Abstract
Metabolic disorders are prevalent worldwide and have recently become public health problems recently. Previous studies have proposed different body composition indices for predicting future cardiovascular risks. We hypothesized an association among fat-to-muscle ratio (FMR), metabolic syndrome (MetS), hypertension (HTN), prediabetes, type 2 diabetes mellitus (DM), and cardiovascular risk in an adult population. A total of 66829 eligible subjects composed of 34182 males and 32647 females aged 20 years or older were obtained from health examinations in the Tri-Service General Hospital from 2011 to 2017. The body composition indices included fat and muscle mass measured by bioelectrical impedance analysis. A multivariable regression model was performed in a large population-based cross-sectional study. FMR was significantly associated with MetS, prediabetes, DM and HTN in all models of both genders. Based on quartile analysis, higher FMR had higher predictive ability for adverse health outcomes. The association between different definitions of MetS and the Framingham risk score was analyzed, and FMR-incorporated MetS was more useful for predicting higher Framingham risk scores than traditional definitions. FMR was a useful indicator for the presence of adverse cardiometabolic risks. Compared to traditional definition of MetS, FMR-incorporated MetS had a greater ability to predict incident cardiovascular risks. FMR seemed to be a simple and effective index for the early prevention and management of cardiometabolic events.
Introduction
The current worldwide prevalence of obesity has increased progressively. As a major public health problem in the world, an increasing number of individuals have been diagnosed with obesity and metabolic syndrome (MetS) in Taiwan with high risks for the development of diabetes mellitus (DM) and hypertension (HTN)[1]. An emerging concept called “sarcopenic obesity”, which reflect a combination of age-associated skeletal muscle loss and fat mass accumulation[2], was also recognized as a critical public health risk in the aging society. Previous studies have proposed an association between sarcopenic obesity and MetS in both sexes[3] and between sarcopenic obesity and insulin resistance in the adult population[4].
Increased total fat mass and its distribution were significantly associated with insulin resistance, glucose intolerance and high risks of DM and cardiovascular diseases[5], wthile loss of skeletal muscle was reported to contribute to MetS and DM in the adult population[6, 7]. However, the associations among simultaneous skeletal muscle mass loss, fat mass accumulation and metabolic disorders have not been well established. The ratio of visceral fat to thigh muscle area was considered as a single anthropometric index for insulin resistance and glucose metabolism[8]. Park et al. suggested muscle-to-fat ratio as a useful indicator for predicting MetS[9].
Although different types of body composition indices have valid predictions for metabolic dysfunction, there is no comprehensive index that can be used simultaneously for the risk of cardiometabolic disorders. The objective of this cross-cohort analysis was to critically examine whether fat-to-muscle ratio (FMR) was associated with the presence of MetS, prediabetes, DM and HTN and to develop sound definitions of MetS.
Methods
Study design and participants
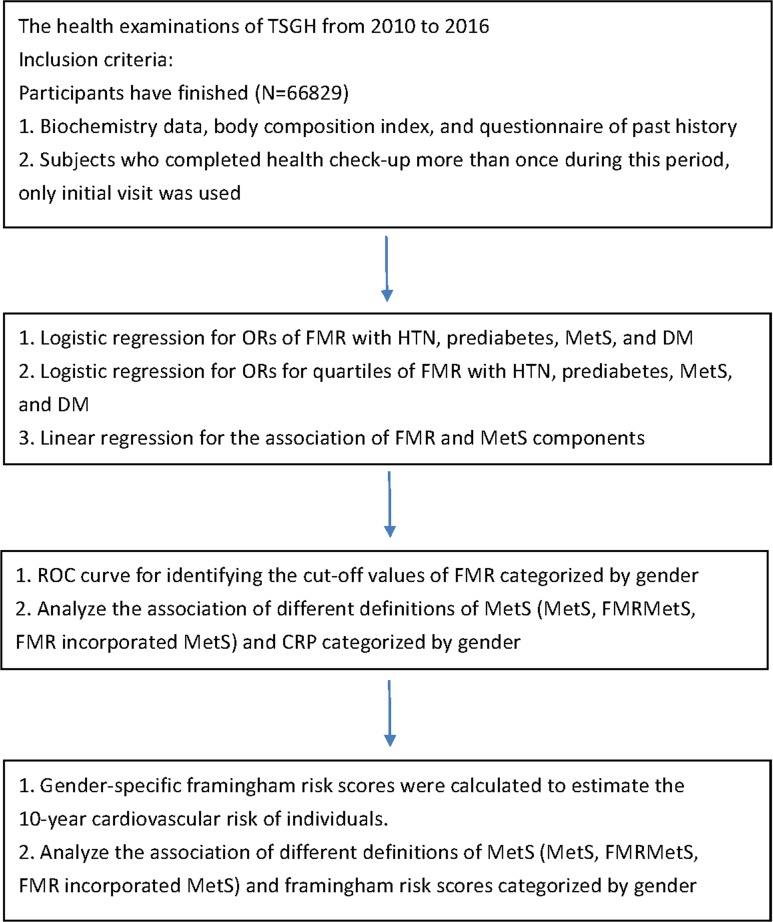
All data were derived from health examinations in the Tri-Service General Hospital from 2010 to 2016. The study design met the requirements of the Helsinki Declaration and the design was approved by the institutional review board of Tri-Service General Hospital. Because the data were analyzed anonymously, the institutional review board of Tri-Service General Hospital waived the need to acquire individual informed consent. Based on the flow chart of the study shown in Fig 1, subjects who attended the health check-up and finished comprehensive examinations, including laboratory biochemistry tests, body composition exams and questionnaires of the personal history were included in this study. 66829 eligible subjects were analyzed in a step-by-step manner in the following orders. First, the ORs of FMR in males and females for the presence of MetS, prediabetes, DM and HTN were conducted by multivariate logistic regression. Next, FMR was divided into quartiles to analyze its association with the presence of adverse health outcomes. Third, multivariable linear regression was used to assess the association between FMR and individual MetS components. Last, we calculated the optimal cut-off values of FMR for MetS in both genders and then created different definitions of MetS to compare the effect of inflammatory process with the traditional MetS criteria. In addition, we analyzed the association between different definitions of MetS and the Framingham risk score by using multivariable linear regression.
Fig 1. Flow chart which represented the steps of analysis performed in the study.
Measurement of body composition
Percentage of skeletal muscle mass and percentage of body fat were measured by bioelectric impedance analysis (BIA) (InBody720, Biospace, Inc., Cerritos, CA, USA) in the present study. BIA has been proven to be one of the most practical procedures to estimate body composition among different groups because of its ready accessibility, quick assessment, low cost, and its high validity against DEXA as the reference method[10]. FMR was defined as the ratio of fat mass to lean muscle mass.
General definition of MetS
According to the Taiwan Health Promotion Administration of the Ministry of Health and Welfare in 2007, the diagnosis of MetS was defined if an individual manifested 3 or more of the following components: (1) waist circumference>90 cm for male participants and >80 cm for female participants.; (2) systolic blood pressure≥130 mmHg, diastolic blood pressure≥80 mmHg, or self-reported hypertension (3) triglyceride≥150 mg/dL (1.7 mmol/L); (4) fasting plasma glucose≥100 mg/dL, a past history of diabetes status, or the use of antidiabetic agents; and (5) HDL-C<40 mg/dL (1.03 mmol/L) for male participants and <50 mg/dL (1.3 mmol/L) for female participants.
Different definitions of MetS
In our study, we created two different definitions of MetS to compare the effects of the inflammatory process with the traditional MetS. To assess the cut-off values of FMR for MetS, a receiver operating characteristic (ROC) curve analysis was performed. In males, the AUROC value was 0.673 (95%CI: 0.660–0.686), and the optimal cut-off value was 0.76 using the maximal Youden index, with a sensitivity of 0.763 and a specificity of 0.494. In females, the AUROC value was 0.701 (95%CI: 0.685–0.717), and the optimal cut-off value was 1.51 with a sensitivity of 0.792 and a specificity of 0.509. Subjects who had FMR above the cut-off values (males: 0.76; females: 1.51) were categorized as “MetFMR”.
First, “FMRMetS” was defined as participants with “MetFMR” along with at least two of four components of MetS except waist circumference. Second, “FMR incorporated MetS” was defined as MetFMR along with at least three out of five components of MetS.
Definition of Type 2 DM
Type 2 DM was defined base on the American Diabetes Association criteria as follows: fasting plasma glucose ≥126 mg/dL; hemoglobin A1c test ≥6.5%; random plasma glucose ≥200 mg/dL; and past history of diabetes status, or use of antidiabetic agents[11].
Definition of HTN
Based on the guidelines of the Taiwan Society of Cardiology and the Taiwan Hypertension Society for the management of hypertension, HTN was defined as blood pressure being higher than 140/90 mmHg or subjects taking antihypertensive agents[12].
Measurement o covariates
The regular health examinations included standard evaluations of comprehensive biochemistry tests and anthropometric measurements. The body mass index (BMI) was obtained based on the formula in which the weight of the subject in kilograms is divided by the square of their height in meters(kg/m2). The waist circumference was measured at the mid-level between the iliac crest and the lower border of the 12th rib while the subject stood with feet 25–30 cm apart. Hemodynamic status included systolic blood pressure (SBP) and diastolic blood pressure (DBP) estimated when the participants were seated. Biochemical analysis was conducted by drawing blood samples from subjects after fasting for at least 8 hours. The fasting plasma glucose (FPG) was detected using a glucose oxidase method. Serum levels of lipid profiles such as total cholesterol (TC), triglycerides (TG) and high-density lipoprotein cholesterol (HDL-C), were measured using an enzymatic colorimetric method.
Statistical analysis
All statistical estimations were performed using the Statistical Package for the Social Sciences, version18.0 (SPSS Inc., Chicago, IL, USA) for Windows. Student’s t-tests and Pearson's chi-square tests were performed to examine the differences between the gender groups in terms of demographic information and laboratory data. A two-sided p-value of ≤ 0.05 was regarded as the threshold for statistical significance. The extend-model approach was performed in the study with multivariable adjustment for pertinent clinical variables. Linear regression with beta coefficients was conducted for the association of FMR with MetS components, inflammation and the Framingham risk score. Logistic regression for ORs was used to examine the association between FMR and the presence of MetS, prediabetes, DM and HTN in a cross-sectional analysis. A receiver operating characteristic (ROC) curve analysis was calculated for the area under the ROC (AUROC), 95% confidence intervals (CI), sensitivity and specificity to assess the cut-off values of FMR.
Results
Characteristics of the study population
All data were obtained from the annual health examinations conducted in the Tri-Service General Hospital (TSGH) from 2010 to 2016. There were 34182 eligible males and 32647 eligible females enrolled in the study after excluding those with missing data. The mean age of male subjects was 42.35±16.14 years old, and the mean age of female was 42.63±15.95 years old. The prevalence of MetS, FMRMetS, and FMR-incorporated MetS were significantly higher in males than females (P<0.05). All demographic characteristics listed in Table 1, such as body composition index, components of MetS and laboratory biochemistry data, had significant difference.
Table 1. Characteristics of entire groups of participants with or without metabolic syndrome.
| Variables | Male | Female | P-value |
|---|---|---|---|
| Continuous Variables, mean (SD) | |||
| Age | 42.35 (16.14) | 42.63 (15.95) | 0.029 |
| Percentage of lean mass (%) | 31.03 (4.60) | 20.74 (3.05) | <0.001 |
| PBF (%) | 25 (6.33) | 31.94 (6.66) | <0.001 |
| FMR | 0.82 (0.24) | 1.57 (0.38) | <0.001 |
| BMI (kg/m2) | 24.93 (3.80) | 22.94 (4.09) | <0.001 |
| WC (cm) | 84.63 (9.90) | 74.46 (10.36) | <0.001 |
| SBP (mmHg) | 123.58 (16.11) | 115.09 (17.84) | <0.001 |
| DBP (mmHg) | 77.18 (11.76) | 71.40 (11.41) | <0.001 |
| TG (mg/dL) | 129.82 (106.55) | 95.35 (63.51) | <0.001 |
| HDL-C (mg/dL) | 48.67 (11.67) | 60.55 (14.11) | <0.001 |
| FPG (mg/dL) | 95.09 (23.28) | 91.67 (19.91) | <0.001 |
| TC (mg/dL) | 183.38 (35.14) | 185.66 (35.50) | <0.001 |
| UA (mg/dL) | 6.39 (1.32) | 4.74 (1.09) | <0.001 |
| Cr (mg/dL) | 0.96 (0.35) | 0.68 (0.21) | <0.001 |
| AST (mg/dL) | 22.47 (14.55) | 18.99 (13.58) | <0.001 |
| Albumin (mg/dL) | 4.54 (0.31) | 4.43 (0.29) | <0.001 |
| hsCRP (mg/dL) | 0.25 (0.54) | 0.21 (0.42) | <0.001 |
| Category Variables, (%) | |||
| Framingham Score | 6.97 (7.63) | 1.18 (2.65) | <0.001 |
| MetS | 4106 (65.5) | 16696 (50.8) | <0.001 |
| FMRMetS | 1981 (66.9) | 5504 (51.5) | <0.001 |
| FMR-incorporated MetS | 1500 (64.7) | 5975 (52.8) | <0.001 |
| Proteinuria | 8597 (29.9) | 7213 (29.0) | 0.004 |
| Smoking | 4971 (42.0) | 712 (9.0) | 0.006 |
| Drinking | 6103 (60.9) | 1868 (28.4) | 0.007 |
PBF, percentage body fat; FMR, fat-muscle ratio; BMI, body mass index; WC, waist circumference; SBP, systolic blood pressure; DBP, diastolic blood pressure; TG, triglyceride; HDL-C, high density lipoprotein cholesterol; FPG, fasting plasma glucose; TC, total cholesterol; UA, uric acid; Cr, creatinine; AST, aspartate aminotransferase; hsCRP, high sensitivity C-reactive protein
Association between FMR and the presence of MetS, Prediabetes, DM and HTN
Table 2 represents the odd ratios (ORs) of the presence of MetS, prediabetes, DM and HTN in male and female participants with FMR. The ORs of MetS were higher than other adverse health outcomes in all adjusted models of male (ORs = 5.69, 4.63, 4.20; 95%CI = 4.05–7.99, 3.26–6.56, 2.91–6.05, respectively). In females, the ORs of MetS and DM were similar in all models. The FMR tended to have more predictive ability for the presence of DM in fully adjusted model (ORs = 1.92; 95%CI = 1.14–3.23).
Table 2. Association between fat-muscle ratio and the presence of MetS, prediabetes, DM and HTN.
| Sex | Variable | Model a 1 OR (95% CI) |
P Value |
Model a 2 OR (95% CI) |
P Value |
Model a 3 OR (95% CI) |
P Value |
|---|---|---|---|---|---|---|---|
| Male | MetS | 5.69 (4.05–7.99) | <0.001 | 4.63 (3.26–6.56) | <0.001 | 4.20 (2.91–6.05) | <0.001 |
| Prediabetes | 1.85 (1.28–2.68) | <0.001 | 1.50 (1.02–2.19) | 0.037 | 1.53 (1.04–2.23) | 0.030 | |
| DM | 2.35 (1.39–3.99) | 0.002 | 2.30 (1.35–3.93) | 0.002 | 2.37 (1.38–4.06) | 0.002 | |
| HTN | 3.21 (2.28–4.53) | <0.001 | 2.68 (1.89–3.80) | <0.001 | 2.70 (1.91–3.83) | <0.001 | |
| Female | MetS | 2.59 (1.96–3.42) | <0.001 | 1.92 (1.42–2.59) | <0.001 | 1.86 (1.36–2.56) | <0.001 |
| Prediabetes | 2.00 (1.47–2.72) | <0.001 | 1.62 (1.17–2.23) | 0.003 | 1.63 (1.18–2.25) | 0.003 | |
| DM | 2.31 (1.40–3.81) | <0.001 | 1.92 (1.14–3.24) | 0.014 | 1.92 (1.14–3.23) | 0.015 | |
| HTN | 1.67 (1.24–2.26) | <0.001 | 1.44 (1.05–1.97) | 0.023 | 1.45 (1.06–1.98) | 0.021 |
a Adjusted covariates:
Model 1 = age
Model 2 = Model 1 + proteinuria,TC, UA, Cr, AST, albumin, hsCRP
Model 3 = Model 2 + history of smoking, drinking
Association between quartiles of FMR and the presence of MetS, Prediabetes, DM and HTN
In Table 3, the FMR in each gender was divided into quartiles and the higher quartiles (Q2, Q3 and Q4) were compared to baseline (Q1) in subgroups to analyze the association between the FMR and the presence of adverse health outcomes. The intervals of FMR in quartiles were <0.66, 0.66–0.81, 0.81–0.96, and >0.96 in males and <1.30, 1.30–1.55, 1.55–1.80, and >1.80 in females from Q1 to Q4, respectively. Obviously, the higher quartile of FMR had more predictive ability for the presence of MetS, prediabetes, DM and HTN in male and female participants.
Table 3. Association between fat-muscle ratio in quartiles and the presence of MetS, prediabetes, DM and HTN.
| Sex | Variable | Quartiles | Model a 1 OR (95% CI) |
P Value |
Model a 2 OR (95% CI) |
P Value |
Model a 3 OR (95% CI) |
P Value |
|---|---|---|---|---|---|---|---|---|
| Male | MetS | Q2 v.s. Q1 | 2.47 (1.88–3.25) | <0.001 | 2.17 (1.65–2.87) | <0.001 | 2.19 (1.66–2.90) | <0.001 |
| Q3 v.s. Q1 | 3.11 (2.39–4.07) | <0.001 | 2.54 (1.93–3.34) | <0.001 | 2.57 (1.95–3.38) | <0.001 | ||
| Q4 v.s. Q1 | 4.48 (3.45–5.81) | <0.001 | 3.45 (2.63–4.53) | <0.001 | 3.54 (2.69–4.66) | <0.001 | ||
| Prediabetes | Q2 v.s. Q1 | 1.67 (1.25–2.23) | <0.001 | 1.40 (1.04–1.88) | 0.028 | 1.40 (1.04–1.89) | 0.026 | |
| Q3 v.s. Q1 | 1.98 (1.49–2.62) | <0.001 | 1.39 (1.03–1.86) | 0.030 | 1.39 (1.04–1.87) | 0.028 | ||
| Q4 v.s. Q1 | 2.50 (1.90–3.28) | <0.001 | 1.50 (1.12–2.01) | 0.006 | 1.51 (1.13–2.03) | 0.005 | ||
| DM | Q2 v.s. Q1 | 1.40 (0.85–2.30) | 0.183 | 1.27 (0.77–2.11) | 0.355 | 1.26 (0.76–2.09) | 0.377 | |
| Q3 v.s. Q1 | 2.20 (1.39–3.49) | <0.001 | 1.75 (1.09–2.82) | 0.022 | 1.73 (1.08–2.80) | 0.024 | ||
| Q4 v.s. Q1 | 3.21 (2.07–5.00) | <0.001 | 1.99 (1.25–3.18) | 0.004 | 1.99 (1.25–3.19) | 0.004 | ||
| HTN | Q2 v.s. Q1 | 1.42 (1.09–1.86) | 0.010 | 1.24 (0.95–1.63) | 0.115 | 1.26 (0.96–1.65) | 0.101 | |
| Q3 v.s. Q1 | 2.22 (1.72–2.87) | <0.001 | 1.74 (1.34–2.26) | <0.001 | 1.76 (1.35–2.28) | <0.001 | ||
| Q4 v.s. Q1 | 2.82 (2.20–3.61) | <0.001 | 2.00 (1.55–2.60) | <0.001 | 2.03 (1.56–2.64) | <0.001 | ||
| Female | MetS | Q2 v.s. Q1 | 2.27 (1.50–3.45) | <0.001 | 1.63 (1.05–2.52) | 0.028 | 1.65 (1.06–2.54) | 0.025 |
| Q3 v.s. Q1 | 3.94 (2.66–5.84) | <0.001 | 2.30 (1.52–3.50) | <0.001 | 2.31 (1.52–3.51) | <0.001 | ||
| Q4 v.s. Q1 | 6.12 (4.17–8.99) | <0.001 | 2.48 (1.63–3.77) | <0.001 | 2.51 (1.65–3.82) | <0.001 | ||
| Prediabetes | Q2 v.s. Q1 | 1.80 (1.10–2.95) | 0.020 | 1.35 (0.82–2.23) | 0.243 | 1.36 (0.82–2.26) | 0.228 | |
| Q3 v.s. Q1 | 3.77 (2.39–5.93) | <0.001 | 2.35 (1.47–3.75) | <0.001 | 2.36 (1.47–3.77) | <0.001 | ||
| Q4 v.s. Q1 | 5.36 (3.44–8.34) | <0.001 | 2.40 (1.50–3.84) | <0.001 | 2.42 (1.51–3.88) | <0.001 | ||
| DM | Q2 v.s. Q1 | 4.62 (1.36–15.71) | 0.014 | 3.61 (1.04–12.54) | 0.043 | 3.64 (1.05–12.64) | 0.042 | |
| Q3 v.s. Q1 | 7.38 (2.25–24.19) | <0.001 | 4.56 (1.35–15.33) | 0.014 | 4.60 (1.37–15.48) | 0.014 | ||
| Q4 v.s. Q1 | 12.18 (3.80–39.05) | <0.001 | 4.76 (1.43–15.88) | 0.011 | 4.79 (1.43–15.99) | 0.011 | ||
| HTN | Q2 v.s. Q1 | 1.76 (1.14–2.71) | 0.010 | 1.30 (0.83–2.04) | 0.244 | 1.31 (0.84–2.04) | 0.243 | |
| Q3 v.s. Q1 | 2.64 (1.75–3.97) | <0.001 | 1.60 (1.04–2.45) | 0.033 | 1.58 (1.03–2.43) | 0.035 | ||
| Q4 v.s. Q1 | 4.61 (3.11–6.83) | <0.001 | 1.94 (1.27–2.97) | 0.002 | 1.95 (1.28–2.99) | 0.002 |
a Adjusted covariates:
Model 1 = age
Model 2 = Model 1 + proteinuria, TC, UA, Cr, AST, albumin, hsCRP
Model 3 = Model 2 + history of smoking, drinking
Association between different definitions of MetS and Framingham risk score
We analyzed the association of MetS, FMRMetS and FMR-incorporated MetS with the Framingham risk score listed in Table 4. All definitions of MetS had significant association with increased Framingham risk score. FMR-incorporated MetS (β = 3.64, 95%CI = 3.25–4.03) was more closely associated with the Framingham risk score than MetS (β = 3.59, 95%CI = 3.26–3.92) in the fully adjusted model in males. However, in females, not only FMR-incorporated MetS (fully adjusted model: β = 2.10, 95%CI = 1.84–2.35) but also FMRMetS (fully adjusted model: β = 1.90, 95%CI = 1.66–2.15) were more closely associated with the Framingham risk score than MetS (fully adjusted model: β = 1.74, 95%CI = 1.53–1.96) in all models.
Table 4. Association between the Framingham risk score and different definitions of MetS.
| Model a 1 βb (95% CI) |
P Value |
Model a 2 βb (95% CI) |
P Value |
Model a 3 βb (95% CI) |
P Value |
||
|---|---|---|---|---|---|---|---|
| Male | MetS | 4.47 (4.06–4.89) | <0.001 | 4.24 (3.84–4.63) | <0.001 | 3.59 (3.26–3.92) | <0.001 |
| FMRMetS | 4.01 (3.54–4.47) | <0.001 | 3.73 (3.29–4.17) | <0.001 | 3.25 (2.89–3.61) | <0.001 | |
| FMR + MetS | 4.29 (3.78–4.79) | <0.001 | 4.15 (3.67–4.63) | <0.001 | 3.64 (3.25–4.03) | <0.001 | |
| Female | MetS | 1.86 (1.64–2.07) | <0.001 | 1.76 (1.54–1.98) | <0.001 | 1.74 (1.53–1.96) | <0.001 |
| FMRMetS | 2.05 (1.80–2.29) | <0.001 | 1.95 (1.70–2.20) | <0.001 | 1.90 (1.66–2.15) | <0.001 | |
| FMR + MetS | 2.23 (1.97–2.49) | <0.001 | 2.15 (1.88–2.41) | <0.001 | 2.10 (1.84–2.35) | <0.001 |
a Adjusted covariates:
Model 1 = age
Model 2 = Model 1 + proteinuria, TC, UA, Cr, AST, albumin, hsCRP
Model 3 = Model 2 + history of smoking, drinking
Association between FMR and individual components of MetS
Multivariable linear regressions of FMR and MetS components performed with the adjusted extend-model approach are shown in S1 Table. As expected, the FMR was significantly associated with higher blood pressure, central obesity, hypertriglyceridemia, hyperglycemia and lower HDL.
Association between different definitions of MetS with inflammation
Multivariable beta coefficients regression was performed for the association between different definitions of MetS and levels of CRP, as shown in S2 Table. It was surprising that different definitions of MetS including MetS, MetFMR, FMRMetS and FMR-incorporated MetS had significant associations with increased levels of CRP in both sexes, except MetS in the fully adjusted model in males.
Discussion
In the cross-sectional study of data from the annual health examinations of a medical center in Taiwan for the general population, a novel indicator, FMR, was suggested as an excellent body composition index for predicting the presence of MetS, prediabetes, DM and HTN. FMR was significantly associated with adverse health outcomes and a substantial dose dependent effect was noted in both genders. Furthermore, FMR-incorporated MetS had better predictive ability for the Framingham risk score than other definitions, particularly in females, indicating the possibility that FMR might have the potential capacity for predicting the incident risks of cardiovascular disease mortality.
In a Korean study composed of 264 adults, an increased visceral fat-to-thigh muscle ratio was significantly associated with MetS with an OR of 6.72 (95%CI = 1.60–28.14)[13]. Another finding obtained from a Korean cohort study indicated that the ratio of skeletal muscle mass to visceral fat was associated with MetS with an OR of 5.43 (95%CI = 2.56–13.34)[14]. Ezch et al. demonstrated that adverse body composition characterized by the ratio of whole body fat to lean mass was independently associated with metabolic dysfunction in women with polycystic ovary syndrome[15]. Compared to the above different body composition indices, our findings suggested that FMR was a useful indicator for predicting the presence of MetS, prediabetes, DM and HTN in the general population. To the best of our knowledge, the present study was the first to propose that FMR was strongly associated with adverse health outcomes in both males and females in a large-scale cross-sectional observational study.
Accumulated evidence has supported the relationship between fat mass and cardiometabolic outcomes. The distribution of body fat is associated with MetS in elderly adults, especially those with normal body weight[16]. In a longitudinal cohort study, those with more visceral fat had higher risks for developing incident MetS during a five-year follow-up[17]. Neeland et al. demonstrated that a higher amount of visceral fat was more useful in predicting the incident prediabetes and DM than other indices in a longitudinal study[5]. Visceral fat was considered an important predictor of insulin resistance in the non-diabetic population[18]. In a cohort study of 903 normotensive participants examining the development of HTN, visceral adipose tissue was associated with incident hypertension (relative risk: 1.22; 95%CI: 1.06–1.39) after multivariable adjustment[19]. Collectively, the above results were consistent with our findings that increased fat mass was associated with the presence of MetS, prediabetes, DM and HTN. Several studies have proposed the important role of fat tissue in cardiometabolic risks through different pathways. Dysfunction in adipose tissue, such as excessive free fatty acid metabolism changes, was caused by fat tissue accumulation[20]. Adipose alternation might lead to the impairment of hepatic metabolism[21]. It could also contribute to degradation of insulin, reduced degradation of apolipoprotein B, and increased hepatic glucose production, leading to hyperinsulinemia, hypertriglyceridemia and eventually DM[22, 23]. Another mechanism was the inflammation of adipose tissue caused by adipocyte hypertrophy, adipose tissue stresses and apoptosis[24]. Impaired insulin sensitivity and deteriorated glucose and lipid metabolism were related to adipocyte hypertrophy, which was described as a predominant and large volume of adipose tissue[25]. Increasing the secretion of chemoattractants and proinflammatory cytokines, such as MCP-1, TNF-α, IL-1, and IL-6, caused by adipocyte hypertrophy contributed to immune cell infiltration[26]. Increased numbers of macrophages caused by phenotypic switching were related to adaptive immune systems[27]. Changes in T-cell phenotype and the recruitment of B cells and T cells preceded macrophage infiltration[28]. A series of inflammatory changes in adipose tissue induced a chronic inflammation strongly implicated in the mechanisms underpinning whole-body metabolic dysregulation.
A progressive loss of muscle mass and an increment of fat mass were prevalent in the aging process. Excessive loss of appendicular lean mass was associated with Type 2 DM in community-dwelling older adults, particularly undiagnosed cases[7]. An inverse association was found between skeletal muscle mass with insulin resistance and the risk of prediabetes. In a recent Taiwanese study composed of 394 middle-aged and elderly adults, lower muscle mass was associated with the risk of metabolic syndrome, especially in the aging female population[6]. Emerging studies have proposed an association between sarcopenia and metabolic dysfunction. Chung et al. reported that the sarcopenic obese group showed close associations with insulin resistance, MetS, and cardiovascular disease risk factors in the elderly population[29]. Subjects with sarcopenia obesity were considered to have a greater risk of hypertension than simply obesity[30]. The significant associations between sarcopenia, defined in terms of muscle mass, sarcopenic obesity and MetS were observed in both men (RR = 1.31, 95%CI = 1.10–1.56) and women (RR = 1.17, 95%CI = 1.10–1.25)[3]. The mechanisms of the relationship between muscle mass and cardiometabolic risks were unclear. There were several plausible explanations, as follows. As an organ of an insulin-responsive target, the loss of muscle mass contributed to insulin resistance, MetS and HTN[31]. Levels of HOMA-IR were higher in sarcopenia participants than in control subjects[30]. The pathophysiology of DM caused an atrophy of muscles and included declines in the activity of anabolic hormones (e.g. IGF-I, testosterone, ghrelin)[32], and increased protein degradation caused by elevated expression of acrogens[33]. The reported loss of lean mass was caused by decreased responsiveness to insulin for the stimulation of muscle protein synthesis and for inhibiting protein breakdown[34]. Macrophage infiltration, one of the potential pathways of adipose dysfunction, was also related to inflammation in muscle mass[35]. Increased levels of IL-6 and CRP induced by elevated numbers of macrophages were significantly associated with the loss of total appendicular lean muscle mass[36]. Elderly adults with higher inflammatory levels such as TNF-α revealed the strongest associations and might be important markers of loss of muscle mass and strength[37]. In a recent study, the negative effects of CRP on muscle mass were identified by a reduction in the size of human myotubes along with a reduction in muscle protein synthesis[38]. Increased CRP levels reduced the phosphorylation of Akt, the major upstream regulator of the mTOR cascade involved in the regulation of muscle growth, and contributed to the impairment of muscle protein synthesis[39]. Another pathway was CRP-mediated cellular energy stress that increased the upregulation of AMPK, leading to the suppression of mTORC1 activity[40].
Interestingly, the gender difference is noted in the association between different definitions of MetS and Framingham risk score in the present study. FMRMetS is more closely associated with the risk score than MestS in females, but not in males. Several studies have reported that females have substantially greater body fat percentage, while males have greater visceral fat[41, 42]. This difference might be associated with the sexual dimorphism of body fat distribution and sex hormones[43].
The strengths of our study were a large population-based survey, and we proposed novel findings for the effect of a body composition index on cardiometabolic events. However, there were several potential limitations among our study. First, causal inference was not suitable because the present study was a cross-sectional design; thus, we could not explain whether FMR affected metabolic dysfunction. Second, the data for insulin resistance and HOMA-IR were not accessible in the health examination. If we could examine the association between insulin resistance and fat and muscle, interesting findings could be uncovered. Third, BIA is quite variable and it is not regarded by many as providing an accurate measure of body composition. Dehydration is an important factor affecting accuracy of BIA measurement that it causes an increase in the body's electrical resistance and an overestimation of body fat[44]. Exercise before BIA measurement contributes to an underestimation of body fat percentage and overestimation of fat-free mass because of reduced impedance[45]. Next, the information regarding drug use for DM, HTN, and dyslipidemia is not available in the study because these data is not assessing in the health examinations that may confound findings. Finally, the dataset was derived from only an Asian population. Thus, the limited ethnicity diversity in the participants might not reflect the association between FMR and metabolic risk factors in terms of racial differences.
Conclusion
The present study highlighted a significant association between FMR and MetS, prediabetes, DM and HTN. FMR might be incorporated in newly constructed MetS definitions, which were better able to predict the incident cardiovascular risks than traditional criteria. We provided a simple and useful body composition indicator for the early prevention and management of cardiometabolic risks and improvement of public health. Further studies should focus more effort on the underlying mechanisms of the interaction between body composition and metabolic alternation.
Supporting information
(DOCX)
(DOCX)
Data Availability
The data set is owned by the Institutional Review Board (IRB) of Tri-Service General Hospital (TSGH). TSGH IRB only approved the data analysis in our study and did not approve data sharing. Therefore, we do not have permission to share the data set. Interested researchers can submit data access requests to the Tri-Service General Hospital IRB using the following email address: tsghirb@ndmctsgh.edu.tw. Others would be able to access these data in the same manner as the authors and the authors also did not have any special access privileges.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Hwang LC, Bai CH, Chen CJ. Prevalence of obesity and metabolic syndrome in Taiwan. Journal of the Formosan Medical Association = Taiwan yi zhi. 2006;105(8):626–35. Epub 2006/08/29. 10.1016/S0929-6646(09)60161-3 . [DOI] [PubMed] [Google Scholar]
- 2.Stenholm S, Harris TB, Rantanen T, Visser M, Kritchevsky SB, Ferrucci L. Sarcopenic obesity—definition, etiology and consequences. Current opinion in clinical nutrition and metabolic care. 2008;11(6):693–700. 10.1097/MCO.0b013e328312c37d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee J, Hong YP, Shin HJ, Lee W. Associations of Sarcopenia and Sarcopenic Obesity With Metabolic Syndrome Considering Both Muscle Mass and Muscle Strength. Journal of preventive medicine and public health = Yebang Uihakhoe chi. 2016;49(1):35–44. Epub 2016/02/05. 10.3961/jpmph.15.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim TN, Park MS, Lim KI, Choi HY, Yang SJ, Yoo HJ, et al. Relationships between sarcopenic obesity and insulin resistance, inflammation, and vitamin D status: the Korean Sarcopenic Obesity Study. Clinical endocrinology. 2013;78(4):525–32. Epub 2012/05/09. 10.1111/j.1365-2265.2012.04433.x . [DOI] [PubMed] [Google Scholar]
- 5.Neeland IJ, Turer AT, Ayers CR, Powell-Wiley TM, Vega GL, Farzaneh-Far R, et al. Dysfunctional adiposity and the risk of prediabetes and type 2 diabetes in obese adults. Jama. 2012;308(11):1150–9. Epub 2012/09/20. 10.1001/2012.jama.11132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ou YC, Chuang HH, Li WC, Tzeng IS, Chen JY. Gender difference in the association between lower muscle mass and metabolic syndrome independent of insulin resistance in a middle-aged and elderly Taiwanese population. Archives of gerontology and geriatrics. 2017;72:12–8. Epub 2017/05/16. 10.1016/j.archger.2017.04.006 . [DOI] [PubMed] [Google Scholar]
- 7.Park SW, Goodpaster BH, Lee JS, Kuller LH, Boudreau R, de Rekeneire N, et al. Excessive loss of skeletal muscle mass in older adults with type 2 diabetes. Diabetes care. 2009;32(11):1993–7. Epub 2009/06/25. 10.2337/dc09-0264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim CS, Nam JY, Park JS, Kim DM, Yoon SJ, Ahn CW, et al. The correlation between insulin resistance and the visceral fat to skeletal muscle ratio in middle-aged women. Yonsei medical journal. 2004;45(3):469–78. Epub 2004/07/01. 10.3349/ymj.2004.45.3.469 . [DOI] [PubMed] [Google Scholar]
- 9.Park J, Kim S. Validity of muscle-to-fat ratio as a predictor of adult metabolic. J Phys Ther Sci. 2016;28(3):1036–45. 10.1589/jpts.28.1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun G, French CR, Martin GR, Younghusband B, Green RC, Xie YG, et al. Comparison of multifrequency bioelectrical impedance analysis with dual-energy X-ray absorptiometry for assessment of percentage body fat in a large, healthy population. The American journal of clinical nutrition. 2005;81(1):74–8. Epub 2005/01/11. 10.1093/ajcn/81.1.74 . [DOI] [PubMed] [Google Scholar]
- 11.Standards of Medical Care in Diabetes-2016: Summary of Revisions. Diabetes care. 2016;39 Suppl 1:S4–5. Epub 2015/12/24. 10.2337/dc16-S003 . [DOI] [PubMed] [Google Scholar]
- 12.Chiang CE, Wang TD, Ueng KC, Lin TH, Yeh HI, Chen CY, et al. 2015 guidelines of the Taiwan Society of Cardiology and the Taiwan Hypertension Society for the management of hypertension. Journal of the Chinese Medical Association: JCMA. 2015;78(1):1–47. Epub 2014/12/31. 10.1016/j.jcma.2014.11.005 . [DOI] [PubMed] [Google Scholar]
- 13.Lim KI, Yang SJ, Kim TN, Yoo HJ, Kang HJ, Song W, et al. The association between the ratio of visceral fat to thigh muscle area and metabolic syndrome: the Korean Sarcopenic Obesity Study (KSOS). Clinical endocrinology. 2010;73(5):588–94. Epub 2010/07/14. 10.1111/j.1365-2265.2010.03841.x . [DOI] [PubMed] [Google Scholar]
- 14.Kim TN, Park MS, Lim KI, Yang SJ, Yoo HJ, Kang HJ, et al. Skeletal muscle mass to visceral fat area ratio is associated with metabolic syndrome and arterial stiffness: The Korean Sarcopenic Obesity Study (KSOS). Diabetes research and clinical practice. 2011;93(2):285–91. Epub 2011/07/15. 10.1016/j.diabres.2011.06.013 . [DOI] [PubMed] [Google Scholar]
- 15.Ezeh U, Pall M, Mathur R, Azziz R. Association of fat to lean mass ratio with metabolic dysfunction in women with polycystic ovary syndrome. Hum Reprod. 2014;29(7):1508–17. Epub 2014/05/13. 10.1093/humrep/deu096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goodpaster BH, Krishnaswami S, Harris TB, Katsiaras A, Kritchevsky SB, Simonsick EM, et al. Obesity, regional body fat distribution, and the metabolic syndrome in older men and women. Archives of internal medicine. 2005;165(7):777–83. Epub 2005/04/13. 10.1001/archinte.165.7.777 . [DOI] [PubMed] [Google Scholar]
- 17.Kwon H, Kim D, Kim JS. Body Fat Distribution and the Risk of Incident Metabolic Syndrome: A Longitudinal Cohort Study. Scientific reports. 2017;7(1):10955 Epub 2017/09/10. 10.1038/s41598-017-09723-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Usui C, Asaka M, Kawano H, Aoyama T, Ishijima T, Sakamoto S, et al. Visceral fat is a strong predictor of insulin resistance regardless of cardiorespiratory fitness in non-diabetic people. Journal of nutritional science and vitaminology. 2010;56(2):109–16. Epub 2010/05/25. . [DOI] [PubMed] [Google Scholar]
- 19.Chandra A, Neeland IJ, Berry JD, Ayers CR, Rohatgi A, Das SR, et al. The relationship of body mass and fat distribution with incident hypertension: observations from the Dallas Heart Study. Journal of the American College of Cardiology. 2014;64(10):997–1002. Epub 2014/09/06. 10.1016/j.jacc.2014.05.057 . [DOI] [PubMed] [Google Scholar]
- 20.Despres JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444(7121):881–7. Epub 2006/12/15. 10.1038/nature05488 . [DOI] [PubMed] [Google Scholar]
- 21.Mittelman SD, Van Citters GW, Kirkman EL, Bergman RN. Extreme insulin resistance of the central adipose depot in vivo. Diabetes. 2002;51(3):755–61. Epub 2002/03/02. . [DOI] [PubMed] [Google Scholar]
- 22.Bjorntorp P. Abdominal obesity and the development of noninsulin-dependent diabetes mellitus. Diabetes/metabolism reviews. 1988;4(6):615–22. Epub 1988/09/01. . [DOI] [PubMed] [Google Scholar]
- 23.Bjorntorp P. "Portal" adipose tissue as a generator of risk factors for cardiovascular disease and diabetes. Arteriosclerosis (Dallas, Tex). 1990;10(4):493–6. Epub 1990/07/01. . [PubMed] [Google Scholar]
- 24.Cancello R, Henegar C, Viguerie N, Taleb S, Poitou C, Rouault C, et al. Reduction of macrophage infiltration and chemoattractant gene expression changes in white adipose tissue of morbidly obese subjects after surgery-induced weight loss. Diabetes. 2005;54(8):2277–86. Epub 2005/07/28. . [DOI] [PubMed] [Google Scholar]
- 25.Hoffstedt J, Arner E, Wahrenberg H, Andersson DP, Qvisth V, Lofgren P, et al. Regional impact of adipose tissue morphology on the metabolic profile in morbid obesity. Diabetologia. 2010;53(12):2496–503. Epub 2010/09/11. 10.1007/s00125-010-1889-3 . [DOI] [PubMed] [Google Scholar]
- 26.McArdle MA, Finucane OM, Connaughton RM, McMorrow AM, Roche HM. Mechanisms of obesity-induced inflammation and insulin resistance: insights into the emerging role of nutritional strategies. Frontiers in endocrinology. 2013;4:52 Epub 2013/05/16. 10.3389/fendo.2013.00052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sell H, Habich C, Eckel J. Adaptive immunity in obesity and insulin resistance. Nature reviews Endocrinology. 2012;8(12):709–16. Epub 2012/08/01. 10.1038/nrendo.2012.114 . [DOI] [PubMed] [Google Scholar]
- 28.Guilherme A, Virbasius JV, Puri V, Czech MP. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol. 2008;9(5):367–77. Epub 2008/04/11. 10.1038/nrm2391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chung JY, Kang HT, Lee DC, Lee HR, Lee YJ. Body composition and its association with cardiometabolic risk factors in the elderly: a focus on sarcopenic obesity. Archives of gerontology and geriatrics. 2013;56(1):270–8. Epub 2012/10/20. 10.1016/j.archger.2012.09.007 . [DOI] [PubMed] [Google Scholar]
- 30.Han K, Park YM, Kwon HS, Ko SH, Lee SH, Yim HW, et al. Sarcopenia as a determinant of blood pressure in older Koreans: findings from the Korea National Health and Nutrition Examination Surveys (KNHANES) 2008–2010. PloS one. 2014;9(1):e86902 Epub 2014/02/04. 10.1371/journal.pone.0086902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dogan MH, Karadag B, Ozyigit T, Kayaoglu S, Ozturk AO, Altuntas Y. Correlations between sarcopenia and hypertensive target organ damage in a Turkish cohort. Acta clinica Belgica. 2012;67(5):328–32. Epub 2012/11/30. 10.2143/ACB.67.5.2062685 . [DOI] [PubMed] [Google Scholar]
- 32.Morley JE. Diabetes, sarcopenia, and frailty. Clinics in geriatric medicine. 2008;24(3):455–69, vi. Epub 2008/08/02. 10.1016/j.cger.2008.03.004 . [DOI] [PubMed] [Google Scholar]
- 33.Vignaud A, Ramond F, Hourde C, Keller A, Butler-Browne G, Ferry A. Diabetes provides an unfavorable environment for muscle mass and function after muscle injury in mice. Pathobiology: journal of immunopathology, molecular and cellular biology. 2007;74(5):291–300. Epub 2007/09/25. 10.1159/000105812 . [DOI] [PubMed] [Google Scholar]
- 34.Lee CG, Boyko EJ, Strotmeyer ES, Lewis CE, Cawthon PM, Hoffman AR, et al. Association Between Insulin Resistance and Lean Mass Loss and Fat Mass Gain in Older Men without Diabetes Mellitus. Journal of the American Geriatrics Society. 2011;59(7):1217–24. 10.1111/j.1532-5415.2011.03472.x PMC3716256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hevener AL, Olefsky JM, Reichart D, Nguyen MT, Bandyopadyhay G, Leung HY, et al. Macrophage PPAR gamma is required for normal skeletal muscle and hepatic insulin sensitivity and full antidiabetic effects of thiazolidinediones. The Journal of clinical investigation. 2007;117(6):1658–69. Epub 2007/05/26. 10.1172/JCI31561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alemán H, Esparza J, Ramirez FA, Astiazaran H, Payette H. Longitudinal evidence on the association between interleukin-6 and C-reactive protein with the loss of total appendicular skeletal muscle in free-living older men and women. Age and Ageing. 2011;40(4):469–75. 10.1093/ageing/afr040 [DOI] [PubMed] [Google Scholar]
- 37.Schaap LA, Pluijm SM, Deeg DJ, Harris TB, Kritchevsky SB, Newman AB, et al. Higher inflammatory marker levels in older persons: associations with 5-year change in muscle mass and muscle strength. The journals of gerontology Series A, Biological sciences and medical sciences. 2009;64(11):1183–9. Epub 2009/07/23. 10.1093/gerona/glp097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wahlin-Larsson B, Wilkinson DJ, Strandberg E, Hosford-Donovan A, Atherton PJ, Kadi F. Mechanistic Links Underlying the Impact of C-Reactive Protein on Muscle Mass in Elderly. Cellular physiology and biochemistry: international journal of experimental cellular physiology, biochemistry, and pharmacology. 2017;44(1):267–78. Epub 2017/11/14. 10.1159/000484679 . [DOI] [PubMed] [Google Scholar]
- 39.Favier FB, Benoit H, Freyssenet D. Cellular and molecular events controlling skeletal muscle mass in response to altered use. Pflugers Archiv: European journal of physiology. 2008;456(3):587–600. Epub 2008/01/15. 10.1007/s00424-007-0423-z . [DOI] [PubMed] [Google Scholar]
- 40.Bolster DR, Crozier SJ, Kimball SR, Jefferson LS. AMP-activated protein kinase suppresses protein synthesis in rat skeletal muscle through down-regulated mammalian target of rapamycin (mTOR) signaling. The Journal of biological chemistry. 2002;277(27):23977–80. Epub 2002/05/09. 10.1074/jbc.C200171200 . [DOI] [PubMed] [Google Scholar]
- 41.Bloor ID, Symonds ME. Sexual dimorphism in white and brown adipose tissue with obesity and inflammation. Hormones and behavior. 2014;66(1):95–103. Epub 2014/03/05. 10.1016/j.yhbeh.2014.02.007 . [DOI] [PubMed] [Google Scholar]
- 42.Wells JC. Sexual dimorphism of body composition. Best practice & research Clinical endocrinology & metabolism. 2007;21(3):415–30. Epub 2007/09/19. 10.1016/j.beem.2007.04.007 . [DOI] [PubMed] [Google Scholar]
- 43.Wu C-J, Kao T-W, Chen Y-Y, Yang H-F, Chen W-L. Peripheral fat distribution versus waist circumference for predicting mortality in metabolic syndrome. Diabetes/Metabolism Research and Reviews. 0(0):e3116 10.1002/dmrr.3116 [DOI] [PubMed] [Google Scholar]
- 44.Lukaski HC, Bolonchuk WW, Hall CB, Siders WA. Validation of tetrapolar bioelectrical impedance method to assess human body composition. Journal of applied physiology (Bethesda, Md: 1985). 1986;60(4):1327–32. Epub 1986/04/01. 10.1152/jappl.1986.60.4.1327 . [DOI] [PubMed] [Google Scholar]
- 45.Kushner RF, Gudivaka R, Schoeller DA. Clinical characteristics influencing bioelectrical impedance analysis measurements. The American journal of clinical nutrition. 1996;64(3 Suppl):423s–7s. Epub 1996/09/01. 10.1093/ajcn/64.3.423S [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
Data Availability Statement
The data set is owned by the Institutional Review Board (IRB) of Tri-Service General Hospital (TSGH). TSGH IRB only approved the data analysis in our study and did not approve data sharing. Therefore, we do not have permission to share the data set. Interested researchers can submit data access requests to the Tri-Service General Hospital IRB using the following email address: tsghirb@ndmctsgh.edu.tw. Others would be able to access these data in the same manner as the authors and the authors also did not have any special access privileges.