Abstract
Nutritional intervention of fruit juice supplementation is able to maximize exercise performance. Watermelon [Citrullus lanatus (Thunb.) Matsum. and Nakai] contains high L-citrulline content and consumption of watermelon juice may promote ergogenic effects. The aim of the present study was to investigate the role of 100% flesh watermelon juice and 100% rind watermelon juice supplementation for 14 days on swimming performance in rats. Twenty four male Sprague-Dawley rats were randomly divided into four groups: Cx group of rats supplemented with filtered tap water (negative control), L-cit group of rats supplemented with L-citrulline (positive control), FR group of rats supplemented with 100% flesh watermelon juice, and RR group of rats supplemented with 100% rind watermelon juice. Each group was supplemented for 14 days ad libitum prior to swimming exercise protocol. The rats were performed swimming exercise for 3 days and swimming time until exhaustion was measured. Plasma samples were collected to measure lactate concentration, ammonia concentration, and nitric oxide production. Rats supplemented with 100% flesh watermelon juice demonstrated significantly prolonged of swimming time until exhaustion, reduction of lactate and ammonia concentrations, and increased of nitric oxide production compared to Cx and L-cit groups (P<0.05). These findings postulate that supplementation with 100% flesh watermelon juice improves endurance in swimming performance.
Keywords: watermelon juice, Citrullus lanatus, L-citrulline, swimming performance
INTRODUCTION
Developing an effective nutritional supplement that improves exercise performance is a major focus for researchers and sport professionals. One of the most popular types of nutritional supplements are legal ergogenic aids, including L-citrulline (1). L-citrulline supplementation acts as a source of energy during exercise and is involved in removal of excess metabolites from the body (2). Pérez-Guisado and Jakeman (3) reported that supplementation of 8 g of citrulline malate can reduce muscle soreness at 24 and 48 h following anaerobic exercise. An in vivo study by Meneguello et al. (4) investigated supplementation of mice with a single dose of supplement containing 0.26 g/kg body weight L-citrulline, 0.4 g/kg body weight L-arginine, and 0.2 g/kg body weight L-ornithine lowered blood ammonia accumulation after exercise and prolonged the time until exhaustion in swimming exercises. These findings suggest that the beneficial functions of L-citrulline in accelerating metabolite waste removal, improving endurance performance, and promoting faster recovery after exercise.
Recent studies demonstrate that L-citrulline supplementation effectively increases L-arginine concentration than oral consumption of pure L-arginine (5–7). However, the extent to which L-citrulline may lead to excess Larginine production remains unknown. A study by Evans et al. (8) reported that L-arginine consumption causes gastrointestinal discomfort, nausea, and diarrhoea suggesting it is unsuitable for daily intake. These side effects may be due to rapid and elevated nitric oxide (NO) production in the gastrointestinal tract, causing body discomfort (9). Emerging researchers are focusing on exploring safer alternative nutritional regimens that contains L-citrulline with other nutrients to improve exercise performance (10–12). Evidence suggests that fruit juice rich in L-citrulline is beneficial for improving exercise performance (10,13,14).
Watermelon (Citrullus lanatus (Thunb.) Matsum. and Nakai) is a member of the Cucurbitaceae plant family and is widely cultivated in Malaysia (15). Watermelon is naturally rich in amino acids including L-citrulline, phytonutrients such as carotenoids (lycopene and β-carotene), polyphenolics, and vitamins (12,15). Our recent report demonstrated that rind watermelon juice contains a higher L-citrulline content compared to flesh watermelon juice (45.02 mg/g compared to flesh watermelon juice, 43.81 mg/g, respectively) (16). Similarly, findings by Rimando and Perkins-Veazie (17) and Jayaprakasha et al. (18) supported that rind watermelon juice contains higher L-citrulline content than flesh thus may suggest that both flesh and rind watermelon juices potentially offer similar benefits to pure L-citrulline for improving exercise performance (10–12). However, there is little evidence to support that watermelon juices could provide such improvements.
Considering that acute watermelon juice supplementation appears to be ineffective in improving exercise performance as reported by Cutrufello et al. (11), longer term supplementation may avert such drawbacks. To date, only a paucity of researches has investigated the effects of longer-term supplementation of watermelon juices on swimming performance. Therefore, the purpose of the current in vivo study is to investigate the role of 100% flesh watermelon juice and 100% rind watermelon juice supplementation for 14 days on swimming performance in rats. To this end, we measured endurance performance parameters including swimming exercise time until exhaustion, lactate measurement, ammonia measurement, and nitric oxide production.
MATERIALS AND METHODS
Materials
L-citrulline was purchased from NOW Foods (Bloomingdale, IL, USA). Lactate assay kit was purchased from Randox Laboratories Ltd. (Kearneysville, WV, USA). Ammonia assay kit was purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). QuantiChromTM Nitric Oxide Assay Kit was purchased from BioAssay Systems (Hayward, CA, USA).
Watermelon juices preparation
Medium sized, ripe, 75~80 days old locally harvested red seedless watermelon were obtained from Selangor Fruit Valley, Rawang, Selangor, Malaysia. The watermelons were cleaned with filtered tap water and peeled to obtain the red flesh and white rind. The flesh and rind processed using a commercial juice maker that automatically separates the pulp and 100% juices. The 100% flesh watermelon juice and 100% rind watermelon juice were prepared freshly on a daily basis.
Animal handling and study design
This study was conducted in accordance with the criteria of the Universiti Teknologi MARA Committee of Animal Research and Ethics (UiTM CARE) guidelines concerning the use of experimental animals [Reference number: 600-FF(PS. 17/2/1)]. Twenty four, healthy, six-week-old male Sprague-Dawley rats were obtained from the Laboratory Animal Facility and Management, Faculty of Pharmacy, UiTM Puncak Alam. The animals were acclimatized for 14 days with a normal pellet diet and filtered tap water ad libitum. Rats was assigned into 4 groups of 6 rats: negative control (Cx) group (rats supplemented with filtered tap water ad libitum for 14 days twice daily), positive control (L-cit) group (rats supplemented with L-citrulline, 500 mg of L-citrulline dissolved in 100 mL filtered tap water ad libitum twice daily for 14 days), FR group (rats supplemented with 100% flesh watermelon juice ad libitum twice daily for 14 days), and RR group (rats supplemented with 100% rind watermelon juice ad libitum twice daily for 14 days). The amount of L-citrulline in 100% flesh watermelon juice and 100% rind watermelon juice were 2.46 and 3.13 mg per 1 mL of juice, respectively, quantified using the reversed phase-highperformance liquid chromatography (RP-HPLC) method in accordance to our previous method with a slight modification (16). All solutions were changed twice daily and the volumes consumed by each rat were recorded. The average of the total amounts of solutions, amount of L-citrulline consumed, and rat body weight changes were calculated after 14 days ad libitum supplementation. Body weight changes were calculated by subtracting final body weights from initial body weights, allowing the net gain in body weight to be standardized and statistically compared. Rats underwent swimming exercise following supplementation of 14 days.
Swimming exercise protocol
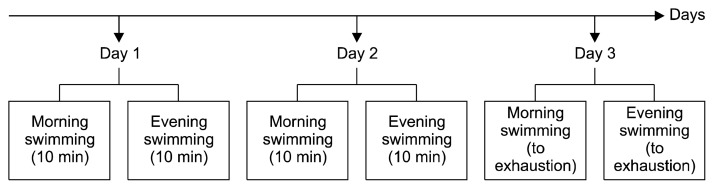
The swimming exercise protocol was carried out in a tank (42×64×38 cm) filled with water to a 30-cm depth and maintained at room temperature. Rats were assessed swimming twice daily for 3 days, as showed in Fig. 1. On the first and second days, rats were assessed swimming for 10 min in the morning and 10 min in the evening. Whilst on third day, rats were assessed swimming until exhaustion in the morning and the evening. The time taken for the rats to swim until exhaustion was recorded. Rats were considered to have reached exhaustion when they were unable to raise their faces to the surface of the water to inhale within 15 s.
Fig. 1.
Experimental protocol of swimming exercise.
Sampling
Twenty four hours after swimming on day 3, rats were anesthetized with ketamine-xylaxine (0.2 to 0.3 mL per 200 to 300 g according to rats’ weights). Rats were euthanized by cardiac puncture and blood samples were collected. Blood samples were immediately stored at 4°C on ice and centrifuged at 3,500 rpm for 10 min to separate plasma from blood cells. Plasma was stored at −80 °C prior to further analysis.
Lactate measurement
Lactate concentration was determined using a lactate assay kit (Randox Laboratories Ltd.). Reagent blank, standard and plasma samples (10 μL) were mixed with 1,000 μL L-lactate reagent (PAP) Randox (LC 2389) in a cuvette. The samples were incubated for 10 min at room temperature. The absorbances of all solutions were measured using a Perkin Elmer LAMBDATM Bio+ Spectrophotometer at a wavelength of 550 nm. Plasma lactate concentration were calculated based on the following formula:
where standard concentration is 4.4 mmol/L.
Ammonia measurement
Ammonia concentration was determined using an Ammonia assay kit (Sigma-Aldrich Co.). Standard and plasma samples (10 μL) were mixed with 90 μL of working reagent mix, consisting of 90 μL ammonia assay buffer, 4 μL reagent A and 4 μL of reagent B in 96 well plates. Reactions then incubated for 15 min in the dark at room temperature. The fluorescence intensities were measured using a POLARstar Omega Reader (ex=360 nm; em= 450 nm). Plasma ammonia concentration was calculated based on the standard curve and the following formula:
where F is fluorescence intensity.
NO assay
NO concentration was determined using a Quanti-ChromTM Nitric Oxide Assay Kit (BioAssay System) in accordance with manufacturer’s instructions. Briefly, 100 μL of deproteinated plasma samples were added to 200 μL working reagent, formulated from 100 μL Reagent A, 4 μL Reagent B, and 100 μL Reagent C. The solution was incubated at 60°C for 10 min. After centrifugation (6,000 g for 10 min), 250 μL supernatant was transferred into 96-well plate and the absorbance calculated at 540 nm using a SPECTROstar MicroPlate Reader (BMG Labtech, Ortenberg, Germany).
Statistical analyses
Mean±standard error of mean (SEM) values were calculated and statistical analysis was undertaken using SPSS version 18.0 (SPSS Inc., Chicago, IL, USA) for Windows. Data were analyzed by one-way analysis of variance (ANOVA), followed by Tukey’s post hoc test for multiple comparisons. Differences were considered statistically significant when P values were less than 0.05.
RESULTS
Effects of treatment on changes in body weight
The average amounts of all solutions and L-citrulline consumed by rats, and body weight changes following supplementation for 14 days ad libitum are shown in Table 1. Rats supplemented with 100% flesh watermelon juice showed the highest solution intake after 14 days, 658.00 mL which is equivalent to 1.62 g of L-citrulline. Rats supplemented with 100% rind watermelon juice consumed 535.36 mL (1.68 g of L-citrulline), and those treated with filtered tap water consumed 424.06 mL while rats supplemented with L-citrulline solution consumed 363.86 mL (1.82 g of L-citrulline). Rats in the experimental groups showed increases in body weight, with significant differences observed between FR and L-cit groups after 14 days supplementation ad libitum. Rats in the FR group had significantly higher changes in body weight (81.67±8.72 g) compared to rats in the L-cit group (55.00±5.63 g) with P<0.05. Rats in the FR group showed a trend for greater changes in body weight compared to RR group (75.83±11.58 g). No significant difference was observed in body weight changes between rats in the FR and RR groups compared to those in the Cx group (60.00±7.75 g).
Table 1.
Averages for total amounts of solution, L-citrulline consumed and body weight changes in rats after 14 days of supplementation ad libitum
| Groups | Average amounts of total solution consumed (mL) | Amounts of L-citrulline consumed (g) | Initial body weight (g) | Final body weight (g) | Body weight changes (g) |
|---|---|---|---|---|---|
| Cx | 424.06 | N/A | 160.00±7.30 | 220.00±4.47 | 60.00±7.75 |
| L-cit | 363.86 | 1.82 | 150.00±0.00 | 205.00±5.63 | 55.00±5.63 |
| FR | 658.00 | 1.62 | 131.67±6.54 | 213.33±8.03 | 81.67±8.72* |
| RR | 535.36 | 1.68 | 139.17±12.68 | 215.00±9.57 | 75.83±11.58 |
Significant difference when compared to L-cit (P <0.05).
Cx, negative control group; L-cit, positive control group; FR, group of rats supplemented with 100% flesh watermelon juice; RR, group of rats supplemented with 100% rind watermelon juice.
N/A, not available.
Swimming time until exhaustion
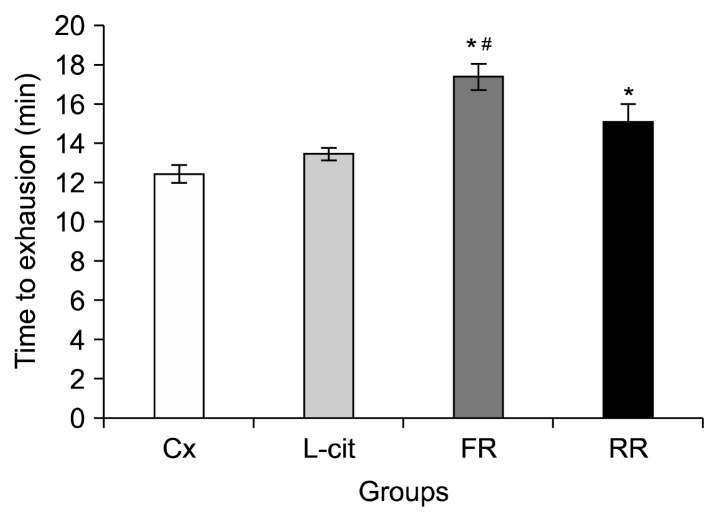
The amounts of time the rats in Cx, L-cit, FR, and RR groups could swim until exhaustion are presented in Fig. 2. Rats in the FR group were able to swim for significantly longer amounts of time before exhaustion than rats in the Cx group (P<0.05). FR group were able to swim for an average of 17.36±0.65 min compared to the Cx group, 12.44±0.44 min and L-cit group, 13.42±0.28 min. Significant differences in swimming time until exhaustion were also observed for the RR group (15.06±0.92 min) compared to Cx group (P<0.05).
Fig. 2.
Time of swimming exercise until exhaustion in groups of rats supplemented with 100% flesh watermelon juice, 100% rind watermelon juice, L-citrulline, and control. The bar chart shows the average duration of swimming exercise time of rats until exhaustion of Cx, L-cit, FR, and RR. Values were expressed as mean±SEM (n=6). Significant difference compared to *Cx and #L-cit (P <0.05). Cx, negative control group; L-cit, positive control group; FR, group of rats supplemented with 100% flesh watermelon juice; RR, group of rats supplemented with 100% rind watermelon juice.
Plasma lactate measurement
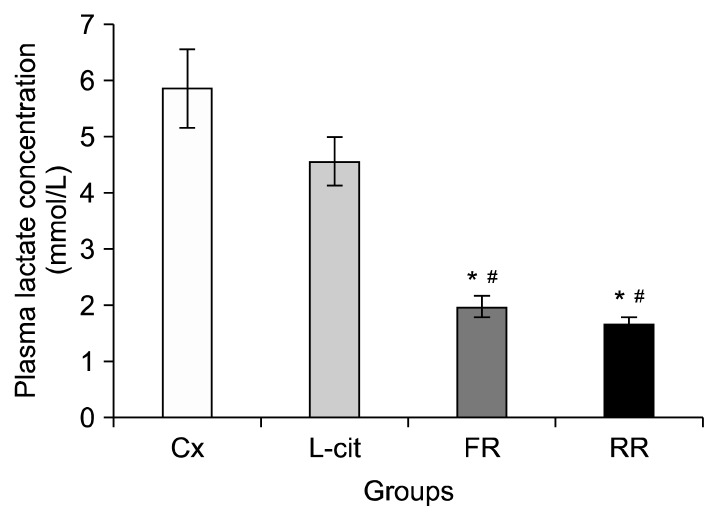
The plasma lactate concentrations of rats following the swimming exercises are presented in Fig. 3. A significantly lower concentration of plasma lactate was observed in the FR group compared to Cx and L-cit groups with P< 0.05. The average lactate concentration for the FR group was 1.96±0.19 mmol/L, whilst the average lactate concentration in the Cx and L-cit groups were 5.84±0.7 mmol/L and 4.56±0.43 mmol/L, respectively. Interestingly, rats in the RR group also showed a significant reduction in plasma lactate concentration, 1.65±0.13 mmol/L compared to those in the Cx and L-cit groups (P<0.05).
Fig. 3.
Plasma lactate concentration in groups of rats supplemented with 100% flesh watermelon juice, 100% rind watermelon juice, L-citrulline, and control. The bar chart shows plasma lactate concentration (mmol/L) in Cx, L-cit, FR, and RR. Values were expressed as mean±SEM (n=6). Significant difference compared to *Cx and #L-cit (P <0.05). Cx, negative control group; L-cit, positive control group; FR, group of rats supplemented with 100% flesh watermelon juice; RR, group of rats supplemented with 100% rind watermelon juice.
Plasma ammonia measurement
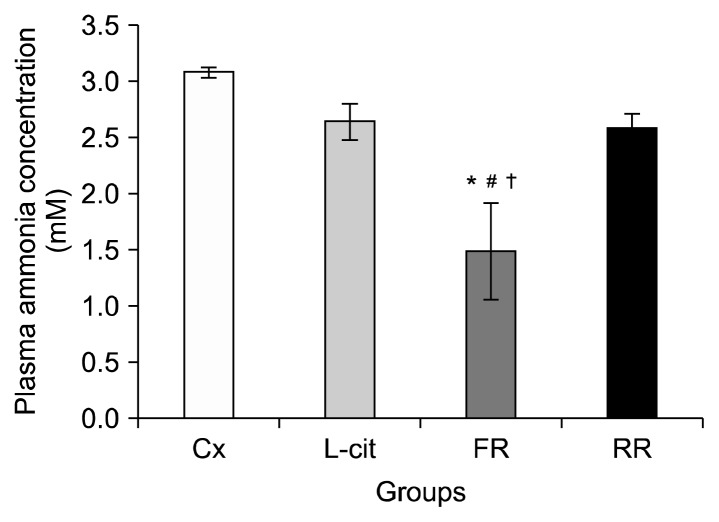
The plasma ammonia concentrations for rats after completing the swimming exercise are presented in Fig. 4. Plasma ammonia concentration were significantly lower in the FR group (1.49±0.43 mM) compared to Cx (3.08 ±0.05 mM), L-cit (2.64±0.16 mM) and RR (2.58±0.13 mM) groups (P<0.05). However, no significant difference was observed in plasma ammonia measurements for rats in the RR group compared to Cx and L-cit groups.
Fig. 4.
Plasma ammonia concentration in groups of rats supplemented with 100% flesh watermelon juice, 100% rind watermelon juice, L-citrulline, and control. The bar chart shows plasma ammonia concentration (mM) in Cx, L-cit, FR, and RR. Values were expressed as mean±SEM (n=6). Significant difference compared to *Cx, #L-cit, and †RR (P <0.05). Cx, negative control group; L-cit, positive control group; FR, group of rats supplemented with 100% flesh watermelon juice; RR, group of rats supplemented with 100% rind watermelon juice.
NO production
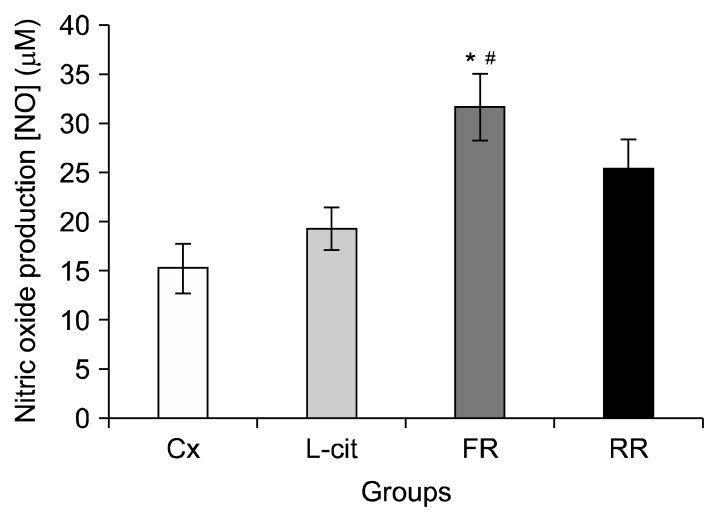
The plasma NO concentrations are presented in Fig. 5. A significant increase in plasma nitric oxide concentration were observed after swimming exercise in the FR group (31.69±3.36 μM) compared to Cx (15.28±2.52 μM) and L-cit (19.28±2.14 μM) groups (P<0.05). However, no significant differences were observed for rats in the RR group (25.42±2.99 μM) compared to Cx and L-cit groups.
Fig. 5.
Plasma nitric oxide (NO) concentration in groups of rats supplemented with flesh watermelon juice, rind watermelon juice, L-citrulline, and control. The bar chart shows NO (μM) in Cx, L-cit, FR, and FY. Values were expressed as mean±SEM (n=6). Significant difference compared to *Cx and #L-cit (P < 0.05). Cx, negative control group; L-cit, positive control group; FR, group of rats supplemented with 100% flesh watermelon juice; RR, group of rats supplemented with 100% rind watermelon juice.
DISCUSSION
The present study was designed to investigate whether nutritional intervention of 100% flesh watermelon juice and 100% rind watermelon juice may improves swimming performance in rats. The main findings of this study was that supplementation of rats with 100% flesh watermelon juice for 14 days may attenuate lactate and ammonia production through increasing NO production leading to prolongation swimming time prior to exhaustion. Whilst, 100% rind watermelon juice decreased plasma lactate concentration and increased swimming time until exhaustion, however no significant differences were observed in other parameters. This study suggests that supplementation of rats with 100% flesh watermelon juice for 14 days may provide similar benefits as supplementation with L-citrulline (19), and could represent an effective method of nutritional intervention for improving endurance swimming performances.
The body weight of rats increased in the experimental groups following supplementation for 14 days ad libitum, as shown in Table 1. Rats supplemented with 100% flesh watermelon juice demonstrated significantly higher changes in body weight compared to the L-cit group. Consumption of watermelon juice rich with fructose may result in lowered energy usage during exercise performance (20). Whilst rats supplemented with L-citrulline exhibited the lowest body weight changes that is in agreement with Kudo et al. (21) reported longer supplementation of 0.5 g/kg body weight/d of L-citrulline in water solution significantly lowered the body weight of high-fat diet Sprague-Dawley rats. The findings from this study suggest that appetite suppression from L-citrulline supplementation may be the main mechanism of body weight reduction in rats.
In the current study, swimming time until exhaustion was chosen as a marker for swimming performance. Time until exhaustion also known as time-to-failure, is defined as the duration of time for maintaining a work rate (power or intensity) before the given work rate cannot be maintained (22). The result presented in Fig. 2 demonstrates that rats supplemented with 100% flesh watermelon juice and 100% rind watermelon juice were able to swim for significantly longer amounts of time until exhaustion compared to Cx group. This finding is consistent with a previous study which stated that long-term supplementation of watermelon juice, a high-fructose fruit juice, may provide a long-term carbohydrate supply to support the energy demands, allowing longer durations of exercise to be sustained (20). Ours results are in contrast to a previous study by Cutrufello et al. (11), which demonstrated no significant change in time until exhaustion following acute supplementation of 710 mL of 100% red watermelon juice (containing ~1 g citrulline) compared to 7.5% sucrose drink containing L-citrulline (6 g) supplementation and a 7.5% sucrose placebo drink (517±104 s, 525±93 s, and 506±93 s, respectively). Recent study by Bailey et al. (7) did not find any significant differences between groups during a severe-intensity ramp incremental cycle exercise test; however, the authors demonstrated longer time until exhaustion following 16 days supplementation of red watermelon juice (providing 3.4 g of L-citrulline per day, 300 mL/d concentrate) with an average time of 550±143 s compared to 539±108 s for placebo and 478±80 s for controls. The significant results observed in the present study compared to previous studies may be due to the duration of the supplementation period, experimental subjects used, and design of the exercise protocol. Supplementation of rats with 100% flesh watermelon juice and 100% rind watermelon juice for 14 days may be effective in improving endurance in swimming exercise.
Plasma lactate concentration provides a useful indicator for endurance swimming performance. Lactate is as main indicator of exhaustion occurring during exercise, and is caused by increased amounts of H+ ions due to accumulation of lactic acid (23). The present result demonstrates that supplementation of rats with 100% flesh watermelon juice and 100% rind watermelon juice for 14 days significantly suppresses plasma lactate concentration after swimming exercise (Fig. 3). This may be because watermelon juices are able to maintain the presence of necessary bioactive nutrients, such as L-citrulline, which are directly involved in suppressing phosphofructokinase (PFK) activation and in mediating the activities of enzymes in the glycolytic system thus inhibiting resynthesis of adenosine triphosphate (ATP) (19). The watermelon juices may therefore be able to prevent excess hydrogen ion concentration built up by balancing the rate of lactate production and removal of metabolic waste (23). This is consistent with Martínez-Sánchez et al. (24) finding that demonstrated watermelon juice supplementation prior to exercise maintains the level of lactate production similar to the basal level. In another study, a significantly lower lactate concentration was observed in the group supplemented with watermelon juice enriched with L-citrulline (3.45 g per 500 mL) immediately after exercise, and this was maintained for 24 to 72 h (1). This may be attributed similar to L-citrulline ingestion, by increasing production of oxidative ATP during exercise and phosphocreatine recovery rate after exercise, which may lead to larger contribution of oxidative ATP synthesis to energy production (19,25). In the current study, 100% flesh watermelon juice and 100% rind watermelon juice supplementation for 14 days marked an improvement from previous studies by Tarazona-Díaz et al. (10) and Bailey et al. (7) that showed no significant differences in lactate concentration were observed after exercise following acute supplementation with watermelon juice.
Consistent with reductions in lactate, significant declines in plasma ammonia concentrations were observed in the FR group compared to Cx, L-cit, and RR groups (Fig. 4). Gosker and Schols (26) suggested that ammonia concentrations are parallelly correlated with lactate concentrations during exercise that consistent with our results. Present result may indicate that supplementation with 100% flesh watermelon juice for 14 days may increase ammonia removal through detoxification of nitrogen (ammonia) in the urea cycle which supported by Martínez-Sánchez et al. (1) that described similar finding. During physical exercise, ammonia is produced rapidly as a product of generated ATP conversion to adenosine monophosphate through deamination process. Accumulation of ammonia generates higher amounts of PFK, which causes reductions in intracellular pH and inhibits pyruvate oxidation to acetyl CoA. This subsequently decreases ATP production in the tricarboxylic acid cycle, leading to muscle exhaustion due to an increase in anaerobic glycolysis (19,27). By buffering ammonia through the urea cycle, supplementation with 100% flesh watermelon juice may be effective in decreasing cellular pH and promoting elimination of ammonia in the form of urea.
NO is a potential modulator of blood flow and endurance during exercise. NO may lead to smooth muscle relaxation during exercise, causing vasodilation and increasing blood perfusion to promote greater removal of metabolites such as lactate and ammonia (7,28,29). In the current study, a plasma NO concentration in the FR group was significantly increased after swimming compared to Cx group (Fig. 5). The plasma NO concentration in FR was increased 2-fold compared to Cx, which strongly suggesting that supplementation with 100% flesh watermelon juice for 14 days attributes to increased NO production. These results are consistent with those reported by Wu et al. (30) which showed that supplementation with watermelon pomace juice containing 0.2% L-citrulline plus L-arginine for 4 weeks increases NO concentration in the serum of Zucker diabetic fatty rats. Similarly, a study by Bailey et al. (7) reported that chronic supplementation with watermelon juice containing 3.4 g/0.42 g of L-citrulline/L-arginine positively elevates plasma NO levels, suggesting improvement in nitric oxide synthesis. The NO produced from the endothelial cell following supplementation with 100% watermelon juice diffuses rapidly into smooth muscle cells where it activates the enzyme guanylate cyclase to converts guanosine triphosphate to cyclic guanosine monophosphate (cGMP) (23). cGMP promotes activation of a calcium pump to induce smooth muscle relaxation and vasodilation, which allows greater distribution of nutrients and oxygen in muscle during exercise (29). Vasodilation subsequently leads greater elimination of metabolites (23). This study therefore suggests that supplementation with 100% flesh watermelon juice for 14 days may effectively generate NO to lead to vasodilatation which in turn facilitates removal of lactate and ammonia for prolonged endurance in swimming performance.
In current study, we did not find any significant differences between the L-cit and Cx groups in terms of swimming endurance, despite observing lower plasma lactate and ammonia concentrations and increased NO production (Fig. 3~5). The results may be due to difference in the supplementation techniques used. A previous study by Takeda et al. (19) reported that trained mice supplemented with 250 mg/kg body weight L-citrulline by oral gavage for 7 days had significantly lower lactate and ammonia levels after swimming exercise compared to the non-supplemented group. Supplementation of L-citrulline ad libitum for 14 days in present study may not achieve the positive effects as demonstrated by Takeda et al. (19). This suggests that differences in supplementation techniques may have difference effectiveness in improving swimming performance. In future studies, a variety of supplementation techniques may be used.
The positive findings were observed in rats supplemented with 100% flesh watermelon juice for 14 days in improving swimming performance compared to supplementation with 100% rind watermelon juice or L-citrulline solution. This may be explained due to the fact that 100% flesh watermelon juice contained high content of major phytonutrients including carotenoids (lycopene and β-carotene) despite 100% rind watermelon juice having markedly higher L-citrulline content than watermelon flesh. It may be possible that these results may not only be dependent on supplementation with either L-citrulline or juice containing a high L-citrulline content, but also required a whole synergistic interaction between the amino acids, carotenoids, micronutrients, and intracellular antioxidant enzymes (31). At this scenario, it is worth to note that the synergistic actions of the bioactive constituents in 100% flesh watermelon juice are working together in improving swimming performance.
The present study demonstrates that supplementation with 100% flesh watermelon juice for 14 days may improve swimming performance in rats. 100% flesh watermelon juice may therefore represent an effective nutritional and natural alternative method for prevention of health-related diseases. The positive findings presented in this study suggest that further investigation in humans may be warranted to explore if similar effects occur across species.
ACKNOWLEDGEMENTS
The study was funded by the Ministry of Higher Education, Malaysia through Fundamental Research Grant Scheme (FRGS Grant No: FRGS/1/2016/WAB01/UITM/02/3) and Institute of Research Management & Innovation (IRMI) of Universiti Teknologi MARA (UiTM), Shah Alam, Malaysia (600-RMI/FRGS 5/3 (0123/2016). Grateful acknowledgment to Integrative Pharmacogenomics Institute (iPROMISE), Atta-ur-Rahman Institute (AuRIns), Centre of Medical Laboratory Technology, and Centre of Postgraduate Study, Faculty of Health Sciences, UiTM Puncak Alam for providing facilities.
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
REFERENCES
- 1.Martínez-Sánchez A, Ramos-Campo DJ, Fernández-Lobato B, Rubio-Arias JA, Alacid F, Aguayo E. Biochemical, physiological, and performance response of a functional watermelon juice enriched in L-citrulline during a half-marathon race. Food Nutr Res. 2017;61:1330098. doi: 10.1080/16546628.2017.1330098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Curis E, Nicolis I, Moinard C, Osowska S, Zerrouk N, Bénazeth S, Cynober L. Almost all about citrulline in mammals. Amino Ac. 2005;29:177–205. doi: 10.1007/s00726-005-0235-4. [DOI] [PubMed] [Google Scholar]
- 3.Pérez-Guisado J, Jakeman PM. Citrulline malate enhances athletic anaerobic performance and relieves muscle soreness. J Strength Cond Res. 2010;24:1215–1222. doi: 10.1519/JSC.0b013e3181cb28e0. [DOI] [PubMed] [Google Scholar]
- 4.Meneguello MO, Mendonça JR, Lancha AH, Jr, Costa Rosa LFBP. Effect of arginine, ornithine and citrulline supplementation upon performance and metabolism of trained rats. Cell Biochem Funct. 2003;21:85–91. doi: 10.1002/cbf.1000. [DOI] [PubMed] [Google Scholar]
- 5.Osowska S, Moinard C, Neveux N, Loï C, Cynober L. Citrulline increases arginine pools and restores nitrogen balance after massive intestinal resection. Gut. 2004;53:1781–1786. doi: 10.1136/gut.2004.042317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwedhelm E, Maas R, Freese R, Jung D, Lukacs Z, Jambrecina A, Spickler W, Schulze F, Böger RH. Pharmacokinetic and pharmacodynamic properties of oral L-citrulline and L-arginine: impact on nitric oxide metabolism. Br J Clin Pharmacol. 2008;65:51–59. doi: 10.1111/j.1365-2125.2007.02990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bailey SJ, Blackwell JR, Williams E, Vanhatalo A, Wylie LJ, Winyard PG, Jones AM. Two weeks of watermelon juice supplementation improves nitric oxide bioavailability but not endurance exercise performance in humans. Nitric Oxide. 2016;59:10–20. doi: 10.1016/j.niox.2016.06.008. [DOI] [PubMed] [Google Scholar]
- 8.Evans RW, Fernstrom JD, Thompson J, Morris SM, Jr, Kuller LH. Biochemical responses of healthy subjects during dietary supplementation with L-arginine. J Nutr Biochem. 2004;15:534–539. doi: 10.1016/j.jnutbio.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 9.Wu G, Meininger CJ. Arginine nutrition and cardiovascular function. J Nutr. 2000;130:2626–2629. doi: 10.1093/jn/130.11.2626. [DOI] [PubMed] [Google Scholar]
- 10.Tarazona-Díaz MP, Alacid F, Carrasco M, Martínez I, Aguayo E. Watermelon juice: potential functional drink for sore muscle relief in athletes. J Agric Food Chem. 2013;61:7522–7528. doi: 10.1021/jf400964r. [DOI] [PubMed] [Google Scholar]
- 11.Cutrufello PT, Gadomski SJ, Zavorsky GS. The effect of L-citrulline and watermelon juice supplementation on anaerobic and aerobic exercise performance. J Sports Sci. 2015;33:1459–1466. doi: 10.1080/02640414.2014.990495. [DOI] [PubMed] [Google Scholar]
- 12.Jayaprakasha GK, Patil BS. A metabolomics approach to identify and quantify the phytochemicals in watermelons by quantitative 1HNMR. Talanta. 2016;153:268–277. doi: 10.1016/j.talanta.2016.02.060. [DOI] [PubMed] [Google Scholar]
- 13.Figueroa A, Wong A, Jaime SJ, Gonzales JU. Influence of L-citrulline and watermelon supplementation on vascular function and exercise performance. Curr Opin Clin Nutr Metab Care. 2017;20:92–98. doi: 10.1097/MCO.0000000000000340. [DOI] [PubMed] [Google Scholar]
- 14.Naderi A, Rezaei S, Moussa A, Levers K, Earnest CP. Fruit for sport. Trends Food Sci Technol. 2018;74:85–98. doi: 10.1016/j.tifs.2018.02.013. [DOI] [Google Scholar]
- 15.Ridwan R, Abduk Razak HR, Adenan MI, Md Saad WM. Separation of L-arginine and L-citrulline in red and yellow crimson watermelon (Citrullus lanatus) juices extract using HPLC gradient mode. Malays J Anal Sci. 2018;22:785–793. [Google Scholar]
- 16.Ridwan R, Abdul Razak HR, Adenan MI, Md Saad WM. Development of isocratic RP-HPLC method for separation and quantification of L-citrulline and L-arginine in watermelons. Int J Anal Chem. 2018;2018 doi: 10.1155/2018/4798530. 4798530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rimando AM, Perkins-Veazie PM. Determination of citrulline in watermelon rind. J Chromatogr A. 2005;1078:196–200. doi: 10.1016/j.chroma.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 18.Jayaprakasha GK, Chidambara Murthy KN, Patil BS. Rapid HPLC-UV method for quantification of L-citrulline in watermelon and its potential role on smooth muscle relaxation markers. Food Chem. 2011;127:240–248. doi: 10.1016/j.foodchem.2010.12.098. [DOI] [Google Scholar]
- 19.Takeda K, Machida M, Kohara A, Omi N, Takemasa T. Effects of citrulline supplementation on fatigue and exercise performance in mice. J Nutr Sci Vitaminol. 2011;57:246–250. doi: 10.3177/jnsv.57.246. [DOI] [PubMed] [Google Scholar]
- 20.Shanely RA, Nieman DC, Perkins-Veazie P, Henson DA, Meaney MP, Knab AM, Cialdell-Kam L. Comparison of watermelon and carbohydrate beverage on exercise-induced alterations in systemic inflammation, immune dysfunction, and plasma antioxidant capacity. Nutrients. 2016;8:E518. doi: 10.3390/nu8080518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kudo M, Yoshitomi H, Momoo M, Suguro S, Yamagishi Y, Gao M. Evaluation of the effects and mechanism of L-citrulline on anti-obesity by appetite suppression in obese/diabetic KK-ay mice and high-fat diet fed SD rats. Biol Pharm Bull. 2017;40:524–530. doi: 10.1248/bpb.b16-01002. [DOI] [PubMed] [Google Scholar]
- 22.Coakley SL, Passfield L. Cycling performance is superior for time-to-exhaustion versus time-trial in endurance laboratory tests. J Sports Sci. 2018;36:1228–1234. doi: 10.1080/02640414.2017.1368691. [DOI] [PubMed] [Google Scholar]
- 23.dos Santos Baião D, Conte CA, Jr, Silva JT, Paschoalin VMF, Alvares TS. L-Arginine supplementation and nitric oxide production: no additional effect when associated to exercise. Food Nutr Sci. 2013;4:779–784. [Google Scholar]
- 24.Martínez-Sánchez A, Alacid F, Rubio-Arias JA, Fernández-Lobato B, Ramos-Campo DJ, Aguayo E. Consumption of watermelon juice enriched in L-citrulline and pomegranate ellagitannins enhanced metabolism during physical exercise. J Agric Food Chem. 2017;65:4395–4404. doi: 10.1021/acs.jafc.7b00586. [DOI] [PubMed] [Google Scholar]
- 25.Baker JS, McCormick MC, Robergs RA. Interaction among skeletal muscle metabolic energy systems during intense exercise. J Nutr Metab. 2010;2010 doi: 10.1155/2010/905612. 905612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gosker HR, Schols AMWJ. Fatigued muscles in COPD but no finishing line in sight. Eur Respir J. 2008;31:693–694. doi: 10.1183/09031936.00015308. [DOI] [PubMed] [Google Scholar]
- 27.Lauer T, Preik M, Rassaf T, Strauer BE, Deussen A, Feelisch M, Kelm M. Plasma nitrite rather than nitrate reflects regional endothelial nitric oxide synthase activity but lacks intrinsic vasodilator action. Proc Natl Acad Sci USA. 2001;98:12814–12819. doi: 10.1073/pnas.221381098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ijomone OM, Olaibi OK, Nwoha PU. Effects of chronic nicotine administration on body weight, food intake and nitric oxide concentration in female and male rats. Pathophysiology. 2014;21:185–190. doi: 10.1016/j.pathophys.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 29.McKinley-Barnard S, Andre T, Morita M, Willoughby DS. Combined L-citrulline and glutathione supplementation increases the concentration of markers indicative of nitric oxide synthesis. J Int Soc Sports Nutr. 2012;12:27. doi: 10.1186/s12970-015-0086-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu G, Collins JK, Perkins-Veazie P, Siddiq M, Dolan KD, Kelly KA, Heaps CL, Meininger CJ. Dietary supplementation with watermelon pomace juice enhances arginine availability and ameliorates the metabolic syndrome in Zucker diabetic fatty rats. J Nutr. 2007;137:2680–2685. doi: 10.1093/jn/137.12.2680. [DOI] [PubMed] [Google Scholar]
- 31.Mohammad MKA, Mohamed MI, Zakaria AM, Abdul Razak HR, Md Saad WM. Juice modulates oxidative damage induced by low dose x-ray in mice. BioMed Res Int. 2014;2014 doi: 10.1155/2014/512834. 512834. [DOI] [PMC free article] [PubMed] [Google Scholar]