Abstract
Objective
To compare 3-dimensional conformal radiotherapy (3D-CRT) and intensity modulated radiotherapy (IMRT) techniques on the target tissue and critical organ doses in terms of dosimetry, during treatment planning of patient’s post-mastectomy radiotherapy (PMRT) to the left chest wall.
Materials and Methods
Twenty breast cancer patients with left-sided post-mastectomy have selected for PMRT both 3D-CRT and IMRT techniques. Dosimetric calculation of dose simulation in Eclipse treatment planning system have been performed. Organs at risk with the maximum dose, minimum dose, mean dose, D95, conformity and homogeneity indexes and total monitor unit for the Planning Target Volume were compared in terms of the critical organ doses.
Results
There was no significant difference between the two treatment planning techniques in terms of maximum, minimum, mean dose and heterogeneity index (p>0.05). At low doses, the dose received at the heart was significantly lower with the 3D-CRT technique, but there was no statistically significant difference between the two techniques at the maximum and average doses in the high dose regions.
Conclusion
For PMRT to the left chest wall, IMRT significantly improves the conformity of plan and reduce the high-dose volumes of ipsilateral lung and heart compared to 3D-CRT, but 3D-CRT is superior in terms of low-dose volume.
Keywords: Left-sided mastectomy, three-dimensional conformal radiotherapy, intensity-modulated radiotherapy, dose simulation, dosimetry
Introduction
Cancer is a health problem that is becoming increasingly prevalent and is the most common cause of death following cardiovascular disease. While the most common type of cancer and the most common cause of death in the world is lung cancer, the most common type of cancer in women is breast cancer (1, 2). Surgery, chemotherapy, and radiotherapy (RT) are used for cancer treatment and new devices and treatment techniques are being developed along with developing technology (3, 4). RT has an important role in the prevention of local and regional recurrences in the curative treatment of early stage and locally advanced breast cancer. However, heart and lung toxicity due to RT can lead to long-term morbidity and mortality (5, 6). Especially in cases of left-sided breast cancer, the contribution of RT to survival can be achieved by meticulous adherence with dose limits to critical organ and coronary artery diseases (Left Anterior Descending-LAD) depending on RT. The goal in RT planning is to be able to protect the neighbouring healthy tissues in the best way while giving the best treatment dose to the target tissue. However, it is not always possible to make ideal planning suitable for the constraints imposed by normal tissues as the target volume to be treated is given in a homogeneous and conformal manner. Several RT techniques can be applied in the treatment of breast cancer (7). In two-dimensional (2D) planning, while the beam is given in one direction and reciprocally; with the three-dimensional (3D) planning system, the beam can be given in more angles by making use of the anatomized space (8). Three-dimensional conformal RT (3D-CRT) technique is used throughout the world in breast RT, owing to improvements in treatment planning technology and the development of multi-leaf collimators (9). Conformal treatment decreases normal tissue doses while increasing target volume confirmation. However, it is still limited to make plans to maintain tissues at adequate levels. The Intensity Modulated Radiotherapy (IMRT) is being used increasingly for a long time. With the IMRT technique, dose distribution and target dose can be better controlled while the plan is made by irradiating from various angles (10).
In this study, we aim to compare target tissue and critical organ doses in 3D-CRT and IMRT plans in RT patients with early-stage left-sided breast cancer, which is receiving radiotherapy on the left chest wall.
Materials and Methods
Patients groups
Twenty breast cancer patients with left-sided post-mastectomy have selected for PMRT both 3D-CRT and IMRT techniques in Eclipse treatment planning system dose calculation simulation. Informed consent was obtained from all patients before the RT procedure. After the procedure, the informed consent and approval ethics committee were not received because of the dosimetric simulation design of the study. Previously untreated post-modified radical mastectomy female breast cancer patients who are older than 18 years with histologically unilateral left-sided breast cancer diagnosed as pathologically early stage invasive breast cancer and axillary lymph node dissection without distant metastasis or second malignancy were included in this study between June 2016 to July 2017 retrospectively. Patients undergoing breast-conserving surgery were excluded. Adjuvant RT was carried out with linear accelerator (Siemens Primus, Germany) 6-MV beam for the left chest wall, including mastectomy scar.
CT imaging
3 mm cross-sectional thickness computed tomography (CT) (Somatom, Siemens, Germany) data which were taken for planning for twenty patients who were diagnosed with left breast cancer. All patients had been treated with the both 3D-CRT and IMRT plan. All patients were immobilized while free breathing using a thermoplastic mould in supine position over a breast board fixed on the couch with both arms extended above their head onto the armrests, abducted and externally rotated. The scar sites, drain sites and breast borders were marked using lead markers. All patients also had IMRT plan. The patients whose surgery and chemotherapy were completed started taking RT within 3 weeks.
Target and risky organ drawing
After the CT of the patients included in the study were contoured, images in Digital Imaging and Communication in Medicine (DICOM) format were transferred to the Eclipse (VARIAN Medical Systems, Palo Alto, CA) treatment planning system. Planning Target Volume (PTV), Clinical Target Volume (CTV), ipsilateral lung, contralateral lung, contralateral breast, medulla spinalis, heart, and whole body were contoured with previously applied methods in the literature. The chest wall was defined as CTV and was limited to 5 mm below the skin. PTV was also limited to the chest wall. CTV, PTV, and organs at risk (OAR) were created according to the protocol of Radiation Therapy Oncology Group (RTOG) 0319 (11).
Treatment planning and dose definition
In the study, two different treatment planning techniques for the chest wall were performed using 6-MV beam photon energy for each patient through Eclipse, V8.9.08 version Varian, USA treatment planning system. Two opposites and 9 non-reciprocal areas in the 3D-CRT technique and IMRT technique were selected respectively, and the treatment plans were made. The PTV was given a total of 50Gy doses at 25 fractions (2Gy/fractions). The treatment of all patients was planned with a goal of 100% volume of PTV to be covered by 95% iso-dose line. The PTV size ranged from 463 cm3 to 1322 cm3 with an average value of 735.4 cm3.
Organs at risk with the maximum dose, minimum dose, mean dose, D95, conformity index (CI) and homogeneity index (HI) and total monitor unit (MU) in the PTV area were compared in terms of the ipsilateral lung (V5, V10, V20 and V30 and mean dose), heart (max, min, mean, D33, V25 and V10) and dosimetric parameters (max, min, mean, D5 and V5).
Doses HI and CI were calculated according to the definition proposed by the International Commission on Radiation Units and Measurements (ICRU) Report 83 (12). HI was defined as the difference between the near-maximum and near-minimum dose normalized to the median dose,
D2%, D98% and D50% is defined as dose taken 2%, 98% and 50% of total volume.
VRI: Reference iso-dose volume and TV: Target volume is defined as.
3D-CRT and IMRT planning
Two most suitable mutual tangential beam fields that best fit PTV breast volume contoured for the 3D-CRT planning were selected. Using BEAM’s Eye View (BEV) area, treatment plans were made with the highest possible dose for PTV, the lowest possible dose for the lung, counterpart breast and heart. The isocentre is determined as the centre of two mutually tangential beam fields. The optimal conformal dose distributions were tried to be obtained by using the field-in-field technique to reduce the dose at 110% and at the maximum hot dose points of the defined dose in the PTV.
Also, in the IMRT planning technique, during PTV used for 3D-CRT, to restrict the misalignment of the target volume due to positioning and breast wall movement, the planning was made with a 1.5 cm margin. Treatment plans were made by selecting the non-reciprocal 9 beam fields with the angles of 0°, 45°, 90°, 118°, 130°, 150°, 290°, 305° and 320° degrees for the left breast tissue.
Organs at risk with the maximum dose, minimum dose, mean dose, D95, CI, and HI and total MU for the (PTV) from the dose volume histograms (DVH) obtained using both treatment planning techniques were compared in terms of the ipsilateral lung (V5, V10, V20 and V30 and mean dose), heart (max, min, mean, D33, V25 and V10) and dosimetric parameters (max, min, mean, D5 and V5). Dx and Vx are defined as %x area dose of the defined volume and %x area volume of the defined dose respectively.
Statistical Analysis
Comparisons of dose-volume data between both planning techniques were made by t-test. Statistical analysis was performed with Statistical Package for the Social Sciences Windows software version 18 (IBM Corp.; Armonk, NY, USA). p value <0.05 was considered statistically significant. To determine the differences of dosimetric parameters obtained for the left chest wall, left lung, heart and the opposite breast was made using IMRT and 3D-CRT were assessed by Student’s t-test.
Results
All patients’ RT plans were designed to receive a total of 50Gy doses in 25 fractions. Comparison of the dosimetric parameters obtained for the left chest wall left lung, heart and the opposite breast was made using IMRT and 3D-CRT techniques. Table 1 presents results of dosimetric analysis and comparison of the left chest wall. There was no significant difference between the two planning techniques in terms of maximum, minimum, mean dose and heterogeneity index.
Table 1.
Dosimetric parameters obtained for Planning Target Volume left chest wall by using IMRT and 3D-CRT techniques
| Dosimetric Values | IMRT | 3D-CRT | p |
|---|---|---|---|
| Maximum Dose (Gy) | 5.579 | 5.529 | 0.51 |
| Minimum Dose (Gy) | 3.900 | 3.887 | 0.85 |
| Mean Dose (Gy) | 4.698 | 5.137 | 0.33 |
| %95 Volume | 4891 | 4717 | 0.04 |
| Conformity index | 1.31 | 1.73 | 0.03 |
| Heterogeneity index | 1.17 | 1.16 | 0.47 |
| Monitor Unit | 477 | 228 | 0.00 |
IMRT: intensity modulated radiotherapy techniques; 3D-CRT: three-dimensional conformal radiotherapy; Gy: gray
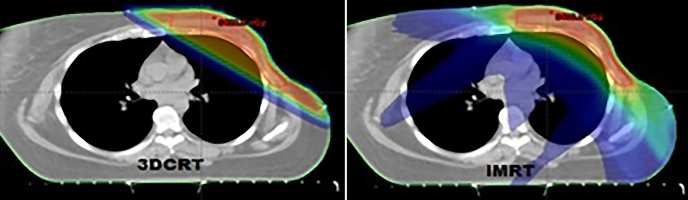
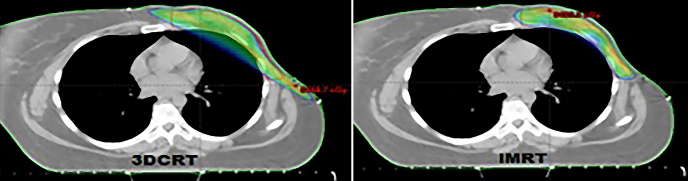
While conformity index was better in treatment plans using IMRT technique (p=0.03); 95% volume (p=0.04) and MU (p=0.00) were found to be better with 3D-CRT. V5 (5Gy and overdose volume) obtained with both techniques and 95% dose distributions of the defined dose are shown in Figure 1 and Figure 2.
Figure 1.
V5 dose distribution patterns obtained for PTV by IMRT and 3D-CRT
Figure 2.
D95 dose distribution patterns obtained for PTV by IMRT and 3D-CRT
Table 2 compares the dosimetric parameters of left lung tissue with both planning techniques. At low doses of V5 and V10, the dose of the left lung was statistically significant with the 3D-CRT technique, while doses of V20 and mean lung dose was similar, whereas higher doses (V30) yielded better results with the IMRT technique.
Table 2.
Dosimetric parameters obtained for left lung by using IMRT and 3D-CRT techniques
| Dosimetric Values | IMRT | 3D-CRT | p |
|---|---|---|---|
| V5 (cm3) | 72 | 31.6 | 0.00 |
| V10 (cm3) | 38 | 27 | 0.02 |
| V20 (cm3) | 21.1 | 23.4 | 0.38 |
| V30 (cm3) | 11.7 | 20.7 | 0.02 |
| Mean Dose (Gy) | 1.30 | 1.21 | 0.45 |
IMRT: intensity modulated radiotherapy techniques; 3D-CRT: three-dimensional conformal radiotherapy; Gy: gray; V: volume; D: dose
Similarly, the doses that the heart took were also compared and shown in Table 3. At low doses, the dose of the heart was significantly lower with the 3D-CRT technique, but there was no statistically significant difference between the two techniques at the maximum and average doses in the high dose regions.
Table 3.
Dosimetric parameters obtained for heart tissue by using IMRT and 3D-CRT techniques
| Dosimetric Values | IMRT | 3D-CRT | p |
|---|---|---|---|
| Maximum Dose (Gy) | 4.05 | 4.18 | 0.81 |
| Minimum Dose (Gy) | 1.67 | 0.56 | 0.01 |
| Mean Dose (Gy) | 7.05 | 4.94 | 0.08 |
| D33 (Gy) | 7.02 | 2.30 | 0.00 |
| V25 (cm3) | 1.8 | 6.7 | 0.08 |
| V10 (cm3) | 13.7 | 10 | 0.01 |
IMRT: intensity modulated radiotherapy techniques; 3D-CRT: three-dimensional conformal radiotherapy; Gy: gray; V: volume; D: dose
Comparing the doses of the opposite breast tissue, there were no significant differences between the minimum and maximum dose values, whereas the mean dose, V5, and D5 doses showed significant results for the 3D-CRT technique and values were shown in Table 4.
Table 4.
Dosimetric parameters obtained for opposite breast tissue by using IMRT and 3D-CRT techniques
| Dosimetric Values | IMRT | 3D-CRT | p |
|---|---|---|---|
| Maximum Dose (Gy) | 2.71 | 1.41 | 0.08 |
| Minimum Dose (Gy) | 1.01 | 0.16 | 0.12 |
| Mean Dose (Gy) | 4.00 | 0.67 | 0.05 |
| V5 (cm3) | 0.16 | 0.0 | 0.01 |
| D5 (Gy) | 9.68 | 1.46 | 0.00 |
IMRT: intensity modulated radiotherapy techniques; 3D-CRT: three-dimensional conformal radiotherapy; Gy: gray; V: volume; D: dose
Discussion and Conclusion
A number of studies have demonstrated the dosimetric benefit of IMRT compared to 3DCRT for the whole breast in early breast cancer patients (13). Many studies have reported lower doses to the ipsilateral lung, contralateral lung, contralateral breast, heart, and left anterior descending artery using IMRT technique for whole breast radiotherapy.
There are geometric differences in the breast tissue structures of patients who have been diagnosed with left-sided breast cancer and whose chest wall radiotherapy is applied; and these differences may have an impact on the resulting dose distribution (14). In general, every patient whose breast tissue or chest wall is treated as an optimal plan that protects organs at risk. However, there may be a lot of difference between the dose in the technique available at the RT centre and used for planning purposes and the doses of the exposed tissues that are at risk and are targeted based on the current patient geometry. Along with the technological possibilities that have been developed, many studies have been carried out to show the superiority of one technique over the other. In the dosimetry studies comparing SIB-3D-CRT with SIB-IMRT technique for breast cancer with breath holding technique, it is stated that compared to 3D-CRT, IMRT reduces the maximum dose in the target volume and decreases the dose of organs under risk (15, 16).
The difficulties encountered in 3D-CRT are heterogeneous dose distribution, hot or cold spots due to irregular breast contour, normal tissue protection and difficult of establishing dose consistency and dose homogeneity, however, the 3D-CRT technique is superior to other techniques in low doses regarding normal tissue, integral dose, and duration of treatment (17).
Although dose-adjusted RT with dose escalation, increased homogeneity in PTV, increased dose conformity, protection of critical organs such as the heart, lung, significant reductions in early and late effects, and successful cosmetic results demonstrate that this technique is advantageous; patient positioning, and protection of this position due to increased number of bundles and segments in treatment planning, increase in organ contingency and preparation duration, long planning period, extra QA requirement, higher dose of organs such as opposite breast, counter lung increased patient treatment times are seen as disadvantages of this technique (18).
In this study which aims to fairly compare the 3D-CRT and IMRT technique for plan and dose delivery for breast cancer patients treated after modified radical mastectomy operation, the target volumes were homogenized, and dose distribution was adjusted to the desired limits. However, with the IMRT technique, the volume of the high-dose area of the opposite breast left lung, and heart is lower than that of the 3D-CRT technique. Considering the age of the patient and long-life expectancy, secondary cancer risks that may arise in breast cancer patients are very important in RT applications. In this case, risk assessment of complications that can occur with doses of intact tissues in young patients, especially those with secondary cancer risk, should be done well with both planning techniques. The dose taken by the other breast in breast RT is important for the risk of secondary cancer. Stovall et al. (19), Berrington et al. (20) and have shown that RT does not play a direct role in secondary cancer formation in a study in which they investigated breast cancer risk after breast RT in 2107 patients. However, it was concluded that this risk has occurred in young ladies in the long run; and it was stated that women under 40 years of age are at risk of secondary cancer at a breast dose of over 1Gy. Since the goal in RT applications is to maximize protection of the healthy tissues and organs around the target while giving the highest dose to the target tissue, the dose taken by the heart in treatment plans of patients with left breast cancer should be evaluated very well. In Rancati et al. (21) compilation, it was shown that the increase in the volume of 30Gy and 25Gy for the whole heart is the most important factor causing an increase in cardiac mortality. It has also been suggested that the volume of 25Gy of the heart (V25) in patients with breast cancer in this review should be kept below 10% concerning long-term cardiac mortality. Similarly, the dose taken by the lungs is very important in breast planning.
In the comparisons of Marks LB et al. (22) investigated the radiation dose-volume relationship in the lung. The three-dimensional dose, volume, and outcome data for lung are reviewed in detail. The rate of symptomatic pneumonitis is related to many dosimetric parameters, and there are no evident threshold «tolerance dose-volume” levels. There are strong volume and fractionation effects. In a study by Stewart et al. (23). Patients treated with 3D-CRT compared to the IMRT technique found that radiation-induced heart disease risk was especially reduced in the right-sided breast disease.
Deep-Inspiration-Breath-hold (DIBH) technique, a new technique, has dosimetric advantages to reduce excessive lung doses and pulmonary risk factors. This technique is also successfully implemented and resulted in optimally low heart radiation. But it also brings additional cost and difficulties in application to the patients (24). IMRT at DIBH is considered but normal tissue constraints are not successful with a 3D-CRT approach (25). Due to the lack of the breath hold apparatus in our radiotherapy unit, we could not use this technique. This is a limitation of our study. Further studies need to enhance DIBH techniques and to optimize patient selection.
For post-mastectomy radiotherapy to the left chest wall, IMRT significantly improves the conformity of plan and reduce the high-dose volumes of ipsilateral lung and heart compared to 3D-CRT, but 3D-CRT is superior in terms of low-dose volume. In conclusion, the choice of radiotherapy in breast cancer treatment is a very important factor in the protection of neighbouring normal structures and in the identification of associated risk. For this reason, the patient profile should be evaluated carefully and the method to be used should be decided accordingly.
Acknowledgements
The authors would like to thank Dr. Seyhan Karacavus for individual assistance.
Footnotes
Ethics Committee Approval: N/A.
Informed Consent: N/A.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - S.A.; Design - S.A.; Supervision - S.A., T.İ.; Funding - S.A.; Materials - S.A.; Data Collection and/or Processing - S.A., T.İ.; Analysis and/or Interpretation - S.A., M.A., T.İ.; Literature Review - S.A., T.İ.; Writer - S.A., T.İ.; Critical Review - T.İ., S.A.
Conflict of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.DeSantis CE, Fedewa SA, Goding SA, Kramer JL, Smith RA, Jemal A. Breast cancer statistics, 2015: Convergence of incidence rates between black and white women. CA Cancer J Clin. 2016;66:31–42. doi: 10.3322/caac.21320. [DOI] [PubMed] [Google Scholar]
- 2.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 3.Lin Y, Wang B. Dosimetric absorption of intensity-modulated radiotherapy compared with conventional radiotherapy in breast-conserving surgery. Oncol Lett. 2015;9:9–14. doi: 10.3892/ol.2014.2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu JJ, Brady LW. Decision making in radiation oncology. 1st ed. Vol. 1. Berlin: Springer; 2011. [DOI] [Google Scholar]
- 5.Taylor CW, Kirby AM. Cardiac side effects from breast cancer radiotherapy. Clin Oncol. 2015;27:621–629. doi: 10.1016/j.clon.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 6.Darby SC, Cutter DJ, Boerma M, Constine LS, Fajardo LF, Kodama K, et al. Radiation related heart disease: current knowledge and future prospects. Int J Radiat Oncol Biol Phys. 2010;76:656–665. doi: 10.1016/j.ijrobp.2009.09.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schubert LK, Gondi V, Sengbusch E, Westerly DC, Soisson ET, Paliwal BR, Mackie TR, Mehta MP, Patel RR, Tomé WA, Cannon GM. Dosimetric comparison of left-sided whole breast irradiation with 3D-CRT, forward planned IMRT, inverse planned IMRT, helical tomotherapy, and topotherapy. Radiother Oncol. 2011;100:241–246. doi: 10.1016/j.radonc.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 8.Lu XQ. A three-field breast treatment technique with precise geometric matching using multi leaf collimator equipped linear accelerators. Int J Radiat Oncol Biol Phys. 2003;55:1420. doi: 10.1016/S0360-3016(02)04514-5. [DOI] [PubMed] [Google Scholar]
- 9.Rastogi K, Sharma S, Gupta S, Agarwal N, Bhaskar S, Jain S. Dosimetric comparison of IMRT versus 3DCRT for post-mastectomy chest wall irradiation. Radiat Oncol J. 2018;36:71–78. doi: 10.3857/roj.2017.00381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khan FM. The Physics of radiation therapy. 3th ed. Philadelphia, USA: Lippincott Williams & Wilkins; 2010. [Google Scholar]
- 11.White J, Tai1 A, Arthur D, Buchholz T, MacDonald S, Marks L, Pierce L, Recht A, Rabinovitch R, Taghian A, Vicini F, Woodward W, Li XA Radiation Therapy Oncology Group (RTOG) Breast cancer atlas for radiation therapy planning consensus definitions. Available from: URL: https://www.rtog.org/LinkClick.aspx?fileticket=vzJFhPaBipE=
- 12.Menzel HG. Journal of the ICRU. 1. Vol. 10. Oxford University; 2010. International Commission of Radiological Units (ICRU) Report 83. Report 83. [Google Scholar]
- 13.Vikstrom J, Hjelstuen MH, Mjaaland I, Dybvik KI. Cardiac and pulmonary dose reduction for tangentially irradiated breast cancer, utilizing deep inspiration breath hold with audio-visual guidance, without compromising target coverage. Acta Oncol. 2011;50:42–50. doi: 10.3109/0284186X.2010.512923. [DOI] [PubMed] [Google Scholar]
- 14.Fiorentino A, Ruggieri R, Giaj-Levra N, Sicignano G, Di Paola G, Naccarato S, Fersino S, Mazzola R, Tebano U, Ricchetti F, Alongi F. Three-dimensional conformal versus intensity modulated radiotherapy in breast cancer treatment: is necessary a medical reversal? Radiol Med. 2017;122:146–53. doi: 10.1007/s11547-016-0700-z. [DOI] [PubMed] [Google Scholar]
- 15.Jeba J, Isiah R, Subhashini J, Backianathan S, Thangakunam B, Christopher DJ. Radiation pneumonitis after conventional radiotherapy for breast cancer a prospective study. J Clin Diagn Res. 2015;9:XC01–XC05. doi: 10.7860/JCDR/2015/13969.6211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gokula K, Earnest A, Wong LC. Meta-analysis of incidence of early lung toxicity in 3- dimensional conformal irradiation of breast carcinomas. Radiat Oncol. 2013;8:268. doi: 10.1186/1748-717X-8-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Henson KE, McGale P, Taylor C, Darby SC. Radiation-related mortality from heart disease and lung cancer more than 20 years after radiotherapy for breast cancer. Br J Cancer. 2013;108:179–182. doi: 10.1038/bjc.2012.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao H, He M, Cheng G, Han D, Wu N, Shi D, Zhao Z, Jin J. A comparative dosimetric study of left-sided breast cancer after breast-conserving surgery treated with VMAT and IMRT. Radiat Oncol. 2015;10:231. doi: 10.1186/s13014-015-0531-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stovall M, Smith SA, Langholz BM, Boice JD, Jr, Shore RE, Andersson M, Buchholz TA, Capanu M, Bernstein L, Lynch CF, Malone KE, Anton-Culver H, Haile RW, Rosenstein BS, Reiner AS, Thomas DC, Bernstein JL. Dose to the Contralateral Breast from Radiotherapy and Risk of Second Primary Breast Cancer in the Wecare Study. Int J Radiat Oncol Biol Phys. 2008;72:1021–1030. doi: 10.1016/j.ijrobp.2008.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berrington de Gonzalez A, Curtis RE, Gilbert E, Smith SA, Stovall M, Ron E. Second solid cancers after radiotherapy for breast cancer in SEER cancer registries. Br J Cancer. 2010;102:220–226. doi: 10.1038/sj.bjc.6605435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rancati T, Wennberg B, Lind P, Svane G, Gagliardi G. Early clinical and radiological pulmonary complications following breast cancer radiation therapy: NTCP fit with four different models. Radiother Oncol. 2007;82:308–316. doi: 10.1016/j.radonc.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 22.Marks LB, Bentzen SM, Deasy JO, Kong FM, Bradley JD, Vogelius IS, El Naqa I, Hubbs JL, Lebesque JV, Timmerman RD, Martel MK, Jackson A. Radiation dose-volume effects in the lung. Int J Radiat Oncol Biol Phys. 2010;76:70–76. doi: 10.1016/j.ijrobp.2009.06.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stewart JR, Gajardo LF, Gillette SM, Constine LS. Radiation injury to the heart. Int J Radiat Oncol Biol Phys. 1995;31:1205–1212. doi: 10.1016/0360-3016(94)00656-6. [DOI] [PubMed] [Google Scholar]
- 24.Rice L, Goldsmith C, Green MM, Cleator S, Price PM. An effective deep-inspiration breath-hold radiotherapy technique for left-breast cancer: impact of post-mastectomy treatment, nodal coverage, and dose schedule on organs at risk. Breast Cancer (Dove Med Press) 2017;9:437–446. doi: 10.2147/BCTT.S130090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bergom C, Currey A, Desai N, Tai A, Strauss JB. Deep Inspiration Breath Hold: Techniques and Advantages for Cardiac Sparing During Breast Cancer Irradiation. Front Oncol. 2018;8:87. doi: 10.3389/fonc.2018.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]