Abstract
Objective
Magnetic resonance imaging (MRI) and ultrasonography (US) are commonly used in the pre-surgery determination of tumor size and the follow-up of breast cancer patients treated with neoadjuvant chemotherapy (NAC). The aim of this study was to compare the efficiency of preoperative MRI and US in tumor size evaluation of patients with breast cancer after NAC to guide clinicians on the appropriate treatment plan.
Materials and Methods
The study included a total of 75 patients who had undergone radiological follow-up, surgical treatment and pathological examination in our hospital between 2013 and 2016. Of these, 28 patients were followed-up with MRI and 47 with US. The dimension evaluations in pathology examination and on both MRI and US were based on the longest dimension of the tumor.
Results
There was no statistically significant difference between the tumor size measured pathologically and the size measured preoperatively on MRI (p=0.379). The tumor size measured on US before surgery was significantly smaller than the size measured in pathology (p=0.004). MRI did not overestimate by more than 10 mm in any patient, whereas US overestimated in 4 patients (8.6%). The correlation coefficient of MRI was higher than that of US (0.927 and 0.687, respectively).
Conclusion
MRI is superior to US in preoperative tumor size evaluation of patients receiving NAC.
Keywords: Breast cancer, neoadjuvant chemotherapy, magnetic resonance imaging, ultrasonography
Introduction
Breast cancer is the most common cancer in women and approximately 10%–15% of cases are locally advanced at the time of diagnosis. Neoadjuvant chemotherapy is given before local advanced breast cancer surgery. The aim of this treatment is to reduce the size of the tumor before surgery and thus to make the patient fit for surgery. Neoadjuvant chemotherapy is the current standard treatment for locally advanced breast cancer (1). Studies have shown that neoadjuvant chemotherapy provides a longer life span, prolongs disease-free survival and reduces recurrence risk (2).
In patients receiving neoadjuvant chemotherapy, the tumor size is monitored radiologically prior to surgical treatment to determine the efficacy of the treatment, whether the patient is eligible for surgery, and the appropriate surgical technique to be applied (3). Ultrasonography (US) and magnetic resonance imaging (MRI) are the most commonly used radiological imaging methods for this purpose (4). While the advantages of US are that it is easy to deal with and cheap, it is user-dependent and artefacts especially arising in calcific lesions are disadvantages. MRI has the advantages of high soft tissue resolution and the ability to identify a contrast pattern, but it has the disadvantages of high cost and difficulty of availability (5).
The aim of this study was to compare the efficacy of MRI and US, which are commonly used in the pre-surgery determination of tumor size and the follow-up of breast cancer patients treated with neoadjuvant chemotherapy and to guide clinicians on the appropriate treatment plan.
Material and Methods
Ethics
Approval for this retrospective study was granted by the Hacettepe University School of Medicine Local Ethics Committee and all procedures were conducted in accordance with the Declaration of Helsinki (2000). Informed consent was waived because of the retrospective nature of the study.
Patients
A retrospective review was made of patients who underwent neoadjuvant chemotherapy for breast cancer at our hospital between 2013 and 2016, and who had undergone radiological follow-up, surgical treatment and pathological examination in our hospital. A total of 75 patients were included in the study; all the patients were female. Of the patients, 68 (90.6%) were invasive ductal and 7 (9.4%) were mixed invasive ductal-lobular cancer. All patients received doxorubicin, cyclophosphamide and paclitaxel as chemotherapy regimens. All patients had a partial response to chemotherapy. Complete responders were excluded from the study. The mean time between the first chemotherapy and preoperative imaging was 173.7 days (±5.2). The mean age of the patients was 50 years (±10.27). Of the total 75 patients, 28 were followed-up with MRI and 47 with US. The MRI, US and pathological dimension evaluations were based on the longest dimension of the tumor.
Imaging technique and image analysis
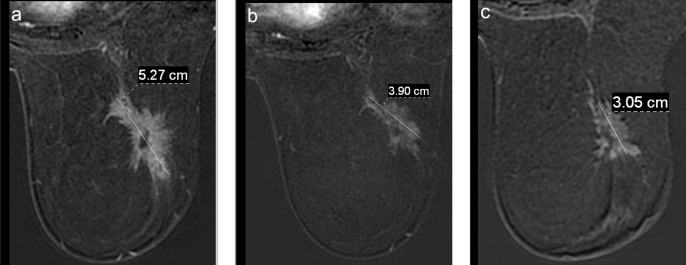
The MRI examinations were performed on a 1.5-Tesla (Signa HD, GE Medical Systems, USA) MRI scanner using a four-channel phased array breast coil. The dynamic breast examination was performed before and after intravenous contrast material injection (Gadovist, Bayer Schering Pharma AG, Germany) through the antecubital vein with a dose of 0.1 mmol/kg using a power injector (Medrad, Bayer HealthCare, Netherlands). Axial T1-weighted non-contrast MR images and the following 5 post-contrast dynamic sequences were obtained at intervals of 90 seconds. Tumor size was measured and recorded from the first post-contrast subtraction images (Figure 1).
Figure 1. a–c.
Initial (a), after first cure chemotherapy (b) and preoperative (c) axial postcontrast subtraction images of a patient followed up with MRI showing a decrease in tumor size in this process
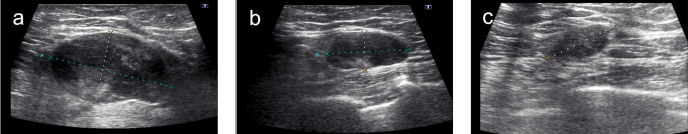
Ultrasonography measurements were made on grayscale images using a 12 MHz probe by Toshiba Aplio 400 device (Toshiba Medical Systems Corporation, Japan). The US images were obtained in both the sagittal and transverse planes. Three measurements were obtained from the tumor in the sagittal, transverse, and anteroposterior planes. The longest dimension was recorded (Figure 2).
Figure 2. a–c.
Images of a patient followed up with US: initial (a), after chemotherapy (b) and before surgery show tumor shrinkage in this process (c)
Both MR and US measurements were made by a radiologist with 15 years of experience in breast radiology. The reviewer was blinded to the physical examination findings, laboratory results, and radiology reports.
Surgical pathology reports were reviewed to determine the longest pathological tumor size. The pathological tumor stage of the primary tumor was recorded according to the American Cancer Committee (AJCC) classification system. The performance of the two imaging modalities was analyzed in respect of the correct staging of the tumors by comparing the determined tumor stage to the pathological tumor stage.
Statistical Analysis
The Statistical Package for Social Sciences version 20.0 (IBM Corp.; Armonk, NY, USA) was used for statistical analysis. Descriptive statistics were given as median (minimum–maximum) and mean±standard deviation. Categorical variables were stated as frequencies and percentages. The Wilcoxon signed rank test was used to compare the data that did not conform to normal distribution according to the normality evaluation with the Kolmogorov-Smirnov and Shapiro-Wilk tests. Spearman and Pearson correlation analyses were used to evaluate the relationship between the longest dimension measured by MR and US and the longest dimension measured in pathology according to normal distribution conformity. A value of p<0.05 was accepted as statistically significant.
Results
There was <10 mm difference in 27 patients (57.4%) in the comparison of the tumor size measured with US before surgery and the size measured in pathology. US underestimated tumor size by >10 mm in 16 (34%) patients and overestimated by >10 mm in 4 patients (8.6%). In the comparison of the tumor size measured by MRI before surgery and the size measured by pathology, there was <10 mm difference in 23 patients (82.1%). MRI underestimated tumor size by >10 mm in 5 (%17.9) patients.
There was no statistically significant difference between the tumor size measured in pathology and the size measured before surgery by MRI (p=0.379). When the same comparison was made for US, it was found that the tumor size measured by US before surgery was statistically significantly smaller than the size measured in pathology (p=0.004).
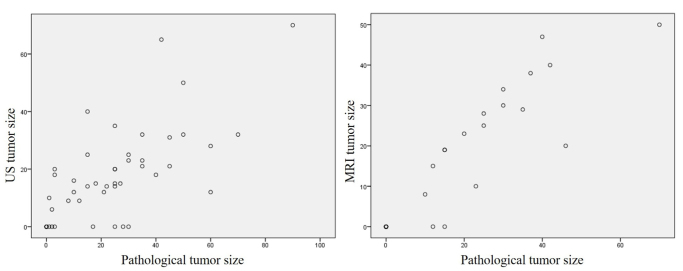
There was a statistically significant relationship between the tumor size measured by US before surgery and pathology size (p<0.001; correlation coefficient: 0.687). This relationship was stronger in the MR measurements than in US (p<0.001; correlation coefficient: 0.927) (Figure 3).
Figure 3.
The difference between MRI, US and pathological tumor size (mm) plotted for pathological tumor size for neoadjuvant chemotherapy and tumor size
According to the AJCC classification, 18 patients measured with US were at a lower T stage compared to the pathology measurements, 26 patients were at the correct stage (accuracy 55.3%) and 3 patients were at a more advanced stage. The MRI measurements showed 5 patients at a lower stage compared to pathology, 22 patients at the correct stage (accuracy 78.5%) and 1 patient at a more advanced stage (Table 1).
Table 1.
Comparison of tumor size measured before surgery by US and MRI with tumor size measured in pathology based on AJCC stage
| Pathological AJCC Stage | Total | ||||||
|---|---|---|---|---|---|---|---|
| T0 | T1 | T2 | T3 | ||||
| US | |||||||
| AJCC Stage | T0 | n | 5 | 4 | 3 | 0 | 12 |
| % | 41.7 | 33.3 | 25.0 | 0.0 | 100.0 | ||
| T1 | n | 0 | 10 | 8 | 1 | 19 | |
| % | 0.0 | 52.6 | 42.1 | 5.3 | 100.0 | ||
| T2 | n | 0 | 2 | 10 | 2 | 14 | |
| % | 0.0 | 14.3 | 71.4 | 14.3 | 100.0 | ||
| T3 | n | 0 | 0 | 1 | 1 | 2 | |
| % | 0.0 | 0.0 | 50.0 | 50.0 | 100.0 | ||
| Total | n | 5 | 16 | 22 | 4 | 47 | |
| % | 10.6 | 34.0 | 46.8 | 8.5 | 100.0 | ||
| MRI | |||||||
| AJCC Stage | T0 | n | 10 | 2 | 0 | 0 | 12 |
| % | 83.3 | 16.7 | 0.0 | 0.0 | 100.0 | ||
| T1 | n | 0 | 4 | 2 | 0 | 6 | |
| % | 0.0 | 66.7 | 33.3 | 0.0 | 100.0 | ||
| T2 | n | 0 | 1 | 8 | 1 | 10 | |
| % | 0.0 | 10.0 | 80.0 | 10.0 | 100.0 | ||
| Total | n | 10 | 7 | 10 | 1 | 28 | |
| % | 35.7 | 25.0 | 35.7 | 3.6 | 100.0 | ||
AJCC: American Cancer Committee; US: Ultrasonography; MRI: Magnetic resonance imaging
Discussion and Conclusion
The main findings of the current study showed that MRI is more sensitive than US in assessing preoperative tumor size in patients with breast cancer receiving neoadjuvant chemotherapy. No statistically significant difference was determined between the tumor size measured by MRI and the pathological dimension. However, the tumor size measured by US before surgery was statistically significantly smaller than the size measured in pathology (p=0.004). In addition, the correlation coefficient of MRI according to the pathology was found to be higher than that of US.
It is known from previous studies that the specificity of breast MRI for breast cancer is not as high as its sensitivity. Thus, additional investigations including repeat MRI and biopsies may be required (6). However, MRI is much better in assessing disease extent, investigating satellite nodules and screening for other cancer foci either in the affected or in the contralateral breast, although it overestimates tumor size (7, 8). In previous studies, tumor size measurement with US has been found to be more accurate than MRI and it has been determined that MRI overestimates tumor size (5, 9–13). Behjatnia et al. (12) and Leddy et al. (11) found that MRI overestimated tumor size in 70% and 68.4% of their patients, respectively. In a study by Leddy et al. (11), when the accuracy of the pathological T stages of patients was compared with modalities using AJCC criteria, it was reported that US (86%) evaluated the T stage more accurately than MRI (77.2%). According to Mennella et al. (14), the main reason for discordance between MRI and pathological dimension is ductal carcinoma in situ (DCIS) histology. The non-mass-like enhancement of DCIS in MRI may be the reason for the overestimation of the size of the invasive tumor on MRI. However, there were no patients with a pathological diagnosis of residual DCIS in our study in which only partial responders after neoadjuvant chemotherapy (NAC) where included.
However, a patient group receiving NAC is quite different from a group without NAC. Similar to the results of the current study, previous studies have shown that MRI is more sensitive than US in assessing preoperative tumor size in patients with breast cancer receiving NAC (15). Segara et al. (16) reported that MRI underestimated tumor size by >10 mm in 11% of patients, whereas it was 22% with USG. There was no statistically significant difference between the size measured by MRI and US and the pathological dimension in the same study. Bhattacharyya et al. (17) compared the correlation coefficients of MRI and US with pathology, and MRI was found to be superior to US. Similar to the study of Segara et al. (16) and Bhattacharyya et al. (17), a difference of 10 mm between pathological dimension and imaging was evaluated in our study. In the current study, MRI underestimated the tumor size by >10 mm in 17.9% of patients whereas it was 34% in US. Furthermore, MRI was found to be superior to US in determining the T stage. The accuracy of MRI to determine the T stage was found to be 78.5%, whereas the accuracy of US was found to be 55.3%. The reason for the superiority of MRI may be related to tumor-infiltrating macrophages (TILs), which are associated with the immune response in breast cancer. In a recent study by Salgado et al. (18), TILs were found to be a prognostic factor in breast cancer. Furthermore, in a MRI study, the authors showed that tumors with high TIL levels tend to represent round shape, circumscribe margin, homogenous enhancement and lack of multifocality (19). We think that these factors are associated with an accurate measurement of residual tumor size.
Contrast-enhanced ultrasonography (CEUS) is also used in the follow-up of patients receiving neoadjuvant chemotherapy (20). In the study performed by Corcioni et al. (21), CEUS was found to have the same sensitivity as MRI in the follow-up of neoadjuvant chemotherapy. In a more recent study by Lee et al. (22), CEUS was found to be as effective as MRI in both the complete pathological response and the noncomplete pathological response group.
In the meta-analysis performed by Marinovich et al. (23), the modalities used in the follow-up of neoadjuvant chemotherapy were compared. In this study, US was underestimated and MRI was overestimated the tumor size after NAC. However, in the same study, it was emphasized that modality selection should be made on a patient-based basis. In addition, in the currently published “RESPONDER” study (24), it was found that 3D US showed a good correlation with MRI.
Previous studies have shown that MRI overestimates the size of the tumor in patients without NAC (11, 12, 14). In the current study, MRI did not overestimate >10 mm in any patient. Similarly, Partridge et al. (25) detected an overestimation on MRI of only 0.9 mm. This could be explained by changes in tumor vascularity in response to chemotherapy. Cytotoxic agents can influence the dynamics of contrast uptake and therefore, the size of the tumor (25). Another reason could be the disappearance of the component of DCIS after NAC, as in the current study there was no DCIS histology.
This study had several limitations. First, the imaging-based measurements were made by a single radiologist, and thus interobserver variability was not evaluated. Second, the nature of the study was retrospective, so it was not possible to measure the size of the same lesion separately on MRI and US and compare these with each other. Therefore, prospective studies are needed to compare the two modalities correctly. On the other hand, our study is still valuable regarding the correlation of the imaging measurements with pathology. Third, the study had a relatively small sample size. Fourth, since there were not enough patients in different histopathological subtypes, the relation of subtypes with tumor size could not be evaluated.
In conclusion, although both modalities have their own advantages and disadvantages, knowing that MRI is more effective in evaluating pre-surgical tumor size in patients with breast cancer who have neoadjuvant chemotherapy, will be a guide for choosing the right modality.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the Ethics Committee of Hacettepe University School of Medicine (GO-17/367-25).
Informed Consent: Informed consent was not received due to the retrospective nature of the study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - O.T., G.D., M.G.A., F.B.D.; Design - O.T., G.D., M.G.A., F.B.D.; Supervision - O.T., G.D., M.G.A., F.B.D.; Resources - O.T., G.D., M.G.A., F.B.D.; Materials - O.T., G.D., M.G.A., F.B.D.; Data Collection and/or Processing - O.T., G.D., M.G.A., F.B.D.; Analysis and/or Interpretation - O.T., G.D., M.G.A., F.B.D.; Literature Search - O.T., G.D., M.G.A., F.B.D.; Writing Manuscript - O.T., G.D., M.G.A., F.B.D.; Critical Review - O.T., G.D., M.G.A., F.B.D.; Other - O.T., G.D., M.G.A., F.B.D.
Conflict of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Hortobagyi GN. Multidisciplinary management of advanced primary and metastatic breast cancer. Cancer. 1994;74:416–423. doi: 10.1002/cncr.2820741329. [DOI] [PubMed] [Google Scholar]
- 2.Kong X, Moran MS, Zhang N, Haffty B, Yang Q. Meta-analysis confirms achieving pathological complete response after neoadjuvant chemotherapy predicts favourable prognosis for breast cancer patients. Eur J Cancer. 2011;47:2084–2090. doi: 10.1016/j.ejca.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 3.Del Turco MR, Ponti A, Bick U, Biganzoli L, Cserni G, Cutuli B, Decker T, Dietel M, Gentilini O, Kuehn T, Mano MP, Mantellini P, Marotti L, Poortmans P, Rank F, Roe H, Scaffidi E, van der Hage JA, Viale G, Wells C, Welnicka-Jaskiewicz M, Wengstöm Y, Cataliotti L. Quality indicators in breast cancer care. Eur J Cancer. 2010;46:2344–2356. doi: 10.1016/j.ejca.2010.06.119. [DOI] [PubMed] [Google Scholar]
- 4.Chagpar AB, Middleton LP, Sahin AA, Dempsey P, Buzdar AU, Mirza AN, Ames FC, Babiera GV, Feig BW, Hunt KK, Kuerer HM, Meric-Bernstam F, Ross MI, Singletary SE. Accuracy of physical examination, ultrasonography, and mammography in predicting residual pathologic tumor size in patients treated with neoadjuvant chemotherapy. Ann Surg. 2006;243:257–264. doi: 10.1097/01.sla.0000197714.14318.6f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berg WA, Gutierrez L, NessAiver MS, Carter WB, Bhargavan M, Lewis RS, Ioffe OB. Diagnostic accuracy of mammography, clinical examination, US, and MR imaging in preoperative assessment of breast cancer. Radiology. 2004;233:830–849. doi: 10.1148/radiol.2333031484. [DOI] [PubMed] [Google Scholar]
- 6.Mann RM, Balleyguier C, Baltzer PA, Bick U, Colin C, Cornford E, Evans A, Fallenberg E, Forrai G, Fuchsjäger MH, Gilbert FJ, Helbich TH, Heywang-Köbrunner SH, Camps-Herrero J, Kuhl CK, Martincich L, Pediconi F, Panizza P, Pina LJ, Pijnappel RM, Pinker-Domenig K, Skaane P, Sardanelli F. Breast MRI: EUSOBI recommendations for women’s information. Eur Radiol. 2015;25:3669–3678. doi: 10.1007/s00330-015-3807-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lehman CD, Gatsonis C, Kuhl CK, Hendrick RE, Pisano ED, Hanna L, Peacock S, Smazal SF, Maki DD, Julian TB, DePeri ER, Bluemke DA, Schnall MD. MRI evaluation of the contralateral breast in women with recently diagnosed breast cancer. N Engl J Med. 2007;356:1295–1303. doi: 10.1056/NEJMoa065447. [DOI] [PubMed] [Google Scholar]
- 8.Van Goethem M, Schelfout K, Kersschot E, Colpaert C, Verslegers I, Biltjes I, Tjalma WA, De Schepper A, Weyler J, Parizel PM. MR mammography is useful in the preoperative locoregional staging of breast carcinomas with extensive intraductal component. Eur J Radiol. 2007;62:273–282. doi: 10.1016/j.ejrad.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 9.Yang WT, Lam WW, Cheung H, Suen M, King WW, Metreweli C. Sonographic, magnetic resonance imaging, and mammographic assessments of preoperative size of breast cancer. J Ultrasound Med. 1997;16:791–797. doi: 10.7863/jum.1997.16.12.791. [DOI] [PubMed] [Google Scholar]
- 10.Boetes C, Mus RD, Holland R, Barentsz JO, Strijk SP, Wobbes T, Hendriks JH, Ruys SH. Breast tumors: comparative accuracy of MR imaging relative to mammography and US for demonstrating extent. Radiology. 1995;197:743–747. doi: 10.1148/radiology.197.3.7480749. [DOI] [PubMed] [Google Scholar]
- 11.Leddy R, Irshad A, Metcalfe A, Mabalam P, Abid A, Ackerman S, Lewis M. Comparative accuracy of preoperative tumor size assessment on mammography, sonography, and MRI: Is the accuracy affected by breast density or cancer subtype? J Clin Ultrasound. 2016;44:17–25. doi: 10.1002/jcu.22290. [DOI] [PubMed] [Google Scholar]
- 12.Behjatnia B, Sim J, Bassett LW, Moatamed NA, Apple SK. Does size matter? Comparison study between MRI, gross, and microscopic tumor sizes in breast cancer in lumpectomy specimens. Int J Clin Exp Pathol. 2010;3:303–309. [PMC free article] [PubMed] [Google Scholar]
- 13.Cortadellas T, Argacha P, Acosta J, Rabasa J, Peiró R, Gomez M, Rodellar L, Gomez S, Navarro-Golobart A, Sanchez-Mendez S, Martinez-Medina M, Botey M, Muoz-Ramos C, Xiberta M. Estimation of tumor size in breast cancer comparing clinical examination, mammography, ultrasound and MRI-correlation with the pathological analysis of the surgical specimen. Gland Surgery. 2017;6:330–335. doi: 10.21037/gs.2017.03.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mennella S, Garlaschi A, Paparo F, Perillo M, Celenza M, Massa T, Rollandi GA, Garlaschi G. Magnetic resonance imaging of breast cancer: factors affecting the accuracy of preoperative lesion sizing. Acta Radiol. 2015;56:260–268. doi: 10.1177/0284185114524089. [DOI] [PubMed] [Google Scholar]
- 15.Gu YL, Pan SM, Ren J, Yang ZX, Jiang GQ. Role of Magnetic Resonance Imaging in Detection of Pathologic Complete Remission in Breast Cancer Patients Treated With Neoadjuvant Chemotherapy: A Meta-analysis. Clin Breast Cancer. 2017;17:245–255. doi: 10.1016/j.clbc.2016.12.010. [DOI] [PubMed] [Google Scholar]
- 16.Segara D, Krop IE, Garber JE, Winer E, Harris L, Bellon JR, Birdwell R, Lester S, Lipsitz S, Iglehart JD, Golshan M. Does MRI predict pathologic tumor response in women with breast cancer undergoing preoperative chemotherapy? J Surg Oncol. 2007;96:474–480. doi: 10.1002/jso.20856. [DOI] [PubMed] [Google Scholar]
- 17.Bhattacharyya M, Ryan D, Carpenter R, Vinnicombe S, Gallagher CJ. Using MRI to plan breast-conserving surgery following neoadjuvant chemotherapy for early breast cancer. Br J Cancer. 2008;98:289–293. doi: 10.1038/sj.bjc.6604171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salgado R, Denkert C, Demaria S, Sirtaine N, Klauschen F, Pruneri G, Wienert S, Van den Eynden G, Baehner FL, Penault-Llorca F, Perez EA, Thompson EA, Symmans WF, Richardson AL, Brock J, Criscitiello C, Bailey H, Ignatiadis M, Floris G, Sparano J, Kos Z, Nielsen T, Rimm DL, Allison KH, Reis-Filho JS, Loibl S, Sotiriou C, Viale G, Badve S, Adams S, Willard-Gallo K, Loi S. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol. 2015;26:259–271. doi: 10.1093/annonc/mdu450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ku YJ, Kim HH, Cha JH, Shin HJ, Baek SH, Lee HJ, Gong G. Correlation Between MRI and the Level of Tumor-Infiltrating Lymphocytes in Patients With Triple-Negative Breast Cancer. AJR Am J Roentgenol. 2016;207:1146–1151. doi: 10.2214/AJR.16.16248. [DOI] [PubMed] [Google Scholar]
- 20.Cao X, Xue J, Zhao B. Potential application value of contrast-enhanced ultrasound in neoadjuvant chemotherapy of breast cancer. Ultrasound Med Biol. 2012;38:2065–2071. doi: 10.1016/j.ultrasmedbio.2012.07.027. [DOI] [PubMed] [Google Scholar]
- 21.Corcioni B, Santilli L, Quercia S, Zamagni C, Santini D, Taffurelli M, Mignani S. Contrast-enhanced US and MRI for assessing the response of breast cancer to neoadjuvant chemotherapy() J Ultrasound. 2008;11:143–150. doi: 10.1016/j.jus.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee SC, Grant E, Sheth P, Garcia AA, Desai B, Ji L, Groshen S, Hwang D, Yamashita M, Hovanessian-Larsen L. Accuracy of Contrast-Enhanced Ultrasound Compared With Magnetic Resonance Imaging in Assessing the Tumor Response After Neoadjuvant Chemotherapy for Breast Cancer. J Ultrasound Med. 2017;36:901–911. doi: 10.7863/ultra.16.05060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marinovich ML, Macaskill P, Irwig L, Sardanelli F, Mamounas E, von Minckwitz G, Guarneri V, Partridge SC, Wright FC, Choi JH, Bhattacharyya M, Martincich L, Yeh E, Londero V, Houssami N. Agreement between MRI and pathologic breast tumor size after neoadjuvant chemotherapy, and comparison with alternative tests: individual patient data meta-analysis. BMC Cancer. 2015;15:662. doi: 10.1186/s12885-015-1664-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Egdom LSE, Lagendijk M, Heijkoop EHM, Koning AHJ, van Deurzen CHM, Jager A, van Lankeren W, Koppert LB. Three-dimensional ultrasonography of the breast; An adequate replacement for MRI in neoadjuvant chemotherapy tumour response evaluation? - RESPONDER trial. Eur J Radiol. 2018;104:94–100. doi: 10.1016/j.ejrad.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 25.Partridge SC, Gibbs JE, Lu Y, Esserman LJ, Sudilovsky D, Hylton NM. Accuracy of MR imaging for revealing residual breast cancer in patients who have undergone neoadjuvant chemotherapy. AJR Am J Roentgenol. 2002;179:1193–1199. doi: 10.2214/ajr.179.5.1791193. [DOI] [PubMed] [Google Scholar]