Abstract
Background
Posttraumatic stress disorder is a debilitating psychiatric disorder characterized by symptoms of intrusive re-experiencing of trauma, avoidance and hyper-arousal. Diagnosis and treatment of PTSD is further complicated by concurrently occurring disorders, the most frequent being major depressive disorder and anxiety disorders. Previous research highlights that attentional processing in posttraumatic stress disorder is associated with substantial interference by emotional stimuli, a phenomenon also observed in these concurrently occurring psychiatric disorders. However, the diagnosis-relevance of this interference remains elusive. Here, we investigated the emotional Stroop interference for diagnosis-related stimuli, generally negative stimuli, and generally positive stimuli in posttraumatic stress disorder, major depressive disorder and anxiety disorders.
Methods
We performed a systematic database search in PubMed (Medline), Cochrane Library and PsycINFO on emotional Stroop performance in individuals with a diagnosis of posttraumatic stress disorder, major depressive disorder or anxiety disorders separately. Mean effect sizes, standard errors and confidence intervals were estimated for each clinical group and healthy control group comparison using random effect models.
Results
As compared to healthy control group, the posttraumatic stress disorder group displayed greater interference by diagnosis-related stimuli and positive stimuli but not for generally negative stimuli. The major depressive disorder and anxiety disorders groups showed greater interference by diagnosis-related and negative stimuli, but not by positive stimuli. The age and sex had no significant impact on interference.
Conclusions
These findings highlight the importance of diagnosis-relevant information on attentional processing in all three clinical populations, posttraumatic stress disorder, major depressive disorder and anxiety disorders. Further, the impact of generally negative stimuli but not generally positive stimuli in major depressive disorder and anxiety disorders indicate impaired attentional bias for mood-congruent stimuli but not for general stimuli. Finally, it remains to be studied whether the influence of generally positive stimuli in posttraumatic stress disorder indicate that positive stimuli are perceived as PTSD related.
Introduction
Individuals diagnosed with posttraumatic stress disorder (PTSD) have been exposed to a traumatic event comprising physical or psychological harm. PTSD is a highly debilitating disorder defined by the direct or indirect exposure to a traumatic event, including symptoms of intrusive re-experience, trauma-related thoughts and feelings and hypervigilance [1]. Hence, PTSD can lead to considerable impairments in individual well-being, from everyday functioning up to suicidal ideation and suicide attempts [2–4].
Symptom severity in PTSD and symptoms of re-experiencing, hypervigilance, avoidance and levels of anxiety have been positively correlated with attentional bias [5,6]. Attentional bias in PTSD usually refers to persistent engagement of attentional processing mechanisms to real or experimentally evoked threatening events. Indeed, attentional processing in PTSD is associated with interference by emotional stimuli [6–17]. Hence, recent clinical trials implemented the existing knowledge on the mechanisms of attentional bias in PTSD into a novel treatment approach, that is, attentional bias modification (ABM) [18,19]. ABM utilizes the detection-of-object (dot) probe task to elicit attentional bias. Studies on ABM treatment reported a decrease in PTSD symptoms with effect sizes similar to those of placebo-pill pharmacotherapy outcomes. Furthermore, ABM treatment did not improve attentional bias [20], even when the ABM approach was tailored to personally relevant bias-evoking stimuli [21]. Given the lack of evidence for ABM as a treatment for PTSD, there is a need to further understand attentional bias in PTSD.
The Stroop task [22] is a well-established cognitive task to investigate attentional processing of simultaneously occurring sensory information in context of selective attention, cognitive set shifting and response inhibition (see for example MacLeod or Stein et al.) [23,24]. In the original version of the color-word Stroop task (CW-Stroop) [25], participants are presented with three different card templates: the first template contains black-on-white printed words of highly distinguishable colors (“blue”, “green”, “red”, “yellow”); the second template depicts rectangles printed in blue, green, red or yellow color (“color-naming” condition); and the last template shows the same words as the first template but printed in a color that does not represent its actual content (“incongruent” condition; e.g., the word “blue” printed in green). Performance on the first template serves as a measure of reading abilities. Then, a Stroop interference index is calculated by subtracting response times (RT) in the color-naming condition from those in the incongruent condition. This index serves as a measure of the attentional engagement and hence the attentional bias. Nowadays, computerized versions of the Stroop paradigm allow for specific modifications in experimental designs that serve individual study purposes (e.g., stimulus presentation in a trial-by-trial fashion instead of a blocked design using physical template cards).
The emotional Stroop task modifies the CW-Stroop rationale by replacing color-words with neutral and emotionally loaded stimuli (e.g., the word “violence” painted in blue compared to a neutral word in the same color) [26]. In healthy participants, the emotional Stroop task does not elicit attentional bias related to threatening stimuli when stimuli were presented in a single-item fashion (i.e., one stimulus at a time) [18]. Individuals diagnosed with PTSD, however, often display attentional bias for negative stimuli as compared to healthy controls at the emotional Stroop task (i.e., threatening or aversive) [9,13]. Hence, emotional interference appears to be related to the thematic relevance of the personally experienced event and not merely on the exposure to threat per se in both civilians [15] and military personnel [5]. In survivors of sexual violence, for instance, an attentional bias was observed for both intimacy-related trauma words (e.g., “rape”) and intimacy-related positive words (e.g., “love”) [5]. However, recent findings on emotional interference, especially in military personnel with acute PTSD, challenge the view of an attentional bias for threatening or trauma-related information. These studies report an attentional shift away from threatening information, suggesting an avoidance pattern [19,27,28]. Given these inconsistencies, a meta-analysis aimed to synthesize findings on emotional Stroop task performance in PTSD. This meta-analysis indicated that individuals with PTSD, as compared to healthy controls, displayed impairments in the emotional Stroop task when processing trauma-related and generally threatening, but not positive information [29].
Attentional bias is also important in patients with major depressive disorder (MDD) or anxiety disorders (AD). During neuropsychological tests, individuals diagnosed with MDD and AD also display abnormal attentional bias to emotionally loaded stimuli [18,30]. This is of particular importance because MDD and AD are among the most concurrent diagnoses in PTSD. Further, these concurrent psychiatric disorders greatly impact and hamper diagnosis and treatment of PTSD [31–33]. For instance, co-occurrence of PTSD and MDD symptoms considerably elevates risk for suicidal ideations and suicide attempts compared to the impact of each disorder on its own [34–37].
The aim of this work is thus to investigate attentional bias in individuals diagnosed with PTSD, MDD and / or AD. Hence, we systematically reviewed findings on the emotional Stroop paradigm addressing task performance in individuals with PTSD, MDD, and /or AD, as compared to healthy controls. We used meta-analytic statistical tools to assess whether interference by stimuli with emotional valence in these groups can be attributed to non-specific emotional valence (generally positive or negative) or diagnosis-relevant emotional words.
Materials and methods
Methodological quality assessment
We conducted this review following PRISMA guidelines [38]. The PRISMA checklist is provided in supplementary material (S1 Table).
Search strategy
We conducted a systematic database search in PubMed (Medline), Cochrane Library and PsycINFO on peer-reviewed articles published between 1986 and 30 April 2018, using combinations of the search strings “posttraumatic stress disorder (PTSD)” or “major depressive disorder (MDD)” or “anxiety disorder (AD)” and “modified/emotional Stroop task”. Detailed search terms are provided in supplementary material (S2 Fig). We limited our search to published peer-reviewed articles as they tend to show higher methodological quality than unpublished studies [39–40]. In the field of psychiatry research, inclusion of grey literature may also increase risk of bias [41]. We applied a filter to identify relevant literature examining humans only. This database search resulted in a total of 900 abstracts for potential inclusion.
Selection criteria
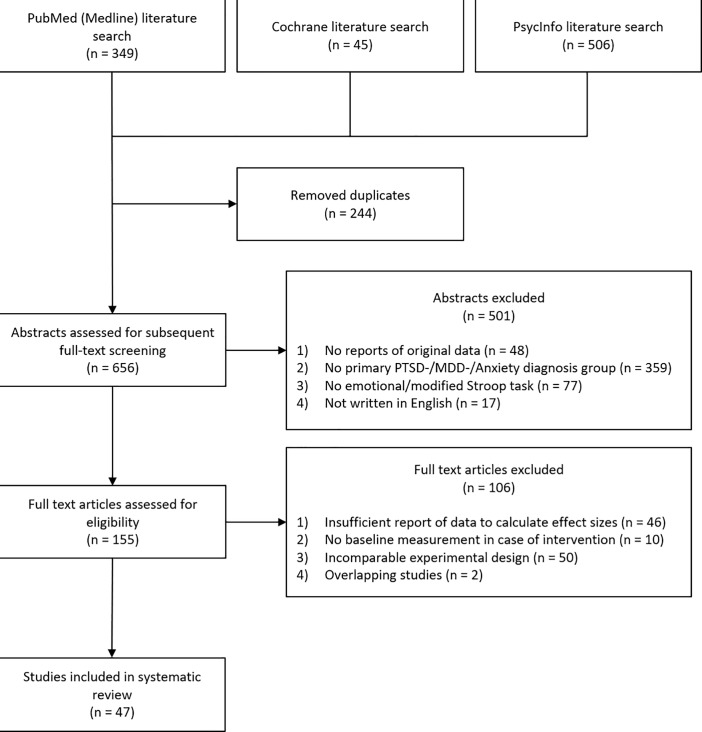
In a first phase of selection, we excluded abstracts (a) not reporting original data (e.g., reviews, meta-analyses, opinion letters), (b) not assessing a group with a primary diagnosis of PTSD, MDD, or AD, (c) not implementing the modified or emotional Stroop task as a cognitive measurement, and (d) not written in English, leaving a total of 155 original studies. In order to conduct coherent statistical analyses, we further excluded 108 of the 155 studies due to the following reasons: (a) no sufficient behavioral data to calculate effect sizes (neither interference scores nor response times to emotional and neutral conditions), (b) experimental manipulation prior to baseline behavioral testing (e.g., assessment of treatment efficacy), (c) experimental designs that were incomparable to designs of the remaining studies (i.e. stimulus presentation in a multi-item instead of a single-item/trial-by-trial design, stroop task with masked presentation mode or with pictures instead of written words) and (d) overlapping studies. Based on the procedure described above, 47 studies [5–7,42–85] were included in statistical analyses (54 datasets: PTSD: 13; MDD: 12; AD: 29) (Fig 1).
Fig 1. Schematic overview of the meta-analytic study selection procedure in this review.
PTSD = Posttraumatic Stress Disorder, MDD = Major Depressive Disorder.
Data extraction
Data extraction was performed by two independent reviewers (TW, JLM). Differences regarding outcome variables were resolved by consensus.
Qualitative data
We collected demographics and experimental design characteristics from all studies including, if available, (a) study type, (b) clinical diagnostics, (c) number of participants, (d) mean age, (e) female/male ratio, (f) medication, (g) comorbidity status, (h) stimulus valence, and (i) response modality. We further assessed potential risk of bias in included studies using the Cochrane risk of bias tool [86]. The Cochrane risk of bias tool allows for inference of studies’ quality via an observer-dependent rating system that scores potential methodological shortcomings as “low”, “high” or “unclear” (S2 Table).
Quantitative data
We extracted mean response times (RT), mean interference scores, as well as respective dispersion indices (Standard Deviation [SD] and Standard Error [SE]) from all studies.
Outcome measures of the emotional Stroop task
Within the emotional Stroop task, participants are asked to identify the color of either neutral or emotionally loaded stimuli while ignoring its actual content. Interference by a specific type of stimulus is calculated by RT difference scores (i.e., subtracting mean RT of the neutral stimuli from that of emotional stimuli). In this regard, positive values mirror longer RT for emotionally loaded stimuli and negative values indicate shorter RT for emotionally loaded stimuli (see for example El Khoury-Malhame et al.) [13]. Interference scores were our primary outcome measure, calculated on RT measurements. Across studies, RT was commonly defined as the latency from stimulus onset to the participant’s response. RTs of the Stroop tasks were reported in milliseconds (ms) and accompanied by statistical dispersion indices (SD, SE).
Stimulus type and response modalities
Stimuli consisted of words and were presented in a trial-by-trial fashion (i.e., one stimulus at a time) in a randomized or quasi-randomized order on a computer monitor. Participants were instructed to respond verbally (e.g., recorded by microphone on a computer) or by pressing a button that was specific to the color of the displayed word. Twenty-nine group comparisons measured RTs of verbal responses (PTSD: n = 8; MDD: n = 6; AD: n = 15) and 25 group comparisons measured RTs by button press (PTSD: n = 5; MDD: n = 6; AD: n = 14).
Arousal ratings and stimulus valence
This section describes how word stimuli were categorized in the studies included in this review. Ratings of stimuli valence and/or arousal were based on participants’ self-reports prior to or after testing (4 studies), ratings in pilot experiments with independent cohorts of participants (6 studies), evaluations by clinicians or otherwise trained professionals (8 studies) or were taken from previous research and validated stimuli databases (27 studies). In four studies, assessment of arousal or stimulus rating was not further specified. Based on stimulus ratings, and for the sake of comparability across studies and psychiatric diagnostic groups, word stimuli included in the present analysis were categorized as (a) diagnosis-relevant (i.e. PTSD-relevant, e.g. war, abuse; MDD-relevant, e.g. sadness, discouraged; AD-relevant, e.g. panic, embarrassed) (b) generally negative (e.g. fraud, divorce), or (c) generally positive (e.g. pleasant, comedy). Diagnosis-relevant cues had the distinction of being related to the trauma inducing-event or to the symptomatology of clinical groups. In all studies, stimuli with neutral valence were utilized for calculation of interference scores.
Statistical data analysis
Statistical analyses were separately performed for each of the three clinical groups (PTSD-groups, MDD-groups, AD-groups). Effect sizes (bias-corrected standardized mean-differences; Hedge’s g) were calculated with the R Metafor package (R 3.5.0; R Development Core Team) for each study contrasting clinical and healthy control groups. Single-study effect sizes were analyzed using meta-analytic random-effect models and the Hedge’s g estimator to derive a meta-analytic overall effect size (g) and tests for heterogeneity (Q and I2) for each group comparison of interest. We also explored whether some variables had an impact on interference using moderator analyses. There were sufficient available data to test mean age of patients, percentage of women in clinical groups, and response modality at the emotional Stroop task. Separate models were fitted to determine the main effects of each variable. In case of large heterogeneity across studies, outliers were identified based on visual inspection of the meta-analytic forest plots. To further test whether any one study was overly influential on effect size estimates, we conducted leave-one-out cross-validation for these group comparisons of interest.
Results
Study types
Case-control studies was the most common study type. For the PTSD groups, articles described case-control studies (11 studies), controlled before-after study (1 study), and randomized controlled trial (1 study). All articles with MDD groups described case-control studies (12 studies). For AD groups, study types were case-control studies (21 studies), controlled before-after study (1 study), randomized controlled trial (1 study), and crossover study (1 study). One article with AD patients also included three experiments, with both case control and before-after designs. In all cases, we extracted data from the baseline condition to avoid any experimental manipulation prior to behavioral testing.
Study groups
Clinical groups
Clinical groups comprised individuals with a primary diagnosis of either (a) PTSD, (b) MDD or (c) AD according to administered clinical interviews and scales (Tables 1–3). Diagnoses of AD included generalized anxiety disorder—not otherwise specified (GAD NOS), panic disorder (PD), social phobia (SoP), specific phobia (SpP) and multiple diagnoses of AD within one study group. To enable statistical group comparisons of interest and provide an overall view of attentional bias in AD in general, all differential AD diagnoses were summarized in a single AD group. In each individual study, clinical assessments were based on the DSM [1] or the International Classification of Disorders (ICD) [87] using the most recent version at the respective time-point of assessment. Across studies, 288 participants were considered in the PTSD-groups, 243 participants in the MDD-groups and 613 participants in the AD-groups.
Table 1. Characteristics of included studies with posttraumatic stress disorder (PTSD) groups.
| Reference | Study typeA | Clinical ScaleB |
N of subject (clinical; HC) |
Age (mean ± SD) |
Female patients (%) | Rx | Comorbidity | Stimulus valenceC | Response modality |
|---|---|---|---|---|---|---|---|---|---|
| Ashley[5] | CC | CPRS | 30; 30 | 32.2 ± 7.9 | 3.3 | - | Yes | GN, GP, PTSD | Verbal |
| Buckley[42] | CC | SCID | 6; 6 | 34.7 ± 7.0 | 100 | - | - | PTSD | Verbal |
| Cassiday[43] | CC | SCID | 12; 12 | 33.2 ± 10.3 | 91.7 | Yes, not specified | - | GN, GP | Verbal |
| Harvey[44] | CC | PTSD-I | 20; 20 | 34.0 ± 10.9 | 70.0 | - | - | PTSD | Verbal |
| Herzog[45] | CC | SCID, CAPS | 28; 28 | 30.6 ± 10.0 | 100 | Yes | Yes | GN, PTSD | Motor |
| Khanna[46] | CC | CAPS | 26; 16 | 33.9 ± 9.0 | 0.0 | - | Yes | GN, PTSD | Verbal |
| El Khoury-Malhame[6] | CBA | MINI | 19; 19 | 45.0 ± 15.0 | 63.2 | Yes | Yes | GN | Motor |
| Martinson[47] | CC | SCID | 33; 35 | 23.6 | 78.8 | - | - | PTSD | Motor |
| McNally[48] | CC | CMISS | 15; 12 | 48.0 ± 13.4 | 100 | - | - | GP, PTSD | Verbal |
| Metzger[49] | CC | SCID | 9; 10 | 37.0 ± 10.0 | 66.7 | No | Yes | GP, PTSD | Motor |
| Paunovic[7] | CC | CAPS | 39; 39 | 35.7 ± 9.7 | 39.3 | Yes | Yes | GP, PTSD | Verbal |
| Thomaes[50] | RCT | CAPS | 29; 22 | 33.5 ± 11.6 | 100 | Yes | Yes | GN, PTSD | Motor |
| Wittekind[51] | CC | SCID | 22; 11 | 71.0 ± 2.4 | 68.2 | - | - | GN, PTSD | Verbal |
A CBA = Controlled before and after study, CC = Case-control, RCT = Randomized Controlled Trial
B CPRS = Computerized Patient Record System, SCID = Structured Clinical Interview for DSM, PTSD-I = PTSD-Interview, CAPS = Clinician Administered PTSD Scale, MINI = Mini-Internal Neuropsychiatric Interview for DSM, CMISS = Mississippi Scale for Combat-Related Posttraumatic Stress Disorder (civilian version)
C GN = Generally negative, GP = Generally positive, PTSD = Posttraumatic stress disorder-relevant; Rx = Prescriptions
Table 3. Characteristics of included studies with anxiety disorder (AD) groups.
| Reference | Study typeA | Clinical ScaleB |
N of subject (clinical; HC) |
Age (mean ± SD) |
Female patients (%) | Rx | Comorbidity | Stimulus valenceC | Response modality |
|---|---|---|---|---|---|---|---|---|---|
| A) Generalized anxiety disorder (GAD NOS) | |||||||||
| Bradley[64] | CC | DSM-III-R | 20; 11 | 37.8 ± 11.3 | 40.0 | - | Yes | GAD | Verbal |
| Chen[65] | CC | DSM-IV-TR | 42; 26 | 34.3 ± 8.0 | 54.8 | Yes | - | GAD, GP | Motor |
| Dozois[54] | CC | SCID | 25; 25 | 38.9 ± 10.4 | - | Yes | - | GN, GP | Verbal |
| Mogg[62] | CC | DSM-III-R | 19; 18 | 38.2 | 73.7 | - | - | GAD, GP | Verbal |
| Price[66] | CC | SCID | 16; 12 | 63.1 ± 3.1 | 68.8 | Yes, not specified | Yes | GN | Motor |
| B) Panic disorder (PD) | |||||||||
| Chen[65] | CC | DSM-IV-TR | 34; 46 | 31.6 ± 8.6 | 67.7 | Yes | - | GP, PD | Motor |
| De Cort[67] | CC | DSM-IV | 32; 30 | 42.5 ± 12.3 | 53.1 | - | - | GN, PD | Motor |
| Deppermann[68] | RCT | DSM-IV-TR | G1: 14; 19 G2: 12; 19 |
38.4 39.1 |
50 75 |
Yes Yes |
Yes Yes |
PD | Motor |
| Dresler[69] | CC | SCID | 20; 23 | 31.7 ± 7.4 | 55.0 | Yes | Yes | PD | Motor |
| Dresler[70] | CC | SCID | 17; 26 | 40.0 ± 11.6 | 58.8 | Yes | Yes | PD | Motor |
| Gropalis[71] | CBA | SCID | 25; 31 | 31.1 ± 8.1 | 64.0 | - | Yes | PD | Motor |
| Kampman[72] | Exp 1–2: CC Exp 3: before after study |
DSM-IV | 18; 18 | 38.9 ± 12.6 | - | - | - | GN, PD | Verbal |
| Lim[57] | CC | ADIS | 33; 33 | 39.7 ± 8.8 | - | Yes | - | GN, GP | Verbal |
| Lundh[73] | CC | ADIS | 35; 35 | 37.2 ± 9.4 | 71.4 | Yes, partially specified | Yes | GN, PD | Verbal |
| Maidenberg[74] | CC | ADIS-R | 15; 15 | Range: 21–49 |
- | No | - | GN, GP, PD | Verbal |
| McNally[75] | CC | ADIS-R | 14; 14 | 36.6 | 64.3 | - | - | GN | Verbal |
| McNally[76] | CC | SCID | 24; 24 | 32.0 ± 9.0 | 91.7 | - | - | GN, GP | Verbal |
| McNally[77] | CC | SCID | 16; 16 | - | 93.7 | - | - | GN, GP, PD | Verbal |
| Reinecke[78] | CC | SCID | 23; 22 | 28.6 ± 8.1 | 69.6 | No | Yes | GN, PD | Verbal |
| Thomas[79] | CC | CIDI | 20; 20 | 36.0 ± 10.0 | 75.0 | Yes | Yes | GN | Motor |
| van den Heuvel[80] | CC | SCID | 15; 19 | 33.7 ± 9.7 | 46.7 | No | No | PD | Motor |
| C) Social phobia (SoP) | |||||||||
| Amir[81] | CC | SCID | 20; 20 | 35.3 ± 13.0 | 45.0 | - | - | GP, SoP | Verbal |
| Boehme[82] | CC | SCID | 16; 16 | 29.1 ± 9.8 | 16.67 | No | Yes | SoP | Motor |
| Maidenberg[74] | CC | ADIS-R | 15; 15 | Range: 19–38 |
- | No | - | GN, GP, SoP | Verbal |
| D) Specific phobia (SpP) | |||||||||
| Britton[83] | CC | SCID | 12; 12 | 25.2 ± 4.5 | 58.3 | No | - | GN, SpP | Motor |
| E) Multiple anxiety diagnoses | |||||||||
| Andrews[84] | Crossover | SCID | 11; 12 | 40.0 ± 7.0 | 45.5 | No | Yes | ANX, GN, GP | Verbal |
| De Cort[67] | CC | DSM-IV | 25; 30 | 36.0 ± 13.6 | 76.0 | - | - | ANX, GN | Motor |
| Quero[85] | CC | DSM-IV | 25; 25 | 29.0 ± 7.0 | 88.0 | - | Yes | PD, SoP, GP | Motor |
A CBA = Controlled before and after study, CC = Case-control, RCT = Randomized Controlled Trial
B DSM-III-R = Diagnostic Manual for DSM Disorders (3rd Edition, revision), DSM-IV-TR = Diagnostic Manual of Mental Disorders (4th Edition, text-revision), SCID = Structured Clinical Interview for DSM, ADIS-R = Anxiety Disorders Interview Schedule (revised), CIDI = Composite International Diagnostic Interview for DSM; G1 = Group 1, G2 = Group 2
C GAD = Generalized anxiety disorder-relevant, GP = Generally positive, GN = Generally negative, PD = Panic disorder-relevant, SoP = Social phobia-relevant, SpP = Specific phobia-relevant; Rx = Prescriptions
Table 2. Characteristics of included studies with major depressive disorder (MDD) groups.
| Reference | Study typeA | Clinical ScaleB |
N of subject (clinical; HC) |
Age (mean ± SD) |
Female patients (%) | Rx | Comorbidity | Stimulus valenceC | Response modality |
|---|---|---|---|---|---|---|---|---|---|
| Broomfield[52] | CC | GDS | 16; 19 | 73.0 ± 6.2 | 56.3 | Yes | - | GN, GP | Motor |
| Constant[53] | CC | SCID | 20; 26 | 47.7 | 60.0 | Yes | - | MDD | Verbal |
| Dozois[54] | CC | SCID | 24; 25 | 38.8 ± 12.7 | - | Yes | - | GN, GP | Verbal |
| Fritzsche[55] | CC | SCID | 20; 20 | 40.6 ± 9.2 | 50.0 | Yes | - | GP, MDD | Verbal |
| Gupta[56] | CC | SCID | 10; 10 | 40.0 ± 8.4 | 50.0 | Yes | No | GN, GP | Motor |
| Lim[57] | CC | ADIS | 33; 33 | 39.7 ± 8.8 | - | Yes | - | GN, GP, MDD | Verbal |
| Markela-Lerenc[58] | CC | SCID | 23; 27 | 41.0 ± 11.4 | 47.8 | Yes | - | GP, MDD | Motor |
| Matsubara[59] | CC | DSM-IV-TR | 16; 20 | 45.4 ± 2.2 | 50.0 | Yes | No | GN, GP, MDD | Motor |
| McNeely[60] | CC | SCID | 15; 14 | 38.5 ± 8.7 | 73.3 | Yes | Yes | GN, GP | Motor |
| Mitterschiffthaler[61] | CC | SCID | 17; 17 | 39.3 ± 9.4 | 82.4 | No | - | MDD | Verbal |
| Mogg[62] | CC | DSM-III-R | 18; 18 | 34.8 | 77.8 | - | - | GP, MDD | Verbal |
| Schlosser[63] | CC | SCID | 31; 37 | 38.7 ± 10.4 | 48.4 | Yes | Yes | GN, MDD | Motor |
A CC = Case-control
B GDS = Geriatric Depression Scale, SCID = Structured Clinical Interview for DSM, ADIS = Anxiety Disorders Interview Schedule, DSM-IV-TR = Diagnostic Manual of Mental Disorders (4th Edition, text-revision), DSM-III-R = Diagnostic Manual for DSM Disorders (3rd Edition, revision)
C GN = Generally negative, GP = Generally positive, MDD = Major depressive disorder-relevant; Rx = Prescriptions
Control groups
All clinical groups were compared to healthy control groups, without psychiatric or neurological disorder. The total number of participants in the healthy control groups was 1068 (260 participants in the PTSD studies; 266 participants in the MDD studies; 542 participants in the AD studies).
Comorbidity and medication status
Comorbidity
Nineteen out of the 47 studies reported secondary diagnoses of other psychiatric and neurological disorders besides the primary diagnosis according to criteria of the DSM or ICD (Tables 1–3). For PTSD groups, 7 studies reported comorbid occurring diagnoses including AD (6 studies), MDD (6 studies), obsessive compulsive disorder (2 studies), personality disorder (2 studies), comorbid AD and MDD (1 study), eating disorder (1 study), somatoform disorder (1 study) and mild traumatic brain injury (1 study). For MDD groups, 2 studies reported comorbid diagnoses: AD (2 studies), dysthymic disorder (1 study), obsessive compulsive disorder (1 study), eating disorder (1 study), somatoform disorder (1 study), PTSD (1 study) and substance use disorder (1 study). For AD groups, 10 studies reported co-occurring disorders, including other AD (8 studies), MDD (6 studies), dysthymic disorder (2 studies), somatoform disorder (2 studies), eating disorder (1 study), personality disorder (1 study) and PTSD (1 study). Data on occurrence of comorbidity were insufficient to conduct moderator analyses to assess whether it has an impact on interference.
Medication status
In 22 out of the 47 selected studies, participants were reported to be undergoing pharmacotherapy (Tables 1–3). Individuals in the PTSD-groups received antidepressant (4 studies), anxiolytic (3 studies), first- or second-generation antipsychotics (2 study) and anticonvulsant (1 study) medication. One study reported that individuals in the PTSD-group were on current medication, but no specification on the type of drug was provided. In the MDD-groups, individuals received antidepressants (10 studies), anxiolytics (5 studies), first- or second-generation antipsychotics (3 studies). Individuals in the AD-groups were given antidepressants (7 studies), anxiolytics (5 studies), first- or second-generation antipsychotics (2 studies) or other non-specified medication (1 study). Any discrepancies in the number of clinical groups and studies reporting comorbid diagnoses or psychopharmacological treatment are related to individual studies assessing more than one clinical group (see for example Lim & Kim) [57], and clinical groups with individuals receiving customized (poly-) pharmacotherapy.
Emotional Stroop interference
PTSD-groups
As illustrated in Fig 2, the PTSD group, as compared to healthy control group, showed greater interference for PTSD-relevant and generally positive words (i.e. slowing of responses for PTSD-relevant and generally positive words as compared to neutral words). Effect sizes were strong on interference by PTSD-relevant words (g = .65, p < .001; Table 4) and moderate by generally positive words (g = .38, p < .01). Interference for generally negative words was not significant (g = .33, p = .139). Heterogeneity across studies was large for generally negative words (Q’s p value = .040, I2 = 71.53%). Cross-validation indicated that the interference remained non-significant for generally negative words when the meta-analysis was run multiple times, each time leaving out a different study (all p-values ≥ .068). Moderator analyses indicated that mean age of patients and female proportion did not influence attentional bias (all p-values ≥ .215, S3 Table). Response modality had a significant influence on effect sizes for interference by PTSD-relevant words only (p < .05). Effect sizes were larger for motor than verbal responses.
Fig 2. Effect sizes of emotional vs. neutral stimuli during emotional Stroop task performance for the PTSD-groups.
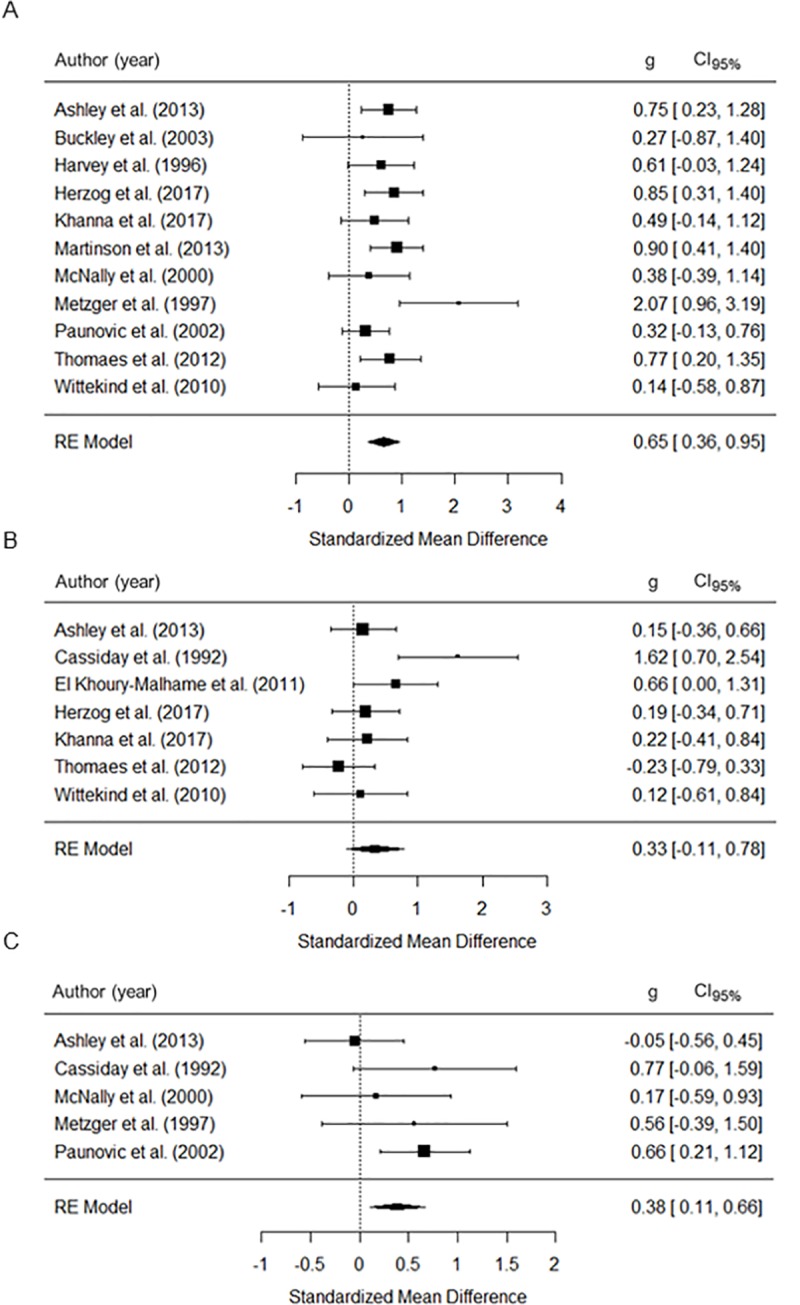
(A) PTSD-specific words, (B) generally negative words, (C) generally positive words; RE = Random effects, g = Standardized mean-difference, CI95% = 95% confidence interval.
Table 4. Summary statistics of overall group comparisons for words with diagnosis-relevant, generally negative and generally positive stimulus valence in the emotional Stroop task.
| Group comparison | Stimulus valence | N | g | g’s p value |
SEM | g’s CI95% |
Q’s p value |
I2 (%) | I2 ‘s CI95% |
|---|---|---|---|---|---|---|---|---|---|
| PTSD vs. HC | PTSD-specific | 11 | .65 | .000 (***) | .15 | [.36, .95] | .209 | 57.91 | [.00, 85.36] |
| Generally negative | 7 | .33 | .139 | .23 | [-.11, .78] | .040 (*) | 71.53 | [.00, 94.08] | |
| Generally positive | 5 | .38 | .007 (**) | .14 | [.11, .66] | .231 | .00 | [.00, 89.22] | |
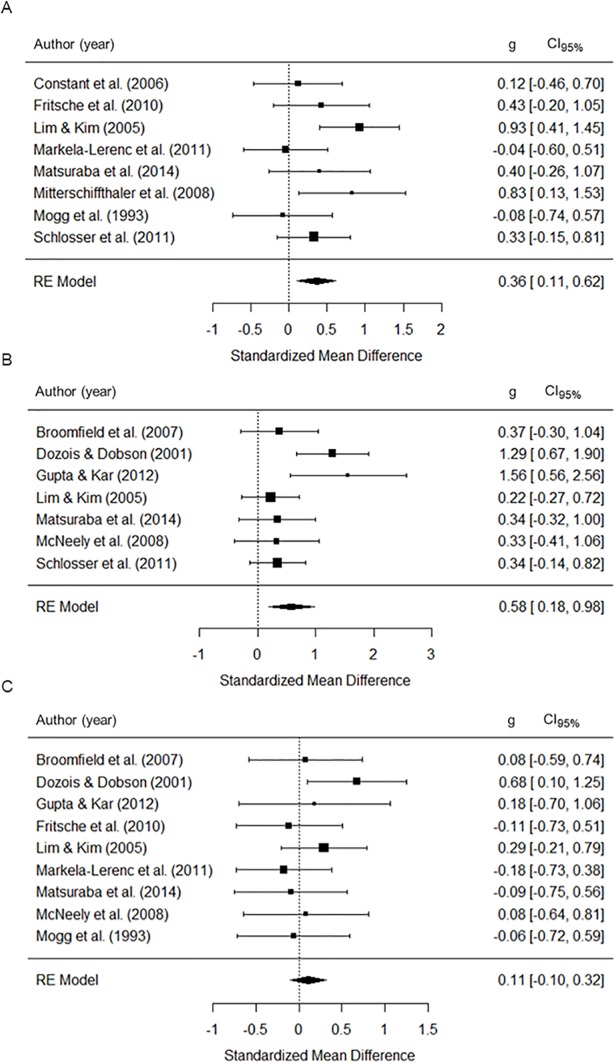
| MDD vs. HC | MDD-specific | 8 | .36 | .005 (**) | .13 | [.11, .62] | .147 | 37.70 | [.00, 84.18] |
| Generally negative | 7 | .58 | .004 (**) | .20 | [.18, .98] | .047 (*) | 63.19 | [.00, 92.57] | |
| Generally positive | 9 | .11 | .293 | .11 | [-.10, .32] | .599 | .00 | [.00, 62.24] | |
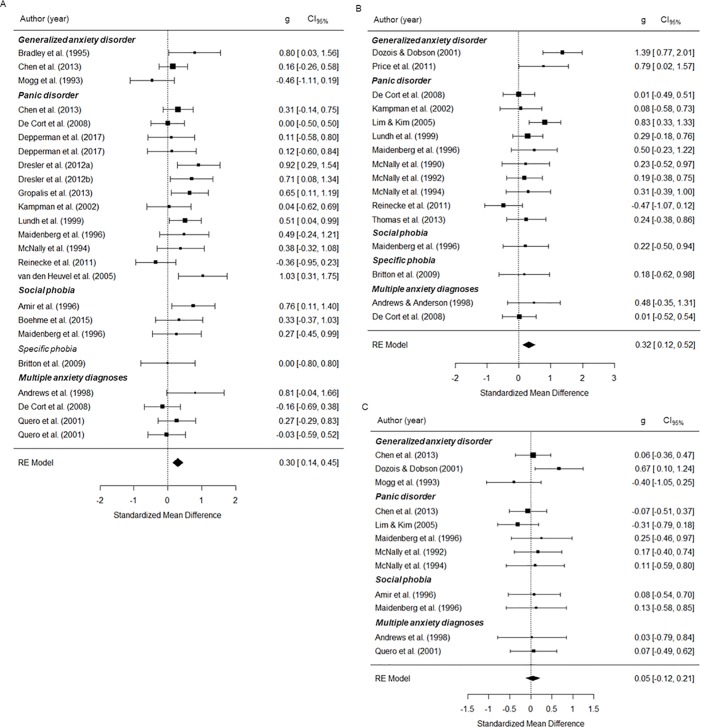
| AD vs. HC | AD-specific | 24 | .30 | .000 (**) | .08 | [.14, .45] | .057 | 35.28 | [.00, 68.11] |
| Generally negative | 16 | .32 | .002 (**) | .10 | [.12, .52] | .022 (*) | 38.52 | [1.50, 76.23] | |
| Generally positive | 12 | .05 | .570 | .08 | [-.12, .21] | .588 | .00 | [.00, 58.57] |
PTSD = Posttraumatic stress disorder; HC = Healthy controls; MDD = Major depressive disorder; AD = Anxiety disorder; N = Number of studies; g = standardized mean-difference; SEM = Standard error of the mean; CI95% = 95% confidence interval; Q = Q-test of study heterogeneity.
(*) p < .05
(**) p < .01
(***) p < .001.
MDD-groups
The group composed of individuals with a primary diagnosis of MDD, as compared to healthy control group, displayed an attentional bias with moderate effect sizes for MDD-relevant words (g = .36, p < .01; Table 4; Fig 3) and generally negative words (g = .58, p < .01), but not for generally positive words (g = .11, p = .293). The Q-test of study heterogeneity was significant for generally negative words (Q’s p value = .047, I2 = 63.19%). The attentional bias for this stimulus valence remained significant when each study was removed one at a time in cross-validation analysis (all p-values ≤ 0.027). None of the moderator variables had a significant effect on the attentional bias in MDD-groups (all p-values ≥ 0.239, S3 Table).
Fig 3. Effect sizes of emotional vs. neutral stimuli during emotional Stroop task performance for the MDD-groups.
(A) MDD-specific words, (B) generally negative words, (C) generally positive words; RE = Random effects, g = Standardized mean-difference, CI95% = 95% confidence interval.
AD-groups
The group composed of individuals with a primary diagnosis of AD, as compared to healthy control group, showed an attentional bias with moderate effect sizes for AD-relevant words (g = .30, p < .001; Table 4; Fig 4) and generally negative words (g = .32, p < .01), but not for generally positive words (g = .05, p = .570). Between-study heterogeneity was large for generally negative words (Q’s p value = .022, I2 = 38.52). The attentional bias for generally negative words remained significant when we performed leave-one-out cross-validation (all p-values ≤ 0.009). None of the moderator variables had a significant effect on the attentional bias in AD-groups (all p-values ≥ 0.062, S3 Table).
Fig 4. Effect sizes of emotional vs. neutral stimuli during emotional Stroop task performance for the AD-groups.
(A) AD-specific words, (B) generally negative words, (C) generally positive words; Generalized anxiety disorder NOS = Generalized anxiety disorder—not otherwise specified, RE = Random effects, g = Standardized mean-difference, CI95% = 95% confidence interval.
Risk of bias assessment
Twenty-five out for the 47 studies had a low risk of bias regarding incomplete outcome data, whereas the remaining 22 studies had an unclear risk of bias, due to insufficient reporting of exclusions or attrition. Thirty-three studies had a low risk of bias concerning the allocation concealment, one had a high risk of bias, and the remaining 13 studies had an unclear risk of bias. Regarding the selective reporting of outcomes, 40 studies had a low risk of bias, whereas 7 studies had an unclear risk of bias. All studies had a low risk of bias regarding blinding of participants and blinding of outcome assessments.
Discussion
Attentional bias to emotionally loaded stimuli has been closely linked to PTSD symptom severity and is considered as one of the core cognitive deficits of PTSD. In this meta-analysis, we examined attentional bias in PTSD and in its most frequently concurrently occurring disorders, that is, MDD and AD. We assessed whether studies using the emotional Stroop task showed consensus findings regarding an attentional bias elicited by emotional stimuli in individuals diagnosed with PTSD, MDD and AD as compared to healthy individuals. The results indicate that PTSD, MDD and AD groups showed greater interference by diagnosis-relevant stimuli (i.e. slowing of responses for diagnosis-relevant stimuli in comparison to neutral stimuli) as compared to healthy control groups. Further, the PTSD-groups displayed greater interference by positive stimuli than the healthy groups, but this was not observed in the MDD- and AD-groups. Finally, the MDD- and AD-groups showed greater interference by generally negative stimuli as compared to the healthy groups, but this effect was not observed in the PTSD-groups.
Results of the current meta-analysis show that the PTSD-groups displayed greater interference by PTSD-relevant and positive stimuli, but not by generally negative stimuli, as compared to healthy controls. They support that patients are sensitive to stimuli related to their concern (see for example Bar Haim et al. or Williams et al.) [18,26]. This is of importance as such attentional bias to self-relevant stimuli may play a role in the maintenance of the disorders [88–90]. They are also similar to some extent to results from the meta-analysis of Cisler et al. [29] which reported greater interference for PTSD-relevant and threatening stimuli in the PTSD group as compared to the control group. Together, these findings support a hypoactive attentional control mechanism, rather than a hyperactive threat detection mechanism. The results also partially support the idea that a general-purpose defense mechanism can be automatically activated by given stimuli (e.g., PTSD-specific) which impacts seemingly irrelevant stimuli (e.g., positive stimuli) [91]. However, we cannot rule out that positive valence in this meta-analysis was not in some instances processed as PTSD-relevant or threatening (e.g., intimacy, love, sex, heal, brave, honor) by a given patient with PTSD would process this as diagnostic-relevant stimuli (e.g., rape or war). Future work should tailor attention bias tasks to individually relevant stimuli to elucidate this. Such interference by positive stimuli in PTSD was not observed in a previous meta-analysis [29]. The lack of significant group differences for interference by generally negative stimuli may suggest preserved attentional processing of negative emotional content unrelated to the traumatic event in PTSD. Similarly, Cisler et al. [29] found no difference between PTSD and healthy control groups regarding interference for generally negative stimuli. Interestingly, Clausen et al. [92] tested for potential correlations between PTSD symptoms and avoid-approach biases of emotional stimuli (happy, disgust, anger). They found a significant correlation indicating that greater symptom severity was associated with greater bias by happy faces. Together these results suggest that attentional processing of positively valenced stimuli might be more impacted than generally negative stimuli in PTSD. However, the present meta-analysis includes a small sample size with high heterogeneity for the generally negative stimuli which may also account for the lack of differences between the PTSD and healthy groups. As such, cross-validation analysis indicated a p-value of 0.068 when one study was removed [50].
In regards to MDD-groups and AD-groups, the findings show that attentional processing is impaired for diagnosis-relevant and negative stimuli, as interference was greater when compared to healthy controls. There were no significant group differences for positive stimuli. These data indicate that general attentional processing is to some extent intact in MDD and AD that fits with traditional models predicting that depression is associated with an attentional bias for mood-congruent stimuli [93,94] or self-relevant stimuli than general stimuli [26,88,90,95]. In regards to AD, the results are similar to those of a previous meta-analysis [18], which reported that patients with AD had greater interference by diagnostic-relevant and negative stimuli, but not by positive stimuli, at the emotional Stroop task. In regards to depression, a previous meta-analysis reported that patients clinically depressed (including dysthymia and minor depression) displayed greater interference by negative, positive, neutral stimuli at the emotional Stroop task, and even greater interference at the Classic Stroop task, when compared to healthy controls [30]. Effect sizes were greater for negative than positive stimuli and greater for positive than neutral stimuli. They also found greater interference in blocked than randomized design. Such impact of the design on interference was also observed in AD [18,26]. It is thus possible that interferences are more content-specific when using a randomized design than a blocked design (minimizing potential mood induction and not eliciting a generic system).
The impact of age and sex on interference by emotional stimuli
We found that age and sex had no significant role in our moderator analyses for the PTSD, MDD and AD groups. Epp et al. [30] also reported no consistent patterns of moderating effects for age and sex in this meta-analysis in patients with depression. Age only had an impact on interference in healthy controls for incongruent versus control stimuli and sex only had an impact in depressed patients for neutral stimuli. In the current meta-analysis, response modality (verbal vs. button-press) had a significant impact for PTSD-relevant words when comparing the PTSD-groups with the healthy controls. Attentional bias was larger with motor than verbal responses. This difference may result from the modality pairing effect, which corresponds to the processing speed advantage for standard modality pairing (e.g. visual stimuli with motor response) as compared to non-standard modality pairing (e.g. visual stimuli with verbal response) [96]. Additional processes in the non-standard modality pairing condition may have diluted the interference effect. However, the lack of influence of the response modality for other stimulus valences in PTSD-groups as well as in MDD- and AD-groups advise caution about this finding.
Lack of data prevented us to use co-morbidity and pharmacological treatments as moderators. We cannot rule out that these factors had no impact on observed interference by emotional stimuli. Among studies included in the meta-analysis, some reported individuals’ current status of psychopharmacological treatment and diagnoses of comorbid disorders, but the majority did not. We cannot rule out that, simply because these covariates have not been reported, individuals were free of any exposure to psychotropic medications and other psychiatric or neurologic disorders that might have affected emotional Stroop task performance in PTSD. In studies comprising individuals with a primary diagnosis of MDD or AD, medication status was reported more frequently. However, groups were administered mono- or poly-pharmacotherapy, or received no medication at all, exacerbating the disentanglement of potential effects medical drug exposure might have had on behavioral outcomes in these cohorts. The same applies to the status of comorbidity in all three clinical groups of interest. When comorbidity was reported systematically, actual diagnoses were highly heterogeneous ranging from DSM axis I- and II-disorders [1] to neurological disorders. Moreover, our design was unsuited to investigate the effects of attentional bias to emotionally valent stimuli between PTSD and other disorders in explicitly comorbid cohorts (e.g., the impact of trauma-related or depression-related words in a group of individuals with a primary diagnosis of PTSD and comorbid MDD). This might come at the cost of potential interplaying effects on attentional bias when individuals are faced with more than a primary diagnosis of PTSD. However, assessing attentional bias in the emotional Stroop task for psychiatric disorders of interest separately might facilitate to disentangle effects that could, in turn, be responsible for attentional deficits in PTSD with frequent concurrent diagnoses. We also refrained from including a group of trauma-exposed, but PTSD-free individuals in our analysis, since the definition of such a control group was too heterogeneous across selected studies. Characterization of a trauma-exposed group might vary in subclinical scores on psychopathological questionnaires, PTSD in remission, as well as the mere presence of traumatic events during lifetime. It is hard to argue in favor of a trauma-exposed group if differentiation from a PTSD-diagnosed group (in terms of clinical features) in one direction and healthy controls (who might have experienced a traumatic event at least once in their life) in the other, if this border is rather transient than sharply defined. Lack of data prevented us to assess whether chronicity (e.g., time since trauma or diagnosis) was linked to greater interference, but previous work reported that greater severity of depression was linked to greater interference [30].
Methodological considerations
This study has limitations to be considered. One limitation is that 32 studies included multiple effect sizes (either by including multiple conditions of the Stroop, multiple groups, or both). Correlations between effect sizes are likely to be stronger within than between studies, which can lead to partially redundant analyses and incorrect estimation of effects. Thus, our approach does not allow direct comparisons of effect sizes across task conditions. Future meta-analyses including multiple conditions should consider using a multivariate model [97]. Also, our number of group comparisons was considerably smaller than that of other meta-analyses assessing attentional bias in psychiatric disorders. This can be accounted for by our inclusion criteria (e.g., stroop task with words only supraliminal, unmasked stimuli), hence reducing variance in experimental designs across studies. On the one hand, this might account for the lack of attentional bias to negative words in PTSD (e.g., a smaller sample size); on the other hand, it might help to further understand the core mechanisms underlying attentional bias in PTSD and related psychiatric disorders. In addition, studies with smaller sample sizes might have introduced positive bias in some group comparisons, as indicated in the funnel plots (S1 Fig). For instance, it might be the case for generally negative words in PTSD- and MDD-groups, where we find studies with large effect sizes outside the funnel. By visual inspection of the corresponding forest plots (Figs 2–3), we identified two studies, that may have contributed to the heterogeneity observed in these group comparisons [43,56]. There was, however, no methodological argument to exclude these studies from our analyses. Moreover, these studies did not lead to increased heterogeneity across group comparisons on other emotionally loaded stimuli (i.e., words with generally positive valence). Cross-validation analyses also indicated that the results remained unchanged when we removed these studies. The considerable level of heterogeneity in effect sizes of studies comparing PTSD-groups and healthy controls on words with generally negative valence could partially explain the lack of statistical significance of this group comparison. Finally, some studies reported that the Stroop interference is reliable (see for example Ebersole et al.) [98], but other studies questioned it and suggest that individual differences play a significant role in such reliability [99,100]. The importance of stimuli choice is nicely discussed in the context of substance-related attentional bias by Field & Christiansen [101]. For instance, a given patient with alcohol use disorders might not respond the same to stimuli of heavy spirits than those of rosé wine. In sum, future work may consider using more than a single attentional bias task, which captures only a subset of processes [100], and add physiological measures such as an eye tracker to gather a more complete picture of bias to emotionally loaded stimuli that are relevant to symptoms of PTSD, MDD and AD.
Conclusions
In sum, this review provides valuable synthesized results regarding an attentional bias for emotional stimuli in PTSD and its most frequently concurrent diagnoses of MDD and AD specifically elicited by the emotional Stroop task. However, it remains to be determined if the results presented here are specific to the emotional Stroop task or reflect a generally applicable cognitive deficit in PTSD and concurrent psychiatric disorders. We believe that additional studies are necessary to address the impact of concurrently occurring psychiatric disorders on the development and persistence of attentional bias in PTSD. Accumulated behavioral data might provide a clearer picture on underlying cognitive mechanisms that directly link to future treatment approaches (such as the ABM).
Supporting information
(TIF)
(TIF)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
The authors would like to thank V. Ashley, R. Gupta and C. E. Wittekind for providing original data sets to be included in the analyses presented here. We also thank Valérie Chénard for her technical help.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
S.F. was supported by the Canada Research Chair in Cognitive Neuroplasticity. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.American Psychiatric Association. Diagnostic and statistical manual of mental disorders 5 ed. Washington, DC: Author; 2013. [Google Scholar]
- 2.Sareen J, Houlahan T, Cox BJ, Asmundson GJ. Anxiety disorders associated with suicidal ideation and suicide attempts in the National Comorbidity Survey. J Nerv Ment Dis. 2005;193(7):450–4. [DOI] [PubMed] [Google Scholar]
- 3.Pagotto LF, Mendlowicz MV, Coutinho ES, Figueira I, Luz MP, Araujo AX, et al. The impact of posttraumatic symptoms and comorbid mental disorders on the health-related quality of life in treatment-seeking PTSD patients. Compr Psychiatry. 2015;58:68–73. 10.1016/j.comppsych.2015.01.002 [DOI] [PubMed] [Google Scholar]
- 4.Wisco BE, Marx BP, Wolf EJ, Miller MW, Southwick SM, Pietrzak RH. Posttraumatic stress disorder in the US veteran population: results from the National Health and Resilience in Veterans Study. J Clin Psychiatry. 2014;75(12):1338–46. 10.4088/JCP.14m09328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ashley V, Honzel N, Larsen J, Justus T, Swick D. Attentional bias for trauma-related words: exaggerated emotional Stroop effect in Afghanistan and Iraq war veterans with PTSD. BMC Psychiatry. 2013;13:86 10.1186/1471-244X-13-86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.El Khoury-Malhame M, Lanteaume L, Beetz EM, Roques J, Reynaud E, Samuelian JC, et al. Attentional bias in post-traumatic stress disorder diminishes after symptom amelioration. Behav Res Ther. 2011;49(11):796–801. 10.1016/j.brat.2011.08.006 [DOI] [PubMed] [Google Scholar]
- 7.Paunovic N, Lundh LG, Öst LG. Attentional and memory bias for emotional information in crime victims with acute posttraumatic stress disorder (PTSD). Anxiety Disorders. 2002;16:675–92. [DOI] [PubMed] [Google Scholar]
- 8.Elsesser K, Sartory G, Tackenberg A. Attention, heart rate, and startle response during exposure to trauma-relevant pictures: a comparison of recent trauma victims and patients with posttraumatic stress disorder. J Abnorm Psychol. 2004;113(2):289–301. 10.1037/0021-843X.113.2.289 [DOI] [PubMed] [Google Scholar]
- 9.Vythilingam M, Blair KS, McCaffrey D, Scaramozza M, Jones M, Nakic M, et al. Biased emotional attention in post-traumatic stress disorder: a help as well as a hindrance? Psychol Med. 2007;37(10):1445–55. 10.1017/S003329170700092X [DOI] [PubMed] [Google Scholar]
- 10.Hayes JP, Labar KS, Petty CM, McCarthy G, Morey RA. Alterations in the neural circuitry for emotion and attention associated with posttraumatic stress symptomatology. Psychiatry Res. 2009;172(1):7–15. 10.1016/j.pscychresns.2008.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hayes JP, Vanelzakker MB, Shin LM. Emotion and cognition interactions in PTSD: a review of neurocognitive and neuroimaging studies. Front Integr Neurosci. 2012;6:89 10.3389/fnint.2012.00089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mueller-Pfeiffer C, Martin-Soelch C, Blair JR, Carnier A, Kaiser N, Rufer M, et al. Impact of emotion on cognition in trauma survivors: what is the role of posttraumatic stress disorder? J Affect Disord. 2010;126(1–2):287–92. 10.1016/j.jad.2010.03.006 [DOI] [PubMed] [Google Scholar]
- 13.El Khoury-Malhame M, Reynaud E, Soriano A, Michael K, Salgado-Pineda P, Zendjidjian X, et al. Amygdala activity correlates with attentional bias in PTSD. Neuropsychologia. 2011;49(7):1969–73. 10.1016/j.neuropsychologia.2011.03.025 [DOI] [PubMed] [Google Scholar]
- 14.Felmingham KL, Rennie C, Manor B, Bryant RA. Eye tracking and physiological reactivity to threatening stimuli in posttraumatic stress disorder. J Anxiety Disord. 2011;25(5):668–73. 10.1016/j.janxdis.2011.02.010 [DOI] [PubMed] [Google Scholar]
- 15.Fleurkens P, Rinck M, van Minnen A. Specificity and generalization of attentional bias in sexual trauma victims suffering from posttraumatic stress disorder. J Anxiety Disord. 2011;25(6):783–7. 10.1016/j.janxdis.2011.03.014 [DOI] [PubMed] [Google Scholar]
- 16.Fani N, Jovanovic T, Ely TD, Bradley B, Gutman D, Tone EB, et al. Neural correlates of attention bias to threat in post-traumatic stress disorder. Biol Psychol. 2012;90(2):134–42. 10.1016/j.biopsycho.2012.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fani N, Tone EB, Phifer J, Norrholm SD, Bradley B, Ressler KJ, et al. Attention bias toward threat is associated with exaggerated fear expression and impaired extinction in PTSD. Psychol Med. 2012;42(3):533–43. 10.1017/S0033291711001565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bar-Haim Y, Lamy D, Pergamin L, Bakermans-Kranenburg MJ, van IMH. Threat-related attentional bias in anxious and nonanxious individuals: a meta-analytic study. Psychol Bull. 2007;133(1): 1–24. 10.1037/0033-2909.133.1.1 [DOI] [PubMed] [Google Scholar]
- 19.Bar-Haim Y, Holoshitz Y, Eldar S, Frenkel TI, Muller D, Charney DS, et al. Life-threatening danger and suppression of attention bias to threat. Am J Psychiatry. 2010;167(6): 694–8. 10.1176/appi.ajp.2009.09070956 [DOI] [PubMed] [Google Scholar]
- 20.Schoorl M, Putman P, Van Der Does W. Attentional bias modification in posttraumatic stress disorder: a randomized controlled trial. Psychother Psychosom. 2013;82(2): 99–105. 10.1159/000341920 [DOI] [PubMed] [Google Scholar]
- 21.Schoorl M, Putman P, Mooren TM, Van Der Werff S, Van Der Does W. Attentional bias modification in Dutch veterans with posttraumatic stress disorder—a case series with a personalized treatment version. J Trauma Stress. 2014;27(2): 240–3. 10.1002/jts.21896 [DOI] [PubMed] [Google Scholar]
- 22.Stroop JR. Studies of Interference in Serial Verbal Reactions. Journal of Experimental Psychology. 1935;18(643–662). [Google Scholar]
- 23.MacLeod CM. Half a century of research on the Stroop effect: an integrative review. Psychol Bull. 1991;109(2):163–203. [DOI] [PubMed] [Google Scholar]
- 24.Stein MB, Kennedy CM, Twamley EW. Neuropsychological Function in Female Victims of Intimate Partner Violence with and without Posttraumatic Stress Disorder. Biol Psychiatry. 2002;52:1079–88. [DOI] [PubMed] [Google Scholar]
- 25.Golden CJ, Freshwater SM. Stroop Color and Word Test: A Manual for Clinical and Experimantal Uses. Chicago, Illinois: Skoelting; 1978. [Google Scholar]
- 26.Williams JM, Mathews A, MacLeod C. The emotional Stroop task and psychopathology. Psychol Bull. 1996;120(1):3–24. [DOI] [PubMed] [Google Scholar]
- 27.Wald I, Lubin G, Holoshitz Y, Muller D, Fruchter E, Pine DS, et al. Battlefield-like stress following simulated combat and suppression of attention bias to threat. Psychol Med. 2011;41(4):699–707. 10.1017/S0033291710002308 [DOI] [PubMed] [Google Scholar]
- 28.Sipos ML, Bar-Haim Y, Abend R, Adler AB, Bliese PD. Postdeployment threat-related attention bias interacts with combat exposure to account for PTSD and anxiety symptoms in soldiers. Depress Anxiety. 2014;31(2):124–9. 10.1002/da.22157 [DOI] [PubMed] [Google Scholar]
- 29.Cisler JM, Wolitzky-Taylor KB, Adams TG Jr., Babson KA, Badour CL, Willems JL. The emotional Stroop task and posttraumatic stress disorder: a meta-analysis. Clin Psychol Rev. 2011;31(5):817–28. 10.1016/j.cpr.2011.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Epp AM, Dobson KS, Dozois DJ, Frewen PA. A systematic meta-analysis of the Stroop task in depression. Clin Psychol Rev. 2012;32(4):316–28. 10.1016/j.cpr.2012.02.005 [DOI] [PubMed] [Google Scholar]
- 31.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593–602. 10.1001/archpsyc.62.6.593 [DOI] [PubMed] [Google Scholar]
- 32.Stander VA, Thomsen CJ, Highfill-McRoy RM. Etiology of depression comorbidity in combat-related PTSD: a review of the literature. Clin Psychol Rev. 2014;34(2):87–98. 10.1016/j.cpr.2013.12.002 [DOI] [PubMed] [Google Scholar]
- 33.Gallagher MW, Brown TA. Bayesian Analysis of Current and Lifetime Comorbidity Rates of Mood and Anxiety Disorders In Individuals with Posttraumatic Stress Disorder. J Psychopathol Behav Assess. 2015;37(1):60–6. 10.1007/s10862-014-9436-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramsawh HJ, Fullerton CS, Mash HB, Ng TH, Kessler RC, Stein MB, et al. Risk for suicidal behaviors associated with PTSD, depression, and their comorbidity in the U.S. Army. J Affect Disord. 2014;161:116–22. 10.1016/j.jad.2014.03.016 [DOI] [PubMed] [Google Scholar]
- 35.Debeer BB, Kimbrel NA, Meyer EC, Gulliver SB, Morissette SB. Combined PTSD and depressive symptoms interact with post-deployment social support to predict suicidal ideation in Operation Enduring Freedom and Operation Iraqi Freedom veterans. Psychiatry Res. 2014;216(3):357–62. 10.1016/j.psychres.2014.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stevens D, Wilcox HC, MacKinnon DF, Mondimore FM, Schweizer B, Jancic D, et al. Posttraumatic stress disorder increases risk for suicide attempt in adults with recurrent major depression. Depress Anxiety. 2013;30(10):940–6. 10.1002/da.22160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pukay-Martin ND, Pontoski KE, Maxwell MA, Calhoun PS, Dutton CE, Clancy CP, et al. The influence of depressive symptoms on suicidal ideation among U.S. Vietnam-era and Afghanistan/Iraq-era veterans with posttraumatic stress disorder. J Trauma Stress. 2012;25(5):578–82. 10.1002/jts.21741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 6(7): e1000097 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Egger M, Jüni P, Bartlett C, Holenstein F, Sterne J. How important are comprehensive literature searches and the assessment of trial quality in systematic reviews? Empirical study. Health technol assess. 2003;7(1):1–4. [PubMed] [Google Scholar]
- 40.Mahood Q, Van Eerd D, Irvin E. Searching for grey literature for systematic reviews: challenges and benefits. Res Synth Methods. 2014;5(3):221–34. 10.1002/jrsm.1106 [DOI] [PubMed] [Google Scholar]
- 41.Martin JL, Pérez V, Sacristán M, Alvarez E. Is grey literature essential for a better control of publication bias in psychiatry? An example from three meta-analyses of schizophrenia. Eur Psychiatry. 2005;20(8):550–3. 10.1016/j.eurpsy.2005.03.011 [DOI] [PubMed] [Google Scholar]
- 42.Buckley TC, Galovski T, Blanchard EB, Hickling EJ. Is the Emotional Stroop Paradigm Sensitive to Malingering? A Between-Groups Study With Professional Actors and Actual Trauma Survivors. J Trauma Stress. 2003;16(1):59–66. 10.1023/A:1022063412056 [DOI] [PubMed] [Google Scholar]
- 43.Cassiday KL, McNally RJ, Zeitlin SB. Cognitive Processing of Trauma Cues in Rape Victims with Post-Traumatic Stress Disorder. Cognit Ther Res. 1992;16(3):283–95. [Google Scholar]
- 44.Harvey AG, Bryant RA, Rapee RM. Preconscious Processing of Threat in Posttraumatic Stress Disorder. Cognit Ther Res. 1996;20:613–23. [Google Scholar]
- 45.Herzog JI, Niedtfeld I, Rausch S, Thome J, Mueller-Engelmann M, Steil R, et al. Increased recruitment of cognitive control in the presence of traumatic stimuli in complex PTSD. Eur Arch Psychiatry Clin Neurosci. 2017. [DOI] [PubMed] [Google Scholar]
- 46.Khanna MM, Badura-Brack AS, McDermott TJ, Embury CM, Wiesman AI, Shepherd A, et al. Veterans with post-traumatic stress disorder exhibit altered emotional processing and attentional control during an emotional Stroop task. Psychol Med. 2017;47:2017–27. 10.1017/S0033291717000460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martinson AA, Sigmon ST, Craner J, Rothstein E, McGillicuddy M. Processing of intimacy-related stimuli in survivors of sexual trauma: the role of PTSD. J Interpers Violence. 2013;28(9):1886–908. 10.1177/0886260512469104 [DOI] [PubMed] [Google Scholar]
- 48.McNally RJ, Clancy SA, Schacter DL, Pitman RK. Cognitive processing of trauma cues in adults reporting repressed, recovered, or continuous memories of childhood sexual abuse. J Abnorm Psychol. 2000;109(3):355–9. [PubMed] [Google Scholar]
- 49.Metzger LJ, Orr SP, Lasko NB, McNally RJ, Pitman RK. Seeking the source of emotional Stroop interference effects in PTSD: a study of P3s to traumatic words. Integr Physiol Behav Sci. 1997;32(1):43–51. [DOI] [PubMed] [Google Scholar]
- 50.Thomaes K, Dorrepaal E, Draijer N, de Ruiter MB, Elzinga BM, van Balkom AJ, et al. Treatment effects on insular and anterior cingulate cortex activation during classic and emotional Stroop interference in child abuse-related complex post-traumatic stress disorder. Psychol Med. 2012;42(11):2337–49. 10.1017/S0033291712000499 [DOI] [PubMed] [Google Scholar]
- 51.Wittekind CE, Jelinek L, Kellner M, Moritz S, Muhtz C. Intergenerational transmission of biased information processing in posttraumatic stress disorder (PTSD) following displacement after World War II. J Anxiety Disord. 2010;24(8):953–7. 10.1016/j.janxdis.2010.06.023 [DOI] [PubMed] [Google Scholar]
- 52.Broomfield NM, Davies R, MacMahon K, Ali F, Cross SM. Further evidence of attention bias for negative information in late life depression. Int J Geriatr Psychiatry. 2007;22(3):175–80. 10.1002/gps.1655 [DOI] [PubMed] [Google Scholar]
- 53.Constant EL, Adam S, Seron X, Bruyer R, Seghers A, Daumerie C. Hypothyroidism and major depression: A common executive dysfunction? J Clin Exp Neuropsychol. 2006;28:790–807. 10.1080/13803390591000990 [DOI] [PubMed] [Google Scholar]
- 54.Dozois DJ, Dobson KS. Information processing and cognitive organization in unipolar depression: specificity and comorbidity issues. J Abnorm Psychol. 2001;110(2):236–46. [DOI] [PubMed] [Google Scholar]
- 55.Fritzsche A, Dahme B, Gotlib IH, Joormann J, Magnussen H, Watz H, et al. Specificity of cognitive biases in patients with current depression and remitted depression and in patients with asthma. Psychol Med. 2010;40(5):815–26. 10.1017/S0033291709990948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gupta R, Kar BR. Attention and memory biases as stable abnormalities among currently depressed and currently remitted individuals with Unipolar Depression. Front Psychiatry. 2012;3(99):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lim SL, Kim JH. Cognitive processing of emotional information in depression, panic, and somatoform disorder. J Abnorm Psychol. 2005;114(1):50–61. 10.1037/0021-843X.114.1.50 [DOI] [PubMed] [Google Scholar]
- 58.Markela-Lerenc J, Kaiser S, Golz T, Fiedler P, Mundt C, Weisbrod M. Attentional bias in depressive patients and the moderating effect of concurrent anxiety. Psychopathology. 2011;44(3):193–200. 10.1159/000319370 [DOI] [PubMed] [Google Scholar]
- 59.Matsubara T, Matsuo K, Nakashima M, Nakano M, Harada K, Watanuki T, et al. Prefrontal activation in response to emotional words in patients with bipolar disorder and major depressive disorder. Neuroimage. 2014;85 Pt 1:489–97. [DOI] [PubMed] [Google Scholar]
- 60.McNeely HE, Lau MA, Christensen BK, Alain C. Neurophysiological evidence of cognitive inhibition anomalies in persons with major depressive disorder. Clin Neurophysiol. 2008;119(7):1578–89. 10.1016/j.clinph.2008.03.031 [DOI] [PubMed] [Google Scholar]
- 61.Mitterschiffthaler MT, Williams SC, Walsh ND, Cleare AJ, Donaldson C, Scott J, et al. Neural basis of the emotional Stroop interference effect in major depression. Psychol Med. 2008;38(2):247–56. 10.1017/S0033291707001523 [DOI] [PubMed] [Google Scholar]
- 62.Mogg K, Bradley BP, Williams R, Mathews A. Subliminal processing of emotional information in anxiety and depression. J Abnorm Psychol. 1993;102(2):304–11. [DOI] [PubMed] [Google Scholar]
- 63.Schlosser N, Mensebach C, Rullkotter N, Schaffrath C, Driessen M, Beblo T, et al. Selective attention in depression: influence of emotionality and personal relevance. J Nerv Ment Dis. 2011;199(9):696–702. 10.1097/NMD.0b013e318229d6cf [DOI] [PubMed] [Google Scholar]
- 64.Bradley BP, Mogg K, Millar N, White J. Selective processing of negative information: effects of clinical anxiety, concurrent depression, and awareness. J Abnorm Psychol. 1995;104(3):532–6. [DOI] [PubMed] [Google Scholar]
- 65.Chen J, Wang Z, Wu Y, Cai Y, Shen Y, Wang L, et al. Differential attentional bias in generalized anxiety disorder and panic disorder. Neuropsychiatr Dis Treat. 2013;9:73–80. 10.2147/NDT.S36822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Price RB, Eldreth DA, Mohlman J. Deficient prefrontal attentional control in late-life generalized anxiety disorder: an fMRI investigation. Transl Psychiatry. 2011;1:e46 10.1038/tp.2011.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.De Cort K, Hermans D, Spruyt A, Griez E, Schruers K. A specific attentional bias in panic disorder? Depress Anxiety. 2008;25(11):951–5. 10.1002/da.20376 [DOI] [PubMed] [Google Scholar]
- 68.Deppermann S, Vennewald N, Diemer J, Sickinger S, Haeussinger FB, Dresler T, et al. Neurobiological and clinical effects of fNIRS-controlled rTMS in patients with panic disorder/agoraphobia during cognitive-behavioural therapy. Neuroimage Clin. 2017;16:668–77. 10.1016/j.nicl.2017.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dresler T, Ehlis AC, Hindi Attar C, Ernst LH, Tupak SV, Hahn T, et al. Reliability of the emotional Stroop task: an investigation of patients with panic disorder. J Psychiatr Res. 2012;46(9):1243–8. 10.1016/j.jpsychires.2012.06.006 [DOI] [PubMed] [Google Scholar]
- 70.Dresler T, Hindi Attar C, Spitzer C, Lowe B, Deckert J, Buchel C, et al. Neural correlates of the emotional Stroop task in panic disorder patients: an event-related fMRI study. J Psychiatr Res. 2012;46(12):1627–34. 10.1016/j.jpsychires.2012.09.004 [DOI] [PubMed] [Google Scholar]
- 71.Gropalis M, Bleichhardt G, Hiller W, Witthoft M. Specificity and modifiability of cognitive biases in hypochondriasis. J Consult Clin Psychol. 2013;81(3):558–65. 10.1037/a0028493 [DOI] [PubMed] [Google Scholar]
- 72.Kampman M, Keijsers GPJ, Verbraak MJPM, Näring G, Hoogduin CAL. The Emotional Stroop: A Comparison of Panic Disorder Patients, Obsessive-Compulsive Patients, and Normal Controls, in Two Experiments. Anxiety Disorders. 2002;16:425–41. [DOI] [PubMed] [Google Scholar]
- 73.Lundh LG, Wikstrom J, Westerlund J, Ost LG. Preattentive bias for emotional information in panic disorder with agoraphobia. J Abnorm Psychol. 1999;108(2):222–32. [DOI] [PubMed] [Google Scholar]
- 74.Maidenberg E, Chen E, Craske M, Bohn P, Bystritsky A. Specificity of Attentional Bias in Panic Disorder and Social Phobia. Journal of Anxiety Disorders. 1996;10(6):529–41. [Google Scholar]
- 75.McNally RJ, Riemann BC, Kim E. Selective processing of threat cues in panic disorder. Behav Res Ther. 1990;28(5):407–12. [DOI] [PubMed] [Google Scholar]
- 76.McNally RJ, Riemann BC, Louro CE, Lukach BM, Kim E. Cognitive processing of emotional information in panic disorder. Behav Res Ther. 1992;30(2):143–9. [DOI] [PubMed] [Google Scholar]
- 77.McNally RJ, Amir N, Louro CE, Lukach BM, Riemann BC, Calamari JE. Cognitive processing of idiographic emotional information in panic disorder. Behav Res Ther. 1994;32(1):119–22. [DOI] [PubMed] [Google Scholar]
- 78.Reinecke A, Cooper M, Favaron E, Massey-Chase R, Harmer C. Attentional bias in untreated panic disorder. Psychiatry Res. 2011;185(3):387–93. 10.1016/j.psychres.2010.07.020 [DOI] [PubMed] [Google Scholar]
- 79.Thomas SJ, Gonsalvez CJ, Johnstone SJ. Neural time course of threat-related attentional bias and interference in panic and obsessive-compulsive disorders. Biol Psychol. 2013;94(1):116–29. 10.1016/j.biopsycho.2013.05.012 [DOI] [PubMed] [Google Scholar]
- 80.van den Heuvel OA, Veltman DJ, Groenewegen HJ, Witter MP, Merkelbach J, Cath DC, et al. Disorder-specific neuroanatomical correlates of attentional bias in obsessive-compulsive disorder, panic disorder, and hypochondriasis. Arch Gen Psychiatry. 2005;62(8):922–33. 10.1001/archpsyc.62.8.922 [DOI] [PubMed] [Google Scholar]
- 81.Amir N, Freshman M, Foa E. Enhanced Stroop Interference for Threat in Social Phobia. Anxiety Disorders. 2002;16:1–9. [DOI] [PubMed] [Google Scholar]
- 82.Boehme S, Ritter V, Tefikow S, Stangier U, Strauss B, Miltner WH, et al. Neural correlates of emotional interference in social anxiety disorder. PLoS One. 2015;10(6):e0128608 10.1371/journal.pone.0128608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Britton JC, Gold AL, Deckersbach T, Rauch SL. Functional MRI study of specific animal phobia using an event-related emotional counting stroop paradigm. Depress Anxiety. 2009;26(9):796–805. 10.1002/da.20569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Andrews TM, Anderson IM. Information processing in anxiety: a pilot study of the effect of manipulating 5-HT function. J Psychopharmacol. 1998;12(2):155–60. 10.1177/026988119801200207 [DOI] [PubMed] [Google Scholar]
- 85.Quero S, Baños RM, Botella C. Cognitive biases in panic disorder: a comparison between computerised and card stroop task. Psychology in Spain. 2001;5(1):26–32. [Google Scholar]
- 86.Higgins JP, Altman DG, Gotzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomized trials. BMJ. 2011;343:d5928 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.World Health Organization. The ICD-10 classification of mental and behavioural disorders: clinical descriptions and diagnostic guidelines: Geneva: World Health Organization; 1992. [Google Scholar]
- 88.Dalgleish T, Watts FN. Biases of attention and memory in disorders of anxiety and depression. Clin Psychol Rev. 1990;10:589–604. [Google Scholar]
- 89.Gotlib IH, Roberts JE, Gilboa E. Cognitive interference in depression In Sarason I. G., Pierce G. R., & Sarason B. R. (Eds.), Cognitive interference: Theories, methods, and findings. 1996:347–377. Hillsdale, NJ, England: Lawrence Erlbaum Associates Inc. [Google Scholar]
- 90.Mogg K, Bradley BP. Attentional bias in generalized anxiety disorder versus depressive disorder. Cognit Ther Res. 2005;29(1):29–45. [Google Scholar]
- 91.Algom D, Chajut E, Lev S. A rational look at the emotional stroop phenomenon: a generic slowdown, not a stroop effect. J Exp Psychol Gen. 2004;133(3):323–38. 10.1037/0096-3445.133.3.323 [DOI] [PubMed] [Google Scholar]
- 92.Clausen AN, Youngren W, Sisante JF, Billinger SA, Taylor C, Aupperle RL. Combat PTSD and Implicit Behavioral Tendencies for Positive Affective Stimuli: A Brief Report. Front Psychol. 2016;7(758):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Beck AT, Rush A, Shaw B, Emery G. Cognitive Therapy of Depression. New York: The Guilford Press; 1979. [Google Scholar]
- 94.Bower GH. Mood and memory. American Psychologist. 1981;36:129–148. [DOI] [PubMed] [Google Scholar]
- 95.Mathews A, MacLeod C. Cognitive approaches to emotion and emotional disorders. Annu Rev Psychol. 1994;45:25–50. 10.1146/annurev.ps.45.020194.000325 [DOI] [PubMed] [Google Scholar]
- 96.Hazeltine E, Ruthruff E. Modality pairing effects and the response selection bottleneck. Psychol Res. 2006;70(6):504–13. 10.1007/s00426-005-0017-3 [DOI] [PubMed] [Google Scholar]
- 97.Riley RD, Thompson JR, Abrams KR. An alternative model for bivariate random-effects meta-analysis when the within-study correlations are unknown. Biostatistics. 2008;9(1):172–86. 10.1093/biostatistics/kxm023 [DOI] [PubMed] [Google Scholar]
- 98.Ebersole CR, Olivia E. Atherton OE, Belanger AL, Skulborstad HM, Allend JM et al. Labs 3: Evaluating participant pool quality across the academic semester via replication. J Exp Soc Psychol. 2016;67:68–82. [Google Scholar]
- 99.Ataya AF, Adams S, Mullings E, Cooper RM, Attwood AS, Munafò MR. Internal reliability of measures of substance-related cognitive bias. Drug Alcohol Depend. 2012;121(1–2):148–51. 10.1016/j.drugalcdep.2011.08.023 [DOI] [PubMed] [Google Scholar]
- 100.Hedge C, Powell G, Sumner P. The reliability paradox: Why robust cognitive tasks do not produce reliable individual differences. Behav Res Methods. 2017;50(3):1166–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Field M, Christiansen P. Commentary on Ataya et al. (2012), 'Internal reliability of measures of substance-related cognitive bias' (2012). Drug Alcohol Depend. 2012;124(3): 189– 10.1016/j.drugalcdep.2012.02.009 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
(TIF)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.