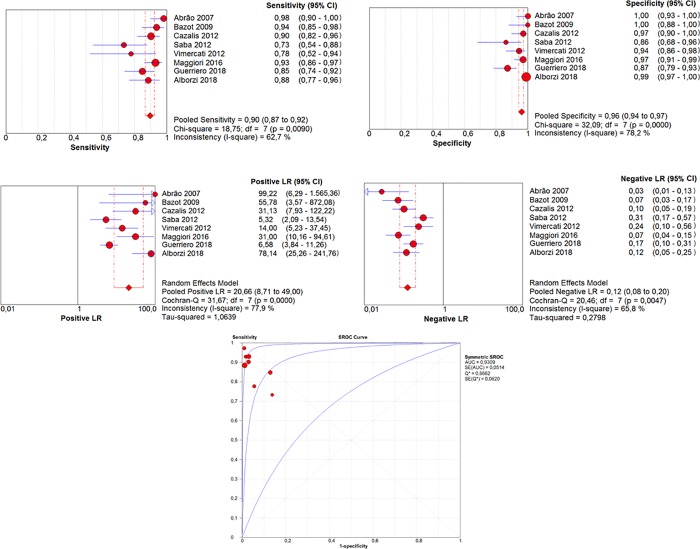
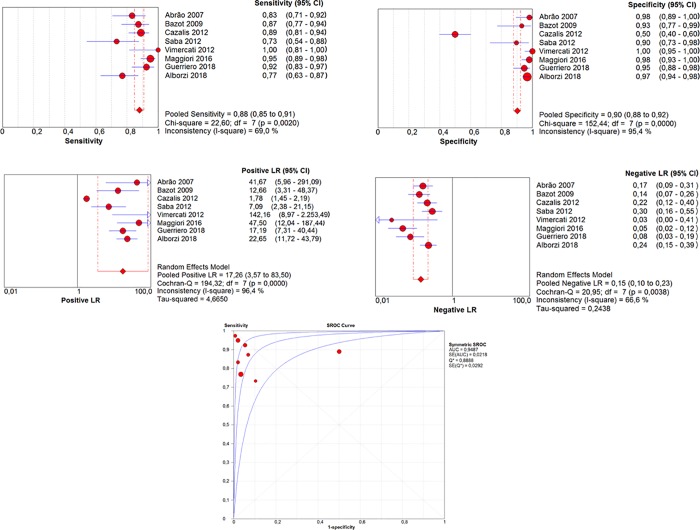
Eight studies (n = 1132) were included in the meta-analysis. The pooled sensitivity, specificity, LR+, and LR- values of MRI for RE were 90% (95% CI, 87–92%), 96% (95% CI, 94–97%), 17.26 (95% CI, 3.57–83.50), and 0.15 (95% CI, 0.10–0.23); values of TVS were 90% [95% CI, 87–92%], 96% (95% CI, 94–97%), 20.66 (95% CI, 8.71–49.00) and 0.12 (95% CI, 0.08–0.20), respectively. Areas under the S-ROC curves (AUC) showed no statistically significant differences between MRI (AUC = 0.948) and TVS (AUC = 0.930) in the diagnosis of RE (P = 0.13). Moreover, considering the average prevalence among the studies of 47.3%, both methods demonstrated similarly high positive post-test probabilities (93.9% for TVS and 94.8% for MRI), and the combined use of them yielded a post-test probability of 99.6%.
Conclusion
MRI and TVS have similarly high accuracy and positive post-test probabilities in the noninvasive diagnosis of RE. Combination of MRI and TVS may increase even further the positive post-test probabilities to near 100%.
Introduction
Endometriosis is defined as the presence of endometrial-like tissue (glands and/or stroma) outside the uterine cavity [1]. It is one of the most common benign diseases in women, affecting about 10% of all women of reproductive age and 20–50% of infertile women [2]. Noninvasive diagnosis is important, as patients with this condition may go through numerous consultations and examinations, with the time from symptom onset to final diagnosis extending up to 7 years [3].
Superficial endometriosis (also called peritoneal endometriosis) occurs with peritoneal infiltration of less than 5 mm depth; ovarian endometriosis includes superficial ovarian implants and endometriomas; deep endometriosis is characterized by foci of depth greater than 5 mm affecting the retrocervix, paracervix, rectovaginal septum, various portions of the digestive tract (e.g., rectosigmoid), ureter, bladder and can obliterate vesicouterine or retouterine pouchs [4, 5]. Exceptionally, endometriotic implants can be found at more distant sites, including the lung, liver, diaphragm, and operative scars.
Bowel endometriosis occurs in 3%-37% of cases [6], and in 90% of them the rectum or sigmoid colon is involved [7, 8], highlighting the relevance of this particular anatomical region, which can be easily approached and assessed by means of transvaginal sonography (TVS) and magnetic resonance imaging (MRI), the most used noninvasive modalities diagnosis and preoperative mapping of endometriotic lesions [9–15].
In the last two decades, several studies have examined the accuracy of imaging modalities such as TVS and MRI for the diagnosis of deep endometriosis, although just a small subset of them separately addressed the rectosigmoid region [16, 17]. Given the heterogeneity of such studies and their results, this systematic review and meta-analysis were conducted to compare the accuracy of TVS and MRI in the diagnosis of rectosigmoid endometriosis (RE) using only data from studies that compared such modalities within the same set of patients, in order to avoid potential biases compromising external validity when both tests had not been compared within the same population (e.g., reference bias, patient cohort bias, etc). Although a meta-analysis on this subject has been recently published [18], it followed a distinct methodology and selection criteria for included studies, with a smaller number of patients and lower pre-test probability, therefore justifying the addition of a different meta-analysis on this theme to the literature.
Methods
Protocol and registration
This systematic review and meta-analysis were conducted in accordance with the PRISMA guidelines [19] (S1 File). The protocol was registered in the PROSPERO international database (www.crd.york.ac.uk/prospero/; no. CRD42017064378).
Eligibility criteria
The review included cross-sectional studies comparing the accuracy of TVS and MRI for the diagnosis of rectosigmoid endometriosis in patients with suspected deep endometriosis based on clinical history and/or physical examination. Eligible studies applied both modalities to the same patients, followed by surgical and histological confirmation. We imposed no restriction related to details of the technique (e.g., with or without intestinal preparation, introduction of contrast medium by the vaginal and/or rectal route). The main outcome measures were accuracy, sensitivity, specificity, positive and negative predictive values (PPV and NPV), and positive and negative likelihood ratios (LR+ and LR-).
Literature search
Three independent researchers (APCM, WMB, and RS) searched the MEDLINE (via PubMed), Embase, and Latin American and Caribbean Health Science Literature (LILACS) electronic databases for literature published in Portuguese, English, Spanish, or French through February 2019. The following search strategy was used for the MEDLINE databases (Box 1):
Box 1. Search strategy used for the MEDLINE databases
(Endometriosis OR Endometrioses OR Endometrioma OR Endometriomas) AND (Ultrasonography OR Ultrasound OR Ultrasounds OR Sonography OR Echography OR Ultrasonic) AND (Ressonance Magnetic Imaging OR NMR Imaging OR MRI Scan OR MRI Scans OR Imaging, Magnetic Resonance OR MRI) AND (sensitiv*[Title/Abstract] OR sensitivity and specificity[MeSH Terms] OR diagnose[Title/Abstract] OR diagnosed[Title/Abstract] OR diagnoses[Title/Abstract] OR diagnosing[Title/Abstract] OR diagnosis[Title/Abstract] OR diagnostic[Title/Abstract] OR diagnosis[MeSH:noexp] OR diagnostic * [MeSH:noexp] OR diagnosis,differential[MeSH:noexp] OR diagnosis[Subheading:noexp]).
For Embase and LILACS, the search was conducted using the term "endometriosis AND diagnostic." In addition, the reference lists of the selected articles have been manually verified in order to identify potential relevant articles missed in the first step.
Study selection
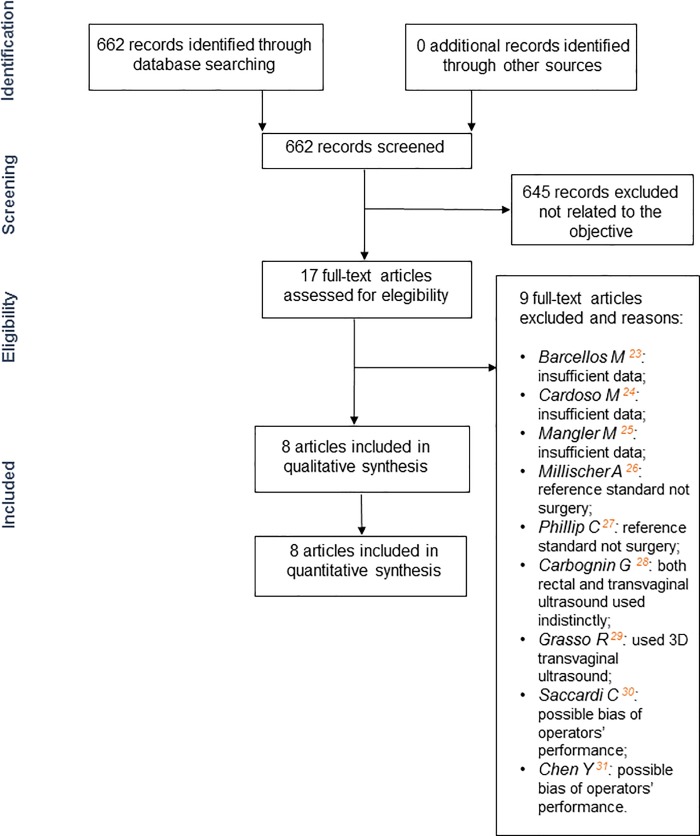
The same three researchers (APCM, WMB and RS) independently evaluated the titles and abstracts of identified publications to assess eligibility for inclusion in the review. They then critically evaluated the full texts of original articles. Disagreements were resolved by consensus. The selection process is summarized in Fig 1.
Fig 1. Flowchart of selection process.
Data collection
One reviewer (APCM) recorded data from each study using an extraction table for diagnostic studies, and a second reviewer (WMB) checked all extracted data. The following data were extracted: number of patients included, study design, patient characteristics, inclusion and exclusion criteria, TVS and MRI results (number of patients with RE based on the surgical findings and histological confirmation), interval between TVS and MRI examinations, interval between TVS/MRI and gold standard examinations, primary outcomes (including true positive, true negative, false positive and false negative), and secondary outcomes (including accuracy, sensitivity, specificity, PPV, NPV, and LR+/LR- for the diagnosis of RE).
Risk of bias assessment
To verify the validity of eligible cross-sectional studies, two reviewers (APCM and WMB) independently analyzed the risk of bias using the QUADAS-2 tool [20]. This tool guides assessment of the risk of bias and applicability to the research question in four domains: patient selection, index test, reference (“gold”) standard, and flow (time between test indices and "gold standard" application). The risk of bias was classified as "low," "high," or "unclear". Studies with "low” risk ratings in at least three of the four domains were considered to be of high quality, and those with "high" or "unclear" risk ratings in at least three of the four domains were considered to be of low quality. Studies with all other combinations of ratings were considered to be of moderate quality.
Analysis
The meta-analysis was performed using the RevMan software (ver. 5.3) [21], obtained from the website of Cochrane Informatics and Department of Knowledge Management. Meta-Disc software (ver. 1.4) [22], available from the Ramón y Cajal University Hospital website (http://www.hrc.es), was used to calculate summary receiving operator characteristic (S-ROC) curves. Both the pretest probability (prevalence) and post-test probability (the chance of RE lesion of deep endometriosis, calculated using meta-analysis data) were also analyzed throughout the studies.
Results
Study selection
The initial search generated 662 citations, and 645 articles were excluded after preliminary review of titles and abstracts because they did not address the main objective. Among the 17 articles that fulfilled the eligibility criteria (comparison of the performance of TVS and MRI in the diagnosis of intestinal endometriosis), five were excluded because they did not provide sufficient data (n = 3) [23–25] or did not use surgery as the reference standard (n = 2) [26, 27]; one study was excluded because vaginal and/or rectal sonography were used indistinctly, precluding the verification of the accuracy of TVS separately [28]; one study was excluded because only tridimensional (rather than conventional) TVS was used [29]; finally, other two articles [30, 31] were excluded because of the possibility of radiologic bias of performers’ experience [32, 33].
Eight studies published between 2007 and 2018 finally met the inclusion criteria, all of them having included surgery and histological analysis as the gold standards [34–41].
Study characteristics
The eight studies included in the analysis gathered 1132 women who underwent TVS and MRI for suspected endometriosis, based on clinical history (pelvic pain or infertility) and/or physical examination (pain and nodulation on palpation). The main characteristics of the studies are summarized in Table 1.
Table 1. Eight final completed studies with their characteristics.
| Study | Year | Country | Design of Study | Number of Patients | Gold Standard | Interval | Interval | Inclusion Criteria | Methods | Bowel Preparation |
|---|---|---|---|---|---|---|---|---|---|---|
| TVUS x MRI | TVUS/MRI x Reference Standard | |||||||||
| Abrão | 2007 | Brazil | Transversal | 104 | Surgery Histology | Not Cited | 3 months | clinical suspicion of endometriosis | Vaginal examination, TVS and MRI | TVS: yes MRI: no |
| Bazot | 2009 | France | Transversal | 92 | Surgery Histology | Not Cited | Not Cited | clinical suspicion of endometriosis | Vaginal and rectal examination, TVS, MRI, RES | TVS: no MRI: no |
| Cazalis | 2012 | France | Transversal | 25 | Surgery Histology | Not Cited | Not Cited | clinical suspicion of endometriosis | TAS, TVS and MRI | TVS: no MRI: no |
| Saba | 2012 | Italy | Transversal | 59 | Surgery Histology | Not Cited | 8 days | clinical suspicion or suspected endometriosis physical examination | TVS and MRI | TVS: yes MRI: no |
| Vimercati | 2012 | Italy | Transversal | 90 | Surgery Histology | Not Cited | Not Cited | clinical suspicion or suspected endometriosis image examination | TVS and ColonoMRI | TVS: no MRI: yes |
| Maggiore | 2016 | Italy | Transversal | 286 | Surgery Histology | Not Cited | 3 months | clinical suspicion of endometriosis | TVS with rectal enema and MRI with rectal enema | TVS: yes MRI: no |
| Guerriero | 2018 | Spain / Italy | Transversal | 159 | Surgery Histology | 30 days | 30 days | clinical suspicion of endometriosis | 2D and 3D TVS and MRI | TVS: no MRI: no |
| Alborzi | 2018 | Iran | Transversal | 317 | Surgery Histology | Not Cited | Not Cited | clinical suspicion or suspected endometriosis physical examination | TVS, TRS, MRI | TVS: yes MRI: no |
TVS: transvaginal sonography; MRI: magnetic resonance imaging; RES: rectal endoscopic sonography; TRS: transrectal sonography
The design, performance, and analysis of results were similar among studies. Examinations in all studies were conducted independently, and the examiners were not aware of the results of physical examination (when appropriate) or other procedures. The protocols used in TVS and MRI in the six selected studies are summarized in Tables 2 and 3, respectively.
Table 2. Transvaginal ultrasound protocol.
| Study | Transducer (MHz) | Number of Examiners | Bowel Preparation | Bowel Opacification | Vaginal Opacification |
|---|---|---|---|---|---|
| Abrão | 5,0–9,0 | 1 | Yes | N/S | N/S |
| Bazot | 5,0–9,0 | 1 | No | N/S | N/S |
| Cazalis | N/S | N/S | No | N/S | N/S |
| Saba | 6,5–7,0 | 1 | N/S | N/S | N/S |
| Vimercati | 5,0–9,0 | N/S | No | No | No |
| Maggiore | N/S | 1 | Yes | Yes | N/S |
| Guerriero | 5,0–9,0 | 1 | N/S | No | No |
| Alborzi | 7,5 | 1 | Yes | N/S | N/S |
N/S: not stated)
Table 3. Magnetic resonance imaging protocol.
| Study | Tesla | Bobine | Number of Examiners | Bowel Preparation | Bowel Opacification | Vaginal Opacification | Fast | Antispasmodic | Gadolinium | Resumed Protocol |
|---|---|---|---|---|---|---|---|---|---|---|
| Abrão | 1,5 | Phased Array | 1 | No | N/S | Yes | 4 hours | Yes | Yes | N/S |
| Bazot | 1,5 | N/S | 1 | Yes | N/S | N/S | 3 hours | Yes | Yes | T2 ax, T2 sag; T1 GE with and without fatsat |
| Cazalis | N/S | N/S | N/S | Yes | N/S | No | N/S | N/S | Yes | T2 ax, T2 sag, T2 cor; T1 with and without fatsat |
| Saba | 1,5 | Phased Array | N/S | Yes | N/S | N/S | 6 hours | Yes | Yes | T2 ax, T2 sag, T2 cor; T1 ax, T1 sag, T1 cor; T1 fatsat with and without Gd |
| Vimercati | 1,5 | Phased Array (4 channels) | N/S | Yes | Yes | N/S | N/S | Yes | Yes | T2 ax, T2 sag, T2 cor; T1 ax, T1 sag |
| Maggiore | 1,5 | Phased Array (8 channels) | 1 | N/S | Yes | N/S | N/S | No | Yes | T2 ax, T2 sag; T1 fatsat cor and sag; T1 cor; DWI Ax; FIESTA cor |
| Guerriero | 1,5 | Body Coil | 1 | N/S | N/S | N/S | 3 hours | Yes | Yes | T2 ax, T2 sag, T2 cor; T1 ax, T1 sag, T1 cor; T1 fatsat with and without Gd |
| Alborzi | 1,5 | Body Coil | 1 | N/S | N/S | Yes | 4 hours | Yes | Yes | T2 ax, T2 sag, T2 cor; T1 ax, T1 sag, T1 cor; T1 fatsat ax and sag with and without Gd |
N/S: not stated
Risk of bias
Overall, the quality of the studies was good (Tables 4 and 5). According to summary QUADAS-2 ratings, seven studies were classified as of high quality and one study was classified as of moderate quality. The risks of bias in the index test and reference standard domains were similar for all studies. In terms of timing and flow, the interval between TVS and MRI examinations was not reported in any study and the interval between the TVS/MRI and reference standard examinations was not reported in three studies [35, 36, 38]; these omissions may have introduced some bias.
Table 4. Bias risk according to QUADAS.
| Abrão | Bazot | Cazalis | Saba | Vimecarti | Maggiore | Guerriero | Alborzi | |||
|---|---|---|---|---|---|---|---|---|---|---|
|
PATIENT SELECTION |
Signaling questions | Was a consecutive or random sample of patients enrolled? | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes |
| Was a case-control design avoided? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | ||
| Did the study avoided inappropriate exclusions? | Yes | Unclear | Unclear | Yes | Yes | Yes | Yes | Yes | ||
| Risk of bias | Could the selection of patients have introduced bias? | Low | Low | Moderate | Low | Low | Low | Low | Low | |
| Concerns regarding applicability | Are there concerns that the included patients do not match the review question? | Low | Low | Low | Low | Low | Low | Low | Low | |
|
INDEX TEST |
Signaling questions | Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| If a threshold was used, was it prespecified? | Yes | Yes | Unclear | Yes | Yes | Yes | Yes | Yes | ||
| Risk of bias | Could the conduct or interpretation of the index test have introduced bias? | Low | Low | Low | Low | Low | Low | Low | Low | |
| Concerns regarding applicability | Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low | Low | Low | Low | Low | low | Low | Low | |
|
REFERENCE STANDARD |
Signaling questions | Is the reference standard likely to correctly classify the target condition? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Were the references standard results interpreted without knowledge of the results of the index test? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | ||
| Risk of bias | Could the reference standard, its conduct, or its interpretation have introduced bias? | Low | Low | Low | Low | Low | Low | Low | Low | |
| Concerns regarding applicability | Are there concerns that the target condition as defined by the reference standard does not match the review question? | Low | Low | Low | Low | Low | Low | Low | Low | |
| Signaling questions | Was there an appropriate interval between index test(s) and reference standard? | Yes | Unclear | Unclear | Yes | Unclear | Yes | Yes | Unclear |
Table 5. Summary of bias risk according to QUADAS-2 (L: low risk, H: high risk, M: moderate risk).
| Study | Patient Selection | Index Test | Reference Standard | Flow and Timing | Study Quality |
|---|---|---|---|---|---|
| Abrão | L | L | L | L | High |
| Bazot | L | L | L | M | High |
| Cazalis | H | L | L | M | Moderate |
| Saba | L | L | L | L | High |
| Vimercati | L | L | L | M | High |
| Maggiore | L | L | L | L | High |
| Guerriero | L | L | L | L | High |
| Alborzi | L | L | L | M | High |
Results of quantitative analysis
Table 6 lists the results of quantitative analysis. Prevalence (pretest probability) values ranged from 16.4 to 76%. Sensitivity values ranged from 73.3% to 98.1% for TVS and from 73.3% to 100.0% for MRI. Specificity values ranged from 66.7% to 100.0% for TVS and from 50.0% to 100.0% for MRI.
Table 6. Diagnostic measures of transvaginal sonography and magnetic resonance imaging in the diagnosis of deep rectosigmoid endometriosis.
| Study | Abrão | Bazot | Cazalis | Saba | Vimercati | Maggiore | Guerriero | Alborzi |
|---|---|---|---|---|---|---|---|---|
| Prevalence | 51,9 | 68,5 | 76 | 50,8 | 20 | 52,8 | 42 | 16,4 |
| Sensitivity | ||||||||
| TVS | 98.1 | 93.6 | 73.7 | 73.3 | 77.8 | 92.7 | 84.8 | 88.4 |
| MRI | 83.3 | 87.3 | 89.5 | 73.3 | 100.0 | 95.3 | 92.4 | 76.9 |
| Specificity | ||||||||
| TVS | 100.0 | 100.0 | 66.7 | 86.2 | 94.4 | 97.0 | 87.1 | 98.8 |
| MRI | 98.0 | 93.1 | 50.0 | 89.6 | 100.0 | 97.7 | 94.6 | 96.7 |
| PPV | ||||||||
| TVS | 100.0 | 100.0 | 87.5 | 84.6 | 77.8 | 97.2 | 82.4 | 93.9 |
| MRI | 97.8 | 96.5 | 85.0 | 88.0 | 100.0 | 97.9 | 92.4 | 81.63 |
| NPV | ||||||||
| TVS | 98.0 | 87.9 | 44.4 | 75.7 | 94.4 | 92.2 | 89.0 | 97.8 |
| MRI | 84.4 | 77.1 | 60.0 | 76.5 | 100.0 | 94.9 | 94.6 | 95.5 |
| LR+ | ||||||||
| TVS | 2.21 | 5.32 | 14.0 | 31.29 | 6.57 | 78.14 | ||
| MRI | 41.67 | 12.66 | 1.79 | 7.09 | 42.91 | 17.2 | 22.65 | |
| LR- | ||||||||
| TVS | 0.02 | 0.06 | 0.39 | 0.31 | 0.24 | 0.08 | 0.174 | 0.12 |
| MRI | 0.17 | 0.14 | 0.21 | 0.30 | 0.00 | 0.05 | 0.08 | 0.24 |
| Accuracy | ||||||||
| TVS | 99.0 | 95.6 | 72.0 | 79.6 | 91.1 | 94.7 | 86.1 | 97.2 |
| MRI | 90.3 | 89.1 | 80.0 | 81.3 | 100.0 | 96.5 | 93.7 | 93.4 |
TVS: transvaginal sonography; MRI: magnetic resonance imaging
Comparative accuracy of TVS and MRI
Sensitivity, specificity, LR+, and LR- values are depicted as forest plots and S-ROC curves (Figs 2 and 3). The areas under the S-ROC curves (AUC) reflected similar accuracy of MRI (AUC = 0.948) and VS (AUC = 0.930) in the diagnosis of RE (P = 0.13). Post-test probability values (for positive test results) were 93.9% for TVS, 94.8% for MRI, and 99.6% for the combination of both examinations.
Fig 2. Results of TVS for the diagnosis of rectosigmoid endometriosis.
Fig 3. Results of MRI for the diagnosis of rectosigmoid endometriosis.
Discussion
Interest in the noninvasive diagnosis of deep endometriosis using laboratory tests (serological markers) or imaging examinations is increasing [42]. In recent decades, TVS and MRI have been shown to be more accurate than other imaging methods (i.e., transrectal sonography, barium enema, computed tomography colonography) in the detection of RE; they are also less invasive and do not require sedation. Few studies, however, have compared the accuracy of TVS and MRI in the diagnosis of RE in the same set of patients. Comparison of these methods by applying them inhomogeneously (just one or another) in different populations in order to subsequently pooling results and comparing accuracy values across studies is methodologically problematic because each study may have inherent biases (e.g., reference bias, patient cohort bias, etc) that, despite not limiting their internal validity, may compromise their external validity. Moreover, some of these studies were performed with small sample sizes, which limit their statistical power and makes it a meta-analytic approach desirable.
This meta-analysis included eight studies with a total of 1132 patients who underwent TVS and MRI, enabling comparison of the performance of these modalities in the same population. It showed that MRI and TVS have similar performance in the diagnosis of rectosigmoid endometriosis. Moreover, the post-test probability findings for TVS and MRI did not differ. The study findings suggest that the combined use of TVS and MRI is reasonable, as the chance of noninvasively and accurately diagnosing RE rises to practically 100% when both examinations yield positive results.
Three recent systematic reviews [18, 42, 43] have involved the comparison of noninvasive diagnostic tests for endometriosis, demonstrating the interest of the scientific community in identifying accurate modalities for this purpose. Nisenblat et al. [42], despite presenting separate mean estimates for each imaging modality (TVS, 14 studies, 15 data sets, 1616 participants, sensitivity of 0.90 and specificity of 0.96; MRI, six studies, seven data sets, 612 participants, sensitivity of 0.92 and specificity of 0.96) have not meta-analyzed studies directly comparing these modalities.
Guerriero et al. [18], compared TVS and MRI findings from studies employing the same population. For MRI detection of RE, they found nearly similar sensitivity and specificity values in comparison to us (of 0.85 and 0.95, respectively, versus the values of 0.88 and 0.90 found in our study). For TVS, these values were of 0.85 and 0.96, respectively, against 0.90 and 0.96 found in our study. Methodological differences between the two meta-analyses, however, must be taken into account, especially in terms of study selection. Given the recent date of our meta-analysis, for example, we included three new additional studies, by Maggiore et al. [39], Guerriero et al. [40] and Alborzi et al. [41]. Maggiore et al. [39] and Alborzi et al. [41] published the two largest series of patients to date (n = 286 and 317, respectively). Therefore, our meta-analysis encompassed a total of 1132 patients in a 12-year timeframe (from 2007–2018), which means 62.6% more than Guerriero et al.’s study [18]. Finally, despite having found no significant statistical difference between such methods in terms of pooled sensitivity and specificity, these authors did not perform comparisons of the AUC values for both modalities, nor estimated the impact of combining such modalities on the positive post-test probabilities of a RE diagnosis, as did we.
Finally, Bazot et al. [43] provided an overview of published reviews and discussed the imaging protocols, the definition of endometriosis per se, and the need for laparoscopic and histological confirmation in the diagnosis of the disease.
The studies included in this review and meta-analysis have some concerns that should be addressed. The first issue is bowel preparation, which has been shown to increase the accuracy of intestinal lesion detection [44]. No study included in this analysis involved intestinal preparation for both TVS and MRI; this procedure was performed for one examination in some studies [34, 37–39] and for neither examination in others [35, 36]. Even without standard bowel preparation, however, the rate of rectosigmoid endometriosis detection was quite high in these studies. The second concern regards the intestinal endometriosis represented only by rectosigmoid disease, preventing that we could extend the scope of such meta-analysis to other intestinal locations beyond the rectosigmoid. Other locations, especially those in the right iliac fossa (cecum, appendix, and ileum) were not mentioned in any study. In all studies, only the rectosigmoid colon was investigated, which represents a limitation in the evaluation of all intestinal endometriotic lesions. The third issue is that no study involved the comparison of lesion characteristics, such as size (along three axes), maximum depth of the affected layer, and circumference. These characteristics have great impact on surgical planning, including the selection of intestinal resection type (linear, discoidal, or segmental). The last issue regards to the prevalence of rectosigmoid endometriosis, which ranged from 16.4 to 76%. Selection bias may have affected the diagnostic performance of the imaging examinations, as reported by Guerriero et al. [18].
Regarding the choice between MRI and US for diagnosing rectosigmoid endometriosis, some aspects should be considered. US is a safe, low cost and widespread technique, with similar diagnostic accuracy and post-test probabilities in comparison to MRI in terms of available published data. However, US is also operator-dependent, and errors in technique, equipment quality, and limitations of operator’s experience are all factors that may lead to misinterpretations and misdiagnoses [45]. MRI, on the other hand, despite its higher costs, has useful advantages in the context of deep pelvic endometriosis, such as the capacity of obtaining multiplanar sequences with distinct relaxation times, allowing an unmatched ability to differentiate normal from diseased tissues [45]; moreover, the standardized obtained images by the MRI scanner can be interpreted remotely by an expert (rising the accuracy), while with US the process of obtaining the diagnostic images depends inherently on the ability and experience of the operator. Therefore, the rationale behind the choice of these methods should take into account a range of variables, such as local characteristics of the institution, availability of each method, costs, available budget, radiologists’ experience with each method, etc. In an ideal scenario (an institution with high expertise in imaging of endometriosis), we think that TVS could be used as an initial method, while MRI could be reserved in doubtful cases or to rise the post-test probabilites to near 100%. Moreover, MRI could be used in the preoperative planning of patients selected for surgical treatment.
Conclusion
The noninvasive diagnosis of RE can be made based on MRI and TVS with good sensitivity and specificity. The review and meta-analysis revealed that both methods have high and similar values of diagnostic accuracy and positive post-test probabilities. The state of art in the diagnostic imaging management of RE should combine the two methods. Both examinations can be performed on the same day, requiring a single bowel preparation, which we believe is important to increase the detection rate of small lesions.
Supporting information
(DOC)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Clement MD. Diseases of the peritoneum. (including endometriosis). In: Kurman. RJ, ed. Blaustein's pathology of the female genital tract. 5th ed.
- 2.Wheeler JM. Epidemiology of endometriosis-associated infertility. J Reprod Med. 1989;34:41–6. [PubMed] [Google Scholar]
- 3.Arruda MS, Petta CA, Abrao MS, Benetti-Pinto CL. Time elapsed from onset of symptoms to diagnosis of endometriosis in a cohort study of Brazilian women. Hum Reprod 2003;18:756–759. [DOI] [PubMed] [Google Scholar]
- 4.Nisolle M, Donnez J. Peritoneal endometriosis, ovarian endometriosis, and adenomyotic nodules of the rectovaginal septum are three different entities. Fertil Steril 1997;68:585–596. [DOI] [PubMed] [Google Scholar]
- 5.Cornillie FJ, Oosterlynck D, Lauweryns JM, Koninckx PR. Deeply infiltrating pelvic endometriosis: histology and clinical significance. Fertil Steril 1990;53:978–983. [DOI] [PubMed] [Google Scholar]
- 6.Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil Steril 1997;67:817–821. [DOI] [PubMed] [Google Scholar]
- 7.Bailey HR, Ott MT, Hartendorp P. Aggressive surgical management for advanced colorectal endometriosis. Dis Colon Rectum 1994;37:747–753. [DOI] [PubMed] [Google Scholar]
- 8.Campagnacci R, Perretta S, Guerrieri M, Paganini AM, De Sanctis A, Ciavattini A, et al. Laparoscopic colorectal resection for endometriosis. Surg Endosc 2005;19:662–664. 10.1007/s00464-004-8710-7 [DOI] [PubMed] [Google Scholar]
- 9.Piketty M, Chopin N, Dousset B, Millischer-Bellaische AE, Roseau G, Leconte M, et al. Preoperative work-up for patients with deeply infiltrating endometriosis: transvaginal ultrasonography must definitely be the first-line imaging examination. Hum Reprod 2009;24:602–607. 10.1093/humrep/den405 [DOI] [PubMed] [Google Scholar]
- 10.Bazot M, Darai E, Biau DJ, Ballester M, Dessolle L. Learning curve of transvaginal ultrasound for the diagnosis of endometriomas assessed by the cumulative summation test (LC-CUSUM). Fertil Steril 2011;95:301–303. 10.1016/j.fertnstert.2010.08.033 [DOI] [PubMed] [Google Scholar]
- 11.Bazot M, Malzy P, Cortez A, Roseau G, Amouyal P, Darai E. Accuracy of transvaginal sonography and rectal endoscopic sonography in the diagnosis of deep infiltrating endometriosis. Ultrasound Obstet Gynecol 2007;30:994–1001. 10.1002/uog.4070 [DOI] [PubMed] [Google Scholar]
- 12.Arrive L, Hricak H, Martin MC. Pelvic endometriosis: MR imaging. Radiology 1989;171:687–692. 10.1148/radiology.171.3.2717739 [DOI] [PubMed] [Google Scholar]
- 13.Bis KG, Vrachliotis TG, Agrawal R, Shetty AN, Maximovich A, Hricak H. Pelvic endometriosis: MR imaging spectrum with laparoscopic correlation and diagnostic pitfalls. Radiographics 1997;17:639–655. 10.1148/radiographics.17.3.9153703 [DOI] [PubMed] [Google Scholar]
- 14.Siegelman ES, Outwater E, Wang T, Mitchell DG. Solid pelvic masses caused by endometriosis: MR imaging features. AJR Am J Roentgenol 1994;163:357–361. 10.2214/ajr.163.2.8037030 [DOI] [PubMed] [Google Scholar]
- 15.Bazot M, Darai E, Hourani R, Thomassin I, Cortez A, Uzan S, et al. Deep pelvic endometriosis: MR imaging for diagnosis and prediction of extension of disease. Radiology 2004;232:379–389. 10.1148/radiol.2322030762 [DOI] [PubMed] [Google Scholar]
- 16.Goncalves MO, Dias JA Jr., Podgaec S, Averbach M, Abrao MS. Transvaginal ultrasound for diagnosis of deeply infiltrating endometriosis. Int J Gynaecol Obstet 2009;104:156–160. 10.1016/j.ijgo.2008.10.005 [DOI] [PubMed] [Google Scholar]
- 17.Hudelist G, Oberwinkler KH, Singer CF, Tuttlies F, Rauter G, Ritter O, et al. Combination of transvaginal sonography and clinical examination for preoperative diagnosis of pelvic endometriosis. Hum Reprod 2009;24:1018–1024. 10.1093/humrep/dep013 [DOI] [PubMed] [Google Scholar]
- 18.Guerriero S, Saba L, Pascual MA, Ajossa S, Rodriguez I, Mais V, et al. Transvaginal ultrasound vs magnetic resonance imaging for diagnosing deep infiltrating endometriosis: systematic review and meta-analysis. Ultrasound Obstet Gynecol 2018;51:586–595. 10.1002/uog.18961 [DOI] [PubMed] [Google Scholar]
- 19.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 2009;62:e1–34. 10.1016/j.jclinepi.2009.06.006 [DOI] [PubMed] [Google Scholar]
- 20.Whiting P, Rutjes AW, Reitsma JB, Bossuyt PM, Kleijnen J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol 2003;3:25 10.1186/1471-2288-3-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.The Nordic Cochrane Centre. (2014). Review Manager. Cochrane Collaboration.
- 22.Zamora J, Abraira V, Muriel A, Khan K, Coomarasamy A. Meta-DiSc: a software for meta-analysis of test accuracy data. BMC Med Res Methodol 2006;6:31 10.1186/1471-2288-6-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barcellos MB, Lasmar B, Lasmar R. Agreement between the preoperative findings and the operative diagnosis in patients with deep endometriosis. Arch Gynecol Obstet 2016;293:845–850. 10.1007/s00404-015-3892-x [DOI] [PubMed] [Google Scholar]
- 24.Cardoso MM, Werner Junior H, Berardo PT, Coutinho Junior AC, Domingues MNA, Gasparetto EL, et al. Evaluation of agreement between transvaginal ultrasonography and magnetic resonance imaging of the pelvis in deep endometriosis with emphasis on intestinal involvement. Radiologia Brasileira 2009;42:89–95. [Google Scholar]
- 25.Mangler M, Medrano N, Bartley J, Mechsner S, Speiser D, Schneider A, et al. Value of diagnostic procedures in rectovaginal endometriosis. Aust N Z J Obstet Gynaecol 2013;53:389–394. 10.1111/ajo.12108 [DOI] [PubMed] [Google Scholar]
- 26.Millischer AE, Salomon LJ, Santulli P, Borghese B, Dousset B, Chapron C. Fusion imaging for evaluation of deep infiltrating endometriosis: feasibility and preliminary results. Ultrasound Obstet Gynecol 2015;46:109–117. 10.1002/uog.14712 [DOI] [PubMed] [Google Scholar]
- 27.Philip CA, Bisch C, Coulon A, de Saint-Hilaire P, Rudigoz RC, Dubernard G. Correlation between three-dimensional rectosonography and magnetic resonance imaging in the diagnosis of rectosigmoid endometriosis: a preliminary study on the first fifty cases. Eur J Obstet Gynecol Reprod Biol 2015;187:35–40. 10.1016/j.ejogrb.2015.02.006 [DOI] [PubMed] [Google Scholar]
- 28.Carbognin G, Girardi V, Pinali L, Raffaelli R, Bergamini V, Pozzi Mucelli R. Assessment of pelvic endometriosis: correlation of US and MRI with laparoscopic findings. Radiol Med 2006;111:687–701. 10.1007/s11547-006-0066-8 [DOI] [PubMed] [Google Scholar]
- 29.Grasso RF, Di Giacomo V, Sedati P, Sizzi O, Florio G, Faiella E, et al. Diagnosis of deep infiltrating endometriosis: accuracy of magnetic resonance imaging and transvaginal 3D ultrasonography. Abdom Imaging 2010;35:716–725. 10.1007/s00261-009-9587-7 [DOI] [PubMed] [Google Scholar]
- 30.Saccardi C, Cosmi E, Borghero A, Tregnaghi A, Dessole S, Litta P. Comparison between transvaginal sonography, saline contrast sonovaginography and magnetic resonance imaging in the diagnosis of posterior deep infiltrating endometriosis. Ultrasound Obstet Gynecol 2012;40:464–469. 10.1002/uog.11102 [DOI] [PubMed] [Google Scholar]
- 31.Chen YH, Wang DB, Guo CS. Accuracy of Physical Examination, Transvaginal Sonography, Magnetic Resonance Imaging, and Rectal Endoscopic Sonography for Preoperative Evaluation of Rectovaginal Endometriosis. Ultrasound Q 2019;35:54–60. 10.1097/RUQ.0000000000000428 [DOI] [PubMed] [Google Scholar]
- 32.Whiting P, Rutjes AW, Reitsma JB, Glas AS, Bossuyt PM, Kleijnen J. Sources of variation and bias in studies of diagnostic accuracy: a systematic review. Ann Intern Med 2004;140:189–202. [DOI] [PubMed] [Google Scholar]
- 33.Rutjes AW, Reitsma JB, Di Nisio M, Smidt N, van Rijn JC, Bossuyt PM. Evidence of bias and variation in diagnostic accuracy studies. CMAJ 2006;174:469–476. 10.1503/cmaj.050090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abrao MS, Goncalves MO, Dias JA Jr., Podgaec S, Chamie LP, Blasbalg R. Comparison between clinical examination, transvaginal sonography and magnetic resonance imaging for the diagnosis of deep endometriosis. Hum Reprod 2007;22:3092–3097. 10.1093/humrep/dem187 [DOI] [PubMed] [Google Scholar]
- 35.Bazot M, Lafont C, Rouzier R, Roseau G, Thomassin-Naggara I, Darai E. Diagnostic accuracy of physical examination, transvaginal sonography, rectal endoscopic sonography, and magnetic resonance imaging to diagnose deep infiltrating endometriosis. Fertil Steril 2009;92:1825–1833. 10.1016/j.fertnstert.2008.09.005 [DOI] [PubMed] [Google Scholar]
- 36.Gauche Cazalis C, Koskas M, Martin B, Palazzo L, Madelenat P, Yazbeck C. [Preoperative imaging of deeply infiltrating endometriosis in: Transvaginal sonography, rectal endoscopic sonography and magnetic resonance imaging]. Gynecol Obstet Fertil 2012;40:634–641. 10.1016/j.gyobfe.2012.09.014 [DOI] [PubMed] [Google Scholar]
- 37.Saba L, Guerriero S, Sulcis R, Pilloni M, Ajossa S, Melis G, et al. MRI and "tenderness guided" transvaginal ultrasonography in the diagnosis of recto-sigmoid endometriosis. J Magn Reson Imaging 2012;35:352–360. 10.1002/jmri.22832 [DOI] [PubMed] [Google Scholar]
- 38.Vimercati A, Achilarre MT, Scardapane A, Lorusso F, Ceci O, Mangiatordi G, et al. Accuracy of transvaginal sonography and contrast-enhanced magnetic resonance-colonography for the presurgical staging of deep infiltrating endometriosis. Ultrasound Obstet Gynecol 2012;40:592–603. 10.1002/uog.11179 [DOI] [PubMed] [Google Scholar]
- 39.Leone Roberti Maggiore U, Biscaldi E, Vellone VG, Venturini PL, Ferrero S. Magnetic resonance enema vs rectal water-contrast transvaginal sonography in diagnosis of rectosigmoid endometriosis. Ultrasound Obstet Gynecol 2017;49:524–532. 10.1002/uog.15934 [DOI] [PubMed] [Google Scholar]
- 40.Guerriero S, Alcazar JL, Pascual MA, Ajossa S, Perniciano M, Piras A, et al. Deep Infiltrating Endometriosis: Comparison Between 2-Dimensional Ultrasonography (US), 3-Dimensional US, and Magnetic Resonance Imaging. J Ultrasound Med 2018;37:1511–1521. 10.1002/jum.14496 [DOI] [PubMed] [Google Scholar]
- 41.Alborzi S, Rasekhi A, Shomali Z, Madadi G, Alborzi M, Kazemi M, et al. Diagnostic accuracy of magnetic resonance imaging, transvaginal, and transrectal ultrasonography in deep infiltrating endometriosis. Medicine (Baltimore) 2018;97:e9536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nisenblat V, Prentice L, Bossuyt PM, Farquhar C, Hull ML, Johnson N. Combination of the non-invasive tests for the diagnosis of endometriosis. Cochrane Database Syst Rev 2016;7:CD012281 10.1002/14651858.CD012281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bazot M, Darai E. Diagnosis of deep endometriosis: clinical examination, ultrasonography, magnetic resonance imaging, and other techniques. Fertil Steril 2017;108:886–894. 10.1016/j.fertnstert.2017.10.026 [DOI] [PubMed] [Google Scholar]
- 44.Ros C, Martinez-Serrano MJ, Rius M, Abrao MS, Munros J, Martinez-Zamora MA, et al. Bowel Preparation Improves the Accuracy of Transvaginal Ultrasound in the Diagnosis of Rectosigmoid Deep Infiltrating Endometriosis: A Prospective Study. J Minim Invasive Gynecol 2017;24:1145–1151. 10.1016/j.jmig.2017.06.024 [DOI] [PubMed] [Google Scholar]
- 45.Coutinho A Jr., Bittencourt LK, Pires CE, Junqueira F, Lima CM, Coutinho E, et al. MR imaging in deep pelvic endometriosis: a pictorial essay. Radiographics 2011;31:549–567. 10.1148/rg.312105144 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.